Properties of Beef Patties with Tenebrio molitor Powder as a Meat Replacer During Storage
Abstract
1. Introduction
2. Materials and Methods
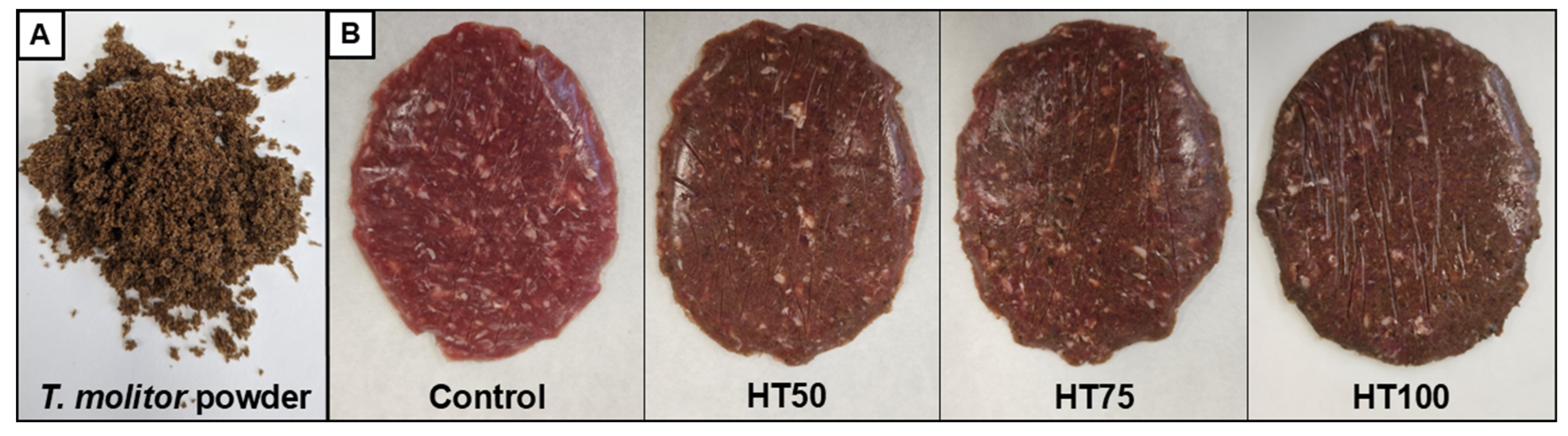
2.1. Raw Materials
2.2. Beef Patties Manufacturing
2.3. Proximate Composition
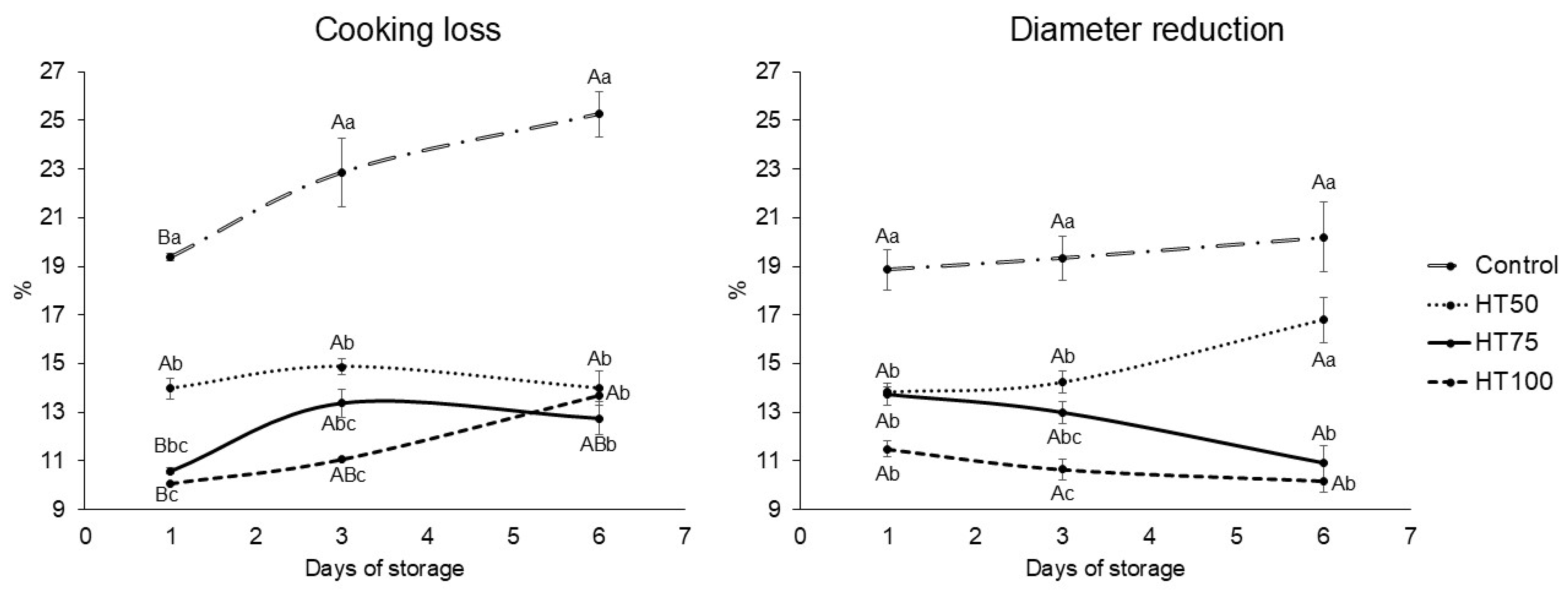
2.4. Cooking Loss and Diameter Reduction
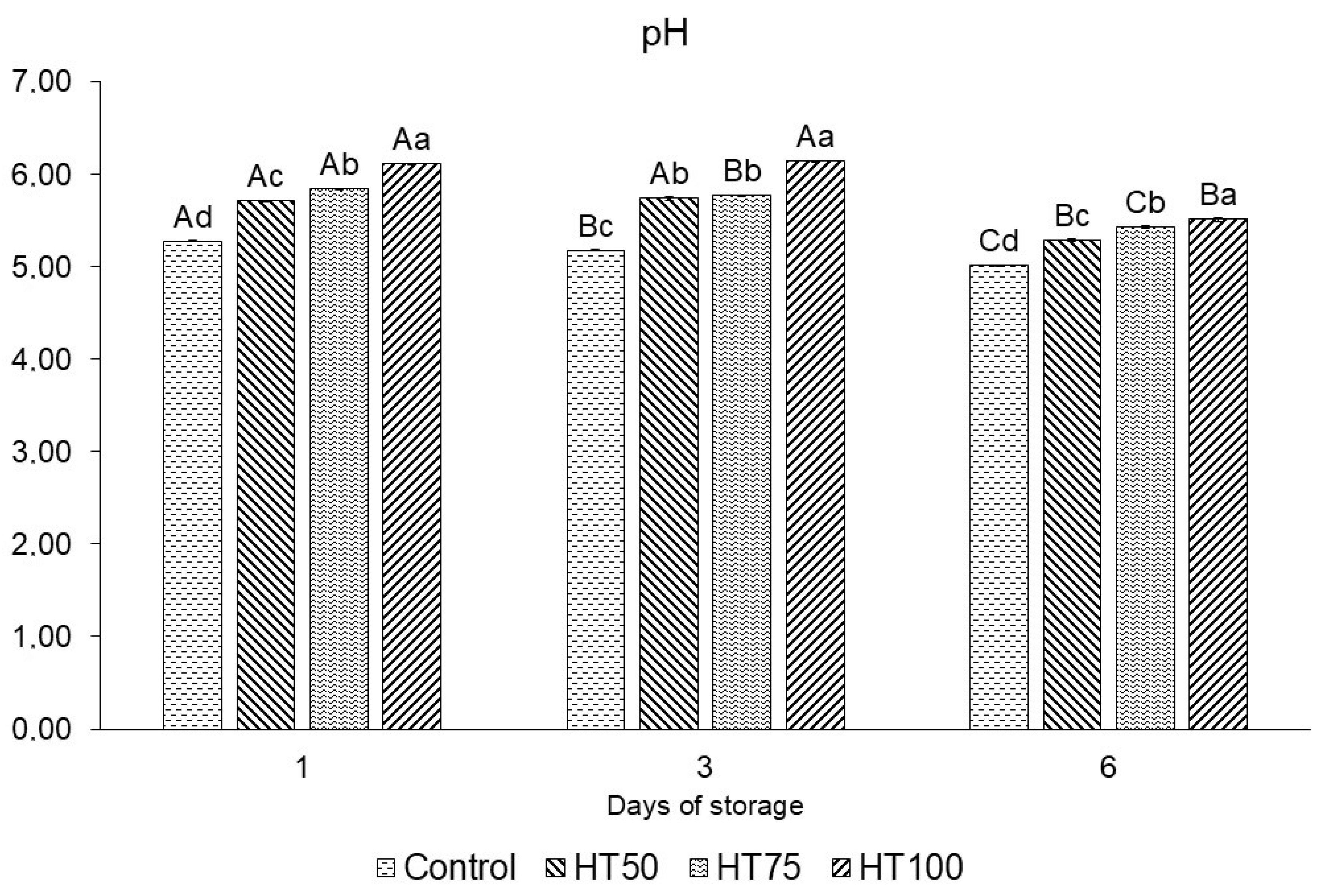
2.5. pH
2.6. Instrumental Color
2.7. Kramer Shear Force
2.8. Lipid Oxidation
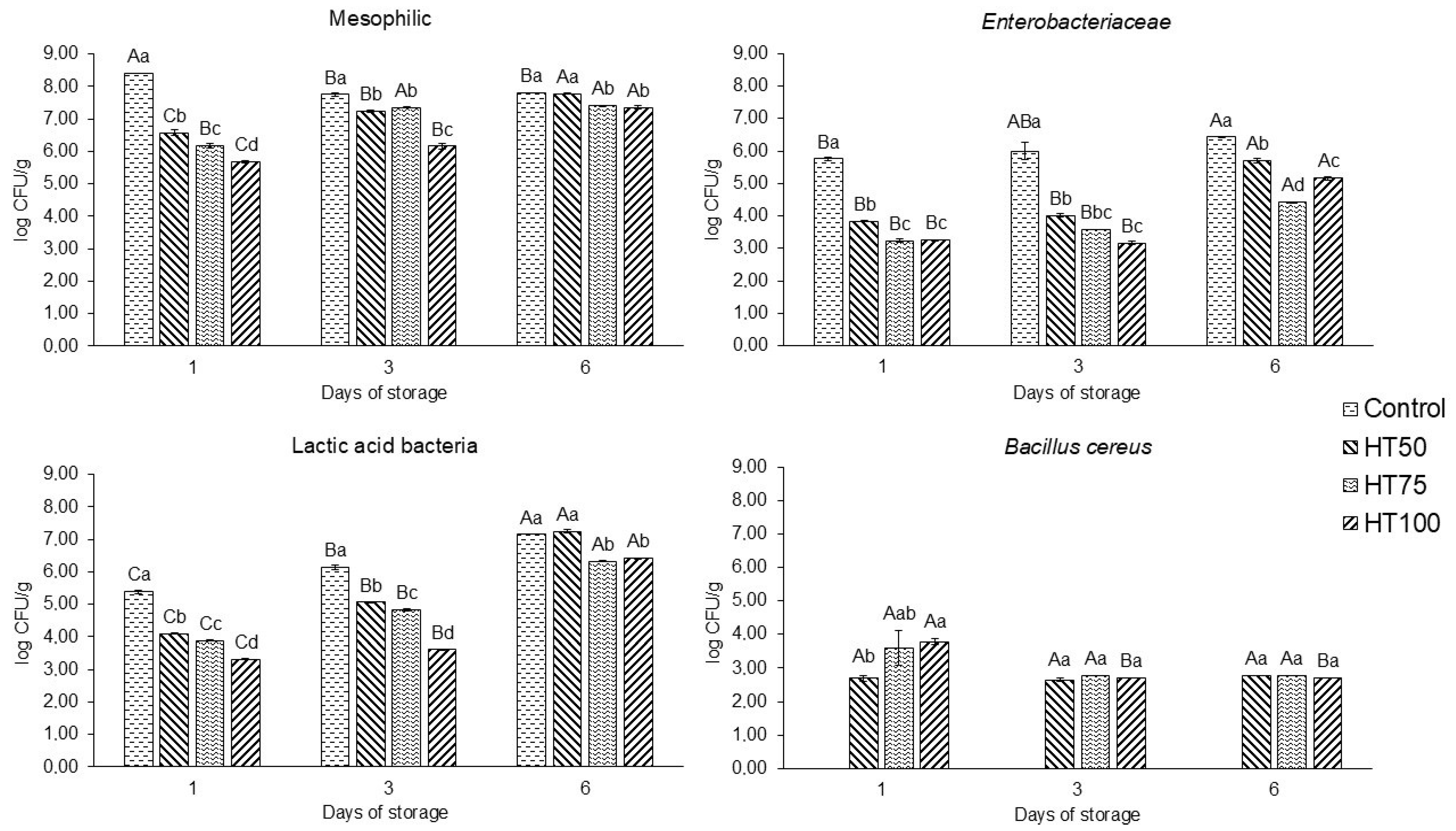
2.9. Microbiological Analysis
2.10. Statistical Analysis
3. Results
3.1. Proximate Composition
3.2. Cooking Loss and Diameter Reduction
3.3. pH
3.4. Instrumental Color
3.5. Kramer Shear Force
3.6. Lipid Oxidation
3.7. Microbiological Analysis
4. Discussion
4.1. Proximate Composition
4.2. Cooking Loss and Diameter Reduction
4.3. pH
4.4. Instrumental Color
4.5. Kramer Shear Force
4.6. Lipid Oxidation
4.7. Microbiological Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMSA | American Meat Science Association |
| AOAC | Association Of Official Analytical Chemists |
| ANOVA | Analysis of Variance |
| APHA | American Public Health Association |
| CFU | Colony-Forming Unit |
| CL | Cooking Loss |
| DR | Diameter Reduction |
| KSF | Kramer Shear Force |
| LAB | Lactic Acid Bacteria |
| MDA | Malondialdehyde |
| TBARS | Thiobarbituric Acid Reactive Substances |
References
- Zhang, F.; Li, X.; Liang, X.; Kong, B.; Sun, F.; Cao, C.; Gong, H.; Zhang, H.; Liu, Q. Feasibility of Tenebrio molitor larvae protein to partially replace lean meat in the processing of hybrid frankfurters: Perspectives on quality profiles and in vitro digestibility. Food Res. Int. 2024, 176, 113846. [Google Scholar] [CrossRef]
- Muazzam, A.; Samad, A.; Alam, A.N.; Hwang, Y.-H.; Joo, S.-T. Microbial Proteins: A Green Approach Towards Zero Hunger. Foods 2025, 14, 2636. [Google Scholar] [CrossRef]
- Lee, D.Y.; Hur, S.J. Gaps and solutions for large scale production of cultured meat: A review on last findings. Curr. Opin. Food Sci. 2025, 61, 101243. [Google Scholar] [CrossRef]
- Tang, W.; Pan, Q.; He, J.; Liu, J. Plant-based meat: The influence on texture by protein–polysaccharide interactions and processing techniques. Food Res. Int. 2025, 202, 115673. [Google Scholar] [CrossRef] [PubMed]
- Sogari, G.; Li, J.; Wang, Q.; Lefebvre, M.; Huang, S.H.; Mora, C.; Gomez, M.I. Toward a reduced meat diet: University North American students’ acceptance of a blended meat-mushroom burger. Meat Sci. 2022, 187, 108745. [Google Scholar] [CrossRef]
- Lin, J.W.X.; Maran, N.; Lim, A.J.; Ng, S.B.; Teo, P.S. Current challenges, and potential solutions to increase acceptance and long-term consumption of cultured meat and edible insects—A review. Future Foods 2025, 11, 100544. [Google Scholar] [CrossRef]
- Muñoz-Seijas, N.; Fernandes, H.; López-Periago, J.E.; Outeiriño, D.; Morán-Aguilar, M.G.; Domínguez, J.M.; Salgado, J.M. Characterization of all life stages of Tenebrio molitor: Envisioning innovative applications for this edible insect. Future Foods 2024, 10, 100404. [Google Scholar] [CrossRef]
- Oliveira, L.A.; Pereira, S.M.S.; Dias, K.A.; Paes, S.S.; Grancieri, M.; Jimenez, L.G.S.; Carvalho, C.W.P.; Oliveira, E.E.; Martino, H.S.D.; Della Lucia, C.M. Nutritional content, amino acid profile, and protein properties of edible insects (Tenebrio molitor and Gryllus assimilis) powders at different stages of development. J. Food Compos. Anal. 2024, 125, 105804. [Google Scholar] [CrossRef]
- Osimani, A.; Garofalo, C.; Milanovic, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Pasquini, M.; Mozzon, M.; Raffaelli, N.; Ruschioni, S.; et al. Insight into the proximate composition and microbial diversity of edible insects marketed in the European Union. Eur. Food Res. Technol. 2017, 243, 1157–1171. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2022/169 of 8 February 2022 authorising the placing on the market of frozen, dried and powder forms of yellow mealworm (Tenebrio molitor larva) as a novel food under Regulation (EU) 2015/2283. Off. J. Eur. Union 2022, L28, 10–16. [Google Scholar]
- van Huis, A.; Rumpold, B.; Maya, C.; Roos, N. Nutritional qualities and enhancement of edible insects. Annu. Rev. Nutr. 2021, 41, 551–576. [Google Scholar] [CrossRef]
- Pastrana-Pastrana, A.J.; Rodríguez-Herrera, R.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C. Plant proteins, insects, edible mushrooms and algae: More sustainable alternatives to conventional animal protein. J. Future Foods 2025, 5, 248–256. [Google Scholar] [CrossRef]
- van Huis, A.; Rumpold, B. Strategies to convince consumers to eat insects? A review. Food Qual. Prefer. 2023, 110, 104927. [Google Scholar] [CrossRef]
- Smart, M.A.; Pontes, N. The role of consumer restraint versus indulgence on purchase intentions of hybrid meat analogues. Food Qual. Prefer. 2023, 104, 104738. [Google Scholar] [CrossRef]
- Borges, M.M.; Costa, D.V.; Trombete, F.M.; Câmara, A.K.F.I. Edible insects as a sustainable alternative to food products: An insight into quality aspects of reformulated bakery and meat products. Curr. Opin. Food Sci. 2022, 46, 100864. [Google Scholar] [CrossRef]
- Scholliers, J.; Steen, L.; Fraeye, I. Partial replacement of meat by superworm (Zophobas morio larvae) in cooked sausages: Effect of heating temperature and insect:meat ratio on structure and physical stability. Innov. Food Sci. Emerg. Technol. 2020, 66, 102535. [Google Scholar] [CrossRef]
- Megido, R.C.; Gierts, C.; Blecker, C.; Brostaux, Y.; Haubruge, E.; Álabi, T.; Francis, F. Consumer acceptance of insect-based alternative meat products in Western countries. Food Qual. Prefer. 2016, 52, 237–243. [Google Scholar] [CrossRef]
- Bessa, L.W.; Pieterse, E.; Marais, J.; Hoffman, L.C. Black soldier fly larvae (Hermetia illucens) as a meat replacer in a burger patty. J. Insects Food Feed 2023, 9, 1211–1222. [Google Scholar] [CrossRef]
- Gomes Martins, V.M.; Milano, P.; Pollonio, M.A.R.; dos Santos, M.; de Oliveira, A.P.; Savay-da-Silva, L.K.; Câmara, A.K.F.I.; Paglarini, C.S. Adding cricket (Gryllus assimilis) flour in hybrid beef patties: Physicochemical, technological and sensory challenges. Int. J. Food Sci. Technol. 2024, 59, 450–459. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Pintado, T.; Sousa, C.C.A.; Leite, J.S.F.; Silva, M.C.A. Potential of cricket (Acheta domesticus) flour as a lean meat replacer in the development of beef patties. Foods 2024, 13, 286. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemistry: Rockville, MA, USA, 2005. [Google Scholar]
- Bligh, E.G.; Dyer, W.J.A. Rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Pematilleke, N.; Kaur, M.; Wai, C.T.R.; Adhikari, B.; Torley, P.J. Effect of the addition of hydrocolloids on beef texture: Targeted to the needs of people with dysphagia. Food Hydrocol. 2021, 113, 106413. [Google Scholar] [CrossRef]
- AMSA. Meat Color Measurement Guidelines; American Meat Science Association: Kearney, MI, USA, 2012. [Google Scholar]
- Triki, M.; Herrero, A.M.; Rodríguez-Salas, L.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Chilled storage characteristics of low-fat. N-3 PUFA-enriched dry fermented sausage reformulated with a healthy oil combination stabilized in a konjac matrix. Food Control 2013, 31, 158–165. [Google Scholar] [CrossRef]
- APHA. Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; APHA: Washington, DC, USA, 2013. [Google Scholar]
- Choi, Y.S.; Kim, T.K.; Choi, H.D.; Park, J.D.; Sung, J.M.; Jeon, K.H.; Kim, Y.-B. Optimization of replacing pork meat with yellow worm (Tenebrio molitor L.) for frankfurters. Korean J. Food Sci. Anim. Resour. 2017, 37, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Pintado, T.; Cruz, T.M.P.; Silva, M.C.A. Cricket (Acheta domesticus) flour as meat replacer in frankfurters: Nutritional, technological, structural, and sensory characteristics. Innov. Food Sci. Emerg. Technol. 2023, 83, 103245. [Google Scholar] [CrossRef]
- Choi, N.; Park, S.; Park, Y.; Park, G.; Oh, S.; Kim, Y.; Lim, Y.; Jang, S.; Kim, Y.; Ahn, K.-S.; et al. Effects of edible insect powders as meat partial substitute on physicochemical properties and storage stability of pork patties. Food Sci. Anim. Resour. 2024, 44, 817–831. [Google Scholar] [CrossRef]
- de Sousa, C.C.A.; Santos, L.J.P.; Silva, M.C.A.; Cavalheiro, C.P. A comprehensive overview of sodium, total and saturated fat content in meat products sold in Brazil. Nutr. Food Sci. 2025, 55, 123–136. [Google Scholar] [CrossRef]
- Sánchez-Zapata, E.; Pérez-Alvarez, J.A.; Fernández-López, J. Effects of tiger nut (Cyperus esculentus) milk liquid co-products on the quality of pork burgers. Int. J. Food Sci. Technol. 2012, 47, 2198–2204. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Mehmood, W.; Qian, S.; Sun, Z.; Zhang, C.; Blecker, C. Effect of storage temperatures on the moisture migration and microstructure of beef. J. Food Qual. 2018, 2018, 3873179. [Google Scholar] [CrossRef]
- Belucci, E.R.B.; dos Santos, J.M.; Carvalho, L.T.; Borgonovi, T.F.; Lorenzo, J.M.; da Silva-Barretto, A.C. Acaí extract powder as natural antioxidant on pork patties during the refrigerated storage. Meat Sci. 2022, 184, 108667. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Wang, Y.; Hao, S.; Zhang, K.; Tian, J.; Jin, Y. Effect of changes in the structure of myoglobin on the color of meat products. Food Mater. Res. 2024, 4, e011. [Google Scholar] [CrossRef]
- Gupta, J.; Bower, C.G.; Sullivan, G.; Cavender, G.A. Effectiveness of different myoglobin states to minimize high pressure induced discoloration in raw ground beef. LWT Food Sci. Technol. 2018, 93, 32–35. [Google Scholar] [CrossRef]
- Pielak, M.; Czarniecka-Skubina, E.; Głuchowski, A. Effect of sugar substitution with steviol glycosides on sensory quality and physicochemical composition of low-sugar apple preserves. Foods 2020, 9, 293. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.I.; Jung, S.; Sung, J.-M.; Jang, H.W.; Choi, Y.-S. Physicochemical and textural properties of emulsions prepared from the larvae of the edible insects Tenebrio molitor, Allomyrina dichotoma, and Protaetia brevitarsis seulensis. J. Anim. Sci. Technol. 2021, 63, 417–425. [Google Scholar] [CrossRef]
- Bernardo, Y.A.A.; Conte-Junior, C.A. Oxidative stability in edible insects: Where is the knowledge frontier? Trends Food Sci. Technol. 2024, 148, 104518. [Google Scholar] [CrossRef]
- Lan, L.; Chen, L.; Zhong, X.; Cao, W.; Zhou, Y.; Wan, J.; Liu, Y.; Zhu, Q. Harmonious regulation flavor and lipid oxidation in dry-cured tenderloin through electrical stimulation: A study on lipase activity and sensory correlations. Food Chem. X 2025, 25, 102189. [Google Scholar] [CrossRef]
- Özvural, E.B.; Huang, Q.; Chikindas, M.L. The comparison of quality and microbiological characteristic of hamburger patties enriched with green tea extract using three techniques: Direct addition, edible coating and encapsulation. LWT-Food Sci. Technol. 2016, 68, 385–390. [Google Scholar] [CrossRef]
- Bedir, T.B.; Kuleaşan, H. A natural approach, the use of a killer toxin produced by Metschnikowia pulcherrima in fresh ground beef patties for shelf life extension. Int. J. Food Microbiol. 2021, 345, 109154. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Leon, M.J.; Montserrat-de la Paz, S. Potential applications of antimicrobial peptides from edible insects in the food supply chain: Uses in agriculture, packaging, and human nutrition. Food Biosci. 2024, 62, 105396. [Google Scholar] [CrossRef]
- Pal, A.; Mann, A.; den Bakker, H.C. Analysis of microbial composition of edible insect products available for human consumption within the United States using traditional microbiological methods and whole genome sequencing. J. Food Prot. 2024, 87, 100277. [Google Scholar] [CrossRef]
| Parameter | Treatment | |||
|---|---|---|---|---|
| Control | HT50 | HT75 | HT100 | |
| Protein | 19.31 ± 0.12 c | 21.51 ± 0.17 b | 21.92 ± 0.15 b | 22.99 ± 0.12 a |
| Moisture | 72.46 ± 0.18 a | 68.69 ± 0.11 b | 68.44 ± 0.30 b | 66.22 ± 0.19 c |
| Fat | 5.65 ± 0.14 a | 6.01 ± 0.10 a | 6.51 ± 0.13 a | 6.63 ± 0.51 a |
| Ash | 2.36 ± 0.03 c | 2.48 ± 0.03 bc | 2.56 ± 0.01 ab | 2.71 ± 0.06 a |
| Parameter | Day | Treatments | |||
|---|---|---|---|---|---|
| Control | HT50 | HT75 | HT100 | ||
| L* | 1 | 42.9 ± 0.5 Ba | 41.2 ± 0.6 Aa | 36.9 ± 0.5 Bb | 36.8 ± 0.6 Ab |
| 3 | 43.4 ± 0.6 Aba | 38.5 ± 0.2 Bb | 37.9 ± 0.6 ABb | 37.5 ± 0.4 Ab | |
| 6 | 45.5 ± 0.6 Aa | 41.1 ± 0.5 Ab | 39.6 ± 0.3 Abc | 38.2 ± 0.3 Ac | |
| a* | 1 | 10.0 ± 0.3 Ba | 6.7 ± 0.3 Bb | 7.6 ± 0.3 Ab | 7.0 ± 0.3 Ab |
| 3 | 13.7 ± 0.4 Aa | 9.2 ± 0.4 Ab | 8.3 ± 0.3 Ab | 6.7 ± 0.2 Ac | |
| 6 | 13.3 ± 0.3 Aa | 9.3 ± 0.2 Ab | 7.4 ± 0.3 Ac | 6.3 ± 0.2 Ac | |
| b* | 1 | 8.1 ± 0.3 Aa | 9.2 ± 0.3 Aa | 8.9 ± 0.3 Aa | 8.5 ± 0.3 Aa |
| 3 | 8.5 ± 0.3 Ab | 9.8 ± 0.2 Aa | 8.5 ± 0.3 Ab | 8.4 ± 0.2 Ab | |
| 6 | 9.1 ± 0.2 Aa | 9.6 ± 0.3 Aa | 8.8 ± 0.1 Aa | 8.7 ± 0.2 Aa | |
| ∆E* | 1 | - | 4.7 ± 0.5 Bb | 6.7 ± 0.7 Aab | 7.2 ± 0.7 Ba |
| 3 | - | 6.9 ± 0.4 Ab | 8.5 ± 0.4 Aab | 9.5 ± 0.4 Aa | |
| 6 | - | 6.2 ± 0.4 Abb | 8.7 ± 0.4 Aa | 10.4 ± 0.4 Aa | |
| Parameter | Day | Treatments | |||
|---|---|---|---|---|---|
| Control | HT50 | HT75 | HT100 | ||
| KSF (N/g) | 1 | 29.38 ± 0.61 Aa | 26.88 ± 0.51 Ba | 28.68 ± 0.59 Ba | 30.32 ± 0.97 Ba |
| 6 | 32.86 ± 0.52 Ab | 34.92 ± 1.42 Ab | 42.45 ± 1.25 Aa | 43.34 ± 0.27 Aa | |
| TBARS (mg MDA/kg) | 1 | 1.63 ± 0.02 Aa | 1.32 ± 0.01 Bb | 1.05 ± 0.02 Bc | 0.92 ± 0.01 Bd |
| 6 | 1.63 ± 0.01 Aa | 1.51 ± 0.01 Ab | 1.44 ± 0.02 Ac | 1.45 ± 0.02 Abc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, C.C.A.d.; Passos, R.S.F.T.; Ruiz-Capillas, C.; Herrero, A.M.; Silva, M.C.A.d.; Cavalheiro, C.P. Properties of Beef Patties with Tenebrio molitor Powder as a Meat Replacer During Storage. Foods 2025, 14, 3707. https://doi.org/10.3390/foods14213707
Sousa CCAd, Passos RSFT, Ruiz-Capillas C, Herrero AM, Silva MCAd, Cavalheiro CP. Properties of Beef Patties with Tenebrio molitor Powder as a Meat Replacer During Storage. Foods. 2025; 14(21):3707. https://doi.org/10.3390/foods14213707
Chicago/Turabian StyleSousa, Camila Cristina A. de, Rafael Sepúlveda F. Trevisan Passos, Claudia Ruiz-Capillas, Ana M. Herrero, Maurício Costa A. da Silva, and Carlos Pasqualin Cavalheiro. 2025. "Properties of Beef Patties with Tenebrio molitor Powder as a Meat Replacer During Storage" Foods 14, no. 21: 3707. https://doi.org/10.3390/foods14213707
APA StyleSousa, C. C. A. d., Passos, R. S. F. T., Ruiz-Capillas, C., Herrero, A. M., Silva, M. C. A. d., & Cavalheiro, C. P. (2025). Properties of Beef Patties with Tenebrio molitor Powder as a Meat Replacer During Storage. Foods, 14(21), 3707. https://doi.org/10.3390/foods14213707








