Bioaccumulation and Biomagnification of Mercury Along the Seafood Chain in Europe: A Systematic Review
Abstract
1. Background
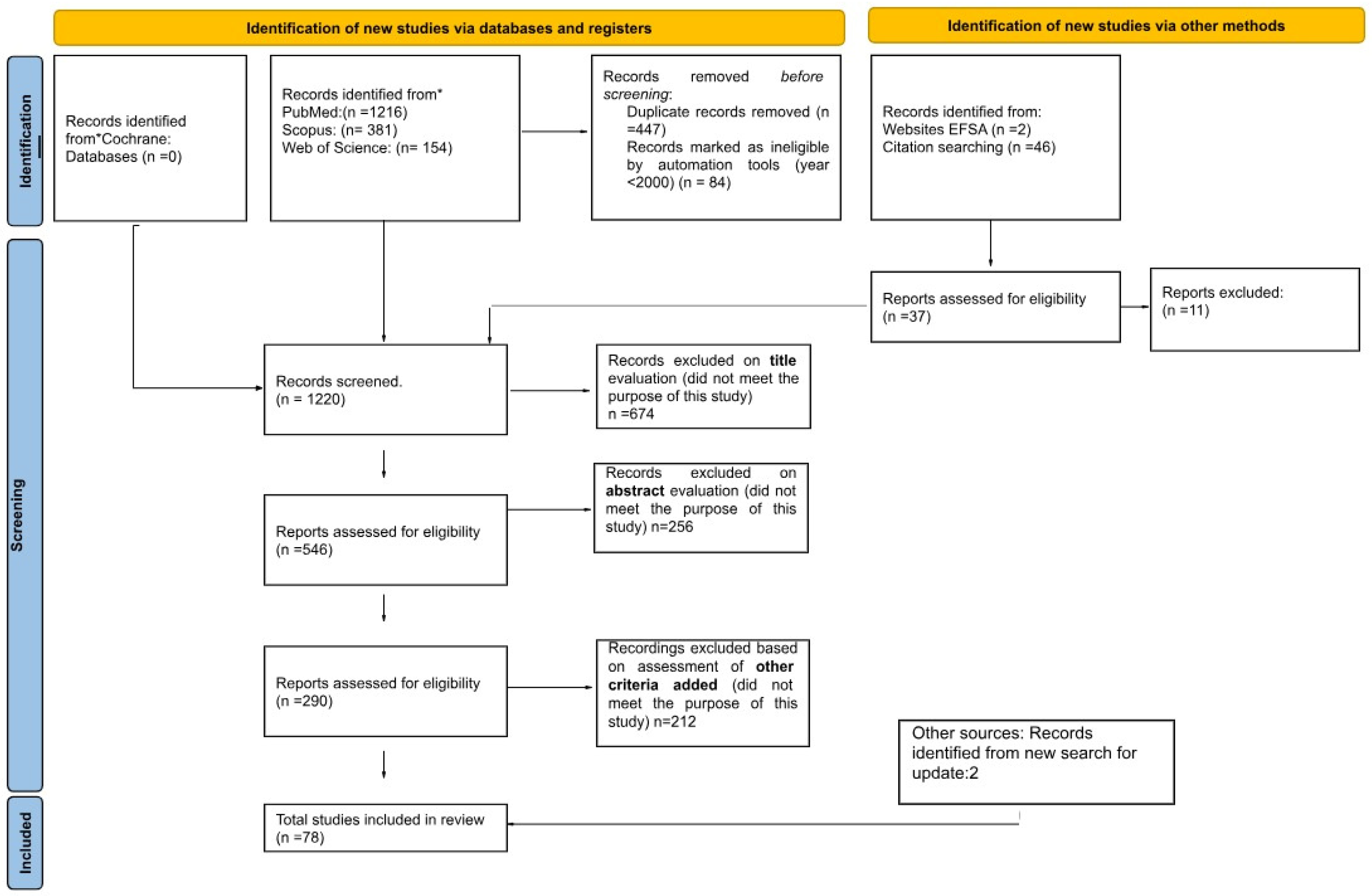
2. Materials and Methods
- Inclusion criteria:
- Studies conducted exclusively in Europe and neighboring countries whose food networks are closely linked to Europe for commercial and geographical reasons.
- Studies conducted on marine fish products.
- Studies conducted only on animals intended for human consumption.
- Exclusion criteria:
- All studies unrelated to Hg concentration.
- All studies related to research on a single organism and/or living beings not intended for human consumption.
- Studies proposing models of heavy metal accumulation.
- Studies conducted on freshwater fish.
- Studies conducted on European territories, but far from the continent itself.
3. Results
3.1. Mediterranean Area Contamination
3.2. Non-Mediterranean Area Contamination
3.3. Farmed Fish
4. Discussion
- Limitations of the Study:
- The vast majority of the selected studies were retrospective in design. This approach may introduce potential biases and limit the ability to establish causal relationships.
- The lack of prospective studies in the selection may restrict the ability to predict future trends or outcomes accurately.
- There was significant variability among the included studies in terms of methodologies, sample sizes, and reporting standards. This heterogeneity makes it challenging to draw consistent conclusions across the entire body of research.
- The initial analysis revealed considerable difficulties in comparing analytical outcomes due to temporal and geographical differences between studies. These disparities may affect the generalizability of the findings and complicate the interpretation of trends over time or across regions.
- Data presentation bias: Some of the included studies exhibited bias in the presentation of data, such as selective aggregation or reporting of results, which may have affected the clarity and interpretability of the findings.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Almeida Rodrigues, P.; Ferrari, R.G.; dos Santos, L.N.; Conte Junior, C.A. Mercury in Aquatic Fauna Contamination: A Systematic Review on Its Dynamics and Potential Health Risks. J. Environ. Sci. 2019, 84, 205–218. [Google Scholar] [CrossRef]
- Scientific Committee on Health and Environmental Risks. Opinion on the Environmental Risks and Indirect Health of Mercury from Dental Amalgam (Update 2014). 2014. Available online: https://health.ec.europa.eu/publications/environmental-risks-and-indirect-health-effects-mercury-dental-amalgam-update-2014_en (accessed on 10 January 2025).
- Marnane, I. Mercury in Europe’s Environment: A Priority for European and Global Action; Publications Office of the European Union: Luxembourg, 2018; ISBN 9789292139841. [Google Scholar]
- Storelli, M.M.; Stuffler, R.G.; Marcotrigiano, G.O. Total and Methylmercury Residues in Tuna-Fish from the Mediterranean Sea. Food Addit. Contam. 2002, 19, 715–720. [Google Scholar] [CrossRef]
- Mille, T.; Bisch, A.; Caill-Milly, N.; Cresson, P.; Deborde, J.; Gueux, A.; Morandeau, G.; Monperrus, M. Distribution of Mercury Species in Different Tissues and Trophic Levels of Commonly Consumed Fish Species from the South Bay of Biscay (France). Mar. Pollut. Bull. 2021, 166, 112172. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on the Risk for Public Health Related to the Presence of Mercury and Methylmercury in Food. EFSA J. 2012, 10, 2985. [CrossRef]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal Seafood Consumption in Pregnancy and Neurodevelopmental Outcomes in Childhood (ALSPAC Study): An Observational Cohort Study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Akinkuolie, A.O.; Wu, J.H.Y.; Ding, E.L.; Gaziano, J.M. Fish Consumption, Omega-3 Fatty Acids and Risk of Heart Failure: A Meta-Analysis. Clin. Nutr. 2012, 31, 846–853. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies); Scientific Opinion on Health Benefits of Seafood (Fish and Shellfish) Consumption in Relation to Health Risks Associated with Exposure to Methylmercury. EFSA J. 2014, 12, 3761. [CrossRef]
- EFSA Scientific Committee. Statement on the Benefits of Fish/Seafood Consumption Compared to the Risks of Methylmercury in Fish/Seafood. EFSA J. 2015, 13, 3982. [Google Scholar] [CrossRef]
- Mantovani, A. Sustainability, Security and Safety in the Feed-to-Fish Chain: Focus on Toxic Contamination. Int. J. Nutr. Food Sci. 2015, 4, 6. [Google Scholar] [CrossRef]
- Thomsen, S.T.; Assunção, R.; Afonso, C.; Boué, G.; Cardoso, C.; Cubadda, F.; Garre, A.; Kruisselbrink, J.W.; Mantovani, A.; Pitter, J.G.; et al. Human Health Risk–Benefit Assessment of Fish and Other Seafood: A Scoping Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7479–7502. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj/eng (accessed on 10 January 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bushman, B.J.; Wang, M.C. Vote-Counting Procedures in Meta-Analysis; Russell Sage Foundation: New York, NY, USA, 2009. [Google Scholar]
- Storelli, M.M.; Marcotrigiano, G.O. Fish for Human Consumption: Risk of Contamination by Mercury. Food Addit. Contam. 2000, 17, 1007–1011. [Google Scholar] [CrossRef]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Storelli, A.; D’Addabbo, R.; Palermo, C.; Marcotrigiano, G.O. Survey of Total Mercury and Methylmercury Levels in Edible Fish from the Adriatic Sea. Food Addit. Contam. 2003, 20, 1114–1119. [Google Scholar] [CrossRef]
- Storelli, M.M.; Barone, G. Toxic Metals (Hg, Pb, and Cd) in Commercially Important Demersal Fish from Mediterranean Sea: Contamination Levels and Dietary Exposure Assessment. J. Food Sci. 2013, 78, T362–T366. [Google Scholar] [CrossRef]
- Llull, R.M.; Garí, M.; Canals, M.; Rey-Maquieira, T.; Grimalt, J.O. Mercury Concentrations in Lean Fish from the Western Mediterranean Sea: Dietary Exposure and Risk Assessment in the Population of the Balearic Islands. Environ. Res. 2017, 158, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Licata, P.; Trombetta, D.; Cristani, M.; Naccari, C.; Martino, D.; Caló, M.; Naccari, F. Heavy Metals in Liver and Muscle of Bluefin Tuna (Thunnus thynnus) Caught in the Straits of Messina (Sicily, Italy). Environ. Monit. Assess. 2005, 107, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Di Lena, G.; Casini, I.; Caproni, R.; Fusari, A.; Orban, E. Total Mercury Levels in Commercial Fish Species from Italian Fishery and Aquaculture. Food Addit. Contam. Part B Surveill. 2017, 10, 118–127. [Google Scholar] [CrossRef]
- Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle. Molecules 2019, 24, 1273. [Google Scholar] [CrossRef] [PubMed]
- Kljaković-Gašpić, Z.; Tičina, V. Mercury and Selenium Levels in Archive Samples of Wild Atlantic Bluefin Tuna from the Mediterranean Sea. Chemosphere 2021, 284, 131402. [Google Scholar] [CrossRef]
- Storelli, M.M.; Marcotrigiano, G.O. Content of Mercury and Cadmium in Fish (Thunnus alalunga) and Cephalopods (Eledone moschata) from the South-Eastern Mediterranean Sea. Food Addit. Contam. 2004, 21, 1051–1056. [Google Scholar] [CrossRef]
- Damiano, S.; Papetti, P.; Menesatti, P. Accumulation of Heavy Metals to Assess the Health Status of Swordfish in a Comparative Analysis of Mediterranean and Atlantic Areas. Mar. Pollut. Bull. 2011, 62, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.; Salvagio Manta, D.; Oliveri, E.; Sprovieri, M.; Basilone, G.; Bonanno, A.; Falco, F.; Traina, A.; Mazzola, S. Mercury in Fishes from Augusta Bay (Southern Italy): Risk Assessment and Health Implication. Food Chem. Toxicol. 2013, 56, 184–194. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.; Cossa, D.; Crochet, S.; Bǎnaru, D.; Letourneur, Y.; Mellon-Duval, C. Difference of Mercury Bioaccumulation in Red Mullets from the North-Western Mediterranean and Black Seas. Mar. Pollut. Bull. 2009, 58, 679–685. [Google Scholar] [CrossRef]
- Di Bella, C.; Traina, A.; Giosuè, C.; Carpintieri, D.; Lo Dico, G.M.; Bellante, A.; Del Core, M.; Falco, F.; Gherardi, S.; Uccello, M.M.; et al. Heavy Metals and PAHs in Meat, Milk, and Seafood From Augusta Area (Southern Italy): Contamination Levels, Dietary Intake, and Human Exposure Assessment. Front. Public Health 2020, 8, 00273. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; Morote, E.; Gil, C.; Ramos-Miras, J.J.; Torrijos, M.; Rodríguez Martin, J.A. Mercury Contents in Relation to Biometrics and Proximal Composition and Nutritional Levels of Fish Eaten from the Western Mediterranean Sea (Almería Bay). Mar. Pollut. Bull. 2018, 135, 783–789. [Google Scholar] [CrossRef]
- Cossa, D.; Harmelin-Vivien, M.; Mellon-Duval, C.; Loizeau, V.; Averty, B.; Crochet, S.; Chou, L.; Cadiou, J.F. Influences of Bioavailability, Trophic Position, and Growth on Methylmercury in Hakes (Merluccius merluccius) from Northwestern Mediterranean and Northeastern Atlantic. Environ. Sci. Technol. 2012, 46, 4885–4893. [Google Scholar] [CrossRef]
- Perugini, M.; Visciano, P.; Manera, M.; Zaccaroni, A.; Olivieri, V.; Amorena, M. Levels of Total Mercury in Marine Organisms from Adriatic Sea, Italy. Bull. Environ. Contam. Toxicol. 2009, 83, 244–248. [Google Scholar] [CrossRef]
- Cirillo, T.; Fasano, E.; Viscardi, V.; Arnese, A.; Amodio-Cocchieri, R. Survey of Lead, Cadmium, Mercury and Arsenic in Seafood Purchased in Campania, Italy. Food Addit. Contam. Part B Surveill. 2010, 3, 30–38. [Google Scholar] [CrossRef]
- Miniero, R.; Abate, V.; Brambilla, G.; Davoli, E.; De Felip, E.; De Filippis, S.P.; Dellatte, E.; De Luca, S.; Fanelli, R.; Fattore, E.; et al. Persistent Toxic Substances in Mediterranean Aquatic Species. Sci. Total Environ. 2014, 494–495, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Marcotrigiano, G.O. Total and Methylmercury Residues in Cartilaginous Fish from Mediterranean Sea. Mar. Pollut. Bull. 2002, 44, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Mancini, L.; Miniero, R.; Beccaloni, E.; di Domenico, K.; Lacchetti, I.; Puccinelli, C.; Cicero, M.R.; Scaini, F.; Carere, M. Mercury (Hg) and Methylmercury (MeHg) in Sediment and Biota: A Case Study in a Lagoon in Central Italy. Mar. Pollut. Bull. 2022, 175, 113308. [Google Scholar] [CrossRef]
- Signa, G.; Mazzola, A.; Tramati, C.D.; Vizzini, S. Diet and Habitat Use Influence Hg and Cd Transfer to Fish and Consequent Biomagnification in a Highly Contaminated Area: Augusta Bay (Mediterranean Sea). Environ. Pollut. 2017, 230, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Prearo, M.; Gavinelli, S.; Pellegrino, M.; Tarasco, R.; Benedetto, A.; Abete, M.C. Heavy Metals in Mugil cephalus (Mugilidae) from the Ligurian Sea (North-West Mediterranean, Italy). Food Addit. Contam. Part B Surveill. 2013, 6, 134–138. [Google Scholar] [CrossRef]
- Miedico, O.; Iammarino, M.; Pompa, C.; Tarallo, M.; Chiaravalle, A.E. Assessment of Lead, Cadmium and Mercury in Seafood Marketed in Puglia and Basilicata (Italy) by Inductively Coupled Plasma Mass Spectrometry. Food Addit. Contam. Part B Surveill. 2015, 8, 85–92. [Google Scholar] [CrossRef]
- Horvat, M.; Degenek, N.; Lipej, L.; Snoj Tratnik, J.; Faganeli, J. Trophic Transfer and Accumulation of Mercury in Ray Species in Coastal Waters Affected by Historic Mercury Mining (Gulf of Trieste, Northern Adriatic Sea). Environ. Sci. Pollut. Res. 2014, 21, 4163–4176. [Google Scholar] [CrossRef]
- Ancora, S.; Mariotti, G.; Ponchia, R.; Fossi, M.C.; Leonzio, C.; Bianchi, N. Trace Elements Levels in Muscle and Liver of a Rarely Investigated Large Pelagic Fish: The Mediterranean Spearfish Tetrapturus Belone (Rafinesque, 1810). Mar. Pollut. Bull. 2020, 151, 110878. [Google Scholar] [CrossRef]
- Di Bella, G.; Potortì, A.G.; Lo Turco, V.; Bua, D.; Licata, P.; Cicero, N.; Dugo, G. Trace Elements in Thunnus Thynnus from Mediterranean Sea and Benefit–Risk Assessment for Consumers. Food Addit. Contam. Part B Surveill. 2015, 8, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; De Roma, A.; La Nucara, R.; Picazio, G.; Gallo, P. Total Mercury Content in Commercial Swordfish (Xiphias gladius) from Different FAO Fishing Areas. Chemosphere 2018, 197, 14–19. [Google Scholar] [CrossRef] [PubMed]
- García, M.Á.; Núñez, R.; Alonso, J.; Melgar, M.J. Total Mercury in Fresh and Processed Tuna Marketed in Galicia (NW Spain) in Relation to Dietary Exposure. Environ. Sci. Pollut. Res. 2016, 23, 24960–24969. [Google Scholar] [CrossRef]
- Rubio, C.; Gutiérrez, Á.; Burgos, A.; Hardisson, A. Total Dietary Intake of Mercury in the Canary Islands, Spain. Food Addit. Contam. Part A 2008, 25, 946–952. [Google Scholar] [CrossRef]
- Nepusz, T.; Petróczi, A.; Naughton, D.P. Food Alert Patterns for Metal Contamination Analyses in Seafoods: Longitudinal and Geographical Perspectives. Environ. Int. 2009, 35, 1030–1033. [Google Scholar] [CrossRef]
- Storelli, M.M.; Giacominelli Stuffler, R.; Storelli, A.; Marcotrigiano, G.O. Total Mercury and Methylmercury Content in Edible Fish from the Mediterranean Sea. J. Food Prot. 2003, 66, 300–303. [Google Scholar] [CrossRef]
- Jureša, D.; Blanuša, M. Mercury, Arsenic, Lead and Cadmium in Fish and Shellfish from the Adriatic Sea. Food Addit. Contam. 2003, 20, 241–246. [Google Scholar] [CrossRef]
- Millour, S.; Noël, L.; Kadar, A.; Chekri, R.; Vastel, C.; Sirot, V.; Leblanc, J.C.; Guérin, T. Pb, Hg, Cd, As, Sb and Al Levels in Foodstuffs from the 2nd French Total Diet Study. Food Chem. 2011, 126, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Biton-Porsmoguer, S.; Bănaru, D.; Harmelin-Vivien, M.; Béarez, P.; Bouchoucha, M.; Marco-Miralles, F.; Marquès, M.; Lloret, J. A Study of Trophic Structure, Physiological Condition and Mercury Biomagnification in Swordfish (Xiphias gladius): Evidence of Unfavourable Conditions for the Swordfish Population in the Western Mediterranean. Mar Pollut Bull 2022, 176, 113411. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Bilbao, E.; Lozano, G.; Jurado-Ruzafa, A.; Hardisson, A.; Rubio, C.; Paz, S.; Martín, V.; Gutiérrez, Á.J. Interdecadal Variations of the Mercury Content in Scomber Colias in Canary Islands. Environ. Sci. Pollut. Res. 2023, 30, 8347–8353. [Google Scholar] [CrossRef] [PubMed]
- Merciai, R.; Rodríguez-Prieto, C.; Torres, J.; Casadevall, M. Bioaccumulation of Mercury and Other Trace Elements in Bottom-Dwelling Omnivorous Fishes: The Case of Diplodus sargus (L.) (Osteichthyes: Sparidae). Mar. Pollut. Bull. 2018, 136, 10–21. [Google Scholar] [CrossRef]
- Milatou, N.; Miliou, H.; Dassenakis, M.; Megalofonou, P. Trace Metal Accumulation in Atlantic Bluefin Tuna and Correlations with Protein-Lipid Composition. Food Chem. 2023, 404, 134691. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.; Bodiguel, X.; Charmasson, S.; Loizeau, V.; Mellon-Duval, C.; Tronczyński, J.; Cossa, D. Differential Biomagnification of PCB, PBDE, Hg and Radiocesium in the Food Web of the European Hake from the NW Mediterranean. Mar. Pollut. Bull. 2012, 64, 974–983. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Caproni, R.; Orban, E. Total Mercury Levels in Crustacean Species from Italian Fishery. Food Addit. Contam. Part B Surveill. 2018, 11, 175–182. [Google Scholar] [CrossRef]
- Stamatis, N.; Kamidis, N.; Pigada, P.; Stergiou, D.; Kallianiotis, A. Bioaccumulation Levels and Potential Health Risks of Mercury, Cadmium, and Lead in Albacore (Thunnus alalunga, Bonnaterre, 1788) from the Aegean Sea, Greece. Int. J. Environ. Res. Public Health 2019, 16, 821. [Google Scholar] [CrossRef] [PubMed]
- Junqué, E.; Garí, M.; Llull, R.M.; Grimalt, J.O. Drivers of the Accumulation of Mercury and Organochlorine Pollutants in Mediterranean Lean Fish and Dietary Significance. Sci. Total Environ. 2018, 634, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, A.; Cardellicchio, N.; Giandomenico, S.; Spada, L. Mercury and Methylmercury Contamination in Mytilus galloprovincialis from Taranto Gulf (Ionian Sea, Southern Italy): Risk Evaluation for Consumers. Food Chem. Toxicol. 2010, 48, 3131–3136. [Google Scholar] [CrossRef]
- Visciano, P.; Scortichini, G.; Suzzi, G.; Diletti, G.; Schirone, M.; Martino, G. Concentrations of Contaminants with Regulatory Limits in Samples of Clam (Chamelea gallina) Collected along the Abruzzi Region Coast in Central Italy. J. Food Prot. 2015, 78, 1719–1728. [Google Scholar] [CrossRef]
- Gutiérrez, A.J.; Lozano, G.; González, T.; Reguera, J.I.; Hardisson, A. Mercury Content in Tinned Molluscs (Mussel, Cockle, Variegated Scallop, and Razor Shell) Normally Consumed in Spain, 2005. J. Food Prot. 2006, 69, 2237–2240. [Google Scholar] [CrossRef]
- Azad, A.M.; Frantzen, S.; Bank, M.S.; Johnsen, I.A.; Tessier, E.; Amouroux, D.; Madsen, L.; Maage, A. Spatial Distribution of Mercury in Seawater, Sediment, and Seafood from the Hardangerfjord Ecosystem, Norway. Sci. Total Environ. 2019, 667, 622–637. [Google Scholar] [CrossRef]
- Blanco, S.L.; González, J.C.; Vieites, J.M. Mercury, Cadmium and Lead Levels in Samples of the Main Traded Fish and Shellfish Species in Galicia, Spain. Food Addit. Contam. Part B Surveill. 2008, 1, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Polak-Juszczak, L. Bioaccumulation of Mercury in the Trophic Chain of Flatfish from the Baltic Sea. Chemosphere 2012, 89, 585–591. [Google Scholar] [CrossRef]
- Jędruch, A.; Bełdowska, M.; Kwasigroch, U. Forms of Mercury in the Baltic Mussel (Mytilus trossulus): Human and Ecosystem Health Risk Assessment. Environ. Res. 2019, 179, 108755. [Google Scholar] [CrossRef]
- Wilman, B.; Bełdowska, M.; Normant-Saremba, M. Labile and Stable Mercury in Harris Mud Crab (Rhithropanopeus harrisii) from the Southern Baltic Sea–Considerations for a Role of Non-Native Species in the Food Web. Mar. Pollut. Bull. 2019, 148, 116–122. [Google Scholar] [CrossRef]
- Biton-Porsmoguer, S.; Bǎnaru, D.; Boudouresque, C.F.; Dekeyser, I.; Bouchoucha, M.; Marco-Miralles, F.; Lebreton, B.; Guillou, G.; Harmelin-Vivien, M. Mercury in Blue Shark (Prionace glauca) and Shortfin Mako (Isurus oxyrinchus) from North-Eastern Atlantic: Implication for Fishery Management. Mar. Pollut. Bull. 2018, 127, 131–138. [Google Scholar] [CrossRef]
- Novakov, N.J.; Mihaljev, Ž.A.; Kartalović, B.D.; Blagojević, B.J.; Petrović, J.M.; Ćirković, M.A.; Rogan, D.R. Heavy Metals and PAHs in Canned Fish Supplies on the Serbian Market. Food Addit. Contam. Part B Surveill. 2017, 10, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Cabañero, A.I.; Carvalho, C.; Madrid, Y.; Batoréu, C.; Cámara, C. Quantification and Speciation of Mercury and Selenium in Fish Samples of High Consumption in Spain and Portugal. Biol. Trace Elem. Res. 2005, 103, 17–35. [Google Scholar] [CrossRef]
- Djermanovic, M.; Baralic, I.; Pejic, S. Total Mercury Levels in Commercial Fish in Market of the Republic of Srpska, Bosnia and Herzegovina. Biol. Trace Elem. Res. 2020, 194, 545–551. [Google Scholar] [CrossRef]
- Magalhães, M.C.; Costa, V.; Menezes, G.M.; Pinho, M.R.; Santos, R.S.; Monteiro, L.R. Intra- and Inter-Specific Variability in Total and Methylmercury Bioaccumulation by Eight Marine Fish Species from the Azores. Mar. Pollut. Bull. 2007, 54, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Besada, V.; Andrade, J.M.; Schultze, F.; González, J.J. Comparison of the 2000 and 2005 Spatial Distributions of Heavy Metals in Wild Mussels from the North-Atlantic Spanish Coast. Ecotoxicol. Environ. Saf. 2011, 74, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, J.; Caetano, M.; Vale, C. Geographical Variation and Partition of Metals in Tissues of Octopus vulgaris along the Portuguese Coast. Sci. Total Environ. 2004, 325, 71–81. [Google Scholar] [CrossRef]
- de Oliveira Novaes, E.; de Oliveira, A.T.; Araruna, L.T.; de Souza, J.S.; de Pinho, J.V.; de Almeida Rodrigues, P.; Vieira, I.R.S.; Conte-Junior, C.A. Mercury Levels in the Worldwide Farmed Fish: A Systematic Review. Biol. Trace Elem. Res. 2025, 203, 4361–4375. [Google Scholar] [CrossRef]
- Lundebye, A.K.; Lock, E.J.; Rasinger, J.D.; Nøstbakken, O.J.; Hannisdal, R.; Karlsbakk, E.; Wennevik, V.; Madhun, A.S.; Madsen, L.; Graff, I.E.; et al. Lower Levels of Persistent Organic Pollutants, Metals and the Marine Omega 3-Fatty Acid DHA in Farmed Compared to Wild Atlantic Salmon (Salmo salar). Environ. Res. 2017, 155, 49–59. [Google Scholar] [CrossRef]
- Pawlaczyk, A.; Przerywacz, A.; Gajek, M.; Szynkowska-Jozwik, M.I. Risk of Mercury Ingestion from Canned Fish in Poland. Molecules 2020, 25, 5884. [Google Scholar] [CrossRef]
- Carrasco, L.; Benejam, L.; Benito, J.; Bayona, J.M.; Díez, S. Methylmercury Levels and Bioaccumulation in the Aquatic Food Web of a Highly Mercury-Contaminated Reservoir. Environ. Int. 2011, 37, 1213–1218. [Google Scholar] [CrossRef]
- Storelli, M.M.; Marcotrigiano, G.O. Bioindicator Organisms: Heavy Metal Pollution Evaluation in the Ionian Sea (Mediterranean Sea-Italy). Environ. Monit. Assess. 2005, 102, 159–166. [Google Scholar] [CrossRef]
- Milatou, N.; Dassenakis, M.; Megalofonou, P. Mercury Concentrations in Reared Atlantic Bluefin Tuna and Risk Assessment for the Consumers: To Eat or Not to Eat? Food Chem. 2020, 331, 127267. [Google Scholar] [CrossRef]
- Costa, F.; Coelho, J.P.; Baptista, J.; Martinho, F.; Pereira, E.; Pardal, M.A. Lifelong Mercury Bioaccumulation in Atlantic Horse Mackerel (Trachurus trachurus) and the Potential Risks to Human Consumption. Mar. Pollut. Bull. 2021, 173, 113015. [Google Scholar] [CrossRef]
- Mauffret, A.; Chouvelon, T.; Wessel, N.; Cresson, P.; Bănaru, D.; Baudrier, J.; Bustamante, P.; Chekri, R.; Jitaru, P.; Le Loc’h, F.; et al. Trace Elements, Dioxins and PCBs in Different Fish Species and Marine Regions: Importance of the Taxon and Regional Features. Environ. Res. 2023, 216, 114624. [Google Scholar] [CrossRef]
- Ervik, H.; Finne, T.E.; Jenssen, B.M. Toxic and Essential Elements in Seafood from Mausund, Norway. Environ. Sci. Pollut. Res. 2018, 25, 7409–7417. [Google Scholar] [CrossRef]
- Kammann, U.; Nogueira, P.; Siegmund, M.; Schmidt, N.; Schmolke, S.; Kirchgeorg, T.; Hasenbein, M.; Wysujack, K. Temporal Trends of Mercury Levels in Fish (Dab, Limanda limanda) and Sediment from the German Bight (North Sea) in the Period 1995–2020. Environ. Monit. Assess. 2022, 195, 73. [Google Scholar] [CrossRef]
- Knowles, T.G.; Farrington, D.; Kestin, S.C. Mercury in UK Imported Fish and Shellfish and UK-Farmed Fish and Their Products. Food Addit. Contam. 2003, 20, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Bank, M.S.; Frantzen, S.; Duinker, A.; Amouroux, D.; Tessier, E.; Nedreaas, K.; Maage, A.; Nilsen, B.M. Rapid Temporal Decline of Mercury in Greenland Halibut (Reinhardtius Hippoglossoides). Environ. Pollut. 2021, 289, 117843. [Google Scholar] [CrossRef] [PubMed]
- Polak-Juszczak, L. Total Mercury and Methylmercury in Garfish (Belone belone) of Different Body Weights, Sizes, Ages, and Sexes. J. Trace Elem. Med. Biol. 2023, 79, 127220. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Marcotrigiano, G.O. Total Mercury Levels in Muscle Tissue of Swordfish (Xiphias Gladius) and Bluefin Tuna (Thunnus Thynnus) from the Mediterranean Sea (Italy). J. Food Prot. 2001, 64, 1058–1061. [Google Scholar] [CrossRef]
- Boquete, M.T.; Aboal, J.R.; Villares, R.; Dorado-García, U.; Fernández, J.Á. High Hg Biomagnification in North Atlantic Coast Ecosystems and Limits to the Use of Δ15N to Estimate Trophic Magnification Factors. Water Res. 2023, 234, 119793. [Google Scholar] [CrossRef]
- Secretariat of the Minamat Convention on Mercury. Minamata Convention on Mercury Text and Annexes, 2024th ed.; Secretariat of the Minamat Convention on Mercury: Geneva, Switzerland, 2024. [Google Scholar]
- Minganti, V.; Drava, G.; De Pellegrini, R.; Siccardi, C. Trace Elements in Farmed and Wild Gilthead Seabream, Sparus Aurata. Mar. Pollut. Bull. 2010, 60, 2022–2025. [Google Scholar] [CrossRef]
- Acquavita, A.; Bettoso, N. Mercury and Selenium in the Grass Goby Zosterisessor ophiocephalus (Pisces: Gobiidae) from a Mercury Contaminated Mediterranean Lagoon. Mar. Pollut. Bull. 2018, 135, 75–82. [Google Scholar] [CrossRef]
- Faganeli, J.; Falnoga, I.; Horvat, M.; Klun, K.; Lipej, L.; Mazej, D. Selenium and Mercury Interactions in Apex Predators from the Gulf of Trieste (Northern Adriatic Sea). Nutrients 2018, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Burioli, E.A.V.; Squadrone, S.; Stella, C.; Foglini, C.; Abete, M.C.; Prearo, M. Trace Element Occurrence in the Pacific Oyster Crassostrea Gigas from Coastal Marine Ecosystems in Italy. Chemosphere 2017, 187, 248–260. [Google Scholar] [CrossRef]
- Carvalho, C.M.L.; Matos, A.I.N.M.; Mateus, M.L.; Santos, A.P.M.; Batoréu, M.C.C. High-Fish Consumption and Risk Prevention: Assessment of Exposure to Methylmercury in Portugal. J. Toxicol. Environ. Health Part A Curr. Issues 2008, 71, 1279–1288. [Google Scholar] [CrossRef]
- Nunes, E.; Cavaco, A.; Carvalho, C. Exposure Assessment of Pregnant Portuguese Women to Methylmercury through the Ingestion of Fish: Cross-Sectional Survey and Biomarker Validation. J. Toxicol. Environ. Health Part A Curr. Issues 2014, 77, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Vasco, E.; Paixão, E.; Alvito, P. Total Mercury in Infant Food, Occurrence and Exposure Assessment in Portugal. Food Addit. Contam. Part B Surveill. 2013, 6, 151–157. [Google Scholar] [CrossRef]
- Soler-Blasco, R.; Murcia, M.; Lozano, M.; Aguinagalde, X.; Iriarte, G.; Lopez-Espinosa, M.J.; Vioque, J.; Iñiguez, C.; Ballester, F.; Llop, S. Exposure to Mercury among 9-Year-Old Spanish Children: Associated Factors and Trend throughout Childhood. Environ. Int. 2019, 130, 104835. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Correig, E.; Marmelo, I.; Marques, A.; la Cour, R.; Sloth, J.J.; Nadal, M.; Marquès, M.; Domingo, J.L. Dietary Exposure to Potentially Toxic Elements through Sushi Consumption in Catalonia, Spain. Food Chem. Toxicol. 2021, 153, 112285. [Google Scholar] [CrossRef]
- Moreno-Ortega, A.; Moreno-Rojas, R.; Martínez-Álvarez, J.R.; González Estecha, M.; Castro González, N.P.; Amaro López, M.Á. Probabilistic Risk Analysis of Mercury Intake via Food Consumption in Spain. J. Trace Elem. Med. Biol. 2017, 43, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Mieiro, C.L.; Pacheco, M.; Duarte, A.C.; Pereira, M.E. Fish Consumption and Risk of Contamination by Mercury-Considerations on the Definition of Edible Parts Based on the Case Study of European Sea Bass. Mar. Pollut. Bull. 2011, 62, 2850–2853. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Coelho, J.P.; Baptista, J.; Martinho, F.; Pereira, M.E.; Pardal, M.A. Mercury Accumulation in Fish Species along the Portuguese Coast: Are There Potential Risks to Human Health? Mar. Pollut. Bull. 2020, 150, 110740. [Google Scholar] [CrossRef] [PubMed]
| Level of Exceedance in Respect to EU Legal Limits: | n. of Samples (%) | |
|---|---|---|
| mg/Kg w.w. | 2000–2011 | 2012–2024 |
| (a) 0.0–0.59 | 21 (26.58%) | 47 (20.61%) |
| (b) 0.60–1.00 | 1 (1.27%) | 3 (1.32%) |
| (c) 1.01–1.49 | 2 (2.53%) | 5 (2.19%) |
| (d) ≥1.50 | 3 (3.80%) | 10 (4.39%) |
| Samples exceeding EU legal limits | 27 (34.18%) | 65 (28.51%) |
| Overall analyzed samples | 79 | 228 |
| Reference (Year) | Sampling Location | Species Analyzed | Sample Size | Range of Maximum THg Level (mg/Kg ww) Detected in Studies |
|---|---|---|---|---|
| Storelli (2000) [16] | Adriatic Sea | Lophius budegassa 1 (Monkfish) | 461 | 0.74–1.26 |
| Storelli (2003) [17] | Adriatic Sea | |||
| Storelli (2013) [18] | Adriatic Sea | |||
| Storelli (2000) [16] | Adriatic Sea | Lophius piscatorius 1 (Monkfish) | 154 | 0.76–1.26 |
| Llull (2017) [19] | Balearic Sea | |||
| Storelli (2002) [4] | Adriatic and Ionian Seas | Thunnus thynnus 2 (Tuna fish) | 368 | 1.02–3.37 |
| Licata (2004) [20] | Tyrrhenian and Ionian Seas | |||
| Di Lena (2017) [21] | Adriatic and Tyrrhenian Seas | |||
| Annibaldi (2019) [22] | Mediterranean Sea | |||
| Kljaković-Gaspić (2021) [23] | Adriatic Sea | |||
| Storelli (2004) [24] | Adriatic Sea | Thunnus alalunga 2 (Tuna fish) | 264 | 1.17–1.56 |
| Storelli (2002) [4] | Adriatic and Ionian Seas | |||
| Damiano (2011) [25] | Northwestern and North-central Atlantic, Tyrrhenian and Ionian Seas | Xiphias gladius 2 (Swordfish) | 34 | 1.04–2.41 |
| Storelli (2013) [18] | Adriatic Sea | Conger conger 1 (Conger) | 180 | 0.56–1.14 |
| Llull (2017) [19] | Balearic Sea | |||
| Bonsignore (2013) [26] | Ionian Sea | Mullus barbatus 2 (Mullet) | 142 | 1.11–1.91 |
| Harmelin-Vivien (2009) [27] | Gulf of Lion and Romanian Black Sea | |||
| Di Bella (2020) [28] | Ionian Sea | |||
| Sánchez-Muros (2018) [29] | Balearic Sea | Merluccius merluccius 1 (Haddock) | 456 | 0.59–1.67 |
| Cossa (2012) [30] | Northeastern Atlantic and Gulf of Lion | |||
| Perugini (2009) [31] | Adriatic Sea |
| Species | No. of Studies | Overall Sample Size (N) | Median (IQR) | Studies with Positive Result (%) | No. of Samples Above ML (%) |
|---|---|---|---|---|---|
| Conger conger 1 (Conger) | 2 | 180 | 1.14 (1.14–1.14) | 2 (100%) | 180 (100%) |
| Lophius budegassa 1 (Monkfish) | 3 | 461 | 0.76 (0.68–0.76) | 3 (100%) | 461 (100%) |
| Lophius piscatorius 1 (Monkfish) | 3 | 160 | 1.26 (1.00–1.26) | 3 (100%) | 154 (96.3%) |
| Merluccius merluccius 1 (Haddock) | 8 | 602 | 1.67 (0.59–1.67) | 3 (37.5%) | 461 (76.6%) |
| Mullus barbatus 2 (Mullet) | 10 | 1271 | 0.31 (0.43–0.70) | 2 (20%) | 138 (10.9%) |
| Thunnus alalunga 2 (Tuna fish) | 3 | 346 | 1.17 (1.17–1.56) | 2 (66,7%) | 264 (76.3%) |
| Thunnus thynnus 2 (Tuna fish) | 9 | 982 | 0.87 (0.86–1.02) | 5 (55.6%) | 384 (39.1%) |
| Xiphias gladius 2 (Swordfish) | 5 | 292 | 0.49 (0.49–0.62) | 2 (40%) | 35 (12%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioravanti, R.; Muzzioli, L.; Maurel, E.; Palma, G.; Calabrese, G.; Angioni, A.; La Rocca, C.; Mantovani, A.; Pezzana, A.; Donini, L.M. Bioaccumulation and Biomagnification of Mercury Along the Seafood Chain in Europe: A Systematic Review. Foods 2025, 14, 3752. https://doi.org/10.3390/foods14213752
Fioravanti R, Muzzioli L, Maurel E, Palma G, Calabrese G, Angioni A, La Rocca C, Mantovani A, Pezzana A, Donini LM. Bioaccumulation and Biomagnification of Mercury Along the Seafood Chain in Europe: A Systematic Review. Foods. 2025; 14(21):3752. https://doi.org/10.3390/foods14213752
Chicago/Turabian StyleFioravanti, Riccardo, Luca Muzzioli, Eleonora Maurel, Giuseppe Palma, Giorgio Calabrese, Alberto Angioni, Cinzia La Rocca, Alberto Mantovani, Andrea Pezzana, and Lorenzo Maria Donini. 2025. "Bioaccumulation and Biomagnification of Mercury Along the Seafood Chain in Europe: A Systematic Review" Foods 14, no. 21: 3752. https://doi.org/10.3390/foods14213752
APA StyleFioravanti, R., Muzzioli, L., Maurel, E., Palma, G., Calabrese, G., Angioni, A., La Rocca, C., Mantovani, A., Pezzana, A., & Donini, L. M. (2025). Bioaccumulation and Biomagnification of Mercury Along the Seafood Chain in Europe: A Systematic Review. Foods, 14(21), 3752. https://doi.org/10.3390/foods14213752









