Boosting Chocolate Nutrition with Sous Vide-Processed White Champignon (Agaricus bisporus) Powder: A Functional and Sustainable Approach
Abstract
1. Introduction
2. Materials and Methods
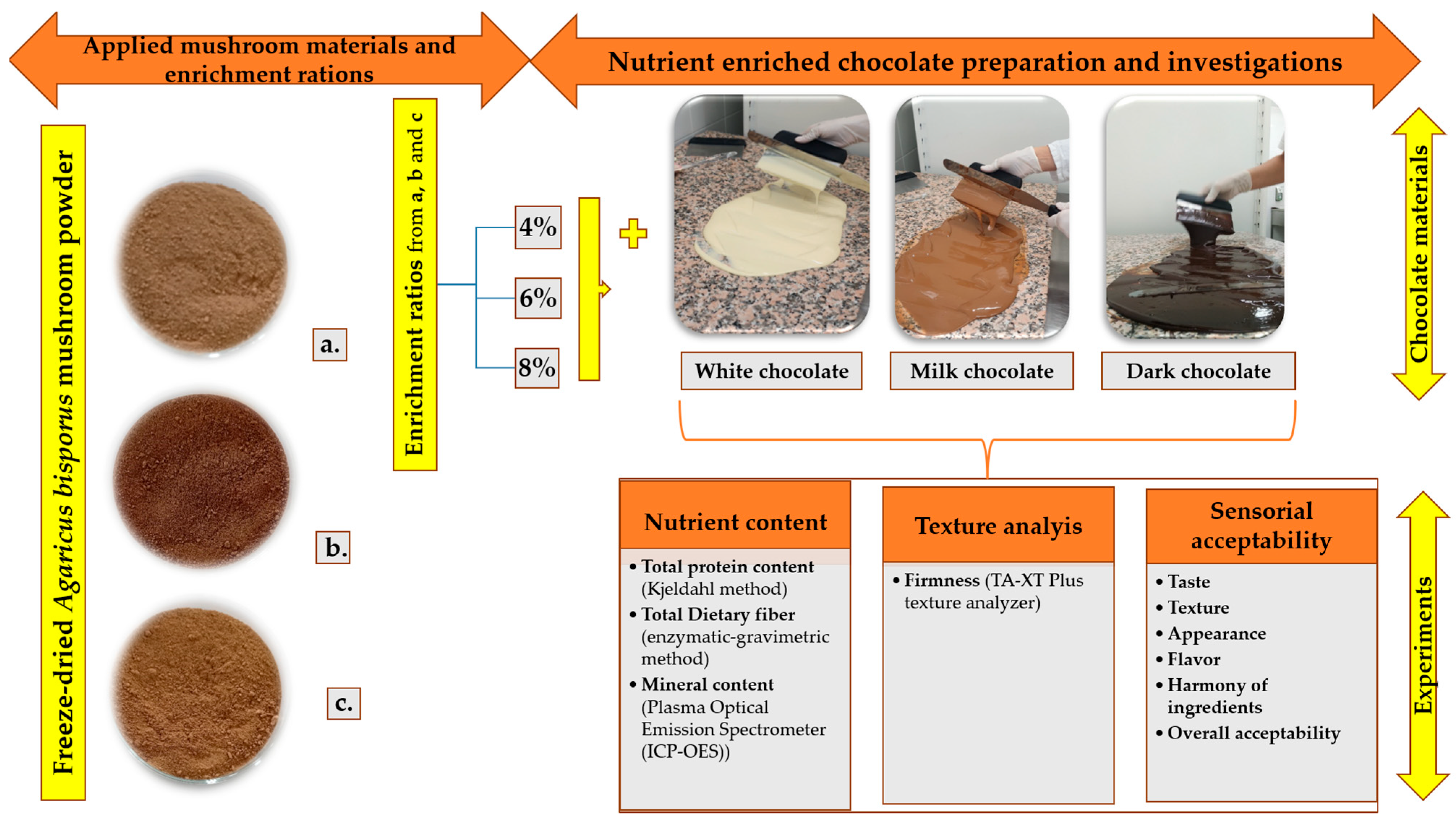
2.1. Experimental Design
2.2. Mushroom Powder Processing and Measurements
2.2.1. Sous-Vide Treatment of Fresh Mushrooms
2.2.2. Freeze-Drying of Cooked Mushroom Samples
2.2.3. Nutritional Characterization of Mushroom Powder
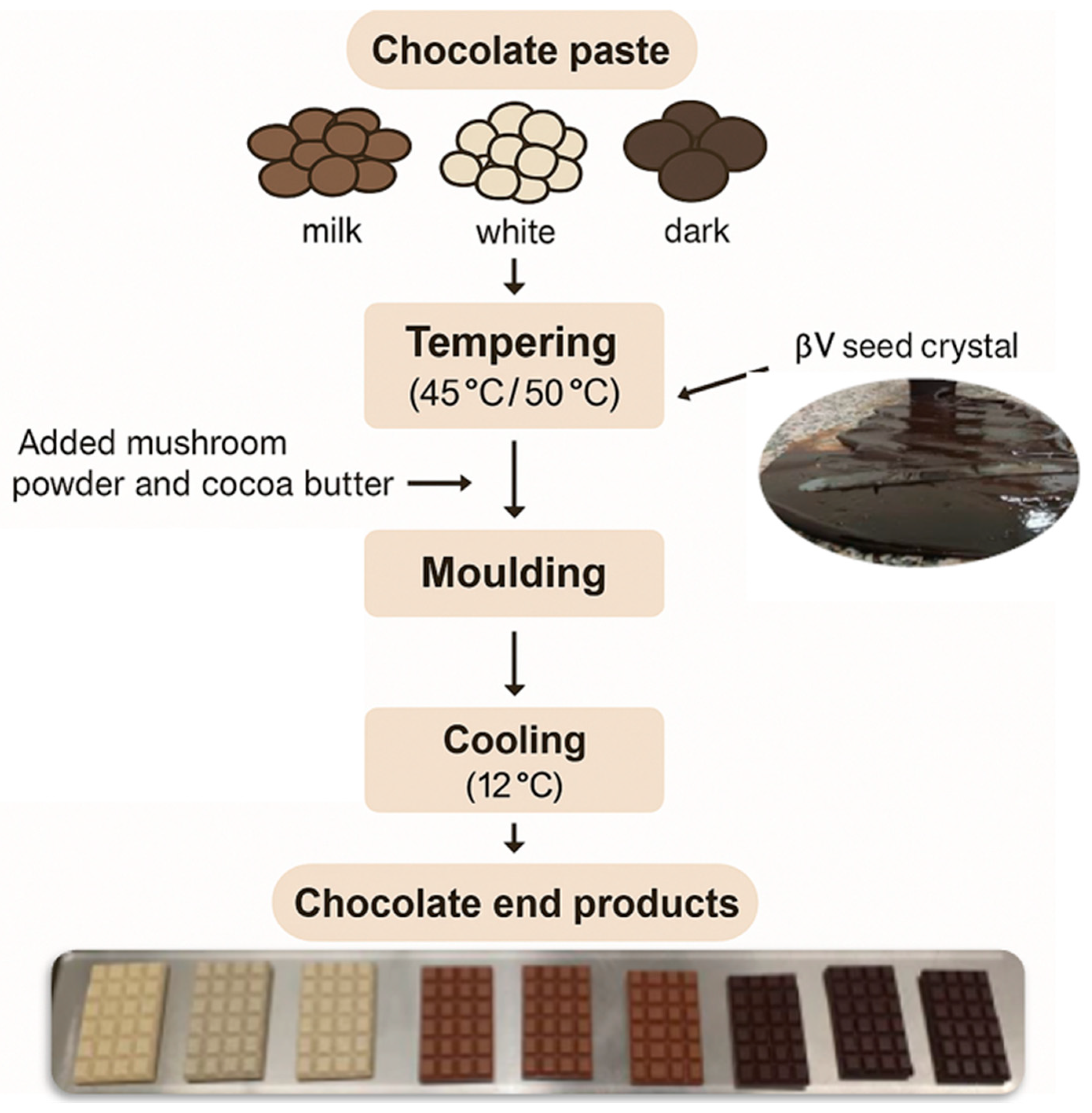
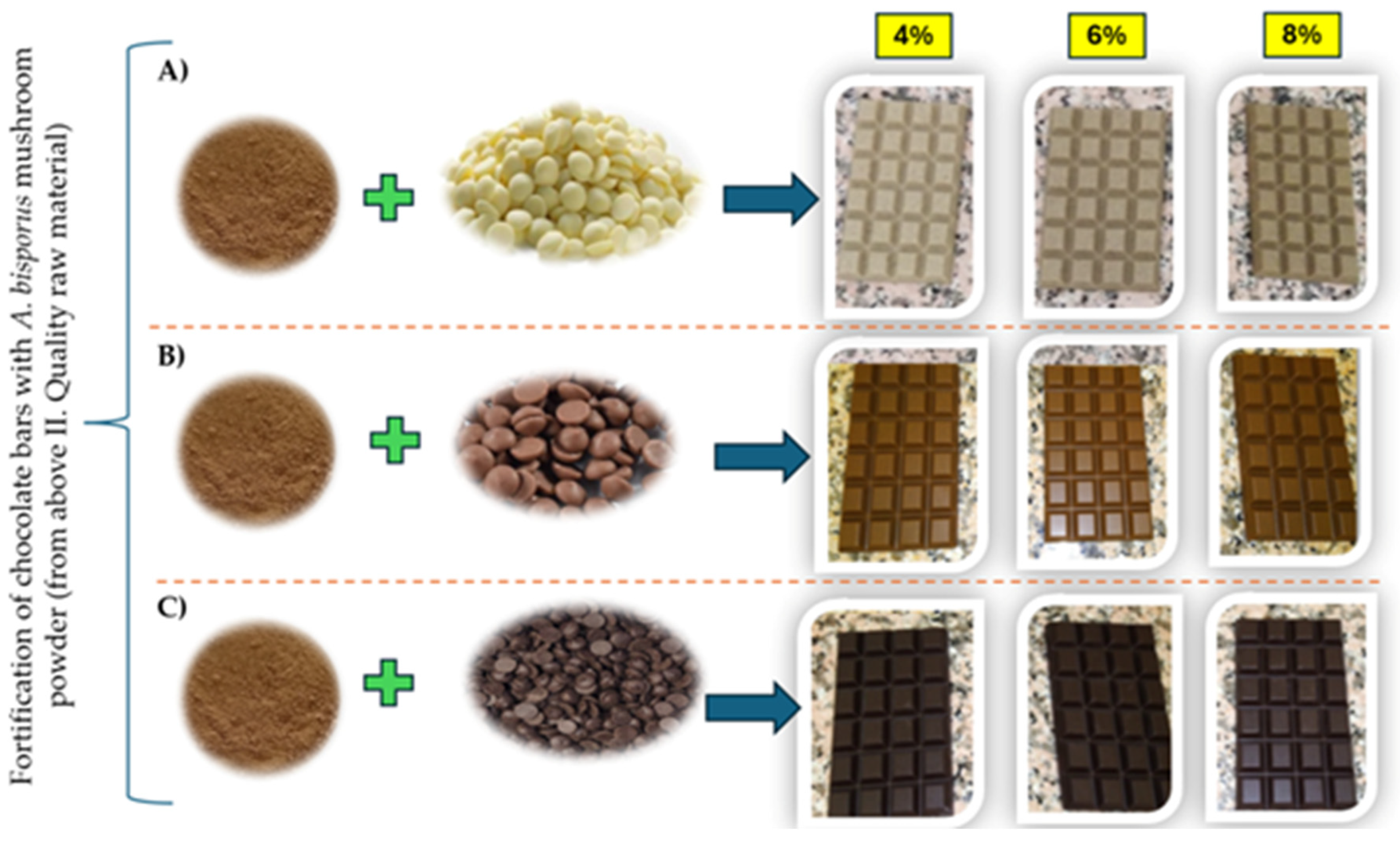
2.3. Chocolate Formulation and Sample Description
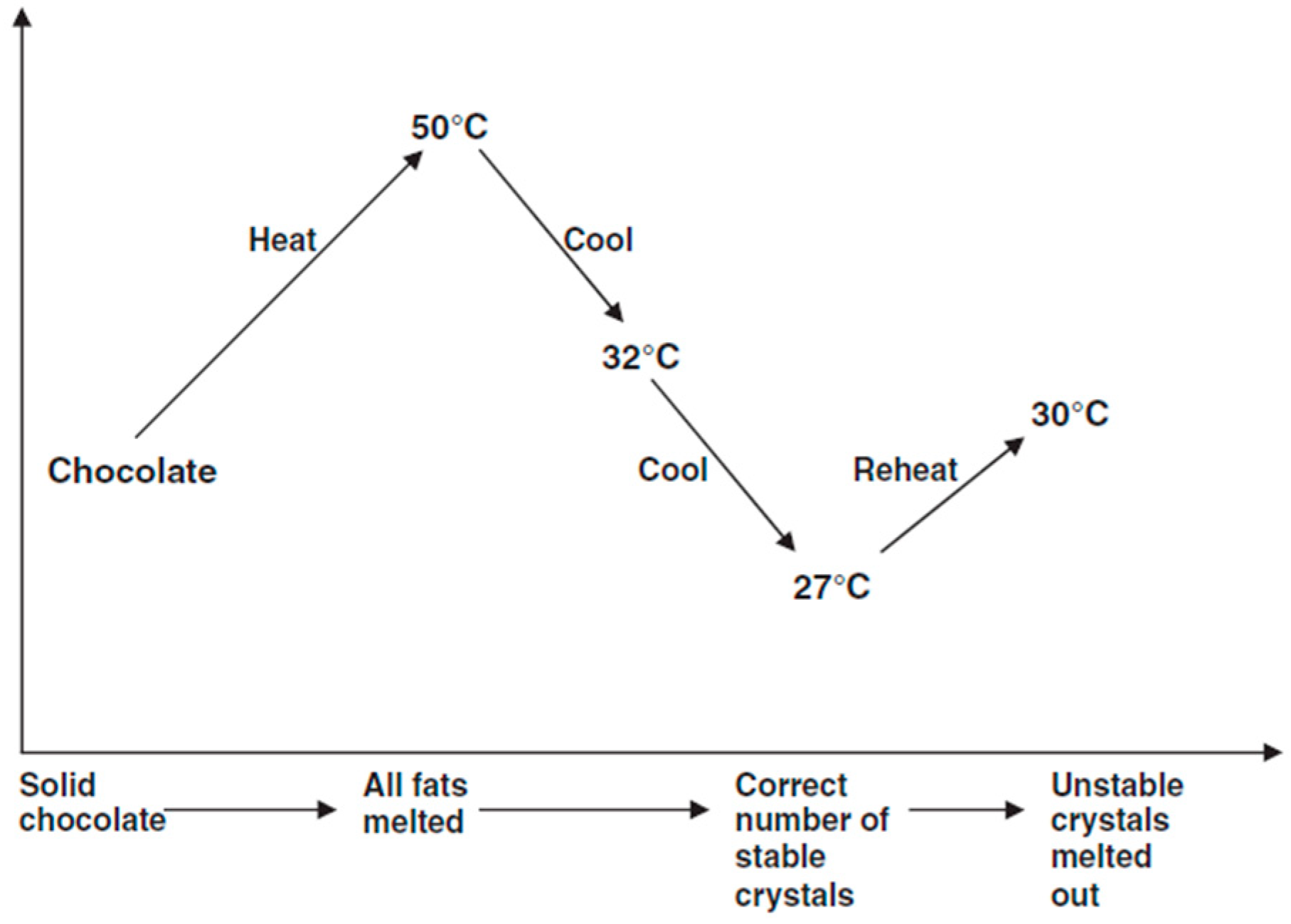
2.3.1. Chocolate Tempering Process
2.3.2. Sample Preparation for Analysis
2.4. Measurements of the Mushroom Powder Fortified Samples
2.4.1. Protein Content Analysis
2.4.2. Dietary Fiber Determination
2.4.3. Mineral Content Determination
2.4.4. Texture Profile Analysis (TPA)
2.4.5. Sensorial Acceptability
2.5. Statistical Analysis
3. Results
3.1. Total Protein Content and Dietary Fiber Content of Chocolate Samples
3.2. Mineral Content of Chocolate Samples
3.3. Results with Texture Profile
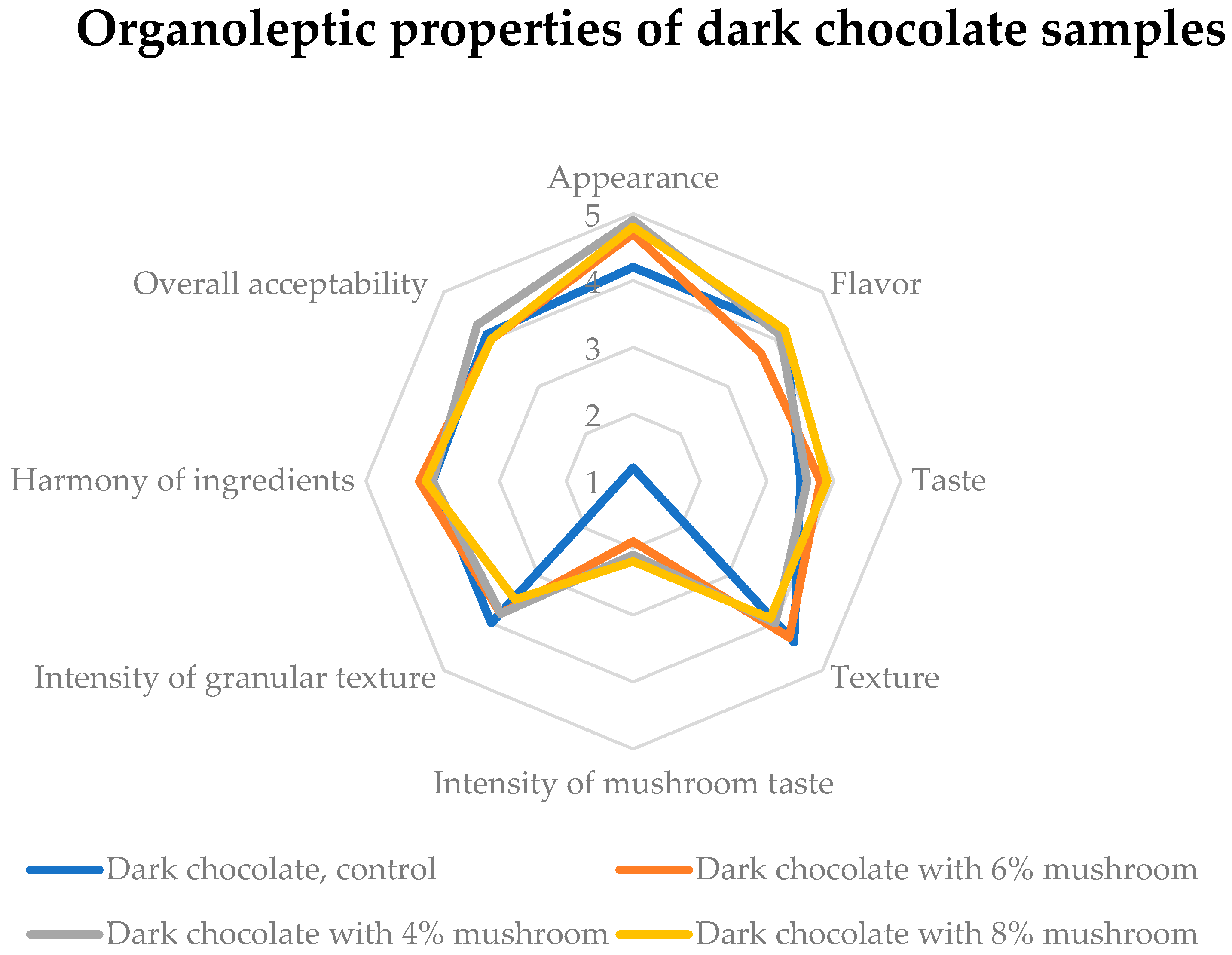
3.4. Sensory Acceptability Profile
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fowler, M.S. Cocoa Beans: From Tree to Factory. In Industrial Chocolate Manufacture and Use; Beckett, S.T., Ed.; Wiley: Hoboken, NJ, USA, 2008; pp. 10–47. ISBN 978-1-4051-3949-6. [Google Scholar]
- Afoakwa, E.O. Chocolate Science and Technology, 1st ed.; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-1-4051-9906-3. [Google Scholar]
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, Supply Chain and Processing of Cocoa—A Review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Wessel, M.; Quist-Wessel, P.M.F. Cocoa Production in West Africa, a Review and Analysis of Recent Developments. NJAS: Wagening J. Life Sci. 2015, 74–75, 1–7. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; van Griensven, L.; Jakovljevic, D.; Todorovic, N.; Wan, W.A.A.Q.I.; Vunduk, J. Mushroom β-Glucan and Polyphenol Formulations as Natural Immunity Boosters and Balancers: Nature of the Application. Food Sci. Hum. Wellness 2023, 12, 378–396. [Google Scholar] [CrossRef]
- Adamson, G.E.; Lazarus, S.A.; Mitchell, A.E.; Prior, R.L.; Cao, G.; Jacobs, P.H.; Kremers, B.G.; Hammerstone, J.F.; Rucker, R.B.; Ritter, K.A.; et al. HPLC Method for the Quantification of Procyanidins in Cocoa and Chocolate Samples and Correlation to Total Antioxidant Capacity. J. Agric. Food Chem. 1999, 47, 4184–4188. [Google Scholar] [CrossRef]
- Hammerstone, J.F.; Lazarus, S.A.; Schmitz, H.H. Procyanidin Content and Variation in Some Commonly Consumed Foods. J. Nutr. 2000, 130, 2086S–2092S. [Google Scholar] [CrossRef]
- Cinquanta, L.; Di Cesare, C.; Manoni, R.; Piano, A.; Roberti, P.; Salvatori, G. Mineral Essential Elements for Nutrition in Different Chocolate Products. Int. J. Food Sci. Nutr. 2016, 67, 773–778. [Google Scholar] [CrossRef]
- Tolve, R.; Tchuenbou-Magaia, F.L.; Verderese, D.; Simonato, B.; Puggia, D.; Galgano, F.; Zamboni, A.; Favati, F. Physico-Chemical and Sensory Acceptability of No Added Sugar Chocolate Spreads Fortified with Multiple Micronutrients. Food Chem. 2021, 364, 130386. [Google Scholar] [CrossRef]
- Al Sunni, A.; Latif, R. Effects of Chocolate Intake on Perceived Stress; a Controlled Clinical Study. Int. J. Health Sci 2014, 8, 393–401. [Google Scholar] [CrossRef]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and Chocolate in Human Health and Disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Casla, V.; Rosales-Conrado, N.; León-González, M.E.; Pérez-Arribas, L.V.; Polo-Díez, L.M. Determination of Serotonin and Its Precursors in Chocolate Samples by Capillary Liquid Chromatography with Mass Spectrometry Detection. J. Chromatogr. A 2012, 1232, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.S.; Santana, L.R.R.D.; Soares, S.E.; Bispo, E.D.S. Effect of the Roasting Temperature and Time of Cocoa Beans on the Sensory Characteristics and Acceptability of Chocolate. Food Sci. Technol. 2017, 37, 522–530. [Google Scholar] [CrossRef]
- Puchol-Miquel, M.; Palomares, C.; Barat, J.M.; Perez-Esteve, É. Formulation and Physico-Chemical and Sensory Characterisation of Chocolate Made from Reconstituted Cocoa Liquor and High Cocoa Content. LWT 2021, 137, 110492. [Google Scholar] [CrossRef]
- Komes, D.; Belščak-Cvitanović, A.; Škrabal, S.; Vojvodić, A.; Bušić, A. The Influence of Dried Fruits Enrichment on Sensory Properties of Bitter and Milk Chocolates and Bioactive Content of Their Extracts Affected by Different Solvents. LWT-Food Sci. Technol. 2013, 53, 360–369. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Karadağ, A.; Duman, Ş.; Özkal, B.; Özçelik, B. Fortification of Dark Chocolate with Spray Dried Black Mulberry (Morus nigra) Waste Extract Encapsulated in Chitosan-Coated Liposomes and Bioaccessability Studies. Food Chem. 2016, 201, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Saini, R.K.; Kumar, A.; Chawla, P.; Kaushik, R. Mushrooms as Nutritional Powerhouses: A Review of Their Bioactive Compounds, Health Benefits, and Value-Added Products. Foods 2025, 14, 741. [Google Scholar] [CrossRef]
- Ashraf Khan, A.; Gani, A.; Masoodi, F.A.; Mushtaq, U.; Silotry Naik, A. Structural, Rheological, Antioxidant, and Functional Properties of β–Glucan Extracted from Edible Mushrooms Agaricus bisporus, Pleurotus ostreatus and Coprinus attrimentarius. Bioact. Carbohydr. Diet. Fibre 2017, 11, 67–74. [Google Scholar] [CrossRef]
- Goyal, R.; Grewal, R.; Goyal, R.K. Vitamin and Mineral Content of Agaricus bisporus (White Button) and Pleurotus sajor-caju (Dhingri) Mushrooms. Int. J. Food Sci. Nutr. 2020, 5, 100–102. [Google Scholar]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Almatrafi, M.; Abdelazez, A.; Helal, M. Evaluation of Antioxidant Activities, Oxidation Enzymes, and Quality of Nano-Coated Button Mushrooms (Agaricus bisporus) during Storage. Coatings 2021, 11, 149. [Google Scholar] [CrossRef]
- Ibrahim, R.M.; Ali, M.I.; Abdel-salam, F.F. Nutritional and Quality Characteristics of Some Foods Fortified with Dried Mushroom Powder as a Source of Vitamin D. Int. J. Food Sci. 2022, 2022, 2792084. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.M.; Nunes, F.M. Agaricus bisporus By-Products as a Source of Chitin-Glucan Complex Enriched Dietary Fibre with Potential Bioactivity. Appl. Sci. 2020, 10, 2232. [Google Scholar] [CrossRef]
- Tirta Ismaya, W.; Tjandrawinata, R.R.; Rachmawati, H. Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials. Molecules 2020, 25, 2368. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B. Dietary Nutrients in Edible Mushroom, Agaricus bisporus and Their Radical Scavenging, Antibacterial, and Antifungal Effects. Process Biochem. 2022, 121, 10–17. [Google Scholar] [CrossRef]
- Agboola, O.O.; Sithole, S.C.; Mugivhisa, L.L.; Amoo, S.O.; Olowoyo, J.O. Growth, Nutritional and Antioxidant Properties of Agaricus bisporus (Crimini and White) Mushrooms Harvested from Soils Collected around Mining Areas in South Africa. Meas. Food 2023, 9, 100078. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, J.; Wen, C.; Sedem Dzah, C.; Chidimma Juliet, I.; Duan, Y.; Zhang, H. Recent Advances in Agaricus bisporus Polysaccharides: Extraction, Purification, Physicochemical Characterization and Bioactivities. Process Biochem. 2020, 94, 39–50. [Google Scholar] [CrossRef]
- Case, S.; O’Brien, T.; Ledwith, A.E.; Chen, S.; Horneck Johnston, C.J.H.; Hackett, E.E.; O’Sullivan, M.; Charles-Messance, H.; Dempsey, E.; Yadav, S.; et al. β-Glucans from Agaricus bisporus Mushroom Products Drive Trained Immunity. Front. Nutr. 2024, 11, 1346706. [Google Scholar] [CrossRef]
- Ozel, N.; Elibol, M. Chitin and Chitosan from Mushroom (Agaricus bisporus) Using Deep Eutectic Solvents. Int. J. Biol. Macromol. 2024, 262, 130110. [Google Scholar] [CrossRef] [PubMed]
- Fadhil, A.; Mous, E.F. Some Characteristics and Functional Properties of Chitin Produced from Local Mushroom Agaricus bisporus. IOP Conf. Ser. Earth Environ. Sci. 2021, 761, 012127. [Google Scholar] [CrossRef]
- Jankov, M.; Léguillier, V.; Gašić, U.; Anba-Mondoloni, J.; Ristivojević, M.K.; Radoičić, A.; Dimkić, I.; Ristivojević, P.; Vidic, J. Antibacterial Activities of Agaricus bisporus Extracts and Their Synergistic Effects with the Antistaphylococcal Drug AFN-1252. Foods 2024, 13, 1715. [Google Scholar] [CrossRef]
- Atila, F.; Nadhim Owaid, M.; Ali Shariati, M. The Nutritional and Medical Benefits of Agaricus bisporus: A Review. J. Microb. Biotech. Food Sci. 2017, 7, 281–286. [Google Scholar] [CrossRef]
- Pashaei, K.H.A.; Irankhah, K.; Namkhah, Z.; Sobhani, S.R. Edible Mushrooms as an Alternative to Animal Proteins for Having a More Sustainable Diet: A Review. J. Health Popul. Nutr. 2024, 43, 205. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, M.; Fang, Z. Valorization of Mushroom By-Products: A Review. J. Sci. Food Agric. 2022, 102, 5593–5605. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, D.; Rajoo, B. Value Addition of Mushrooms by Incorporation in the Food Products: An Overview. Int. J. Food Eng. 2023, 19, 573–591. [Google Scholar] [CrossRef]
- Maphosa, Y.; Jideani, V.A. Dietary Fiber Extraction for Human Nutrition—A Review. Food Rev. Int. 2016, 32, 98–115. [Google Scholar] [CrossRef]
- Verma, A.; Singh, V. Formulation and Quality Evaluation of Mushroom (Oyster Mushroom) Powder Fortified Potato Pudding. Asian J. Dairy Food Res. 2017, 36, 72–75. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Vunduk, J.; Nikšić, M. The Influence of Mushroom Coriolus versicolor and Hazelnuts Enrichment on Antioxidant Activities and Bioactive Content of Dark Chocolate. Food Feed Res. 2020, 47, 23–32. [Google Scholar] [CrossRef]
- Benton, D. The Biology and Psychology of Chocolate Craving. In Coffee, Tea, Chocolate, and the Brain; Nehlig, A., Ed.; Nutrition, Brain and Behavior; CRC Press: Boca Raton, FL, USA, 2004; Volume 20043667, ISBN 978-0-415-30691-1. [Google Scholar]
- Baldwin, D.E. Sous Vide Cooking: A Review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Inés Molina, R.D.; Campos-Silva, R.; Díaz, M.A.; Macedo, A.J.; Blázquez, M.A.; Alberto, M.R.; Arena, M.E. Laurel Extracts Inhibit Quorum Sensing, Virulence Factors and Biofilm of Foodborne Pathogens. LWT 2020, 134, 109899. [Google Scholar] [CrossRef]
- Rey, M.D.L.Á.; Cap, M.; Favre, L.C.; Rodríguez Racca, A.; Dus Santos, M.J.; Vaudagna, S.R.; Mozgovoj, M. Evaluation of PMA-qPCR Methodology to Detect and Quantify Viable Shiga Toxin-producing Escherichia coli in Beef Burgers. J. Food Process. Preserv. 2021, 45, e15338. [Google Scholar] [CrossRef]
- Talbot, G. Chocolate Temper. In Industrial Chocolate Manufacture and Use; Beckett, S.T., Ed.; Wiley: Hoboken, NJ, USA, 2008; pp. 261–275. ISBN 978-1-4051-3949-6. [Google Scholar]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Vieira, J. Effects of Tempering and Fat Crystallisation Behaviour on Microstructure, Mechanical Properties and Appearance in Dark Chocolate Systems. J. Food Eng. 2008, 89, 128–136. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Vieira, J. Influence of Tempering and Fat Crystallization Behaviours on Microstructural and Melting Properties in Dark Chocolate Systems. Food Res. Int. 2009, 42, 200–209. [Google Scholar] [CrossRef]
- Stobbs, J.A.; Ghazani, S.M.; Donnelly, M.-E.; Marangoni, A.G. Chocolate Tempering: A Perspective. Cryst. Growth Des. 2025, 25, 2764–2783. [Google Scholar] [CrossRef]
- Standard’s MSZ EN 12135:1999; Fruit and Vegetable Juices Determination of Nitrogen Content and Calculation of crude Protein Content Kjeldahl Method. Hungarian Standards Institution (MSZT): Budapest, Hungary, 1999.
- Eriksen, J. Measuring Natural Abundance of Stable s Isotopes in Soil by Isotope Ratio Mass Spectrometry. Commun. Soil. Sci. Plant Anal. 1996, 27, 1251–1264. [Google Scholar] [CrossRef]
- Vitorino, C.; Alves, L.; Antunes, F.E.; Sousa, J.J.; Pais, A.A.C.C. Design of a Dual Nanostructured Lipid Carrier Formulation Based on Physicochemical, Rheological, and Mechanical Properties. J. Nanopart Res. 2013, 15, 1993. [Google Scholar] [CrossRef]
- Mardani, M.; Yeganehzad, S.; Ptichkina, N.; Kodatsky, Y.; Kliukina, O.; Nepovinnykh, N.; Naji-Tabasi, S. Study on Foaming, Rheological and Thermal Properties of Gelatin-Free Marshmallow. Food Hydrocoll. 2019, 93, 335–341. [Google Scholar] [CrossRef]
- Toliba, A. Quality Characteristics of Compound Chocolate Enriched with Dried Indian Cherry Fruit Pulp. Suez Canal Univ. J. Food Sci. 2018, 5, 91–97. [Google Scholar] [CrossRef]
- Yu, K.; Wang, Y.; Wang, Y.; Guo, L.; Du, X. Effects of Annealing and Additives on the Gelatinization, Structure, and Textural Characteristics of Corn Starch. Int. J. Food Prop. 2016, 19, 1272–1281. [Google Scholar] [CrossRef]
- Szczesniak, A.S. Texture Is a Sensory Property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Kaltsa, O.; Alibade, A.; Batra, G.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Fortification of Chocolate Using Moringa oleifera Extract Encapsulated in Microemulsions. OCL 2021, 28, 38. [Google Scholar] [CrossRef]
- Abedini, A.; Dakhili, S.; Bazzaz, S.; Kamaladdin Moghaddam, S.; Mahmoudzadeh, M.; Andishmand, H. Fortification of Chocolates with High-value-added Plant-based Substances: Recent Trends, Current Challenges, and Future Prospects. Food Sci. Nutr. 2023, 11, 3686–3705. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J.; Taylor, J.R.N.; Ferruzzi, M.G.; Debelo, H. What Is Food-to-food Fortification? A Working Definition and Framework for Evaluation of Efficiency and Implementation of Best Practices. Comp. Rev. Food Sci. Food Safe 2020, 19, 3618–3658. [Google Scholar] [CrossRef]
- Şahin, O.I. Effect of Spirulina Biomass Fortification for Biscuits and Chocolates. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 583–587. [Google Scholar] [CrossRef]
- Milagres, M.P.; Silva, D.M.; Pereira, I.D.O.; Senhorinho, L.M.; Goulart Sant’Ana, A.E.; Matos, T.B. Health Benefits of Chocolate Consumption with High Concentration of Cocoa Incorporated from Triterpenic Acids, Isolated from Mansoa hirsuta DC. Food Sci. Technol. 2020, 40, 305–311. [Google Scholar] [CrossRef]
- De, S.; Chawla, P.; Dhull, S.B.; Goksen, G.; Arjun, A.D.; Bains, A. Techno-Functional Biochemical Analysis and Food Applications of Edible Mushroom Powder. J. Food Biochem. 2025, 2025, 2888689. [Google Scholar] [CrossRef]
- Blanco, E.; Hodgson, D.J.M.; Hermes, M.; Besseling, R.; Hunter, G.L.; Chaikin, P.M.; Cates, M.E.; Van Damme, I.; Poon, W.C.K. Conching Chocolate: A Prototypical Transition from Frictionally Jammed Solid to Flowable Suspension with Maximal Solid Content. Proc. Natl. Acad. Sci. USA 2019, 116, 10303–10308. [Google Scholar] [CrossRef] [PubMed]
- Chapingo, P.E.C.Y.T.A.U.A.; Espejel-Sánchez, K.I.; Espinosa-Solares, T.; Reyes-Trejo, B.; Chapingo, L.d.P.N.U.A.; Hernández-Rodríguez, G.; Chapingo, I.A.Y.U.I.d.A.U.A.; Cunill-Flores, J.M.; de Puebla, U.P.M.; Guerra-Ramírez, D. Nutritional Value and Thermal Degradation of Bioactive Compounds in Wild Edible Mushrooms. Rev. Chapingo Ser. Cienc. For. Ambient. 2021, 27, 337–354. [Google Scholar] [CrossRef]
- Bazyar, Y.Z.; Rabbani, M.; Azizi, M.H. Effect of Ganoderma lucidum to Produce Functional Chocolate: Physicochemical, Textural and Sensory Properties. Food Sci. Nutr. 2025, 13, e70676. [Google Scholar] [CrossRef]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Galletti, A.; Francis, M.; Wilkowski, J.; Salas-de La Cruz, D.; Hu, X. Protein–Polysaccharide Composite Materials: Fabrication and Applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Kumar, Y.; Malav, O.P.; Sazili, A.Q.; Domínguez, R.; Lorenzo, J.M. Microencapsulation as a Noble Technique for the Application of Bioactive Compounds in the Food Industry: A Comprehensive Review. Appl. Sci. 2022, 12, 1424. [Google Scholar] [CrossRef]
- Blumfield, M.; Abbott, K.; Duve, E.; Cassettari, T.; Marshall, S.; Fayet-Moore, F. Examining the Health Effects and Bioactive Components in Agaricus bisporus Mushrooms: A Scoping Review. J. Nutr. Biochem. 2020, 84, 108453. [Google Scholar] [CrossRef]
- Leiva, F.J.; Saenz-Díez, J.C.; Martínez, E.; Jiménez, E.; Blanco, J. Environmental Impact of Agaricus bisporus Cultivation Process. Eur. J. Agron. 2015, 71, 141–148. [Google Scholar] [CrossRef]
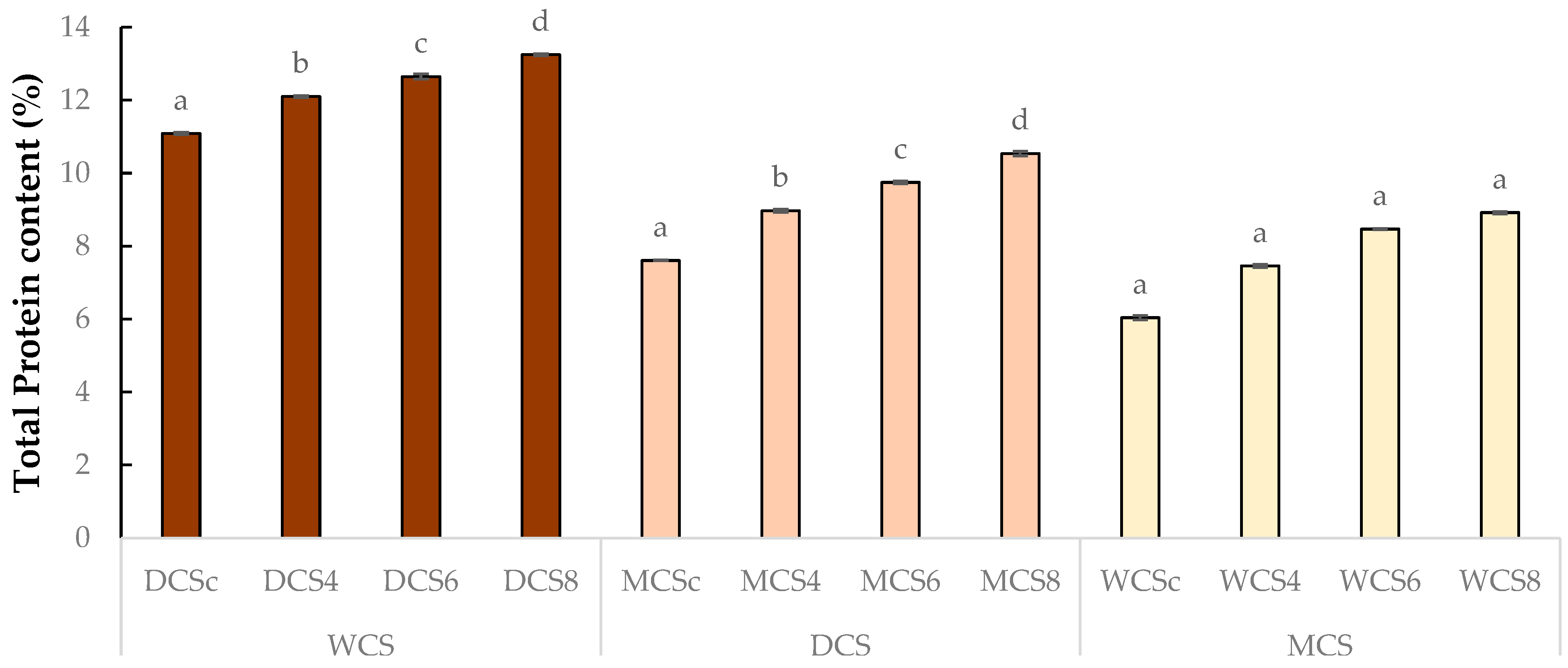
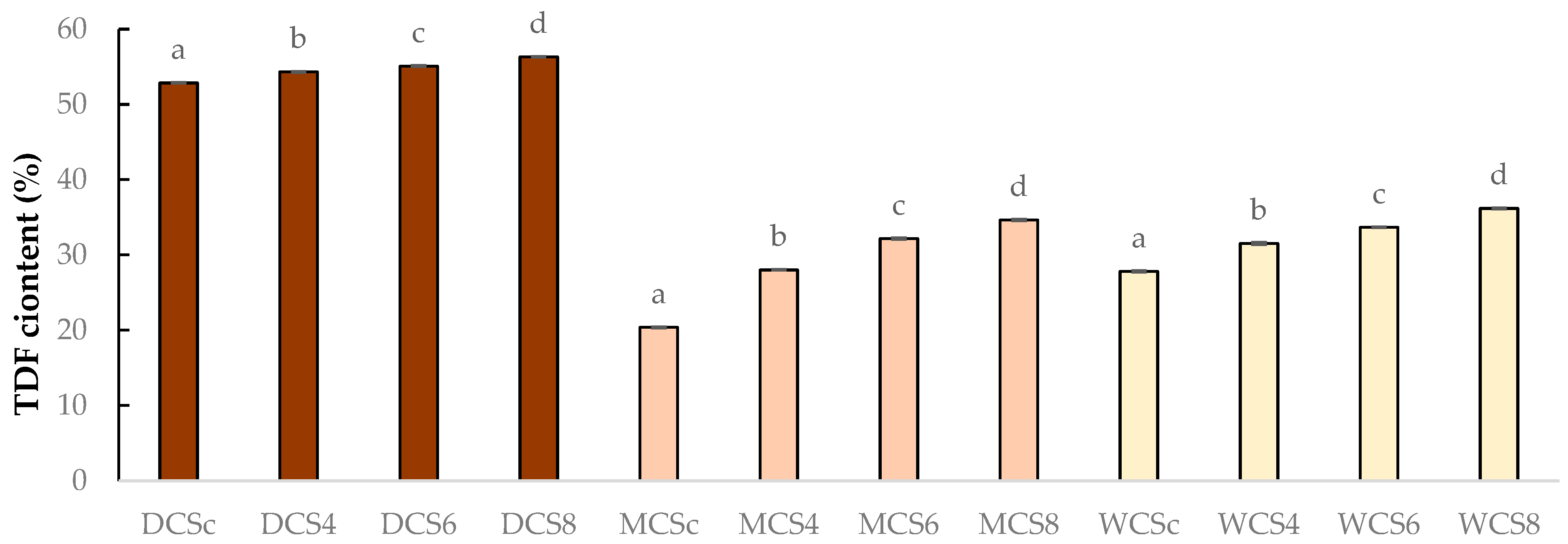



| Tested Parameter | I. Quality | II. Quality | Above II. Quality |
|---|---|---|---|
| Al (mg/kg) | 1.08 ± 0.25 | 0.34 ± 0.12 | 0.17 ± 0.02 |
| B (mg/kg) | 3.55 ± 2.05 | 4.17 ± 0.34 | 1.25 ± 0.41 |
| Ba (mg/kg) | 0.12 ± 0.00 | - | - |
| Ca (mg/kg) | 71.82 ± 33.67 | 64.64 ± 1.88 | 62.84 ± 9.22 |
| Cr (mg/kg) | 0.18 ± 0.00 | 0.20 ± 0.02 | 0.18 ± 0.01 |
| Cu (mg/kg) | 1.26 ± 0.47 | 0.79 ± 0.40 | 0.15 ± 0.40 |
| Fe (mg/kg) | 2.54 ± 0.57 | 2.06 ± 0.37 | 2.46 ± 0.27 |
| K (mg/kg) | 2518.25 ± 82.76 | 2620.00 ± 307.41 | 2604.75 ± 407.51 |
| Samples | TP Content % (m/m) | TDF Content (%) |
|---|---|---|
| DCSc | 11.09 ± 0.03 d | 52.86 ± 0.07 d |
| DCS4 | 12.10 ± 0.03 c | 54.32 ± 0.04 c |
| DCS6 | 12.65 ± 0.07 b | 55.09 ± 0.09 b |
| DCS8 | 13.25 ± 0.03 a | 56.31 ± 0.11 a |
| MCSc | 7.61 ± 0.01 a | 20.39 ± 0.07 d |
| MCS4 | 8.97 ± 0.04 a | 28.02 ± 0.13 c |
| MCS6 | 9.75 ± 0.04 a | 32.17 ± 0.03 b |
| MCS8 | 10.54 ± 0.06 a | 34.65 ± 0.08 a |
| WCSc | 6.04 ± 0.06 d | 27.82 ± 0.05 d |
| WCS4 | 7.46 ± 0.04 c | 31.53 ± 0.07 c |
| WCS6 | 8.47 ± 0.01 b | 33.69 ± 0.07 b |
| WCS8 | 8.92 ± 0.03 a | 36.19 ± 0.03 a |
| Samples | Ca | K | Mg | Na | P | S |
|---|---|---|---|---|---|---|
| DCSc | 1464 ± 9.93 d | 6673 ± 10.89 d | 2579 ± 8.24 d | 208 ± 1.69 c | 3667 ± 5.74 d | 1302 ± 1.64 d |
| DCS4 | 1605 ± 5.63 c | 8175 ± 20.44 c | 2784 ± 7.63 c | 250 ± 0.91 b | 3711 ± 4.92 c | 1376 ± 7.38 c |
| DCS6 | 1784 ± 10.14 b | 8717 ± 7.40 b | 3049 ± 18.50 b | 254 ± 2.97 b | 3949 ± 8.40 b | 1444 ± 7.33 b |
| DCS8 | 2011 ± 2.00 a | 8951 ± 6.67 a | 3184 ± 5.60 a | 277 ± 3.00 a | 4120 ± 12.10 a | 1773 ± 10.05 a |
| MCSc | 2857 ± 9.44 d | 3794 ± 2.90 a | 788 ± 6.40 a | 383 ± 2.19 a | 2294 ± 7.37 a | 857 ± 4.53 a |
| MCS4 | 3257 ± 11.55 c | 4769 ± 2.92 a | 841 ± 5.81 a | 428 ± 1.11 a | 2648 ± 4.24 a | 926 ± 5.47 a |
| MCS6 | 3565 ± 9.60 b | 5138 ± 10.04 a | 941 ± 4.20 a | 556 ± 2.36 a | 2701 ± 5.45 a | 990 ± 6.64 a |
| MCS8 | 2603 ± 5.52 a | 5752 ± 1.21 a | 975 ± 1.82 a | 580 ± 0.92 a | 2982 ± 0.94 a | 1085 ± 4.41 a |
| WCSc | 3251 ± 1.70 d | 2948 ± 21.67 d | 425 ± 8.59 c | 388.8 ± 0.27 a | 2089 ± 3.29 d | 796 ± 5.55 d |
| WCS4 | 3379 ± 13.75 c | 3171 ± 19.90 c | 453 ± 0.49 b | 453.5 ± 2.33 a | 2392 ± 8.88 c | 816 ± 2.98 c |
| WCS6 | 3693 ± 13.11 b | 3363 ± 8.51 b | 477 ± 13.96 b | 471.5 ± 12.91 a | 2490 ± 2.46 b | 859 ± 2.38 b |
| WCS8 | 3826 ± 8.18 a | 4378 ± 5.35 a | 566 ± 4.73 a | 605.4 ± 2.84 a | 2724 ± 7.67 a | 912 ± 4.27 a |
| Samples | Al | B | Ba | Cr | Cu | Fe | Mn | Ni | Zn |
|---|---|---|---|---|---|---|---|---|---|
| DCSc | 43.0 ± 0.20 d | 14.6 ± 0.02 d | 6.07 ± 0.08 c | 1.010 ± 0.01 d | 24.1 ± 0.04 d | 116 ± 0.18 d | 22.3 ± 0.11 d | 4.55 ± 0.18 b | 44.2 ± 0.34 d |
| DCS4 | 49.5 ± 0.07 c | 15.9 ± 0.05 c | 6.61 ± 0.02 b | 1.160 ± 0.01 c | 26.1 ± 0.02 c | 119 ± 0.63 c | 24.2 ± 0.09 c | 4.59 ± 0.05 b | 45.4 ± 0.06 c |
| DCS6 | 55.8 ± 0.29 b | 16.3 ± 0.02 b | 6.70 ± 0.01 b | 1.290 ± 0.02 b | 26.7 ± 0.03 b | 126 ± 0.13 b | 25.3 ± 0.42 b | 5.03 ± 0.08 a | 50.5 ± 0.13 b |
| DCS8 | 64.7 ± 0.02 a | 17.4 ± 0.19 a | 7.36 ± 0.05 a | 1.510 ± 0.03 a | 28.0 ± 0.03 a | 135 ± 0.50 a | 28.2 ± 0.05 a | 5.13 ± 0.02 a | 51.7 ± 0.27 a |
| WCSc | 34.5 ± 0.03 d | 5.04 ± 0.00 d | 1.48 ± 0.00 c | 0.806 ± 0.01 c | 3.25 ± 0.04 d | 26.1 ± 0.05 d | 1.12 ± 0.03 c | 0.363 ± 0.01 d | 14.3 ± 0.07 d |
| WCS4 | 36.0 ± 0.03 c | 5.83 ± 0.02 c | 1.50 ± 0.02 c | 0.988 ± 0.02 b | 18.06 ± 0.02 c | 29.3 ± 0.07 c | 1.38 ± 0.03 b | 0.466 ± 0.01 c | 21.5 ± 0.03 c |
| WCS6 | 38.3 ± 0.07 b | 7.22 ± 0.09 b | 2.04 ± 0.03 b | 0.995 ± 0.01 b | 19.04 ± 0.03 b | 32.4 ± 0.02 b | 1.39 ± 0.02 b | 0.510 ± 0.01 b | 22.3 ± 0.09 b |
| WCS8 | 49.8 ± 0.03 a | 8.17 ± 0.01 a | 2.67 ± 0.05 a | 1.077 ± 0.02 a | 26.23 ± 0.12 a | 36.5 ± 0.04 a | 1.52 ± 0.02 a | 0.543 ± 0.00 a | 24.9 ± 0.03 a |
| MCSc | 24.4 ± 0.12 d | 5.20 ± 0.03 d | 1.49 ± 0.01 d | 0.526 ± 0.01 c | 5.29 ± 0.01 a | 31.7 ± 0.01 b | 4.24 ± 0.08 a | 0.571 ± 0.01 b | 18.4 ± 0.09 a |
| MCS4 | 28.7 ± 0.07 c | 6.33 ± 0.04 c | 2.03 ± 0.16 c | 0.534 ± 0.01 c | 8.46 ± 0.02 a | 51.2 ± 0.05 b | 4.41 ± 0.01 a | 0.808 ± 0.02 b | 19.3 ± 0.13 a |
| MCS6 | 33.0 ± 0.10 b | 7.44 ± 0.01 b | 2.61 ± 0.01 b | 0.704 ± 0.02 b | 9.44 ± 0.05 a | 68.6 ± 0.62 b | 5.07 ± 0.05 a | 1.00 ± 0.08 a | 27.7 ± 0.30 a |
| MCS8 | 47.5 ± 0.02 a | 8.93 ± 0.02 a | 2.86 ± 0.03 a | 0.867 ± 0.01 a | 10.60 ± 0.01 a | 119 ± 0.15 a | 5.48 ± 0.03 a | 1.19 ± 0.01 a | 31.7 ± 0.08 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jevcsák, S.; Törős, G.; Diósi, G.; Llanaj, X.; Prokisch, J. Boosting Chocolate Nutrition with Sous Vide-Processed White Champignon (Agaricus bisporus) Powder: A Functional and Sustainable Approach. Foods 2025, 14, 3808. https://doi.org/10.3390/foods14223808
Jevcsák S, Törős G, Diósi G, Llanaj X, Prokisch J. Boosting Chocolate Nutrition with Sous Vide-Processed White Champignon (Agaricus bisporus) Powder: A Functional and Sustainable Approach. Foods. 2025; 14(22):3808. https://doi.org/10.3390/foods14223808
Chicago/Turabian StyleJevcsák, Szintia, Gréta Törős, Gerda Diósi, Xhensila Llanaj, and József Prokisch. 2025. "Boosting Chocolate Nutrition with Sous Vide-Processed White Champignon (Agaricus bisporus) Powder: A Functional and Sustainable Approach" Foods 14, no. 22: 3808. https://doi.org/10.3390/foods14223808
APA StyleJevcsák, S., Törős, G., Diósi, G., Llanaj, X., & Prokisch, J. (2025). Boosting Chocolate Nutrition with Sous Vide-Processed White Champignon (Agaricus bisporus) Powder: A Functional and Sustainable Approach. Foods, 14(22), 3808. https://doi.org/10.3390/foods14223808







