GA4/GA7 Deficiency and Downregulated Ent-Kaurenoic Acid Oxidase Impair Seedless Mango Fruit Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Detection of Gibberellins
2.3. GA3 and GA4+7 Treatment
2.4. Transcriptome Sequencing and Analysis
2.5. Tissue-Specific Expression Patterns and Heatmap Visualization
2.6. Phylogenetic Tree Construction and Protein Alignment
2.7. GAs and Their Metabolite Genes Correlation Analysis
2.8. Statistical Analysis
3. Results
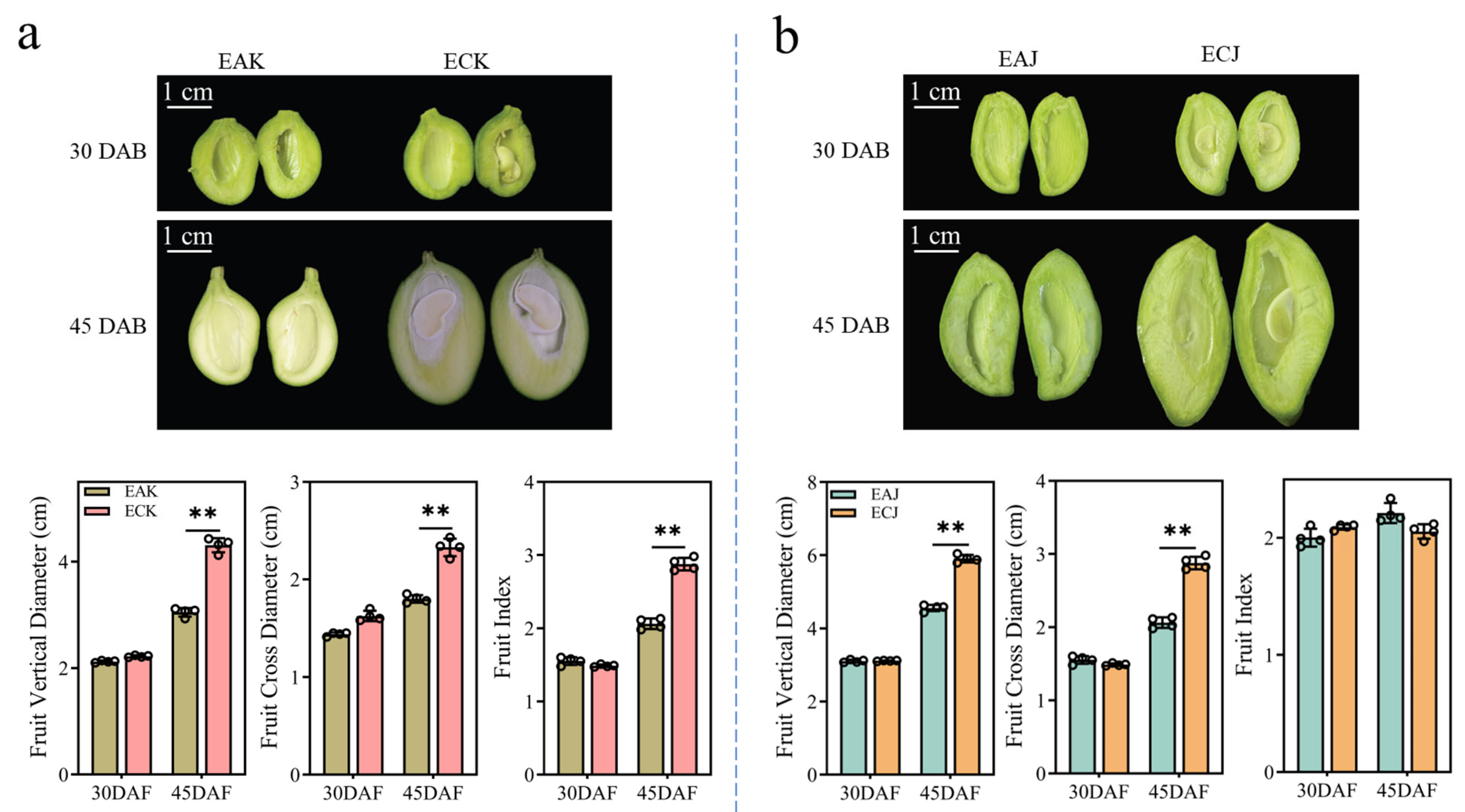
3.1. The Period 30 to 45 Days After Bloom Is Crucial for Seedless Fruit Development
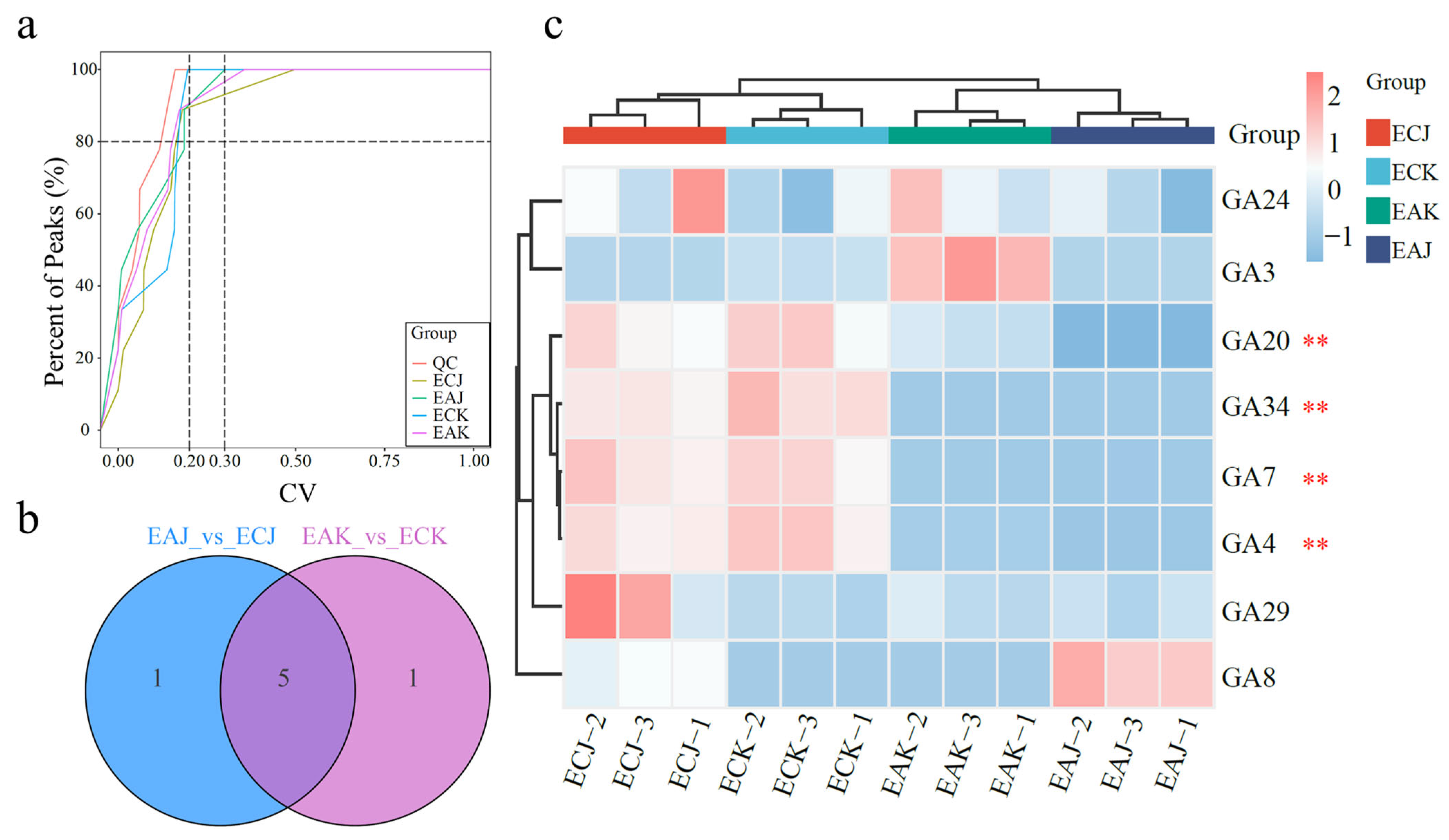
3.2. Targeted Metabolomics of GAs Highlight GA4 and GA7 as Key Drivers of Mango Fruit Development
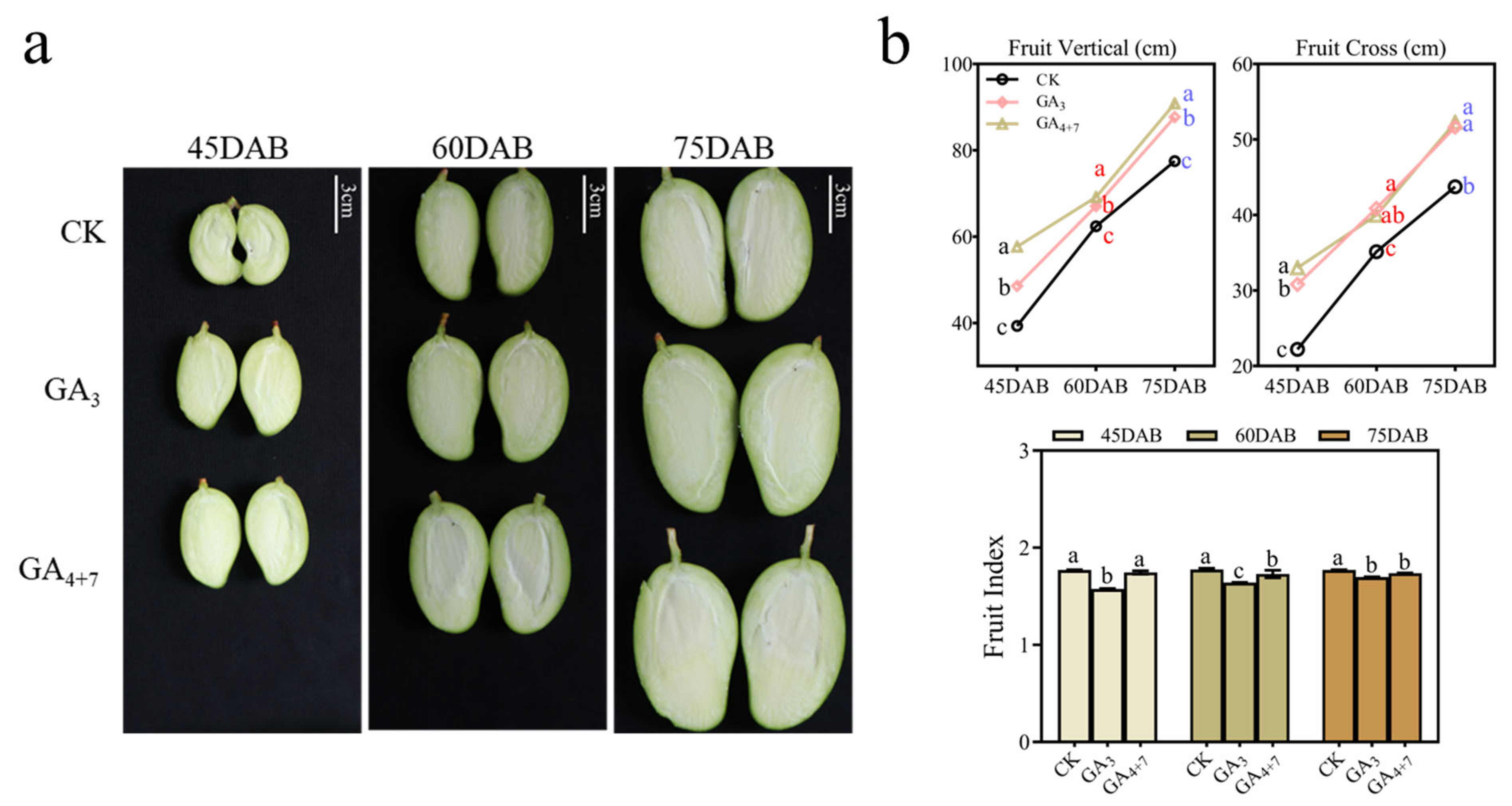
3.3. GA4+7 Outperforms GA3 in Promoting Development of EA ‘Jinhuang’ Mangoes
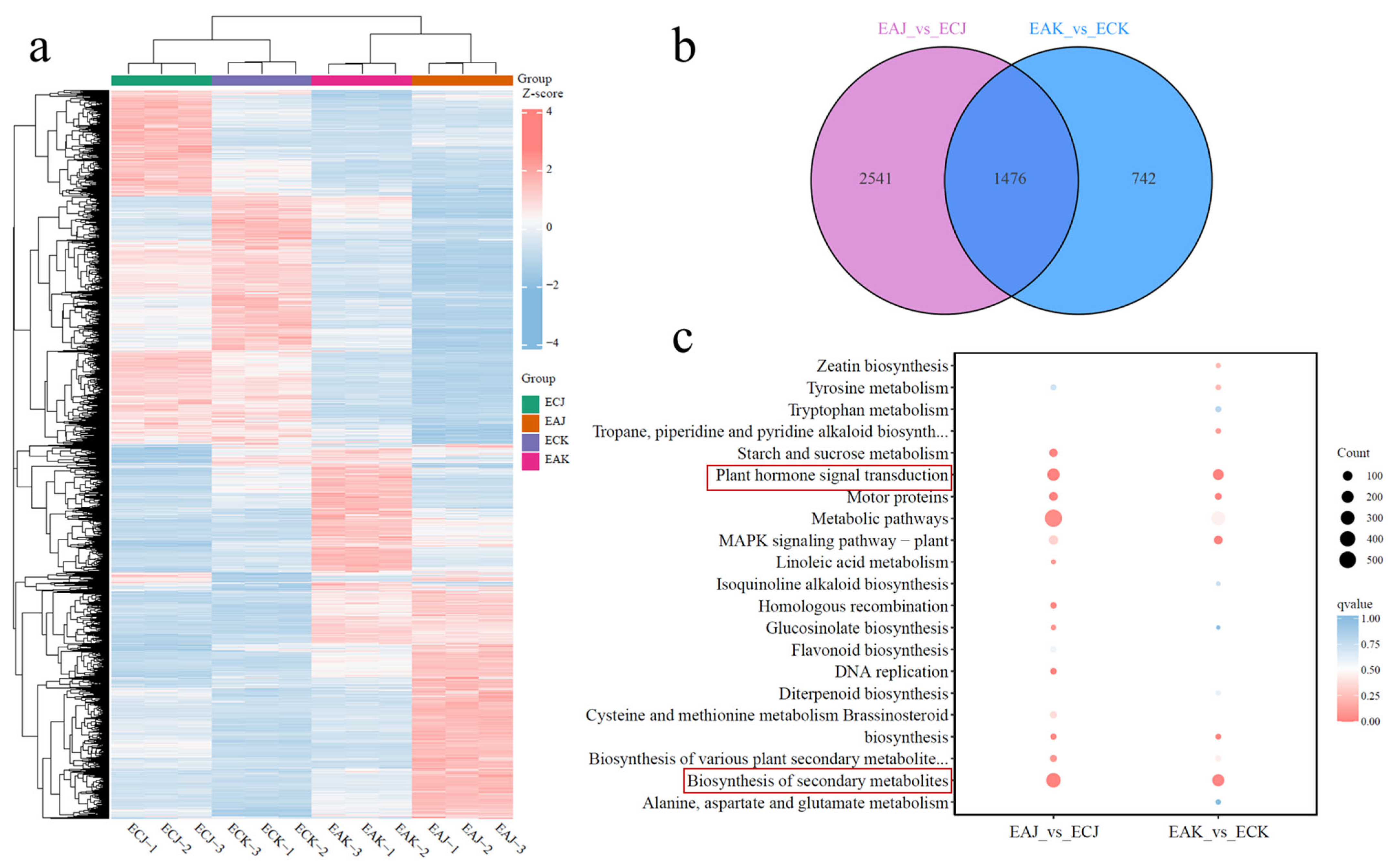
3.4. Differential Transcriptomes of EA and EC Fruits Reveal Enrichment in Hormone Signaling and Secondary Metabolite Biosynthesis
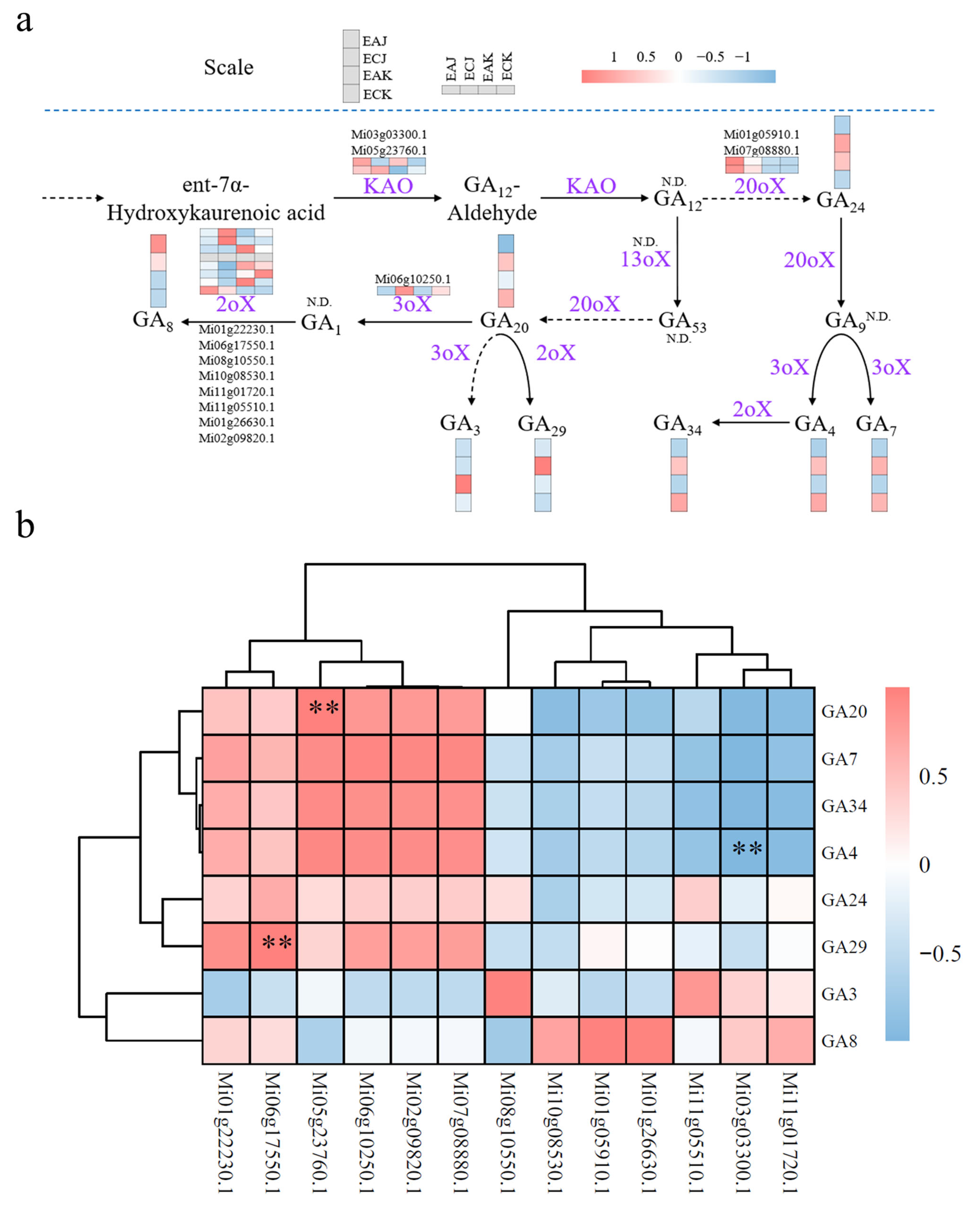
3.5. KAO Expression Associates with Divergent GA Profiles in EA and EC Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GA | Gibberellin |
| DAB | Days after blooming |
| EA | Embryo-absent |
| EC | Embryo-containing |
References
- Ma, X.; Wu, H.; Liu, B.; Wang, S.; Zhang, Y.; Su, M.; Zheng, B.; Pan, H.; Du, B.; Wang, J.; et al. Genomic diversity, population structure, and genome-wide association reveal genetic differentiation and trait improvements in mango. Hortic. Res. 2024, 11, uhae153. [Google Scholar] [CrossRef]
- Diop, A.; Méot, J.-M.; Léchaudel, M.; Chiroleu, F.; Ndiaye, N.D.; Mertz, C.; Cissé, M.; Chillet, M. Impact of Preharvest and Postharvest on Color Changes during Convective Drying of Mangoes. Foods 2021, 10, 490. [Google Scholar] [CrossRef]
- Cong, L.; Yue, R.; Wang, H.; Liu, J.; Zhai, R.; Yang, J.; Wu, M.; Si, M.; Zhang, H.; Yang, C.; et al. 2,4-D-induced parthenocarpy in pear is mediated by enhancement of GA(4) biosynthesis. Physiol. Plant. 2019, 166, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Venkataratnam, L. Hormone induced set and parthenocarpy in mango (Mangifera indica L.). Curr. Sci. 1949, 18, 409. [Google Scholar]
- Zheng, Y.; Shi, J.; Pan, Z.; Cheng, Y.; Zhang, Y.; Li, N. Effect of heat treatment, pH, sugar concentration, and metal ion addition on green color retention in homogenized puree of Thompson seedless grape. LWT Food Sci. Technol. 2014, 55, 595–603. [Google Scholar] [CrossRef]
- Pandit, S.S.; Kulkarni, R.S.; Giri, A.P.; Köllner, T.G.; Degenhardt, J.; Gershenzon, J.; Gupta, V.S. Expression profiling of various genes during the fruit development and ripening of mango. Plant Physiol. Biochem. 2010, 48, 426–433. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Y.; Huang, J.; Gao, S.; Zhu, G.; Dang, Z.; Gai, J.; Yang, M.; Zhu, M.; Zhang, H. The genome evolution and domestication of tropical fruit mango. Genome Biol. 2020, 21, 60. [Google Scholar] [CrossRef]
- Li, L.; Wu, H.X.; Ma, X.W.; Xu, W.T.; Liang, Q.Z.; Zhan, R.L.; Wang, S.B. Transcriptional mechanism of differential sugar accumulation in pulp of two contrasting mango (Mangifera indica L) cultivars. Genomics 2020, 112, 4505–4515. [Google Scholar] [CrossRef]
- Dautt-Castro, M.; López-Virgen, A.G.; Ochoa-Leyva, A.; Contreras-Vergara, C.A.; Sortillón-Sortillón, A.P.; Martínez-Téllez, M.A.; González-Aguilar, G.A.; Casas-Flores, J.S.; Sañudo-Barajas, A.; Kuhn, D.N.; et al. Genome-Wide Identification of Mango (Mangifera indica L.) Polygalacturonases: Expression Analysis of Family Members and Total Enzyme Activity During Fruit Ripening. Front. Plant Sci. 2019, 10, 969. [Google Scholar] [CrossRef] [PubMed]
- Chidley, H.G.; Deshpande, A.B.; Oak, P.S.; Pujari, K.H.; Giri, A.P.; Gupta, V.S. Effect of postharvest ethylene treatment on sugar content, glycosidase activity and its gene expression in mango fruit. J. Sci. Food Agric. 2017, 97, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Mcatee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef]
- Wu, L.; Lan, J.; Xiang, X.; Xiang, H.; Jin, Z.; Khan, S.; Liu, Y. Transcriptome sequencing and endogenous phytohormone analysis reveal new insights in CPPU controlling fruit development in kiwifruit (Actinidia chinensis). PLoS ONE 2020, 15, e0240355. [Google Scholar] [CrossRef]
- Tyagi, K.; Maoz, I.; Kochanek, B.; Sela, N.; Lerno, L.; Ebeler, S.E.; Lichter, A. Cytokinin but not gibberellin application had major impact on the phenylpropanoid pathway in grape. Hortic. Res. 2021, 8, 51. [Google Scholar] [CrossRef]
- Lavee, S. Effect of Gibberellic Acid on Seeded Grapes. Nature 1960, 185, 395. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef]
- Ueguchi-Tanaka, M.; Nakajima, M.; Katoh, E.; Ohmiya, H.; Matsuoka, M. Molecular Interactions of a Soluble Gibberellin Receptor, GID1, with a Rice DELLA Protein, SLR1, and Gibberellin. Plant Cell 2007, 19, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Ueguchi-Tanaka, M.; Ashikari, M.; Nakajima, M.; Itoh, H.; Katoh, E.; Kobayashi, M.; Chow, T.; Hsing, Y.; Kitano, H.; Yamaguchi, I. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 2005, 437, 693–698. [Google Scholar] [CrossRef]
- Zeevaart, J.A.D.; Gage, D.A.; Talon, M. Gibberellin A1 is required for stem elongation in spinach. Proc. Natl. Acad. Sci. USA 1993, 90, 7401–7405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, J.; Wang, X.; Dai, P.; Zhang, H.; Zhou, F.; Yang, C.; Zhai, R.; Wang, Z.; Xu, L. PbDELLA-PbMYB56-PbCYP78A6 module regulates GA4+7-induced pseudo-embryo development and parthenocarpy in pear (Pyrus bretschneideri). Hortic. Res. 2025, 12, uhaf021. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Schuler, M.; Paquette, S.; Werck-Reichhart, D.; Bak, S. Comparative Genomics of Rice and Arabidopsis. Analysis of 727 Cytochrome P450 Genes and Pseudogenes from a Monocot and a Dicot. Plant Physiol. 2004, 135, 756–772. [Google Scholar] [CrossRef]
- Gao, M.; Yang, N.; Shao, Y.; Shen, T.; Li, W.; Ma, B.; Wei, X.; Ruan, Y.-L.; Ma, F.; Li, M. An insertion in the promoter of a malate dehydrogenase gene regulates malic acid content in apple fruit. Plant Physiol. 2024, 0, 1–14. [Google Scholar] [CrossRef]
- Pérez-Herrera, A.; Martínez-Gutiérrez, G.; León-Martínez, F.; Sánchez-Medina, M. The effect of the presence of seeds on the nutraceutical, sensory and rheological properties of Physalis spp. Fruits jam: A comparative analysis. Food Chem. 2020, 302, 125141. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, T.; Liu, J.; Cong, L.; Zhu, Y.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. PbGA20ox2 regulates fruit set and induces parthenocarpy by enhancing GA4 content. Front. Plant Sci. 2020, 11, 113. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Dong, R.; Wang, L.; Yao, J.; van Nocker, S.; Wang, X. The grapevine homeobox gene VvHB58 influences seed and fruit development through multiple hormonal signaling pathways. BMC Plant Biol. 2019, 19, 523. [Google Scholar] [CrossRef]
- Fuentes, S.; Ljung, K.; Sorefan, K.; Alvey, E.; Harberd, N.P.; Østergaard, L. Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell 2012, 24, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Kikuchi, K.; Fukuda, M.; Honda, I.; Imanishi, S. Roles and regulation of cytokinins in tomato fruit development. J. Exp. Bot. 2012, 63, 5569–5579. [Google Scholar] [CrossRef]
- Cruz-Castillo, J.G.; Baldicchi, A.; Frioni, T.; Marocchi, F.; Moscatello, S.; Proietti, S.; Battistelli, A.; Famiani, F. Pre-anthesis CPPU low dosage application increases ‘Hayward’ kiwifruit weight without affecting the other qualitative and nutritional characteristics. Food Chem. 2014, 158, 224–228. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Huang, Y.; Kong, Q.; Bie, Z. Comparative analysis of sugar, acid, and volatile compounds in CPPU-treated and honeybee-pollinated melon fruits during different developmental stages. Food Chem. 2023, 401, 134072. [Google Scholar] [CrossRef]
- Fenn, M.A.; Giovannoni, J.J. Phytohormones in fruit development and maturation. Plant J. 2021, 105, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014, 65, 4561–4575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, M.; Cheng, F.; Han, X.; Cheng, C.; Yu, X.; Chen, J.; Lou, Q. The mutation of ent-kaurenoic acid oxidase, a key enzyme involved in gibberellin biosynthesis, confers a dwarf phenotype to cucumber. Theor. Appl. Genet. 2024, 138, 12. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Wang, S.; Xu, W.; Li, W.; Ma, X. GA4/GA7 Deficiency and Downregulated Ent-Kaurenoic Acid Oxidase Impair Seedless Mango Fruit Development. Foods 2025, 14, 3705. https://doi.org/10.3390/foods14213705
Gao M, Wang S, Xu W, Li W, Ma X. GA4/GA7 Deficiency and Downregulated Ent-Kaurenoic Acid Oxidase Impair Seedless Mango Fruit Development. Foods. 2025; 14(21):3705. https://doi.org/10.3390/foods14213705
Chicago/Turabian StyleGao, Meng, Songbiao Wang, Wentian Xu, Wenxin Li, and Xiaowei Ma. 2025. "GA4/GA7 Deficiency and Downregulated Ent-Kaurenoic Acid Oxidase Impair Seedless Mango Fruit Development" Foods 14, no. 21: 3705. https://doi.org/10.3390/foods14213705
APA StyleGao, M., Wang, S., Xu, W., Li, W., & Ma, X. (2025). GA4/GA7 Deficiency and Downregulated Ent-Kaurenoic Acid Oxidase Impair Seedless Mango Fruit Development. Foods, 14(21), 3705. https://doi.org/10.3390/foods14213705






