Assessment of the Functional Quality of Extra Virgin Olive Oil: Green Extraction of Phenolic Compounds Using Ethyl Lactate
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Olive Oil Samples
2.3. Extraction
2.4. Determination of Total Phenolic Content
2.5. Determination of Radical Scavenging Activity
2.6. HPLC Phenolic Profile Analysis
2.7. Acid Hydrolysis
2.8. Statistical Analyses
3. Results and Discussion
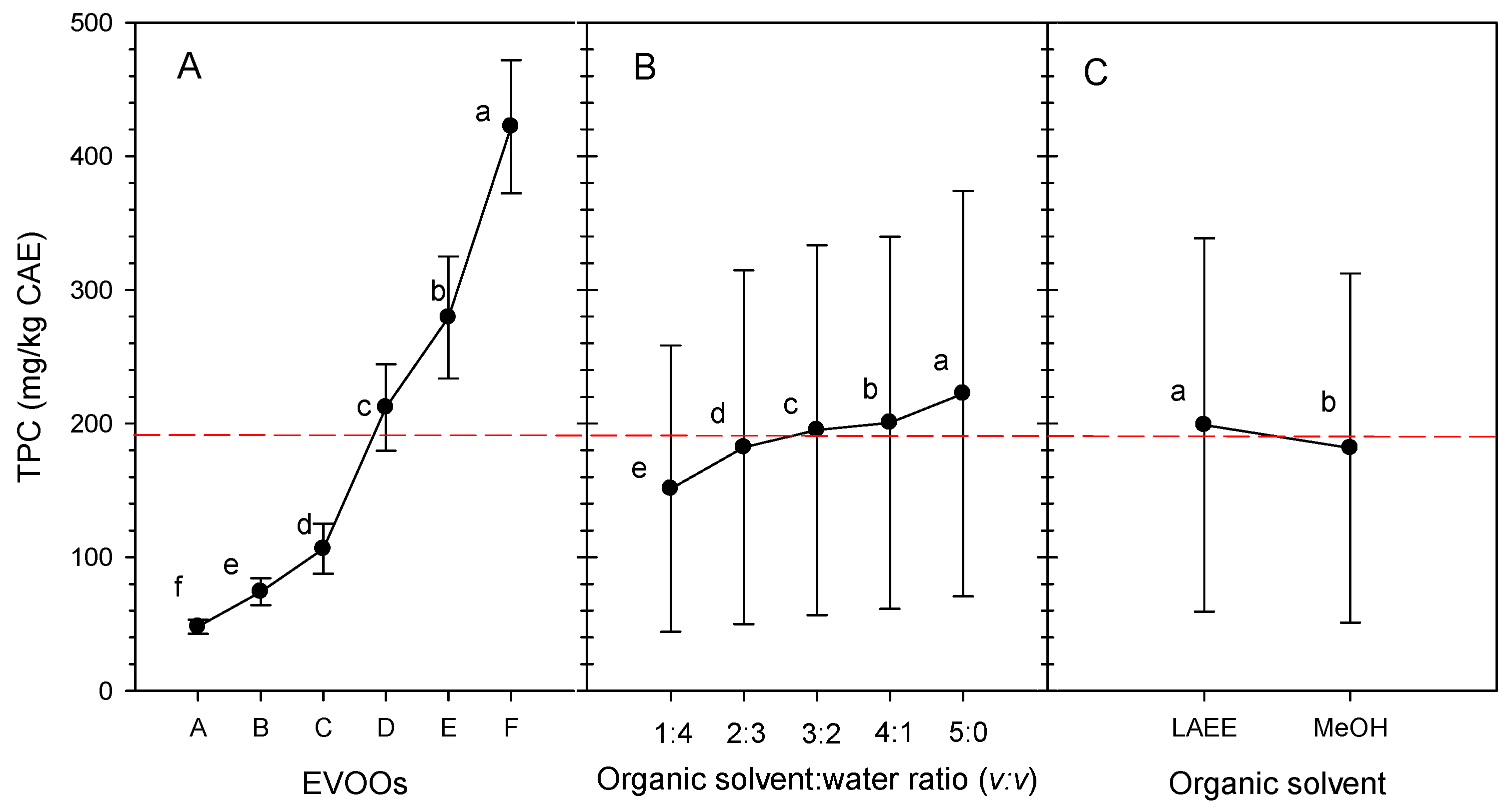
3.1. Total Phenolic Compounds
3.1.1. Effects of EVOOs
3.1.2. Effects of Organic-Solvent-to-Water Ratio
3.1.3. Effect of Organic Solvent
3.1.4. Interaction Effects
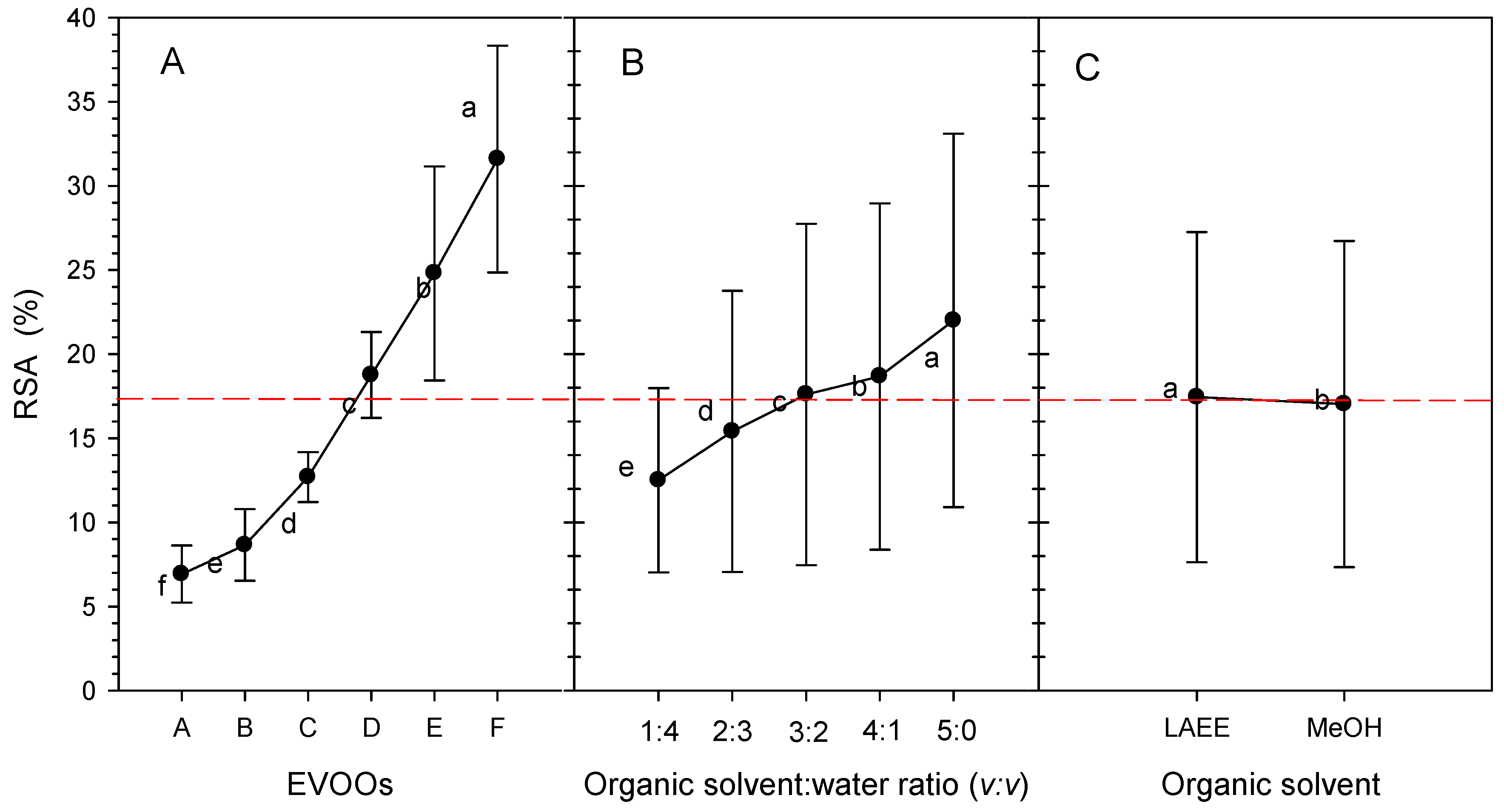
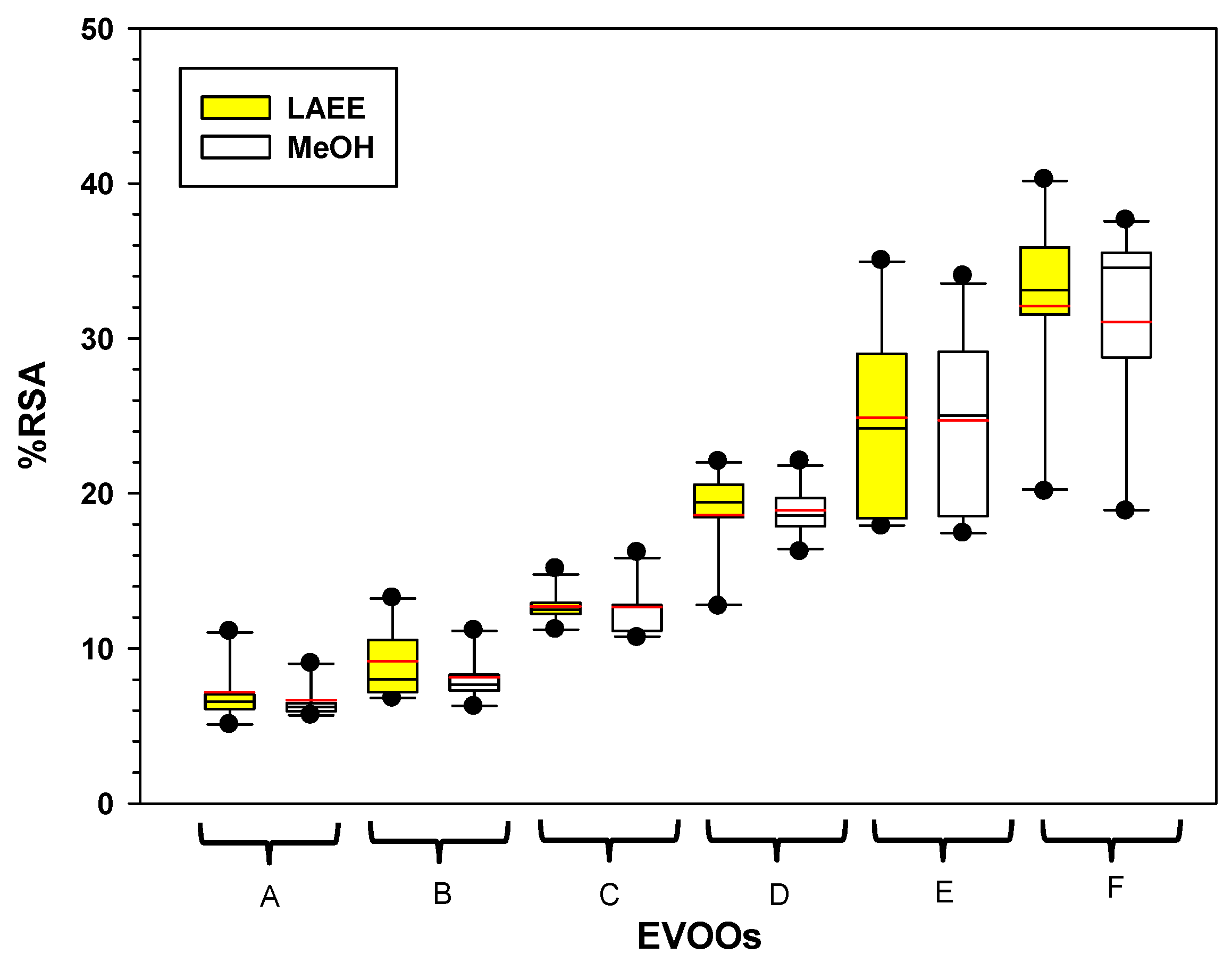
3.2. RSA Antioxidant Activity
3.2.1. Effects of EVOOS
3.2.2. Effects of the Organic-Solvent-to-Water Ratio
3.2.3. Effect of Organic Solvent
3.2.4. Interaction Effects
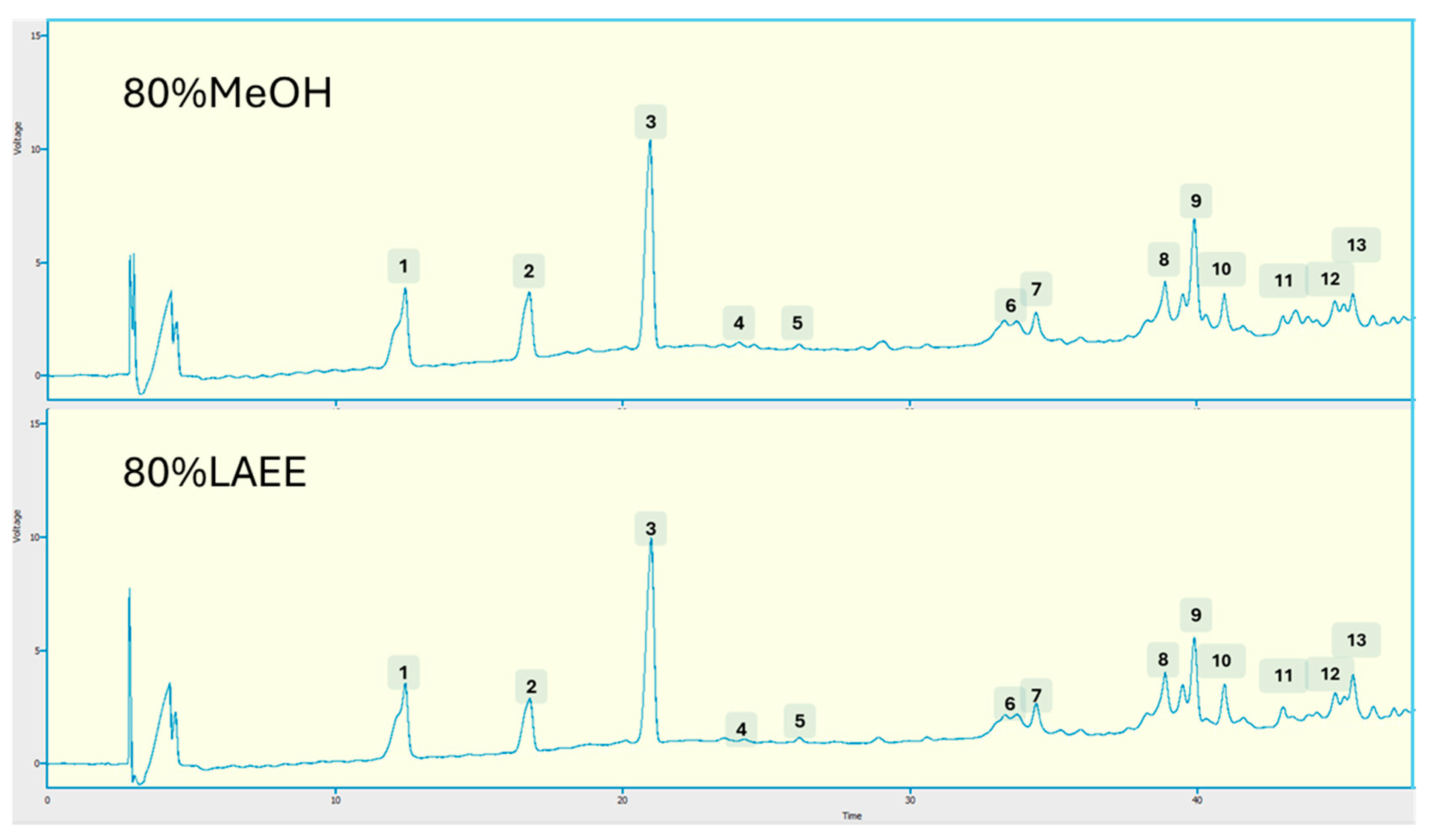
3.3. Phenolic Profiles
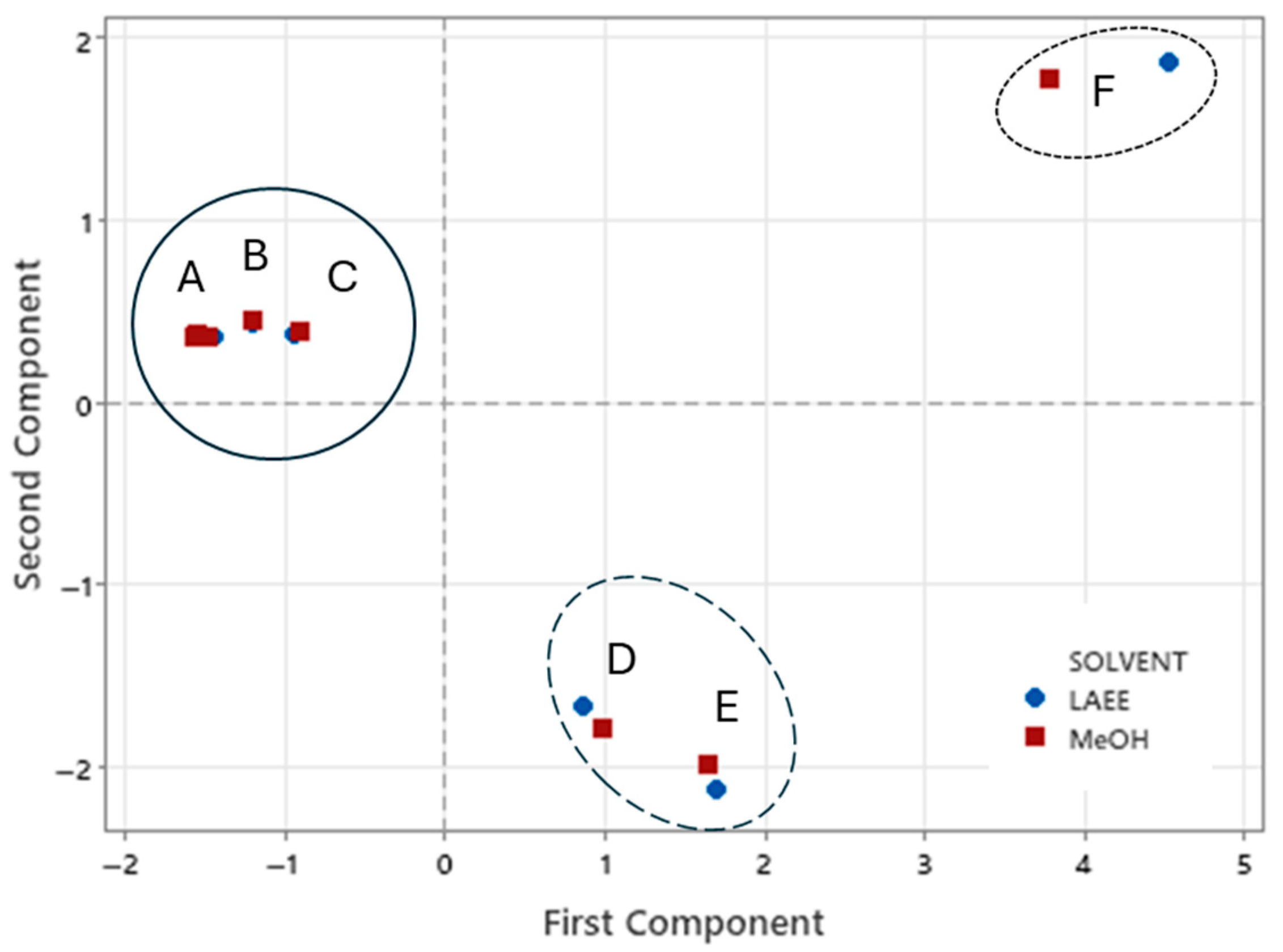
Principal Component Analysis
3.4. Acid Hydrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lores, M.; Pájaro, M.; Álvarez-Casas, M.; Domínguez, J.; García-Jares, C. Use of ethyl lactate to extract bioactive compounds from Cytisus scoparius: Comparison of pressurized liquid extraction and medium scale ambient temperature systems. Talanta 2015, 140, 134–142. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green. Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Clary, J.J.; Feron, V.J.; Van Velthuijsen, J.A. Safety assessment of lactate esters. Regul. Toxicol. Pharmacol. 1998, 27, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Opinion, S. Scientific Opinion on the safety and efficacy of primary aliphatic saturated or unsaturated alcohols/aldehydes/acids/acetals/esters with a second primary, secondary or tertiary oxygenated functional group including aliphatic lactones (chemical group 9) wh. EFSA J. 2012, 10, 2928. [Google Scholar]
- Food Additives Permitted for Direct Addition to Food for Human Consumption; Folic Acid. Final rule. Fed Regist. 2016, 81, 22176–22183. [Google Scholar]
- Tombokan, X.C.; Aguda, R.M.; Danehower, D.A.; Kilpatrick, P.K.; Carbonell, R.G. Three-component phase behavior of the sclareol-ethyl lactate-carbon dioxide system for GAS applications. J. Supercrit. Fluids 2008, 45, 146–155. [Google Scholar] [CrossRef]
- Rebelo, C.S.; Velho, P.; Macedo, E.A. eNRTL modelling and partition of phenolics in the ATPSs {ethyl lactate (1) + potassium sodium tartrate or disodium succinate (2) + water (3)} at 298.2 K and 0.1 MPa. Fluid. Phase Equilib. 2024, 582. [Google Scholar] [CrossRef]
- Kua, Y.L.; Gan, S.; Morris, A.; Ng, H.K. Ethyl lactate as a potential green solvent to extract hydrophilic (polar) and lipophilic (non-polar) phytonutrients simultaneously from fruit and vegetable by-products. Sustain. Chem. Pharm. 2016, 4, 21–31. [Google Scholar] [CrossRef]
- Kamalanathan, I.; Canal, L.; Hegarty, J.; Najdanovic-Visak, V. Partitioning of amino acids in the novel biphasic systems based on environmentally friendly ethyl lactate. Fluid. Phase Equilib. 2018, 462, 6–13. [Google Scholar] [CrossRef]
- Sepulveda, B.; Benites, D.; Albornoz, L.; Simirgiotis, M.; Castro, O.; Garcia-Beltran, O.; Areche, C. Green ultrasound-assisted extraction of lichen substances from Hypotrachyna cirrhata. Ethyl lactate, a better extracting agent than methanol toxic organic solvent? Nat. Prod. Res. 2023, 37, 159–163. [Google Scholar] [CrossRef]
- Ishida, B.K.; Chapman, M.H. Carotenoid extraction from plants using a novel, environmentally friendly solvent. J. Agric. Food Chem. 2009, 57, 1051–1059. [Google Scholar] [CrossRef]
- Vicente, G.; Paiva, A.; Fornari, T.; Najdanovic-Visak, V. Liquid-liquid equilibria for separation of tocopherol from olive oil using ethyl lactate. Chem. Eng. J. 2011, 172, 879–884. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Chemical screening of olive biophenol extracts by hyphenated liquid chromatography. Anal. Chim. Acta 2007, 603, 176–189. [Google Scholar] [CrossRef]
- Fitó, M.; Covas, M.I.; Lamuela-Raventós, R.M.; Vila, J.; Torrents, J.; De La Torre, C.; Marrugat, J. Protective effect of olive oil and its phenolic compounds against low density lipoprotein oxidation. Lipids 2000, 35, 633–638. [Google Scholar] [CrossRef]
- Saija, A.; Trombetta, D.; Tomaino, A.; Lo Cascio, R.; Princi, P.; Uccella, N.; Bonina, F.; Castelli, F. “In vitro” evaluation of the antioxidant activity and biomembrane interaction of the plant phenols oleuropein and hydroxytyrosol. Int. J. Pharm. 1998, 166, 123–133. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Galli, C. Free radical-scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C. Olive Oil Phenols and Their Potential Effects on Human Health. J. Agric. Food Chem. 1998, 46, 4292–4296. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. A simple and rapid electrophoretic method to characterize simple phenols, lignans, complex phenols, phenolic acids, and flavonoids in extra-virgin olive oil. J. Sep. Sci. 2006, 29, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Segura-Carretero, A.; Arráez-Román, D.; Menéndez, J.A.; De La Torre, A.; Fernández-Gutiérrez, A. Tentative characterization of novel phenolic compounds in extra virgin olive oils by rapid-resolution liquid chromatography coupled with mass spectrometry. J. Agric. Food Chem. 2009, 57, 11140–11147. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Macià, A.; Romero, M.P.; Motilva, M.J. Improved liquid chromatography tandem mass spectrometry method for the determination of phenolic compounds in virgin olive oil. J. Chromatogr. A. 2008, 1214, 90–99. [Google Scholar] [CrossRef] [PubMed]
- De La Torre-Carbot, K.; Jauregui, O.; Gimeno, E.; Castellote, A.I.; Lamuela-Raventós, R.M.; López-Sabater, M.C. Characterization and quantification of phenolic compounds in olive oils by solid-phase extraction, HPLC-DAD, and HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 4331–4340. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; Romero, C.; García, A.; Hidalgo, F.J.; Ruiz-Méndez, M.V. Phenolic compounds in olive oils intended for refining: Formation of 4-ethylphenol during olive paste storage. J. Agric. Food Chem. 2004, 52, 8177–8181. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D. Discrimination of olive oils and fruits into cultivars and maturity stages based on phenolic and volatile compounds. J. Agric. Food Chem. 2005, 53, 8054–8062. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and Hydrolyzable Phenolic Compounds in Virgin Olive Oil. 1. Their Extraction, Separation, and Quantitative and Semiquantitative Evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Ferhat, R.; Lekbir, A.; Ouadah, H.; Kahoul, M.A.; Khlalfa, L.; Laroui, S.; Alloui-Lombarkia, O. Effect of extraction solvent on total phenolic content, total flavonoid content, and antioxidant activities of Algerian pomace olive oil. Int. Food Res. J. 2017, 24, 2295–2303. [Google Scholar]
- Jerman Klen, T.; Vodopivec, B.M. Optimisation of olive oil phenol extraction conditions using a high-power probe ultrasonication. Food Chem. 2012, 134, 2481–2488. [Google Scholar] [CrossRef]
- Liang, F.; Li, X.; Zhang, Y.; Wu, Y.; Bai, K.; Agusti, R.; Soleimani, A.; Wang, W.; Yi, S. Recent Progress on Green New Phase Extraction and Preparation of Polyphenols in Edible Oil. Molecules 2023, 28, 8150. [Google Scholar] [CrossRef]
- Palos-Hernández, A.; González-Paramás, A.M.; Santos-Buelga, C. Latest Advances in Green Extraction of Polyphenols from Plants, Foods and Food By-Products. Molecules 2025, 30, 55. [Google Scholar] [CrossRef]
- Judge, M.D.; Aab, C. Ethyl lactate as an environmentally friendly HPLC mobile-phase modifier in the analysis of acetaminophen, caffeine, and ASA. Can. J. Chem. 2013, 91, 352–356. [Google Scholar] [CrossRef]
- Aparicio, S.; Alcalde, R. Insights into the ethyl lactate + water mixed solvent. J. Phys. Chem. B 2009, 113, 14257–14269. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, S.; Halajian, S.; Alcalde, R.; García, B.; Leal, J.M. Liquid structure of ethyl lactate, pure and water mixed, as seen by dielectric spectroscopy, solvatochromic and thermophysical studies. Chem. Phys. Lett. 2008, 454, 49–55. [Google Scholar] [CrossRef]
- Drapeau, J.; Verdier, M.; Touraud, D.; Kröckel, U.; Geier, M.; Rose, A.; Kunz, W. Effective insect repellent formulation in both surfactantless and classical microemulsions with a long-lasting protection for human beings. Chem. Biodivers. 2009, 6, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Guillen, E.; Terrones, H.; de Terrones, T.C.; Simirgiotis, M.J.; Hájek, J.; Cheel, J.; Sepulveda, B.; Areche, C. Microwave-Assisted Extraction of Secondary Metabolites Using Ethyl Lactate Green Solvent from Ambrosia arborescens: LC/ESI-MS/MS and Antioxidant Activity. Plants 2024, 13, 1213. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Delegated Regulation (EU) 2022/2104 of 29 July 2022 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as regards marketing standards for olive oil, and repealing Commission Regulation (EEC) No 2568/91. Off. J. Eur. Union 2022, L284, 1–22. [Google Scholar]
- International Olive Council. Determination of Biophenols in Olive Oils By HPLC; Document No. 29; International Olive Council: Madrid, Spain, 2017; pp. 1–8. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in Wood: Comparison of Different Estimation Methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH.) Tests. J. Am. Oil Chem. Soc. 2002, 79, 1191–1195. [Google Scholar] [CrossRef]
- Fitri, R.A.; Lestari, T.A.; Sari, Y.; Sutriyo, S.; Mun’im, A. Freeze drying of natural deep eutectic solvent (NADES) extract of green coffee bean (Coffea canephora Pierre ex A. Froehner). J. Res. Pharm. 2020, 24, 225–232. [Google Scholar] [CrossRef]
- Mulinacci, N.; Giaccherini, C.; Ieri, F.; Innocenti, M.; Romani, A.; Vincieri, F.F. Evaluation of lignans and free and linked hydroxy-tyrosol and tyrosol in extra virgin olive oil after hydrolysis processes. J. Sci. Food Agric. 2006, 86, 757–764. [Google Scholar] [CrossRef]
- Bayram, B.; Esatbeyoglu, T.; Schulze, N.; Ozcelik, B.; Frank, J.; Rimbach, G. Comprehensive Analysis of Polyphenols in 55 Extra Virgin Olive Oils by HPLC-ECD and Their Correlation with Antioxidant Activities. Plant Foods Hum. Nutr. 2012, 67, 326–336. [Google Scholar] [CrossRef]
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of phenolic compounds and their contribution to sensory properties of olive oil. Molecules 2019, 24, 2041. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Lantano, C.; Chiavaro, E.; Cerretani, L.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Classification of extra virgin olive oils according to their geographical origin using phenolic compound profiles obtained by capillary electrochromatography. Food Res. Int. 2009, 42, 1446–1452. [Google Scholar] [CrossRef]
- Nakbi, A.; Issaoui, M.; Dabbou, S.; Koubaa, N.; Echbili, A.; Hammami, M.; Attia, N. Evaluation of antioxidant activities of phenolic compounds from two extra virgin olive oils. J. Food Compos. Anal. 2010, 23, 711–715. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Machado, J.; Gomes, S.; Lopes, J.; Martins-Lopes, P.; Barros, A.I.R.N.A. Phenolic composition and antioxidant activity of monovarietal and commercial portuguese olive oils. J. Am. Oil Chem. Soc. 2014, 91, 1197–1203. [Google Scholar] [CrossRef]
- Tsolis, T.; Kyriakou, D.; Sifnaiou, E.; Thomos, D.; Glykos, D.; Tsiafoulis, C.G.; Garoufis, A. NMR Analysis of Extra Virgin Olive Oil of the Epirus Region of Greece with Emphasis on Selected Phenolic Compounds. Molecules 2024, 29, 1111. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashmi, Z.H.; Al-Lawati, H.A.; Suliman, F.E.O.; Hassanzadeh, J.; Aal-Thani, G.S.S.; Forqani, A.S.; Al Fahdi, A.R. Quantitative estimation of pharmacologically relevant phenolic compounds in olive oils harvested in Jabal Al Akhdar in Oman. Food Chem. Adv. 2025, 6, 100922. [Google Scholar] [CrossRef]
- Albdady, E.A.; Ghazaly, M.E.; Mansour, N.A.; Abdelrahman, M.; Saad Abd, E.A. Assesment of Total Polyphenolic Contents in Virgin Olive Oil Consumed. Bull. Fac. Sci. Zagazig Univ. 2023, 2023, 129–133. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.A.; Dar, M.M.; Akhter, R.; Sharma, O.C. Subcritical treatment of olive oil: Minor phenolic composition and antioxidant properties of the solvent extracts. LWT 2021, 147, 111584. [Google Scholar] [CrossRef]
- Bartella, L.; Mazzotti, F.; Talarico, I.R.; Santoro, I.; Di Donna, L. Hydroxytyrosol-fortified foods obtained by supercritical fluid extraction of olive oil. Antioxidants 2021, 10, 1619. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertolini, A.; Silva, A.M.; Rodrigues, F.; Gabbia, D.; De Martin, S.; Saba, A.; Bertini, S.; Digiacomo, M.; Macchia, M. Comparative Analysis on Polyphenolic Composition of Different Olive Mill Wastewater and Related Extra Virgin Olive Oil Extracts and Evaluation of Nutraceutical Properties by Cell-Based Studies. Foods 2024, 13, 3312. [Google Scholar] [CrossRef]
- Nenadis, N.; Mastralexi, A.; Tsimidou, M.Z.; Vichi, S.; Quintanilla-Casas, B.; Donarski, J.; Bailey-Horne, V.; Butinar, B.; Miklavčič, M.; García-González, D.L.; et al. Toward a Harmonized and Standardized Protocol for the Determination of Total Hydroxytyrosol and Tyrosol Content in Virgin Olive Oil (VOO). Extraction Solvent. Eur. J. Lipid Sci. Technol. 2018, 120, 1800099. [Google Scholar] [CrossRef]
- Tasioula-Margari, M.; Tsabolatidou, E. Extraction, separation, and identification of phenolic compounds in virgin olive oil by HPLC-DAD and HPLC-MS. Antioxidants 2015, 4, 548. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Juan, E.; Rodríguez-Romero, C.; Fernández-Bolaños, J.; Florido, M.C.; Garcia-Borrego, A. Phenolic compounds from virgin olive oil obtained by natural deep eutectic solvent (NADES): Effect of the extraction and recovery conditions. J. Food Sci. Technol. 2021, 58, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, V.M.; Squeo, G.; Pasqualone, A.; Caponio, F.; Summo, C. An easy and green tool for olive oils labelling according to the contents of hydroxytyrosol and tyrosol derivatives: Extraction with a natural deep eutectic solvent and direct spectrophotometric analysis. Food Chem. 2019, 291, 1–6. [Google Scholar] [CrossRef]
- Pizarro, M.L.; Becerra, M.; Sayago, A.; Beltrán, M.; Beltrán, R. Comparison of Different Extraction Methods to Determine Phenolic Compounds in Virgin Olive Oil. Food Anal. Methods 2013, 6, 123–132. [Google Scholar] [CrossRef]
- Minioti, K.S.; Georgiou, C.A. Comparison of different tests used in mapping the Greek virgin olive oil production for the determination of its total antioxidant capacity. Grasas Aceites 2010, 61, 45–51. [Google Scholar] [CrossRef]
- Rizvi, N.B.; Fatima, A.; Busquets, R.; Khan, M.R.; Ashraf, S.; Khan, M.S.; Oz, F. Effect of the Media in the Folin-Ciocalteu Assay for the Analysis of the Total Phenolic Content of Olive Products. Food Anal. Methods 2023, 16, 1627–1634. [Google Scholar] [CrossRef]
- López-Bascón, M.A.; Moscoso-Ruiz, I.; Quirantes-Piné, R.; del Pino-García, R.; López-Gámez, G.; Justicia-Rueda, A.; Verardo, V.; Quiles, J.L. Characterization of Phenolic Compounds in Extra Virgin Olive Oil from Granada (Spain) and Evaluation of Its Neuroprotective Action. Int. J. Mol. Sci. 2024, 25, 4878. [Google Scholar] [CrossRef] [PubMed]
- Ricciutelli, M.; Marconi, S.; Boarelli, M.C.; Caprioli, G.; Sagratini, G.; Ballini, R.; Fiorini, D. Olive oil polyphenols: A quantitative method by high-performance liquid-chromatography-diode-array detection for their determination and the assessment of the related health claim. J. Chromatogr. A 2017, 1481, 53–63. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Subject Index. Solvents Solvent Eff. Org. Chem. 2010, 677–692. [Google Scholar]
- Dolzhenko, A.V. Ethyl lactate. Green Sustainable Process for Chemical and Environmental Engineering and Science. In Plant-Derived Green Solvents: Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 171–189. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for green solvents. Green. Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Velho, P.; Oliveira, I.; Gómez, E.; MacEdo, E.A. pH Study and Partition of Riboflavin in an Ethyl Lactate-Based Aqueous Two-Phase System with Sodium Citrate. J. Chem. Eng. Data. 2022, 67, 1985–1993. [Google Scholar] [CrossRef]
- Benguennouna, N.; Benabdelmoumene, D.; Dahmouni, S.; Bengharbi, Z.; Bouzouina, M.; Qadi, W.S.M.; Dawoud, E.A.; Al-Olayan, E.; Moreno, A.; Mediani, A. Impact of diverse irrigation water sources on olive oil quality and its physicochemical, fatty acids, antioxidant, and antibacterial properties. Sci. Rep. 2025, 15, 15049. [Google Scholar] [CrossRef]
- Keceli, T.M. Influence of time of harvest on “Adana Topagi”,’Gemlik’olives, olive oil properties and oxidative stability. Acad. Edu. 2013, 1, 52–58. [Google Scholar]
- Atamyradova, N.; Özkılıç, S.Y.; Arslan, D. Blanching of olive fruits before storage at different conditions: Effects on oil yield, lipase activity and oxidation. J. Agric. Food Res. 2024, 18. [Google Scholar] [CrossRef]
- Karakuş, M.; Bayrak, A.; Çalikoǧlu, E.; Kiralan, M. Comparison of oxidation stability of virgin olive oils from different locations of Turkey. Acta Aliment. 2014, 43, 133–141. [Google Scholar] [CrossRef][Green Version]
- Gözüpek, K.; Otağ, M.R. The effects of olive leaf addition and storage conditions on the bioactive components and some quality parameters of “Patos” olive oils. J. Food Process. Preserv. 2022, 46, 16698. [Google Scholar] [CrossRef]
- Rodrigues, N.; Casal, S.; Peres, A.M.; Baptista, P.; Bento, A.; Martín, H.; Asensio-S.-Manzanera, M.C.; Pereira, J.A. Effect of olive trees density on the quality and composition of olive oil from cv. Arbequina. Sci. Hortic. 2018, 238, 222–233. [Google Scholar] [CrossRef]
- Yahia, L.B.; Baccouri, B.; Ouni, Y.; Hamdi, S. Quality, stability and radical scavenging activity of olive oils after Chétoui olives (Olea europaea L.) storage under modified atmospheres. Food Sci. Technol. Int. 2012, 18, 353–365. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, B.Y.; Kim, Y.C.; Shetty, K.; Kim, Y.C. Radical scavenging-linked antioxidant activity of ethanolic extracts of diverse types of extra virgin olive oils. J. Food Sci. 2008, 73. [Google Scholar] [CrossRef]
- Quintero-Flórez, A.; Pereira-Caro, G.; Sánchez-Quezada, C.; Moreno-Rojas, J.M.; Gaforio, J.J.; Jimenez, A.; Beltrán, G. Effect of olive cultivar on bioaccessibility and antioxidant activity of phenolic fraction of virgin olive oil. Eur. J. Nutr. 2018, 57, 1925–1946. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Marini, F.; Frezzini, M.A.; Massimi, L.; Capriotti, A.L.; Montone, C.M.; Canepari, S. Multielement Characterization and Antioxidant Activity of Italian Extra-Virgin Olive Oils. Front. Chem. 2021, 9, 769620. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Rodríguez-Pérez, C.; Gerasopoulos, D.; Segura- Carretero, A. Olive oil enrichment in phenolic compounds during malaxation in the presence of olive leaves or olive mill wastewater extracts. Eur. J. Lipid Sci. Technol. 2017, 119, 1600425. [Google Scholar] [CrossRef]
- Nenadis, N.; Moutafidou, A.; Gerasopoulos, D.; Tsimidou, M.Z. Quality characteristics of olive leaf-olive oil preparations. Eur. J. Lipid Sci. Technol. 2010, 112, 1337–1344. [Google Scholar] [CrossRef]
- Zullo, B.A.; Ciafardini, G. The olive oil oxygen radical absorbance capacity (DPPH assay) as a quality indicator. Eur. J. Lipid Sci Technol. 2008, 110, 428–434. [Google Scholar] [CrossRef]
- Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Maio, I.; Selvaggini, R.; Gobbetti, M.; Di Cagno, R. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria. Int. J. Food Microbiol. 2011, 47, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Fanali, C.; Della Posta, S.; Vilmercati, A.; Dugo, L.; Russo, M.; Petitti, T.; Mondello, L.; De Gara, L. Extraction, analysis, and antioxidant activity evaluation of phenolic compounds in different Italian extra-virgin olive oils. Molecules 2018, 8, 3249. [Google Scholar] [CrossRef] [PubMed]
- Ballus, C.A.; Meinhart, A.D.; De Souza Campos, F.A.; Godoy, H.T. Total phenolics of virgin olive oils highly correlate with the hydrogen atom transfer mechanism of antioxidant capacity. J. Am. Oil Chem. Soc. 2015, 92, 843–851. [Google Scholar] [CrossRef]
- Samaniego Sánchez, C.; Troncoso González, A.M.; García-Parrilla, M.C.; Quesada Granados, J.J.; López García de la Serrana, H.; López Martínez, M.C. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal. Chim. Acta. 2007, 593, 103–107. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; Nicolì, F.; Nutricati, E.; Vergine, M.; Miceli, A.; Blando, F.; Sabella, E.; De Bellis, L. Phenolic Profile and antioxidant activity of Italian Monovarietal extra virgin olive oils. Antioxidants 2019, 8, 161. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233. [Google Scholar] [CrossRef]
- Lammi, C.; Mulinacci, N.; Cecchi, L.; Bellumori, M.; Bollati, C.; Bartolomei, M.; Franchini, C.; Clodoveo, M.L.; Corbo, F.; Arnoldi, A. Virgin olive oil extracts reduce oxidative stress and modulate cholesterol metabolism: Comparison between oils obtained with traditional and innovative processes. Antioxidants 2020, 9, 798. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M.Z. Metrological aspects of a gas-phase DFT/B3LYP quantum-chemical approach to prioritize radical scavenging activity among a group of olive oil phenols. Explor. Foods Foodomics 2024, 2, 326–338. [Google Scholar] [CrossRef]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct measurement of oleocanthal and oleacein levels in olive oil by quantitative 1H NMR. Establishment of a new index for the characterization of extra virgin olive oils. J. Agric. Food Chem. 2012, 60, 11696–11703. [Google Scholar] [CrossRef] [PubMed]
- Sánchez de Medina, V.; Miho, H.; Melliou, E.; Magiatis, P.; Priego-Capote, F.; Luque de Castro, M.D. Quantitative method for determination of oleocanthal and oleacein in virgin olive oils by liquid chromatography–tandem mass spectrometry. Talanta 2017, 162, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Bubola, K.B.; Lukić, M.; Lukić, I.; Koprivnjak, O. Effect of different clarification methods on volatile aroma compound composition of virgin olive oil. Food Technol. Biotechnol. 2019, 57, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Naruszewicz, M.; Czerwinska, M.E.; Kiss, A.K. Oleacein. Translation from Mediterranean diet to potential antiatherosclerotic drug. Curr. Pharm. Des. 2014, 21, 1205–1212. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Ponikowska, M.-G. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Nikou, T.; Liaki, V.; Stathopoulos, P.; Sklirou, A.D.; Tsakiri, E.N.; Jakschitz, T.; Bonn, G.; Trougakos, I.P.; Halabalaki, M.; Skaltsounis, L.A. Leandron Comparison survey of EVOO polyphenols and exploration of healthy aging-promoting properties of oleocanthal and oleacein. Food Chem. Toxicol. 2019, 125, 403–412. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Tsimidou, M.Z. Antioxidants in greek virgin olive oils. Antioxidants 2014, 3, 387–413. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Del Carlo, M.; Gallina-Toschi, T.; Lercker, G.; Compagnone, D.; Fernández-Gutiérrez, A. Evaluation of the antioxidant capacity of individual phenolic compounds in virgin olive oil. J. Agric. Food Chem. 2005, 53, 8918–8925. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 1018/2013 of 23 October 2013 amending Regulation (EU) No 432/2012 establishing a list of permitted health claims made on foods other than those referring to the reduction of disease risk and to children’s development and heal. Off. J. Eur. Union 2013, 56, 43–45. [Google Scholar]
- Caporaso, N.; Savarese, M.; Paduano, A.; Guidone, G.; De Marco, E.; Sacchi, R. Nutritional quality assessment of extra virgin olive oil from the Italian retail market: Do natural antioxidants satisfy EFSA health claims? J. Food Compos. Anal. 2015, 40, 154–162. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.; Tsimidou, M.Z.; Zhang, H. Radical Scavenging Potential of Phenolic Compounds Encountered in O. europaea Products as Indicated by Calculation of Bond Dissociation Enthalpy and Ionization Potential Values. J. Agric. Food Chem. 2004, 53, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Bellumori, M.; Cecchi, L.; Innocenti, M.; Clodoveo, M.L.; Corbo, F.; Mulinacci, N. The EFSA health claim on olive oil polyphenols: Acid hydrolysis validation and total hydroxytyrosol and tyrosol determination in Italian virgin olive oils. Molecules 2019, 24, 2179. [Google Scholar] [CrossRef]
- Mastralexi, A.; Nenadis, N.; Tsimidou, M.Z. Addressing analytical requirements to support health claims on “Olive oil polyphenols” (EC Regulation 432/2012). J. Agric. Food Chem. 2014, 62, 2459–2461. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Sotiroglou, M.; Mastralexi, A.; Nenadis, N.; García-González, D.L.; Gallina Toschi, T. In House Validated UHPLC Protocol for the Determination of the Total Hydroxytyrosol and Tyrosol Content in Virgin Olive Oil Fit for the Purpose of the Health Claim Introduced by the EC Regulation 432/2012 for “Olive Oil Polyphenols”. Molecules 2019, 24, 1044. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]



| Concentration Range (mg/kg) | Determination Coefficient (r2) | Repeatability of Retention Time (%RSD) | LOD | LOQ | |
|---|---|---|---|---|---|
| Luteolin | 0.5–10 | 0.9971 | 0.100 | 0.709 | 2.149 |
| Vanillic acid | 0.05–10 | 1 | 0.129 | 0.037 | 0.111 |
| Hydroxytyrosol | 7.5–100 | 0.9997 | 0.183 | 2.154 | 6.526 |
| Tyrosol | 7.5–100 | 1 | 0.100 | 0.185 | 0.560 |
| Oleacin | 7.5–100 | 0.9961 | 0.197 | 8.422 | 25.520 |
| Oleuropein | 7.5–200 | 0.9981 | 0.089 | 35.479 | 107.512 |
| Oleocanthal | 7.5–100 | 0.9997 | 0.056 | 2.497 | 7.567 |
| Cinnamic acid | 0.1–1 | 0.9987 | 0.111 | 0.048 | 0.144 |
| Vanillin | 0.1–1 | 0.9982 | 0.064 | 0.018 | 0.056 |
| p-Coumaric acid | 0.2–10 | 0.9999 | 0.074 | 0.103 | 0.312 |
| Apigenin | 0.1–5 | 1 | 0.027 | 0.028 | 0.084 |
| Ferulic acid | 0.1–10 | 0.9996 | 0.158 | 0.019 | 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsitsipas, C.; Gerasopoulos, A.; Nenadis, N.; Gerasopoulos, D. Assessment of the Functional Quality of Extra Virgin Olive Oil: Green Extraction of Phenolic Compounds Using Ethyl Lactate. Foods 2025, 14, 3822. https://doi.org/10.3390/foods14223822
Tsitsipas C, Gerasopoulos A, Nenadis N, Gerasopoulos D. Assessment of the Functional Quality of Extra Virgin Olive Oil: Green Extraction of Phenolic Compounds Using Ethyl Lactate. Foods. 2025; 14(22):3822. https://doi.org/10.3390/foods14223822
Chicago/Turabian StyleTsitsipas, Chrysostomos, Athanasios Gerasopoulos, Nikolaos Nenadis, and Dimitrios Gerasopoulos. 2025. "Assessment of the Functional Quality of Extra Virgin Olive Oil: Green Extraction of Phenolic Compounds Using Ethyl Lactate" Foods 14, no. 22: 3822. https://doi.org/10.3390/foods14223822
APA StyleTsitsipas, C., Gerasopoulos, A., Nenadis, N., & Gerasopoulos, D. (2025). Assessment of the Functional Quality of Extra Virgin Olive Oil: Green Extraction of Phenolic Compounds Using Ethyl Lactate. Foods, 14(22), 3822. https://doi.org/10.3390/foods14223822










