A Comprehensive Review on Minimally Destructive Quality and Safety Assessment of Agri-Food Products: Chemometrics-Coupled Mid-Infrared Spectroscopy
Abstract
1. Introduction
2. Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.1. Principle and Instrumentation
2.2. Applications of ATR-FTIR in Agri-Food Products Validation and Compositional Analysis
2.2.1. Fruits and Vegetables
2.2.2. Grains
2.2.3. Others
2.3. Safety Monitoring and Adulteration Detection of Food and Agricultural Products
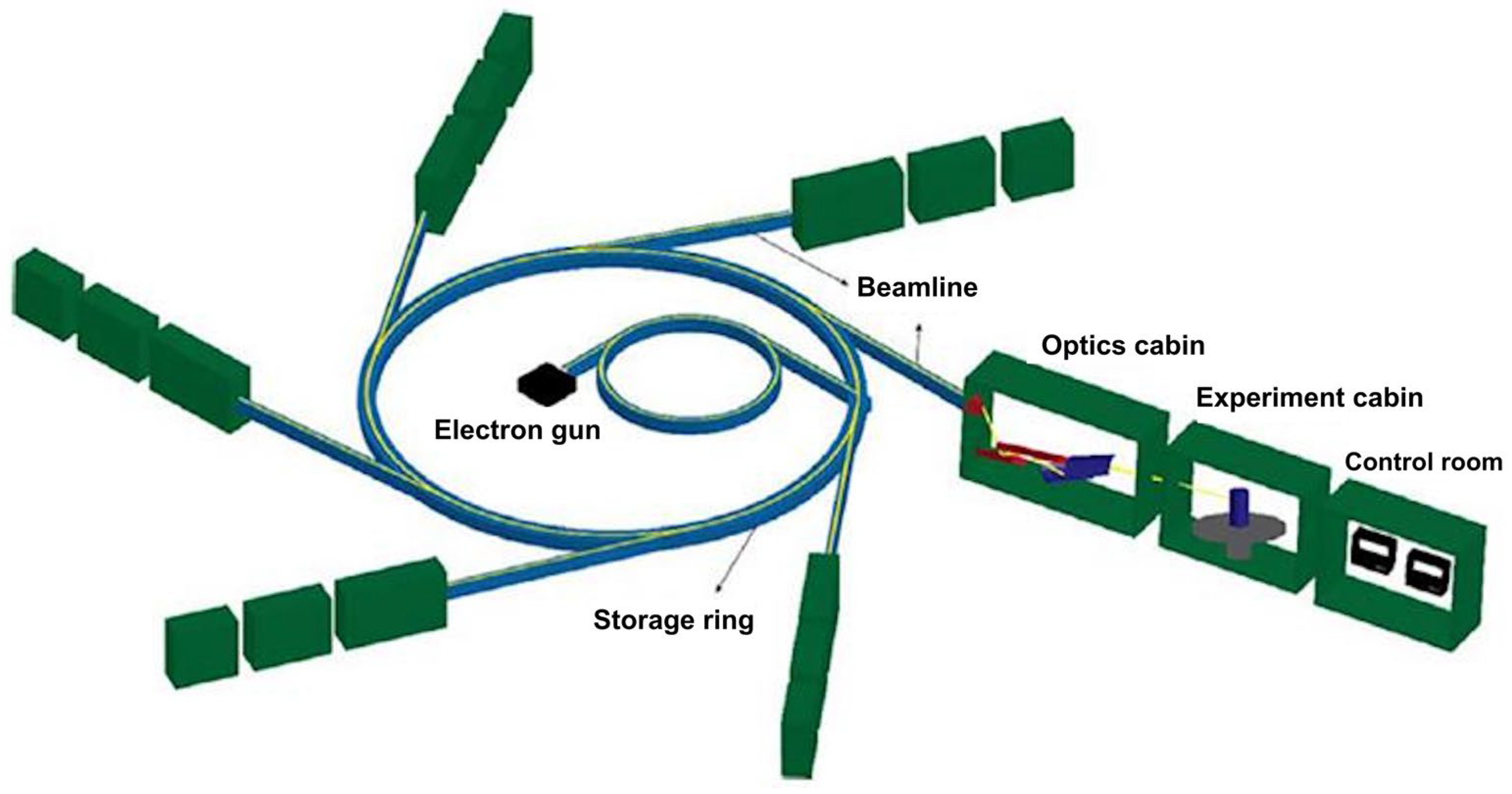
3. Synchrotron Radiation
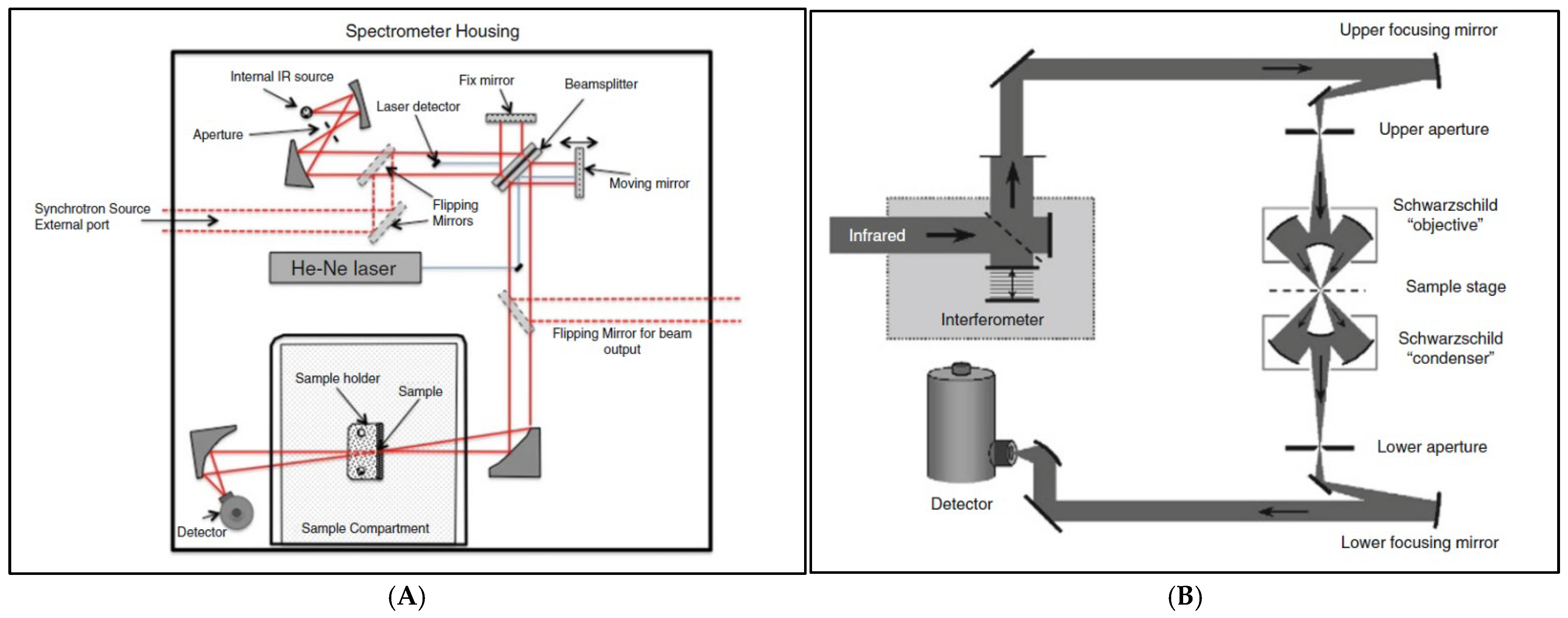
3.1. Synchrotron (SR)-Based FTIR Spectrophotometer
3.2. Applications Using Synchrotron Technology
3.2.1. Quality of Food and Agricultural Products
3.2.2. Safety of Food and Agricultural Products
4. Techniques (ATR-FTIR and SR-FTIR) at a Glance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1D-CNN | One-Dimensional Convolutional Neural Network |
| AFB1 | Aflatoxin B1 |
| AI | Artificial Intelligence |
| AIS | Alcohol-Insoluble Solids |
| ALS | Advanced Light Source |
| ATR | Attenuated Total Reflectance |
| CA | Cluster Analysis |
| FAO | Food and Agriculture Organization |
| FTIR | Fourier Transform Infrared |
| GeV | Giga-Electron Volts |
| HCA | Hierarchical Cluster Analysis |
| IoT | Internet of Things |
| IR | Infrared |
| IRE | Internal Reflection Element |
| IRM | Infrared Microspectroscopy |
| LDA | Linear Discriminant Analysis |
| LINAC | Linear Accelerator |
| MeV | Millions of Electron Volts |
| MIR | Mid-Infrared |
| MLR | Multiple Linear Regression |
| MOD | Moderately Susceptible |
| MTL | Multi-Task Learning |
| NIR | Near-Infrared |
| NSLS | National Synchrotron Light Source |
| OPLS-DA | Orthogonal Projections to Latent Structures Discriminant Analysis |
| PCA | Principal Component Analysis |
| PCA-LDA | Principal Component Analysis–Linear Discriminant Analysis |
| PGA | Potato Growers of Alberta |
| PLS | Partial Least Squares |
| PLS-DA | Partial Least Squares–Discriminant Analysis |
| PLSR | Partial Least Squares Regression |
| QDA | Quadratic Discriminant Analysis |
| RDAR | Results Driven Agricultural Research |
| RES | Reference |
| RMSE | Root Mean Square Error |
| SAS | Statistical Analysis System |
| SCAA | Sulfur-Containing Amino Acids |
| SENT | Sensitive |
| SIA | Sequential Injection Analysis |
| SLRI | Synchrotron Light Research Institute |
| SNR | Signal-to-Noise Ratio |
| SPME-GC-MS | Solid-Phase Microextraction Technique–Gas Chromatography–Mass Spectrometry |
| SR | Synchrotron Radiation |
| SSRF | Shanghai Synchrotron Radiation Facility |
| SUS | Susceptible |
| SVM | Support Vector Machine |
| SWIR | Short-Wave Infrared |
| TOL | Tolerant |
| UK | United Kingdom |
| USA | United States of America |
References
- Mohammed, A.; Bekeko, Z.; Yusufe, M.; Sulyok, M.; Krska, R. Fungal species and multi-mycotoxin associated with post-harvest sorghum (Sorghum bicolor L. Moench) grain in eastern Ethiopia. Toxins 2022, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Mirón, I.J.; Linares, C.; Díaz, J. The influence of climate change on food production and food safety. Environ. Res. 2023, 216, 114674. [Google Scholar] [CrossRef]
- Borchers, A.; Teuber, S.S.; Keen, C.L.; Gershwin, M.E. Food safety. Clin. Rev. Allergy Immunol. 2009, 39, 95–141. [Google Scholar] [CrossRef]
- Dhaulaniya, A.S.; Balan, B.; Yadav, A.; Jamwal, R.; Kelly, S.; Cannavan, A.; Singh, D.K. Development of an FTIR based chemometric model for the qualitative and quantitative evaluation of cane sugar as an added sugar adulterant in apple fruit juices. Food Addit. Contam. Part A 2020, 37, 539–551. [Google Scholar] [CrossRef]
- Singh, D.; Pradhan, M.; Materny, A. (Eds.) Modern Techniques of Spectroscopy: Basics, Instrumentation, and Applications; Springer: Singapore, 2021; ISBN 978-981-33-6083-9. [Google Scholar]
- Penner, M.H. Basic principles of spectroscopy. In Food Analysis. Food Science Text Series; Nielsen, S.S., Ed.; Springer Nature: Cham, Switzerland, 2017; pp. 79–88. [Google Scholar] [CrossRef]
- McHale, J.L. Molecular Spectroscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–458. [Google Scholar] [CrossRef]
- Gupta, P.; Das, S.S.; Singh, N.B. Spectroscopy; Jenny Stanford Publishing: Singapore, 2023. [Google Scholar]
- Ozaki, Y. Infrared spectroscopy—Mid-infrared, near-infrared, and far-infrared/terahertz spectroscopy. Anal. Sci. 2021, 37, 1193–1212. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.H.; Tapp, H.S. Mid-infrared spectroscopy for food analysis: Recent new applications and relevant developments in sample presentation methods. TrAC Trends Anal. Chem. 1999, 18, 85–93. [Google Scholar] [CrossRef]
- Seybold, C.A.; Ferguson, R.; Wysocki, D.; Bailey, S.; Anderson, J.; Nester, B.; Schoeneberger, P.; Wills, S.; Libohova, Z.; Hoover, D. Application of mid-infrared spectroscopy in soil survey. Soil. Sci. Soc. Am. J. 2019, 83, 1746–1759. [Google Scholar] [CrossRef]
- Mendes, E.; Duarte, N. Mid-infrared spectroscopy as a valuable tool to tackle food analysis: A literature review on coffee, dairies, honey, olive oil and wine. Foods 2021, 10, 477. [Google Scholar] [CrossRef]
- Ng, W.; Minasny, B.; Jeon, S.H.; McBratney, A. Mid-infrared spectroscopy for accurate measurement of an extensive set of soil properties for assessing soil functions. Soil Secur. 2022, 6, 100043. [Google Scholar] [CrossRef]
- Moro, M.K.; de Castro, E.V.R.; Romão, W.; Filgueiras, P.R. Data fusion applied in near and mid infrared spectroscopy for crude oil classification. Fuel 2023, 340, 127580. [Google Scholar] [CrossRef]
- Jiménez-Carvelo, A.M.; Osorio, M.T.; Koidis, A.; González-Casado, A.; Cuadros-Rodríguez, L. Chemometric classification and quantification of olive oil in blends with any edible vegetable oils using FTIR-ATR and Raman spectroscopy. LWT 2017, 86, 174–184. [Google Scholar] [CrossRef]
- Ladika, G.; Strati, I.F.; Tsiaka, T.; Cavouras, D.; Sinanoglou, V.J. On the assessment of strawberries’ shelf-life and quality, based on image analysis, physicochemical methods, and chemometrics. Foods 2024, 13, 234. [Google Scholar] [CrossRef]
- Lv, G.; Zhang, W.; Liu, X.; Zhang, J.; Liu, F.; Mao, H.; Song, J. Feasibility of nondestructive soluble sugar monitoring in tomato: Quantified and sorted through ATR-FTIR coupled with chemometrics. Agronomy 2024, 14, 2392. [Google Scholar] [CrossRef]
- Quijano-Ortega, N.; Fuenmayor, C.A.; Zuluaga-Dominguez, C.; Diaz-Moreno, C.; Ortiz-Grisales, S.; García-Mahecha, M.; Grassi, S. FTIR-ATR spectroscopy combined with multivariate regression modeling as a preliminary approach for carotenoids determination in Cucurbita spp. Appl. Sci. 2020, 10, 3722. [Google Scholar] [CrossRef]
- Schorn-García, D.; Giussani, B.; García-Casas, M. Assessment of variability sources in grape ripening parameters by using FTIR and multivariate modelling. Foods 2023, 12, 962. [Google Scholar] [CrossRef] [PubMed]
- Socaciu, C.; Fetea, F.; Ranga, F.; Bunea, A.; Dulf, F.; Socaci, S.; Pintea, A. Attenuated total reflectance-fourier transform infrared spectroscopy (ATR-FTIR) coupled with chemometrics, to control the botanical authenticity and quality of cold-pressed functional oils commercialized in Romania. Appl. Sci. 2020, 10, 8695. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, X.; Wu, W. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Sample Preparation: Material Characterization and Mechanism Investigation. Adv. Sample Prep. 2024, 11, 100122. [Google Scholar] [CrossRef]
- Khalid, S.; Arshad, M.; Mahmood, S.; Siddique, F.; Roobab, U.; Ranjha, M.M.A.N.; Lorenzo, J.M. Extraction and Quantification of Moringa Oleifera Leaf Powder Extracts by HPLC and FTIR. Food Anal. Methods 2023, 16, 787–797. [Google Scholar] [CrossRef]
- Saji, R.; Ramani, A.; Gandhi, K.; Seth, R.; Sharma, R. Application of FTIR Spectroscopy in Dairy Products: A Systematic Review. Food Humanity 2024, 2, 100239. [Google Scholar] [CrossRef]
- Fung, S.H.; Wong, E.S.W.; O, C.Y.; Chan, S.M.N.; Sze, E.T.P.; Tang, W.F.; Li, C.H.; Lee, F.W.F. Sample optimization of fast authentication of concentrated Chinese medicine granules using FTIR-ATR with chemometrics. In International Conference on WorldS4; Springer Nature: Singapore, 2024. [Google Scholar] [CrossRef]
- Fattahi, S.H.; Seyfari, A.K.Y. Accurate detection of safflower adulteration in saffron by atr-ftir spectroscopy and feature selection and machine learning algorithms. J. Food Meas. Charact. 2025, 19, 6295–6309. [Google Scholar] [CrossRef]
- Vigo, F.; Tozzi, A.; Lombardo, F.C.; Eugster, M.; Kavvadias, V.; Brogle, R.; Rigert, J.; Heinzelmann-Schwarz, V.; Kavvadias, T. Binary classification of gynecological cancers based on atr-ftir spectroscopy and machine learning using urine samples. Clin. Exp. Med. 2025, 25, 143. [Google Scholar] [CrossRef]
- Barbora, A.; Karri, S.; Firer, M.A.; Minnes, R. Multifractal analysis of cellular ATR-FTIR spectrum as a method for identifying and quantifying cancer cell metastatic levels. Sci. Rep. 2023, 13, 18935. [Google Scholar] [CrossRef]
- Xian, H.; He, P.; Lan, D.; Qi, Y.; Wang, R.; Lü, F.; Zhang, H.; Long, J. Predicting the elemental compositions of solid waste using ATR-FTIR and machine learning. Front. Environ. Sci. Eng. 2023, 17, 121. [Google Scholar] [CrossRef]
- Durlik-Popińska, K.; Żarnowiec, P.; Konieczna-Kwinkowska, I.; Lechowicz, Ł.; Gawęda, J.; Kaca, W. Correlations between autoantibodies and the ATR-FTIR spectra of sera from rheumatoid arthritis patients. Sci. Rep. 2021, 11, 17886. [Google Scholar] [CrossRef] [PubMed]
- Schiemer, R.; Grant, J.; Shafiee, M.N.; Phang, S.; Furniss, D.; Boitor, R.; Seddon, A.B.; Notingher, I.; Atiomo, W.; Jones, N.W.; et al. Infrared and Raman spectroscopy of blood plasma for rapid endometrial cancer detection. Br. J. Cancer 2025, 133, 194–207. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Santos, E.; Silva, A.A.B.; Faria, R.R.A.; de Almeida Rizzutto, M.; Rodrigues, P.H.S.; Baruque-Ramos, J. Raw Cellulosic Fibers: Characterization and classification by FTIR-ATR spectroscopy and multivariate analysis (PCA and LDA). Mater. Circ. Econ. 2024, 6, 13. [Google Scholar] [CrossRef]
- Parija, S.R.T.; Alam, J.; Roy, H.; Bhuiyan, M.; Khan, M.S.; Rifat, M.R.A.; Ahammed, M.S.; Rahman, M.; Uddin, M.N.; Rahman, I.M.M.; et al. Development of a rapid qualitative and quantitative method for the detection of palm oil adulteration in cow milk from Bangladesh by using ATR-FTIR Spectroscopy. Anal. Methods 2025, 18, 999–1008. [Google Scholar] [CrossRef]
- Marcelli, A.; Cricenti, A.; Kwiatek, W.M.; Petibois, C. Biological applications of synchrotron radiation infrared spectromicroscopy. Biotechnol. Adv. 2012, 30, 1390–1404. [Google Scholar] [CrossRef]
- Ismail, A.A.; van de Voort, F.R.; Sedman, J. Fourier transform infrared spectroscopy: Principles and applications. Tech. Instrum. Anal. Chem. 1997, 18, 93–139. [Google Scholar]
- Bayu, A.; Nandiyanto, D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Tech. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Smith, B. Fundamentals of Fourier Transform Infrared Spectroscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Bhuiyan, M.H.R.; Ngadi, M.O. SR-FTIR microspectroscopy: Emerging 2D structural-chemical analytical technique for food quality and safety monitoring. Food Biosci. 2023, 56, 103392. [Google Scholar] [CrossRef]
- Ahmad, A.; Ayub, H. Quality assessment techniques for the undefined Fourier transform infrared spectroscopy (FTIR) technique for food analysis and authentication. In Nondestructive Quality Assessment Techniques for Fresh Fruits and Vegetable; Springer Nature: Singapore, 2022; pp. 103–142. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Vongsvivut, J.; Ye, Q.; Selomulya, C. On Surface Composition and Stability of β-Carotene Microcapsules Comprising Pea/Whey Protein Complexes by Synchrotron-FTIR Microspectroscopy. Food Chem. 2023, 426, 136565. [Google Scholar] [CrossRef] [PubMed]
- Rana, B.; Tomar, D.; Kaur, H.; Kaur, S.; Jena, K.C.; Kaur, H.; Jena, K.C.; Rana, B.; Tomar, D.; Kaur, S. Fundamentals of ATR-FTIR Spectroscopy and its role for probing in-situ molecular-level interactions. Mod. Tech. Spectrosc. Basics Instrum. Appl. 2021, 13, 3–37. [Google Scholar] [CrossRef]
- Liu, G.; Analyst, S.K. Recent advances and applications to cultural heritage using ATR-FTIR spectroscopy and ATR-FTIR spectroscopic Imaging. Analyst 2022, 147, 1777–1797. [Google Scholar] [CrossRef] [PubMed]
- Bureau, S.; Ruiz, D.; Reich, M.; Gouble, B.; Bertrand, D.; Audergon, J.M.; Renard, C.M. Application of ATR-FTIR for a rapid and simultaneous determination of sugars and organic acids in apricot fruit. Food Chem. 2009, 115, 1133–1140. [Google Scholar] [CrossRef]
- Ouaabou, R.; Hssaini, L.; Ennahli, S.; Alahyane, A. Evaluating the Impact of Storage Time and Temperature on the Stability of Bioactive Compounds and Microbial Quality in Cherry Syrup from the ‘Burlat’ Cultivar. Discov. Food 2024, 4, 83. [Google Scholar] [CrossRef]
- Canteri, M.H.G.; Renard, C.M.G.C.; Le Bourvellec, C.; Bureau, S. ATR-FTIR spectroscopy to determine cell wall composition: Application on a large diversity of fruits and vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef]
- Skolik, P.; McAinsh, M.R.; Martin, F.L. ATR-FTIR Spectroscopy non-destructively detects damage-induced sour rot infection in whole tomato fruit. Planta 2019, 249, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Vermeir, S.; Beullens, K.; Mészáros, P.; Polshin, E.; Nicolaï, B.M.; Lammertyn, J. Sequential injection ATR-FTIR spectroscopy for taste analysis in tomato. Sens. Actuators B Chem. 2009, 137, 715–721. [Google Scholar] [CrossRef]
- Shukla, N.; Deo, M.N.; Gupta, S.; Mishra, S.; Uttam, K.N.; Mishra, A.K. Non-Destructive and Label Free Evaluation of the Biochemicals Lady Finger by Vibrational Spectroscopy (FT-Raman and ATR-FTIR) Technique. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Hssaini, L.; Ouaabou, R.; Razouk, R.; Charafi, J.; Hanine, H.; Houmanat, K.; Ennahli, S.; Lahlali, R. ATR–FTIR spectroscopy combined with the invitro antioxidant activity and chromaticity for rapid discrimination of fig (Ficus carica L.) cultivars. J. Anal. Test. 2021, 5, 270–285. [Google Scholar] [CrossRef]
- Sachadyn-Król, M.; Budziak-Wieczorek, I.; Jackowska, I. The visibility of changes in the antioxidant compound profiles of strawberry and raspberry fruits subjected to different storage conditions using ATR-FTIR and chemometrics. Antioxidants 2023, 12, 1719. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Moriwaki, T.; Gibin, M.S.; Vollmann, A.; Pattaro, M.C.; Giacomelli, M.E.; Antunes, W.C. Classification and prediction by pigment content in lettuce (Lactuca sativa L.) varieties using machine learning and ATR-FTIR spectroscopy. Plants 2022, 11, 3413. [Google Scholar] [CrossRef]
- Losacco, D.; Campanale, C.; Tumolo, M.; Ancona, V.; Massarelli, C.; Uricchio, V.F. Evaluating the influence of nitrogen fertilizers and biochar on Brassica oleracea L. var. botrytis by the use of Fourier transform infrared (FTIR) spectroscopy. Sustainability 2022, 14, 11985. [Google Scholar] [CrossRef]
- Tete, T.K. Convective Drying of Potato Slices: Impact of ethanol pretreatment and time on drying behavior, comparison of thin-layer and artificial neural network modeling, color. Potato Res. 2024, 67, 759–783. [Google Scholar] [CrossRef]
- Masithoh, R.E.; Amanah, H.Z.; Yoon, W.S.; Joshi, R.; Cho, B.K. Determination of protein and glucose of tuber and root flours using NIR and MIR spectroscopy. Infrared Phys. Technol. 2021, 113, 103577. [Google Scholar] [CrossRef]
- Lin, H.; Bean, S.R.; Tilley, M.; Peiris, K.H.S.; Brabec, D. Qualitative and quantitative analysis of sorghum grain composition including protein and tannins using ATR-FTIR spectroscopy. Food Anal. Methods 2021, 14, 268–279. [Google Scholar] [CrossRef]
- Hadjadj, N.; Abdellaoui, Z.; Bougherra, F.; Ramdane, S.; Retimi, M.; Ermis, E.; Hazzit, M.; Mizrak, O.F.; Bostanci, F.; El Enshasy, H.A. Thermal Stabilization of Wheat Germ: Nutritional and Biochemical Characterization of a Valuable Agri-Food By-Product. J. Food Meas. Charact. 2025, 1–17. [Google Scholar] [CrossRef]
- Pro, C.; Basili, D.; Notarstefano, V.; Belloni, A.; Fiorentini, M.; Zenobi, S.; Giorgini, E. A spectroscopic approach to evaluate the effects of different soil tillage methods and nitrogen fertilization levels on the biochemical composition of durum wheat (Triticum turgidum subsp. durum) leaves and caryopses. Agriculture 2021, 11, 321. [Google Scholar] [CrossRef]
- Srinuttrakul, W.; Mihailova, A.; Islam, M.D.; Liebisch, B.; Maxwell, F.; Kelly, S.D.; Cannavan, A. Geographical differentiation of Hom Mali rice cultivated in different regions of Thailand using FTIR-ATR and NIR spectroscopy. Foods 2021, 10, 1951. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Kalogiouri, N.; Hrbek, V.; Hajslova, J. Spelt authenticity assessment using a rapid and simple Fourier transform infrared spectroscopy (FTIR) method combined with advanced chemometrics. Eur. Food Res. Technol. 2023, 249, 441–450. [Google Scholar] [CrossRef]
- Biancolillo, A.; Foschi, M.; Di Micco, M.; Di Donato, F.; D’Archivio, A.A. ATR-FTIR-based rapid solution for the discrimination of lentils from different origins, with a special focus on PGI and Slow Food typical varieties. Microchem. J. 2022, 178, 107327. [Google Scholar] [CrossRef]
- Madurapperumage, A.; Johnson, N.; Thavarajah, P.; Tang, L.; Thavarajah, D. Fourier-transform infrared spectroscopy (FTIR) as a high-throughput phenotyping tool for quantifying protein quality in pulse crops. Plant Phenome J. 2022, 5, e20047. [Google Scholar] [CrossRef]
- Durand, E.; Bourlieu-Lacanal, C.; Michel Salaun, F.; Villeneuve, P. Measurement of peroxide values (PV) in oils by triphenylphosphine/triphenylphosphine oxide (TPP/TPPO) assay coupled with ATR-FTIR spectroscopy. Multidimens. Charact. Diet. Lipids 2024, 185–194. [Google Scholar] [CrossRef]
- Revelou, P.K.; Pappa, C.; Kakouri, E.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. Discrimination of botanical origin of olive oil from selected greek cultivars by SPME-GC-MS and ATR-FTIR spectroscopy combined with chemometrics. J. Sci. Food Agric. 2021, 101, 2994–3002. [Google Scholar] [CrossRef]
- Jha, S.N.; Jaiswal, P.; Kaur, J.; Ramya, H.G. Rapid detection and quantification of aflatoxin B1 in milk using Fourier transform infrared spectroscopy. J. Inst. Eng. Ser. A 2021, 102, 259–265. [Google Scholar] [CrossRef]
- da Silva Bruni, A.R.; de Oliveira, V.M.A.T.; Fernandez, A.S.T.; Sakai, O.A.; Março, P.H.; Valderrama, P. Attenuated total reflectance Fourier transform (ATR-FTIR) spectroscopy and chemometrics for organic cinnamon evaluation. Food Chem. 2021, 365, 130466. [Google Scholar] [CrossRef]
- Kartini, K.; Ariyani, V.M.; Ang, W.; Aini, Q.; Jayani, N.I.E.; Oktaviyanti, N.D.; Setiawan, F.; Azminah, A. A Validated TLC-densitometric analysis of curcumin in eight important Zingiberaceae rhizomes and their ATR-FTIR fingerprint profiles. Food Anal. Methods 2025, 18, 717–731. [Google Scholar] [CrossRef]
- Akram, U.; Sahar, A.; Sameen, A.; Muhammad, N.; Ahmad, M.H.; Khan, M.I.; Usman, M.; Rahman, H.U. ur Use of Fourier transform infrared spectroscopy and multi-variant analysis for detection of butter adulteration with vegetable oil. J. Food Prop. 2023, 26, 167–178. [Google Scholar] [CrossRef]
- Windarsih, A.; Indrianingsih, A.W.; Apriyana, W.; Rohman, A. Rapid Detection of Pork Oil Adulteration in Snakehead Fish Oil Using FTIR-ATR Spectroscopy and Chemometrics for Halal Authentication. Chem. Pap. 2023, 77, 2859–2870. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Y.; Lin, W.; Zhang, Q. Rapid quantitative authentication and analysis of camellia oil adulterated with edible oils by electronic nose and FTIR spectroscopy. Curr. Res. Food Sci. 2024, 8, 100732. [Google Scholar] [CrossRef]
- Limm, W.; Karunathilaka, S.R.; Mossoba, M.M. Fourier transform infrared spectroscopy and chemometrics for the rapid screening of economically motivated adulteration of honey spiked with corn or rice syrup. J. Food Prot. 2023, 86, 100054. [Google Scholar] [CrossRef]
- Miaw, C.S.W.; Assis, C.; Silva, A.R.C.S.; Cunha, M.L.; Sena, M.M.; de Souza, S.V.C. Determination of main fruits in adulterated nectars by ATR-FTIR spectroscopy combined with multivariate calibration and variable selection methods. Food Chem. 2018, 254, 272–280. [Google Scholar] [CrossRef]
- Shannon, M.; Lafeuille, J.L.; Frégière-Salomon, A.; Lefevre, S.; Galvin-King, P.; Haughey, S.A.; Elliott, C.T. The detection and determination of adulterants in turmeric using fourier-transform infrared (FTIR) spectroscopy coupled to chemometric analysis and micro-FTIR imaging. Food Control 2022, 139, 109093. [Google Scholar] [CrossRef]
- Lv, G.; Shan, D.; Ma, Y.; Zhang, W.; Ciren, D.; Jiang, S.; Mao, H. In-situ quantitative prediction of pesticide residues on plant surface by ATR-FTIR technique coupled with chemometrics. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2024, 305, 123432. [Google Scholar] [CrossRef]
- Li, C.; Lu, Y.; Fu, S.; Guo, Y.; Huang, Z.; Wen, L.; Jiang, L. Simultaneous qualitative and quantitative analyses of pesticide residues on fruit peels with ATR-FTIR spectroscopy and multi-task learning. Vib. Spectrosc. 2025, 103807. [Google Scholar] [CrossRef]
- Zhou, R.; Ye, W.; Zhang, Z.; Zhang, Y.; Shen, T.; Wang, D.; Guo, Z.; Gao, S.; Zou, X. Advances in Molecular Vibrational Spectroscopy for Foodborne Pathogen Detection. J. Agric. Food Chem. 2025, 73, 25756–25779. [Google Scholar] [CrossRef] [PubMed]
- Binati, R.L.; Ferremi Leali, N.; Avesani, M.; Salvetti, E.; Felis, G.E.; Monti, F.; Torriani, S. Application of FTIR Microspectroscopy in Oenology: Shedding Light on Cell Wall Composition of Saccharomyces Cerevisiae Strains. Food Bioproc. Tech. 2024, 17, 1596–1609. [Google Scholar] [CrossRef]
- Balerna, A.; Mobilio, S. Introduction to Synchrotron Radiation. In Synchrotron Radiation: Basics, Methods and Applications; Springer, A., Balerna, S.M., Eds.; Springer: Berlin, Germany, 2015; pp. 3–28. [Google Scholar] [CrossRef]
- Cramer, S.P. Synchrotron radiation fundamentals. In X-Ray Spectroscopy with Synchrotron Radiation: Fundamentals and Applications; Springer International Publishing: Cham, Switzerland, 2020; pp. 39–68. [Google Scholar]
- Mobilio, S.; Boscherini, F.; Meneghini, C. Synchrotron radiation: Basics, methods and applications. In Synchrotron Radiation: Basics, Methods and Applications; Springer: Berlin, Germany, 2015; pp. 1–799. [Google Scholar] [CrossRef]
- Sedigh Rahimabadi, P.; Khodaei, M.; Koswattage, K.R. Review on applications of synchrotron-based X-ray techniques in materials characterization. X-Ray Spectrom. 2020, 49, 348–373. [Google Scholar] [CrossRef]
- Vijayan, P.; Willick, I.R.; Lahlali, R.; Karunakaran, C.; Tanino, K.K. Synchrotron radiation sheds fresh light on plant research: The use of powerful techniques to probe structure and composition of plants. Plant Cell Physiol. 2015, 56, 1252–1263. [Google Scholar] [CrossRef]
- Tanino, K.; Willick, I.R.; Hamilton, K.; Vijayan, P.; Jiang, Y.; Brar, G.S.; Yu, P.; Kalcsits, L.; Lahlali, R.; Smith, B.; et al. Chemotyping using synchrotron mid-infrared and X-ray spectroscopy to improve agricultural production. J. Plant Sci. 2017, 97, 982–996. [Google Scholar] [CrossRef]
- Yan, M.; Guevara-Oquendo, V.H.; Rodríguez-Espinosa, M.E.; Yang, J.C.; Lardner, H.; Christensen, D.A.; Feng, X.; Yu, P. Utilization of synchrotron-based and globar-sourced mid-infrared spectroscopy for faba nutritional research about molecular structural and nutritional interaction. Crit. Rev. Food Sci. Nutr. 2022, 62, 1453–1465. [Google Scholar] [CrossRef]
- Dumas, P.; Martin, M.C.; Carr, G.L. IR Spectroscopy and spectromicroscopy with synchrotron radiation. In Synchrotron Light Sources and Free-Electron Lasers; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–55. [Google Scholar] [CrossRef]
- Pax, A.P.; Ong, L.; Vongsvivut, J.; Tobin, M.J.; Kentish, S.E.; Gras, S.L. The Characterisation of Mozzarella Cheese Microstructure Using High Resolution Synchrotron Transmission and ATR-FTIR Microspectroscopy. Food Chem. 2019, 291, 214–222. [Google Scholar] [CrossRef]
- Phansak, P.; Siriwong, S.; Sangpueak, R.; Kanawapee, N.; Thumanu, K.; Buensanteai, N. Screening rice blast-resistant cultivars via synchrotron Fourier transform infrared (SR-FTIR) microspectroscopy. Emir. J. Food Agric. 2021, 33, 726–741. [Google Scholar] [CrossRef]
- Ranathunga, A.; Thumanu, K.; Kiatponglarp, W.; Siriwong, S.; Wansuksri, R.; Suwannaporn, P. Image mapping of biological changes and structure-function relationship during rice grain development via Synchrotron FTIR spectroscopy. Food Chem. Adv. 2023, 2, 100290. [Google Scholar] [CrossRef]
- Kongmon, E.; Jitvisate, M.; Panchaisri, B.; Techarang, J.; Thumanu, K.; Rimjaem, S. Classification of ion-beam-induced traits in Thai jasmine rice mutants using synchrotron radiation FTIR microspectroscopy. Nucl. Instrum. Methods Phys. Res. B 2020, 465, 37–41. [Google Scholar] [CrossRef]
- Indore, N.S.; Karunakaran, C.; Jayas, D.S.; Bondici, V.F.; Vu, M.; Tu, K.; Muir, D. Mapping biochemical and nutritional changes in durum wheat due to spoilage during storage. Heliyon 2023, 9, e22139. [Google Scholar] [CrossRef]
- Xin, H.; Zhang, X.; Yu, P.I. Using synchrotron radiation-based infrared microspectroscopy to reveal microchemical structure characterization: Frost damaged Wheat vs. Normal. Int. J. Mol. Sci. 2013, 14, 16706–16718. [Google Scholar] [CrossRef] [PubMed]
- Thepbandit, W.; Papathoti, N.K.; Daddam, J.R.; Thumanu, K.; Siriwong, S.; Thanh, T.L.; Buensanteai, N. Identification of salicylic acid mechanism against leaf blight disease in Oryza sativa by SR-FTIR microspectroscopic and docking studies. Pathogens 2021, 10, 652. [Google Scholar] [CrossRef]
- Doiron, K.J.; Yu, P.; Christensen, C.R.; Christensen, D.A.; McKinnon, J.J. Detecting molecular changes in Vimy flaxseed protein structure using synchrotron FTIRM and DRIFT spectroscopic techniques: Structural and biochemical characterization. J. Spectrosc. 2009, 23, 307–322. [Google Scholar] [CrossRef]
- Feng, Z.; Morton, J.D.; Maes, E.; Kumar, L.; Serventi, L. Exploring Faba Beans (Vicia faba L.): Bioactive compounds, cardiovascular health, and processing insights. Crit. Rev. Food Sci. Nutr. 2025, 65, 4354–4367. [Google Scholar] [CrossRef]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Faba beans protein as an unconventional protein source for the food industry: Processing influence on nutritional, techno-functionality, and bioactivity. Food Rev. Int. 2024, 40, 1999–2023. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kidwai, M.K.; Noor, R.; Chawla, P.; Rose, P.K. A Review of Nutritional profile and processing of faba bean (Vicia Faba L.). Legume Sci. 2022, 4, e129. [Google Scholar] [CrossRef]
- Rodriguez Espinosa, M.E. Investigation of the Role of Amide I to Amide II Ratio and Alpha Helix to Beta Sheet Ratio of Faba Bean Seeds in the Determination of Microbial Protein Synthesis and Animal Performance and Metabolism in Ruminant Livestock Systems. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2023. [Google Scholar]
- Ashe, P.; Tu, K.; Stobbs, J.A.; Dynes, J.J.; Vu, M.; Shaterian, H.; Kagale, S.; Tanino, K.K.; Wanasundara, J.P.D.; Vail, S.; et al. Applications of synchrotron light in seed research: An array of X-ray and infrared imaging methodologies. Front. Plant Sci. 2024, 15, 1395952. [Google Scholar] [CrossRef]
- Liu, N.; Yu, P. Characterization of the microchemical structure of seed endosperm within a cellular dimension among six barley varieties with distinct degradation kinetics, using ultraspatially resolved synchrotron-based infrared microspectroscopy. J. Agric. Food Chem. 2010, 58, 7801–7810. [Google Scholar] [CrossRef]
- Yu, P. Molecular Chemistry of plant protein structure at a cellular level by synchrotron-based FTIR spectroscopy: Comparison of yellow (Brassica rapa) and brown (Brassica napus) canola seed tissues. Infrared Phys. Technol. 2008, 51, 473–481. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Siriwong, S.; Thumanu, K.; Boonanuntanasarn, S.; Yongsawatdigul, J. Investigating the effect of various sous-vide cooking conditions on protein structure and texture characteristics of tilapia fillet using synchrotron radiation-based FTIR. Foods 2023, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Charoensin, S.; Boonkum, W.; Thumanu, K.; Laopaiboon, B.; Duangjinda, M. Synchrotron fourier transform infrared microspectroscopy and scanning electron microscopy assessment of key physical meat properties of Thai native chickens for selection in breeding programs. Asia-Pac. J. Sci. Technol. 2021, 26, 1–10. [Google Scholar]
- Ong, L.; Pax, A.P.; Ong, A.; Vongsvivut, J.; Tobin, M.J.; Kentish, S.E.; Gras, S.L. The Effect of PH on the Fat and Protein within Cream Cheese and Their Influence on Textural and Rheological Properties. Food Chem. 2020, 332, 127327. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Vongsvivut, J.; Tobin, M.J.; Adhikari, R.; Barrow, C.; Adhikari, B. Investigation of Oil Distribution in Spray-Dried Chia Seed Oil Microcapsules Using Synchrotron-FTIR Microspectroscopy. Food Chem. 2019, 275, 457–466. [Google Scholar] [CrossRef]
- Bouchon, P.; Hollins, P.; Pearson, M.; Pyle, D.L.; Tobin, M.J. Oil distribution in fried potatoes monitored by infrared microspectroscopy. J. Food Sci. 2001, 66, 918–923. [Google Scholar] [CrossRef]
- Foodborne Pathogens|FDA. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/foodborne-pathogens (accessed on 16 August 2025).
- Gourama, H. Foodborne pathogens. In Food Safety Engineering; Springer International Publishing: Cham, Switzerland, 2020; pp. 25–49. [Google Scholar]
- Wang, Y.D.; Li, X.L.; Liu, Z.X.; Zhang, X.X.; Hu, J.; Lu, J.H. Discrimination of foodborne pathogenic bacteria using synchrotron FTIR microspectroscopy. Nucl. Sci. Tech. 2017, 28, 49. [Google Scholar] [CrossRef]
- Mycotoxins. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 16 August 2025).
- Nuankaew, S.; Boonyuen, N.; Thumanu, K.; Pornputtapong, N. Development of a machine learning model for systematics of Aspergillus section Nigri using synchrotron radiation-based fourier transform infrared spectroscopy. Heliyon 2024, 10, e26812. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yu, P. Advanced Synchrotron-Based and Globar-Sourced Molecular (Micro) Spectroscopy contributions to advances in food and feed research on molecular structure, mycotoxin determination, and molecular nutrition. Crit. Rev. Food Sci. Nutr. 2018, 58, 2164–2175. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, B.; Yoon, S.C.; Zhuang, H.; Ni, X.; Guo, B.; Wang, W. Spatio-temporal patterns of Aspergillus flavus infection and aflatoxin B1 biosynthesis on maize kernels probed by SWIR hyperspectral imaging and synchrotron FTIR microspectroscopy. Food Chem. 2022, 382, 132340. [Google Scholar] [CrossRef]
- Sukprasert, J.; Thumanu, K.; Phung-On, I.; Jirarungsatean, C.; Erickson, L.E.; Tuitemwong, P.; Tuitemwong, K. Synchrotron FTIR Light reveals signal changes of biofunctionalized magnetic nanoparticle attachment on Salmonella sp. J. Nanomater. 2020, 2020, 6149713. [Google Scholar] [CrossRef]
- Meneghel, J.; Passot, S.; Jamme, F.; Lefrançois, S.; Lieben, P.; Dumas, P.; Fonseca, F. FTIR Micro-Spectroscopy Using Synchrotron-Based and Thermal Source-Based Radiation for Probing Live Bacteria. Anal. Bioanal. Chem. 2020, 412, 7049–7061. [Google Scholar] [CrossRef]
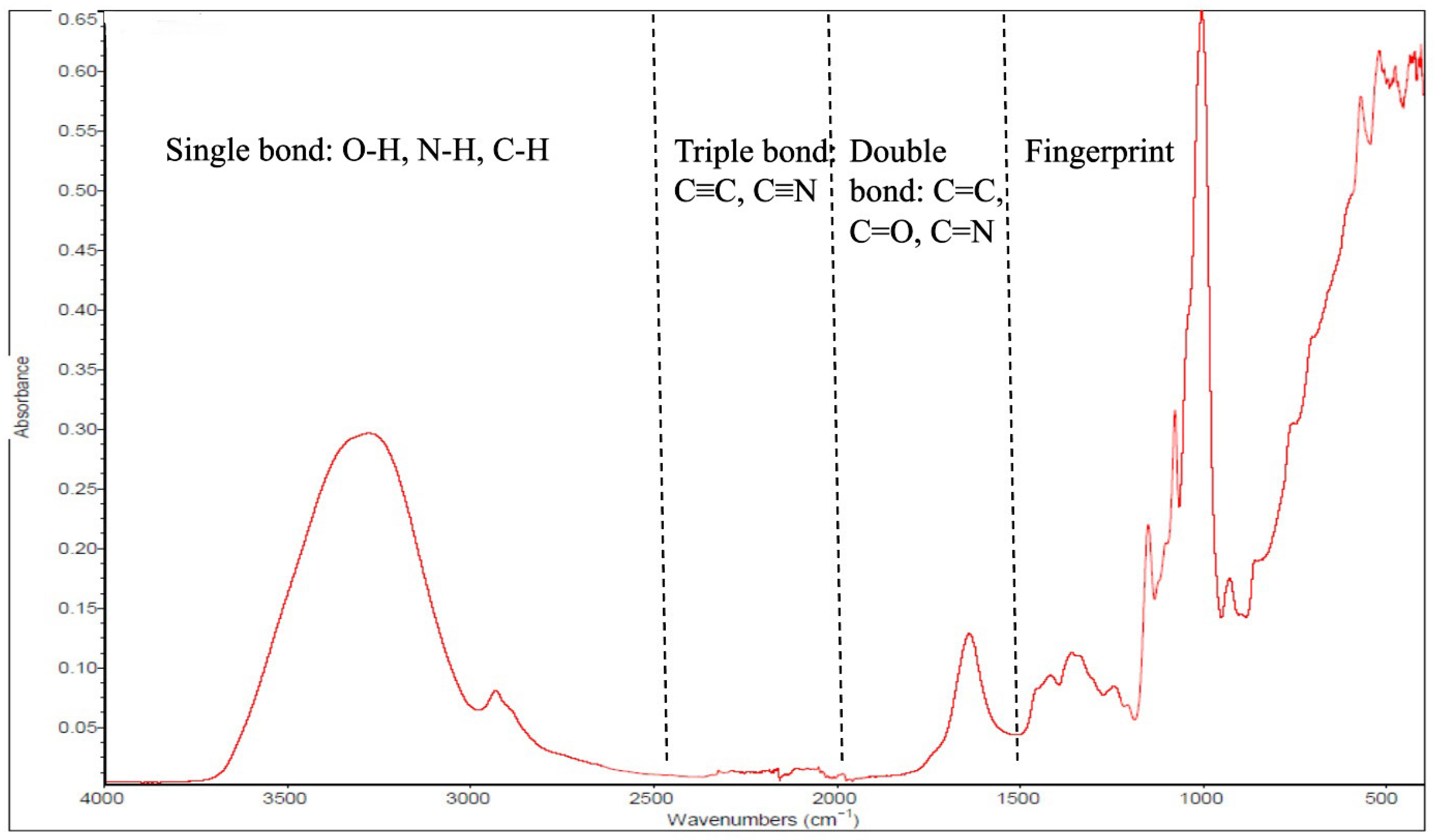
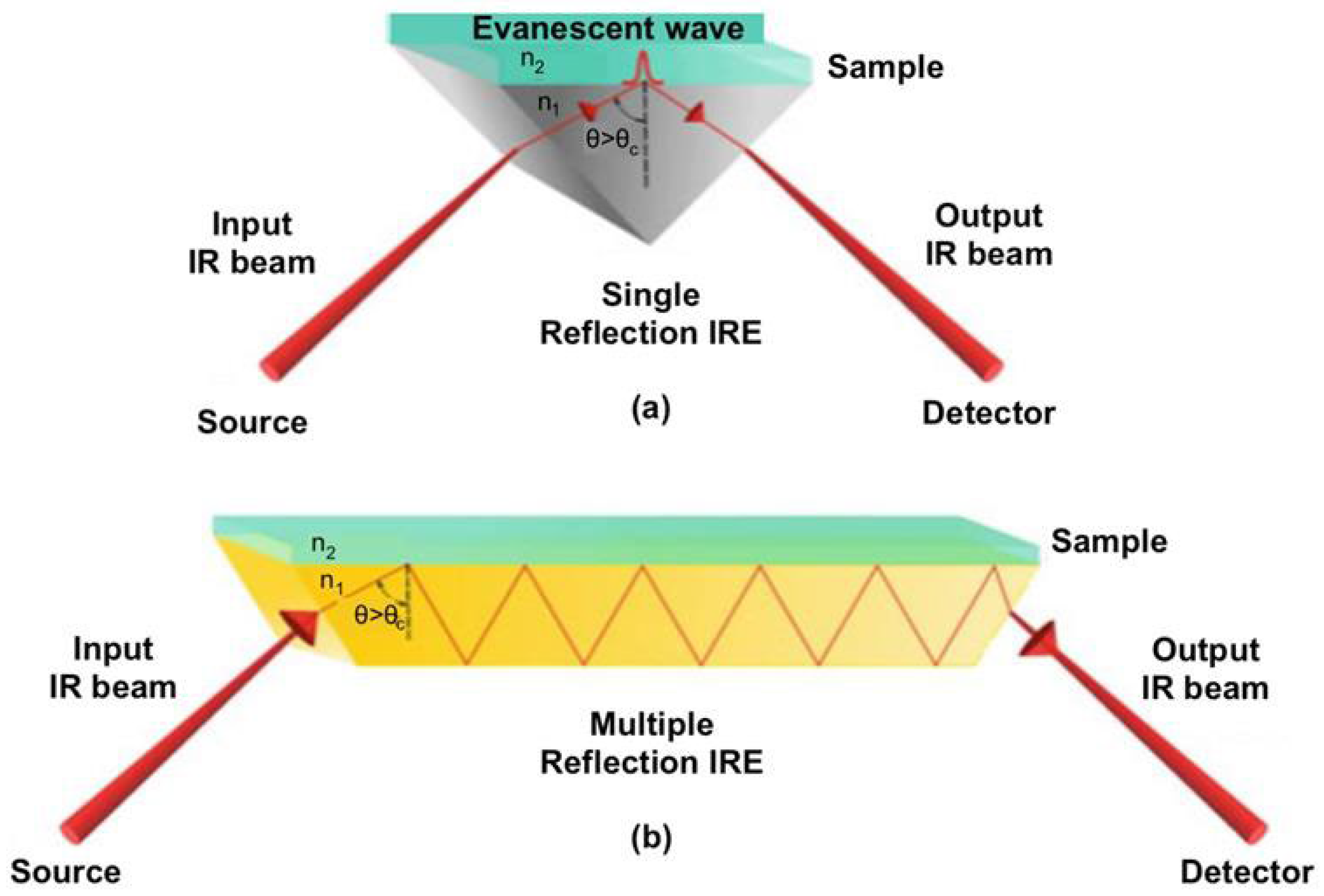
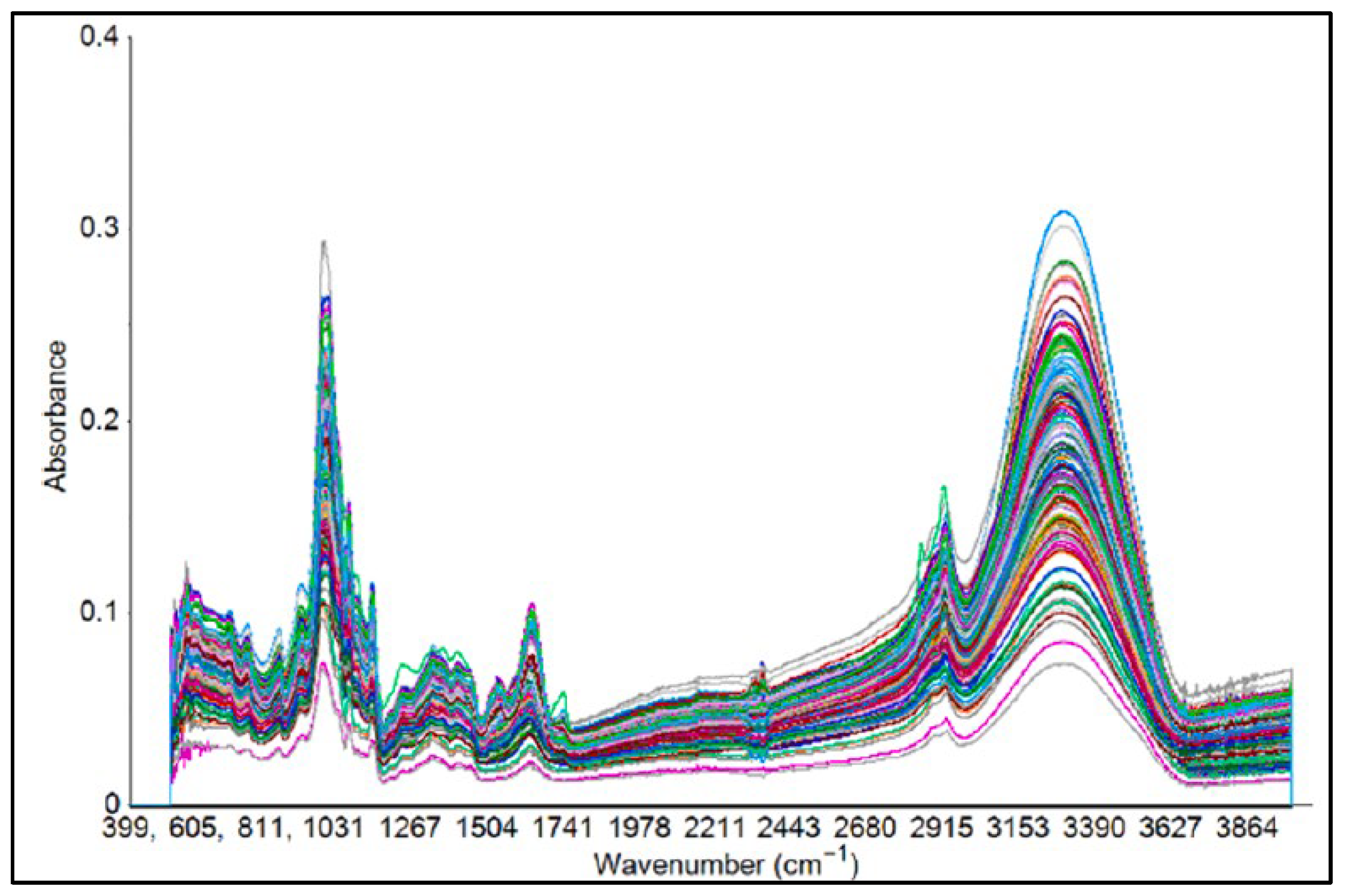
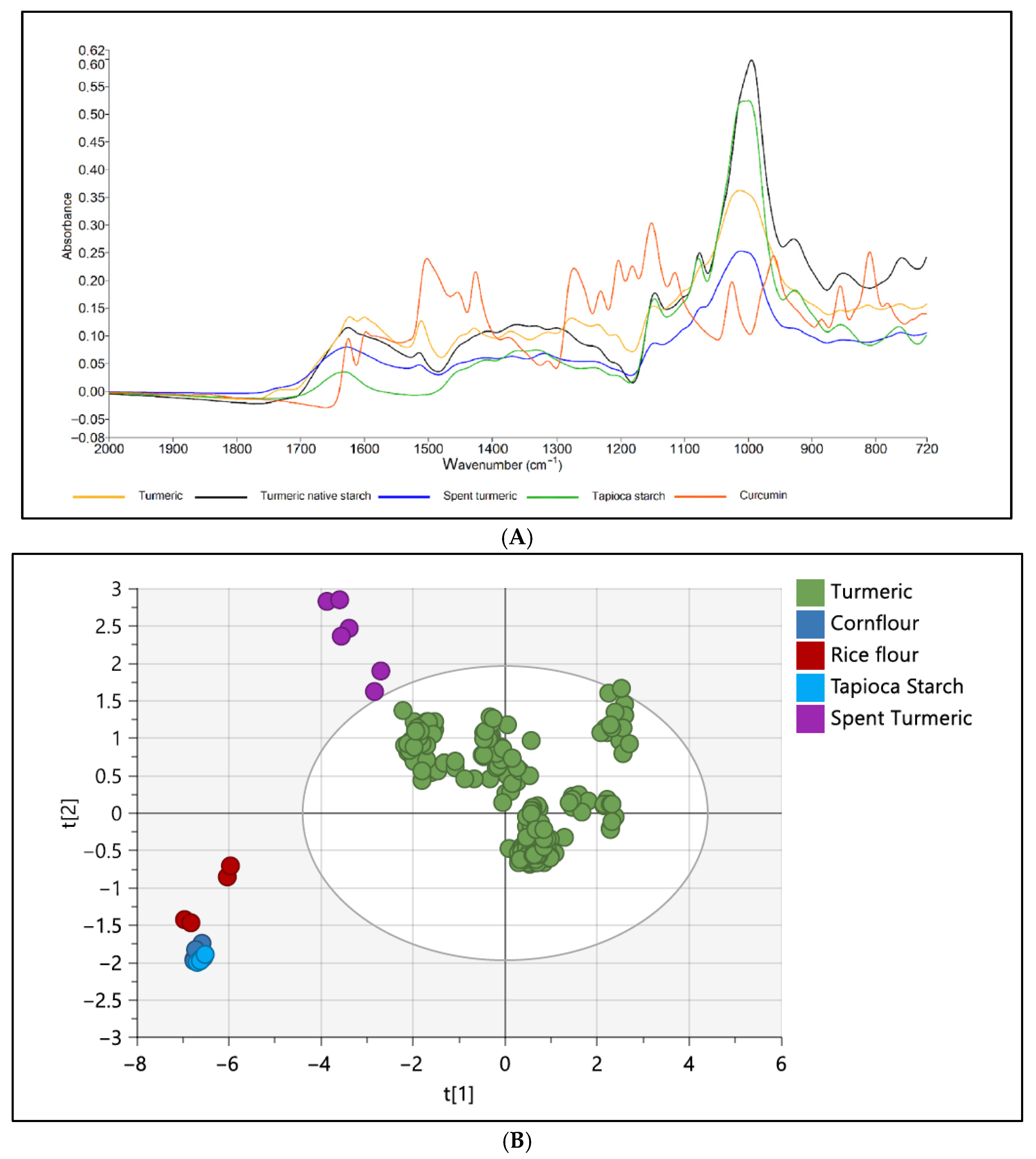
| Target | Category | Sample | Purpose | IR Range (cm−1) | ATR Crystal Type | Number of Scans | Resolution (cm−1) | Chemometric | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Quality | Fruits and vegetables | Apricot fruit slurries | Quantification of sugars (sucrose, glucose, fructose) and organic acids (malic, citric) | 4000–650 | ZnSe | 32 | 4 | PLSR | [42] |
| Fresh fruits and vegetables | Primary components of cell walls | 4000–650 | - | 16 | - | PLS | [44] | ||
| Tomatoes | Soluble sugars in tomatoes | 4000–600 | ZnSe | 32 | 2 | PLSR and PCA | [17] | ||
| Tomatoes | Flavor assessment | 1800–800 | - | - | - | PLS-DA | [46] | ||
| Figs fruit | Antioxidant activity | 4000–450 | Germanium | 128 | 4 | PCA | [48] | ||
| Strawberry and raspberry fruits | Changes in antioxidant compounds | 4500–500 | ZnSe | 30 | 4 | PCA and HCA | [49] | ||
| Strawberries | Shelf life and quality deterioration | - | - | - | - | PCA | [16] | ||
| Grape berries | Variability in grape ripening characteristics | 4000–650 | Diamond | 32 | 8 | PCA and PLSR | [19] | ||
| Cucurbita (squash and pumpkin) | Total carotenoid content | 4000–450 | Diamond | 24 | 4 | PLSR | [18] | ||
| Lettuce leaf | Pigment content | 4000–400 | Diamond | 300 | 4 | PCA and LDA | [50] | ||
| Cauliflower | Biochemical effects of nitrogen fertilizer levels and biochar | 4000–400 | Diamond | 32 | 4 | PCA | [51] | ||
| Potatoes | Impact of ethanol pretreatment and drying time on moisture removal behavior and quality parameters (color, shrinkage, total phenolic content, and antioxidant activity) | 4000–400 | ZnSe | - | 2 | PCA | [52] | ||
| Tuber and roots (arrowroot, canna, taro, cassava, white, yellow, and purple sweet potato) flours | Protein and glucose | 4000–400 | - | 32 | 3 | PCA and PLSR | [53] | ||
| Grains | Sorghum | Grain composition (protein and tannin contents) | 4000–400 | Diamond | - | 4 | Pearson’s correlation Analyses | [54] | |
| Durum wheat leaves and caryopses (grain) | Nitrogen fertilization levels on the macromolecular composition | 4000–650 | ZnSe | 64 | 4 | PCA | [56] | ||
| Hom Mali rice | Regional discrimination | 4000–450 | - | 6 | 1 | OPLS-DA | [57] | ||
| Al. | Spelt | Authenticity assessment | 4000–400 | - | 64 | 2 | PCA and OPLS-DA | [58] | |
| Lentils | Discrimination of place of origin | 4000–400 | Diamond | 10 | 4 | PCA | [59] | ||
| Pulses (chickpea, dry pea, and lentil) | Protein quality (sulfur-containing amino acids concentration) | 4000–650 | Diamond | 100 (for lentil) 64 (for chickpea and dry pea) | 2 (for lentil) 4 (for chickpea and dry pea) | PLS | [60] | ||
| Others | Oil | Protocol for measurement of peroxide value | 4000–400 | - | 16 (proposed) | 4 (proposed) | - | [61] | |
| Olive oil | Botanical origin discrimination | 4000–400 | ZnSe | 100 | 4 | LDA and QDA | [62] | ||
| Organic cinnamon | Evaluation of organic cinnamon from non-organic | 4000–500 | - | 32 | 4 | PARAFAC | [64] | ||
| Zingiberaceae rhizomes | Differentiation of Zingiberaceae rhizomes | 4000–650 | Diamond | - | - | PCA and CA | [65] | ||
| Safety | Lipid-rich foods | Butter | Detection of butter adulteration with vegetable oil | 4000–800 | ZnSe | 16 | 4 | PCA and PLSR | [66] |
| Snakefish oil | Rapid identification of pork oil adulteration in snakehead fish oil | 4000–650 | - | 32 | 8 | PCA and OPLS-DA | [67] | ||
| Camellia oil | Detection of edible oil adulteration in camellia oil | 4000–650 | ZnSe | 32 | 4 | Vector machine regression | [68] | ||
| Honey | Detection of honey adulteration with syrup or invert sugar in particular | 4000–650 | Diamond | 128 | 4 | PLS and PCA | [69] | ||
| Apple juices | Detection of adulteration of apple juices with cane sugar | 4000–400 | ZnSe | 32 | 4 | PCA | [4] | ||
| Nectars | Identification of main fruits in adulterated nectar | 4000–650 | ZnSe | 16 | 4 | PLS | [70] | ||
| Turmeric powder | Detection of adulterants in turmeric powder | 4000–550 | Diamond | 32 | 4 | PCA, OPLS-DA and PLS-DA | [71] |
| Target | Area of Study | Purpose | IR Range (cm−1) | Number of Scans | Resolution (cm−1) | Microspectroscopy Aperture Size/Pixel Size | SR-FTIR Location | Chemometric | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Quality | Rice | Screening and identification of blast-resistant rice cultivars | 4000–600 | 64 | 4 | 10 × 10 λm2 | SLRI, Nakhon Ratchasima, Thailand | PCA and HCA | [85] |
| Rice | Biochemical and functional structural changes during developmental stages (milky, dough, and mature) | 4000–800 | 64 | 6 | 10 × 10 λm2 | SLRI, Nakhon Ratchasima, Thailand | PCA and HCA | [86] | |
| Rice | Biochemical composition of the improved (ion-beam-induced) mutant Thai jasmine rice | 4000–800 | 64 | 4 | 20 × 20 λm2 | SLRI, Nakhon Ratchasima, Thailand | PCA | [87] | |
| Rice | Control of leaf blight infection in rice by salicylic acid-ricemate treatment | - | 64 | 4 | 10 × 10 µm | SLRI, Nakhon Ratchasima, Thailand | [90] | ||
| Wheat | Protein structure (amide I, II, and secondary structures), carbohydrate structure, and functional groups in normal vs. frost-damaged wheat | 4000–800 | 256 | 4 | 10 × 10 µm | NSLS, New York, NY, USA | PCA | [89] | |
| Flaxseed | Molecular and protein structural characterization in flaxseed (cultivar: Vimy) | 4000–800 | 128 | 4 | 10 × 10 λm2 | NSLS, New York, NY, USA | PCA and HCA | [91] | |
| Faba bean | Intrinsic molecular structural characterization of faba bean seed endosperms influenced by pressure toasting (steam) | 4000–750 | 64 | 4 | 10 × 10 µm | ALS, Berkeley, CA, USA | MIXED of SAS 9.4 software | [95] | |
| Barley | Biochemical structure of barley cultivars | 4000–800 | 128 | 4 | 10 × 10 µm | NSLS, New York, NY, USA | MIXED procedure of SAS 9.1.3 | [97] | |
| Canola | Molecular structures of plant proteins in the yellow and brown canola seed tissues | 4000–800 | 64 | 4 | 10 × 10 µm | NSLS in New York, NY, USA | PCA | [98] | |
| Fish | Impact of sous vide cooking parameters on the physicochemical, textural, protein structure degradation, and sensory qualities of tilapia fillets | 4000–400 | 64 | 4 | - | - | PCA | [99] | |
| Chicken | Muscle fibre properties and secondary protein structures | 4000–800 | 64 | 6 | 10 × 10 µm | SLRI, Thailand | Savitzky-Golay method in the Unscrambler X software (version 10.1) | [100] | |
| Cheese | Characterisation of proteins, lipids, and microstructures of mozzarella cheese | 3800–700 | 16 | 4 | 60 × 60 µm | Australian Synchrotron Infrared Microspectroscopy (IRM), Clayton, Australia | PCA and HCA | [84] | |
| Fried potatoes | Oil absorption | 8000–800 | 24 × 24 µm | Synchrotron Radiation Source in Daresbury, UK | [103] | ||||
| Safety | Foodborne disease | Discrimination of foodborne disease-causing bacteria | 4000–650 | 64 | 4 | 20 × 20 μm2 | SSRF, Shanghai, China | PCA | [106] |
| Mycotoxins | Quick identification of Aspergillus species | 4000–400 | 64 | 6 | 10 × 10 µm2 | SLRI, Thailand | 1D-CNN | [108] | |
| Mycotoxins | Spatial and chemical changes in maize kernels infected with A. flavus | 4000–400 | 64 | 4 | 20 × 20 μm2 | SSRF, Shanghai, China | PCA | [110] | |
| Salmonella | Detection of Salmonella in food | 4000–800 | 64 | 4 | 20 × 20 µm2 | SLRI, Thailand | - | [111] |
| Mid-IR Spectroscopy | Mid-Infrared Source | Interaction Mode | Depth of Penetration | Signal-to-Noise Ratio | Instrumentation | Applications |
|---|---|---|---|---|---|---|
| ATR-FTIR | Globar, a conventional thermal infrared Low brightness | Total internal reflection via ATR crystal (diamond, ZnSe, Ge) | Shallow (micron) | Good Limitations in detecting traces | Compact bench-top Portable Commonly accessible | Bulk sample qualitative and quantitative analysis Functional group identification, and Monitoring of chemical modification |
| SR-Based FTIR (With and without ATR) | Synchrotron radiation Very brilliant and Highly collimated 100 to 1000 times brighter than a conventional mid-infrared source | Transmission or reflection mode | Able to penetrate thicker materials | Extremely high Identifies analytes with low concentrations and minimal quantities (down to 1–3 µm in mid-infrared microspectroscopy) | Large-scale synchrotron facilities Expensive infrastructure | High-resolution mapping of complex and heterogeneous materials Micro-domain analysis in biological tissues, and Advanced research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keithellakpam, L.B.; Danielski, R.; Singh, C.B.; Jayas, D.S.; Karunakaran, C. A Comprehensive Review on Minimally Destructive Quality and Safety Assessment of Agri-Food Products: Chemometrics-Coupled Mid-Infrared Spectroscopy. Foods 2025, 14, 3805. https://doi.org/10.3390/foods14223805
Keithellakpam LB, Danielski R, Singh CB, Jayas DS, Karunakaran C. A Comprehensive Review on Minimally Destructive Quality and Safety Assessment of Agri-Food Products: Chemometrics-Coupled Mid-Infrared Spectroscopy. Foods. 2025; 14(22):3805. https://doi.org/10.3390/foods14223805
Chicago/Turabian StyleKeithellakpam, Lakshmi B., Renan Danielski, Chandra B. Singh, Digvir S. Jayas, and Chithra Karunakaran. 2025. "A Comprehensive Review on Minimally Destructive Quality and Safety Assessment of Agri-Food Products: Chemometrics-Coupled Mid-Infrared Spectroscopy" Foods 14, no. 22: 3805. https://doi.org/10.3390/foods14223805
APA StyleKeithellakpam, L. B., Danielski, R., Singh, C. B., Jayas, D. S., & Karunakaran, C. (2025). A Comprehensive Review on Minimally Destructive Quality and Safety Assessment of Agri-Food Products: Chemometrics-Coupled Mid-Infrared Spectroscopy. Foods, 14(22), 3805. https://doi.org/10.3390/foods14223805







