Impact of Ethanol Stress on Yarrowia lipolytica for Sustainable Bioconversion of Agro-Food Oil Wastes into Lipases and Lipids
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strain, Materials, and Culture Conditions
2.2. Ethanol Stress Induction
2.3. Determination of Dry Cell Weight (DCW)
2.4. Lipase Activity Assay
2.5. Determination of Fatty Acid Composition
2.6. Oxidative Stability Determination
2.7. Statistical Analysis
3. Results
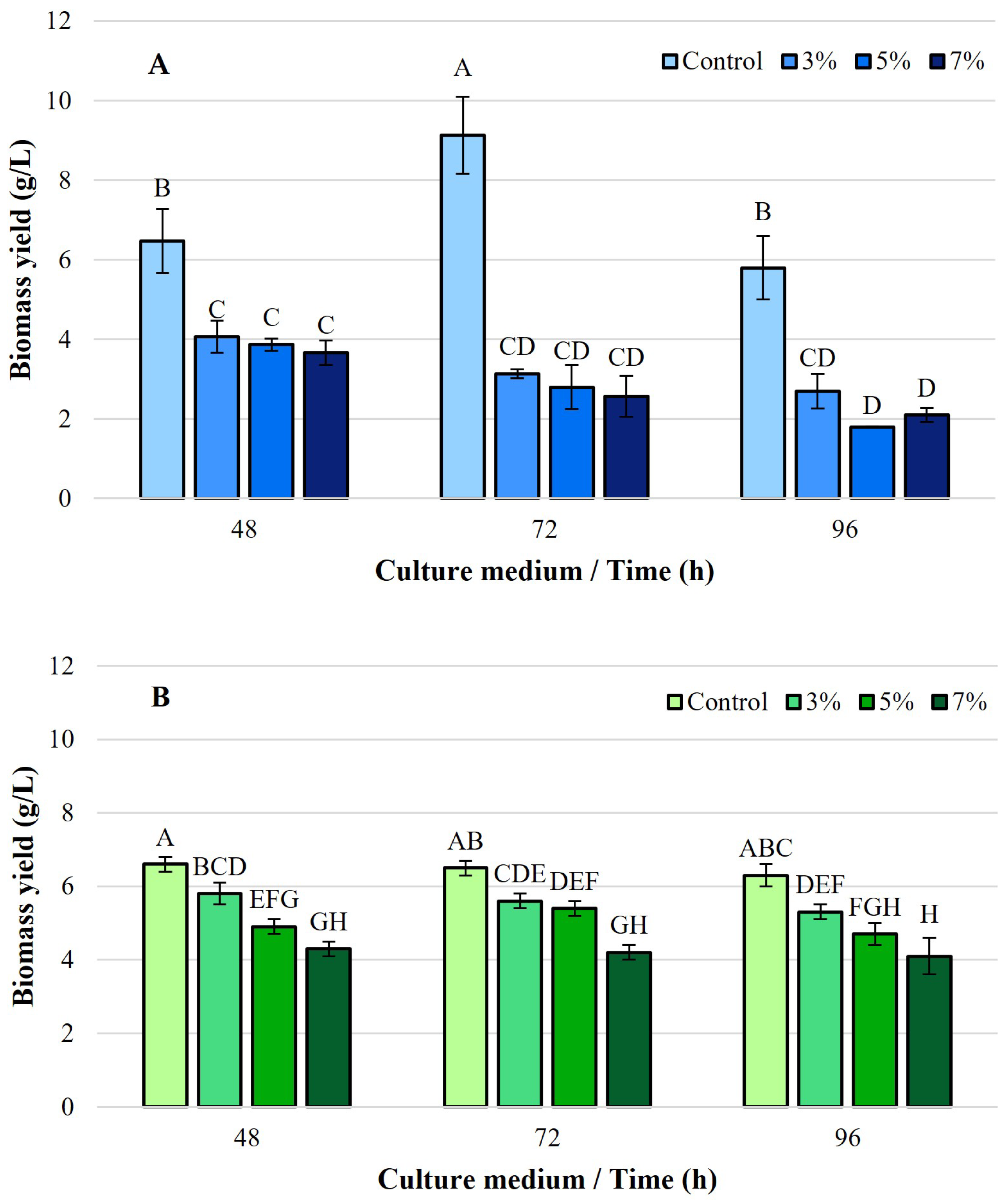
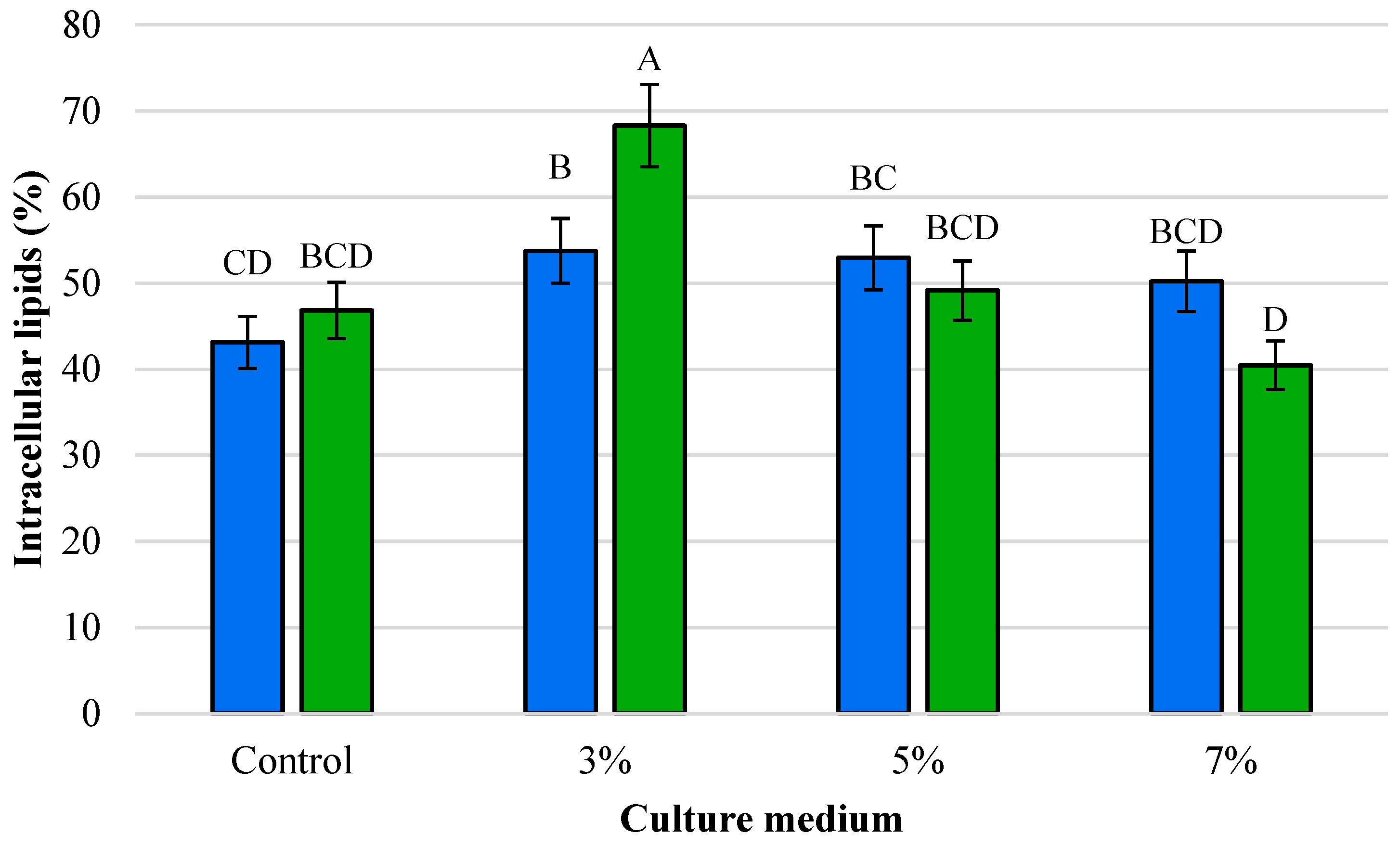
3.1. Dry Cell Weight (DCW) and Intracellular Lipid Yields
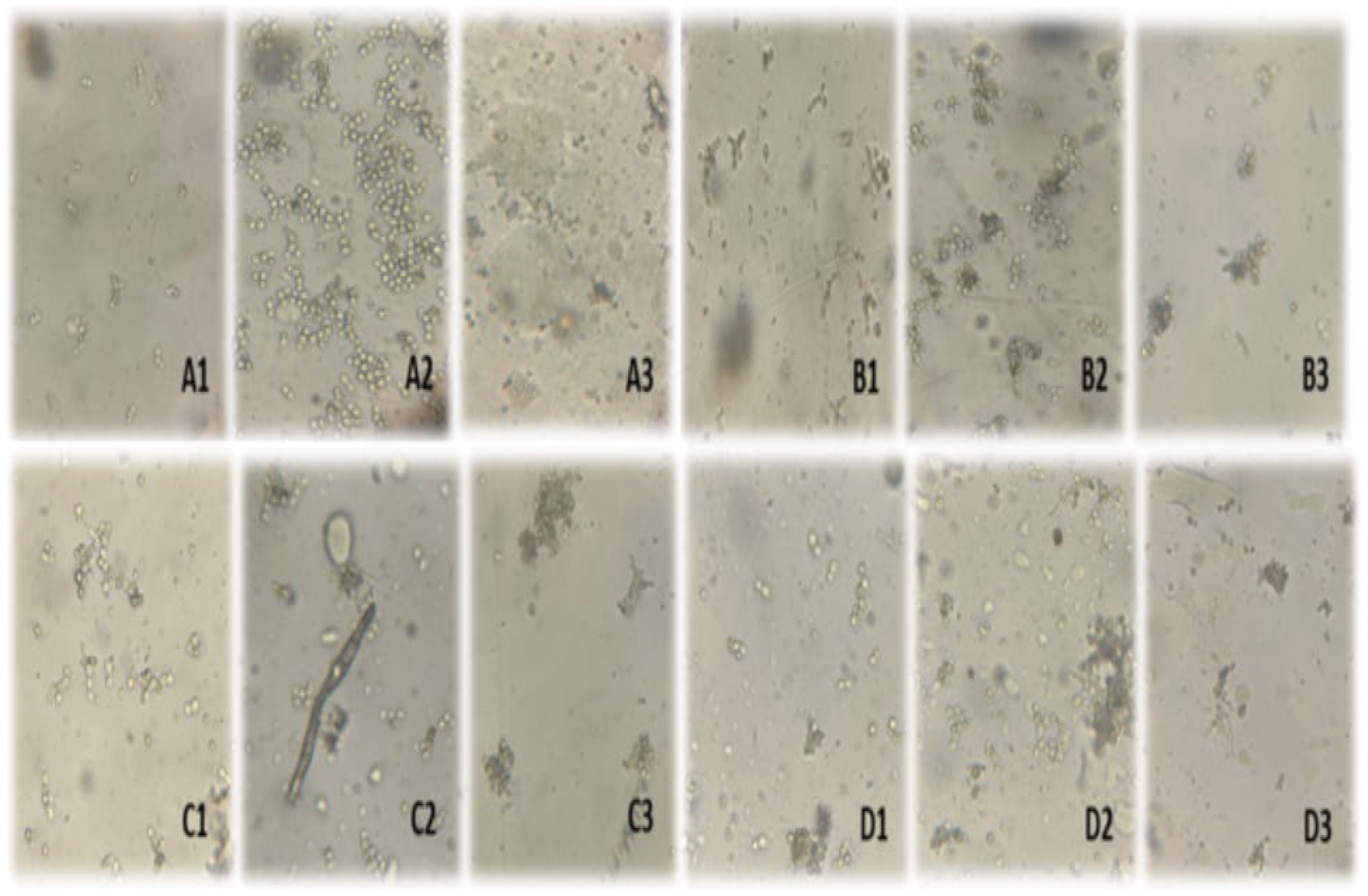
3.2. Morphological Observation of Yeast Under Light Microscopy
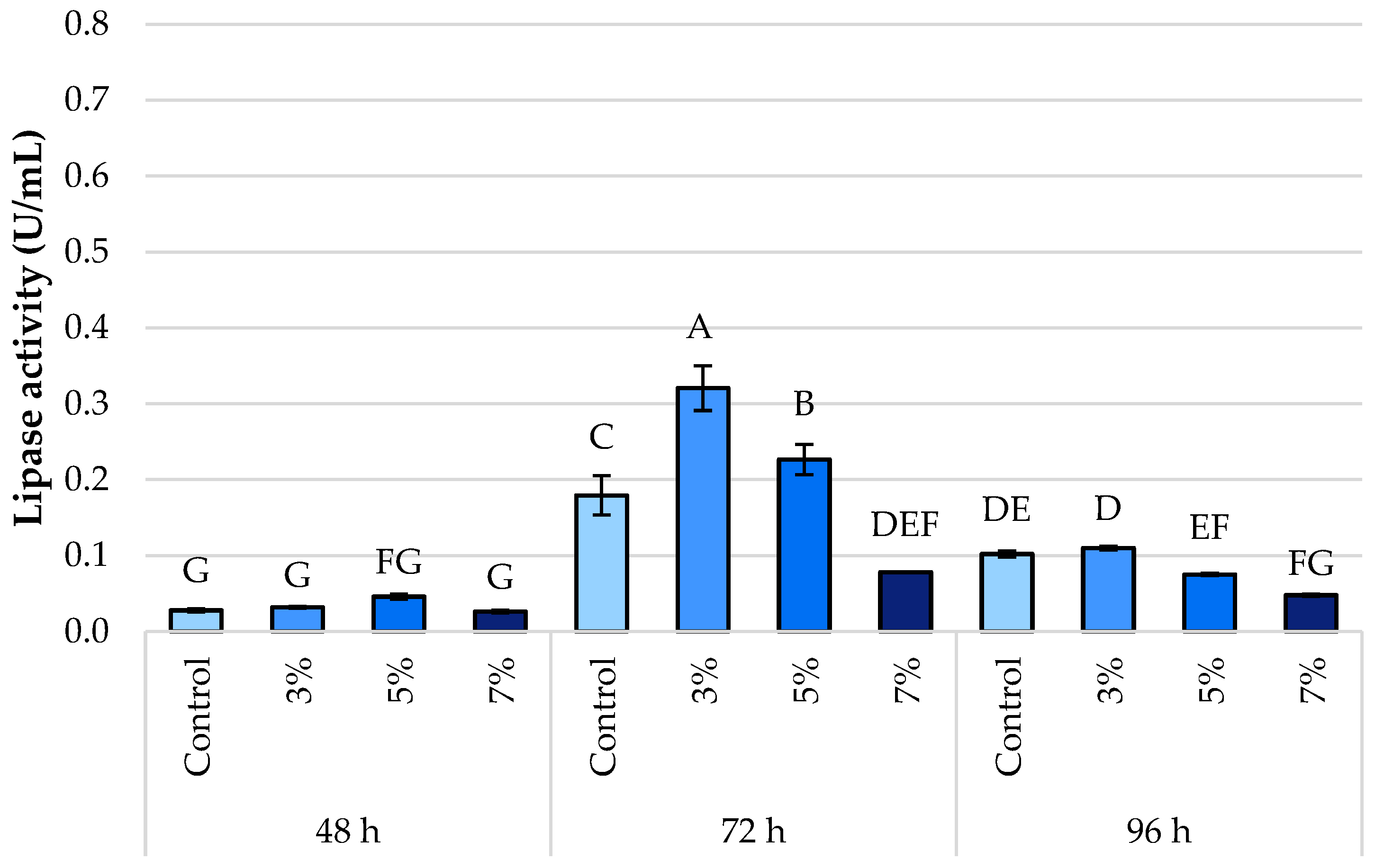
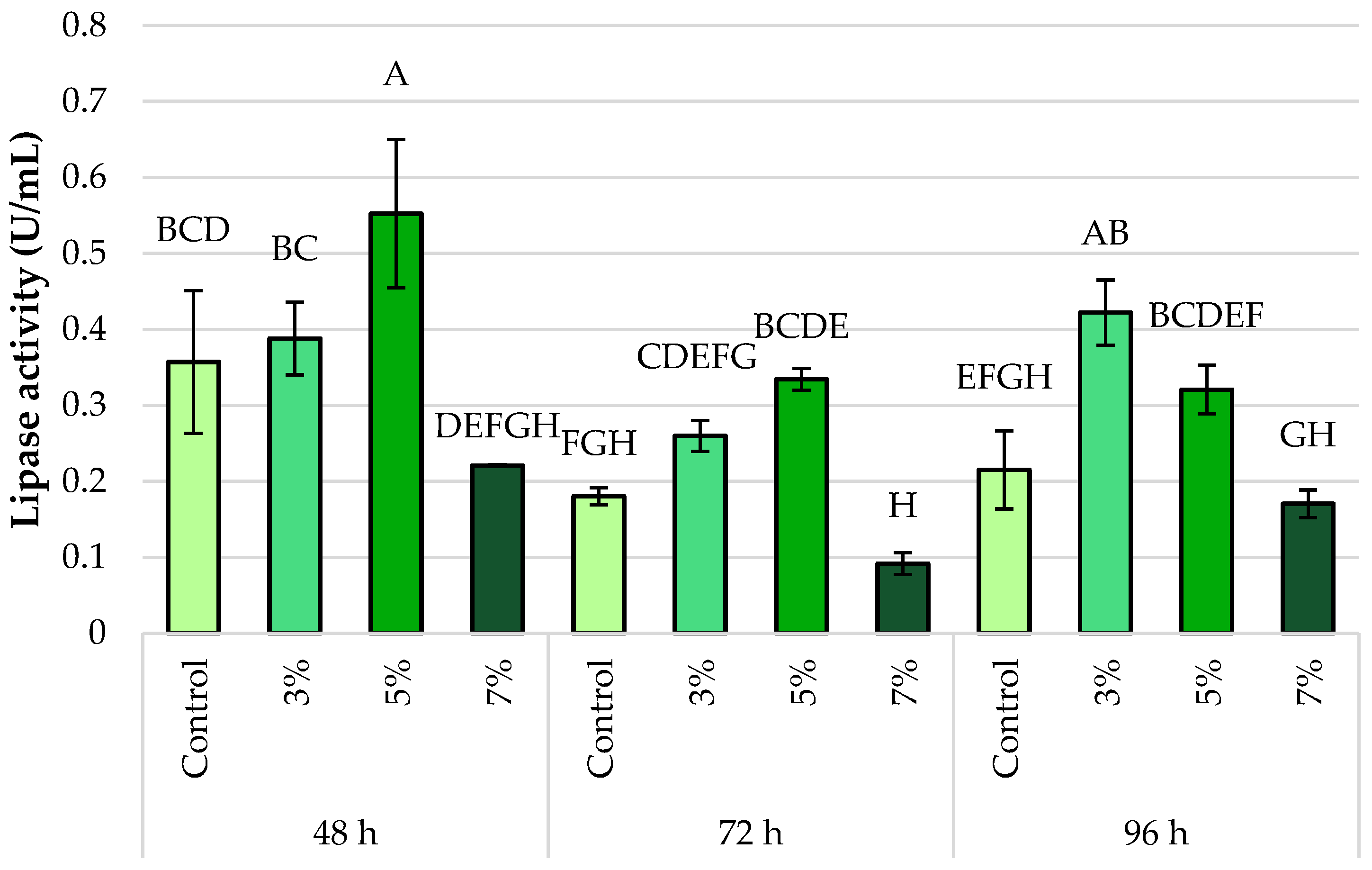
3.3. Lipase Activity
3.4. Fatty Acid Composition of Yeast Lipids
3.5. Oxidative Stability of Microbial Oils by Pressurized Differential Scanning Calorimetry (PDSC)
4. Discussion
4.1. Effect of Ethanol on Growth and Lipogenesis
4.2. Morphological Transitions and Their Implications
4.3. Lipase Activity
4.4. Fatty Acid Composition of Yeast Lipids
4.5. Insights into Oxidative Stability of Microbial Oils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| DCW | Dry Cell Weight |
| DSC | Differential Scanning Calorimetry |
| FA | Fatty Acid |
| FAME | Fatty Acid Methyl Ester |
| FID | Flame Ionization Detector |
| GC | Gas Chromatography |
| HAP1 | Heme-Activated Protein 1 |
| ISO | International Organization for Standardization |
| MUFA | Monounsaturated Fatty Acid |
| OMW | Olive Mill Wastewater |
| OTR | Oxygen Transfer Rate |
| PDSC | Pressure Differential Scanning Calorimetry |
| PUFA | Polyunsaturated Fatty Acid |
| ROS | Reactive Oxygen Species |
| SFA | Saturated Fatty Acid |
| SD | Standard Deviation |
| WFO | Waste Frying Oil |
References
- Gong, G.; Wu, B.; Liu, L.; Li, J.; He, M. Engineering Oleaginous Red Yeasts as Versatile Chassis for the Production of Oleochemicals and Valuable Compounds: Current Advances and Perspectives. Biotechnol. Adv. 2024, 76, 108432. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Costa, A.R.; Pereira, A.S.; Belo, I. Yarrowia lipolytica as a Biorefinery Platform for Effluents and Solid Wastes Valorization—Challenges and Opportunities. Crit. Rev. Biotechnol. 2022, 42, 163–183. [Google Scholar] [CrossRef]
- Sarris, D.; Tsouko, E.; Photiades, A.; Tchakouteu, S.S.; Diamantopoulou, P.; Papanikolaou, S. Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 2: Citric Acid Production and Circular-Oriented Valorization of Glucose-Enriched Olive Mill Wastewaters Using Novel Yarrowia lipolytica Strains. Microorganisms 2023, 11, 2243. [Google Scholar] [CrossRef]
- Laribi, A.; Zieniuk, B.; Bouchedja, D.N.; Hafid, K.; Elmechta, L.; Becila, S. Valorization of Olive Mill Wastewater via Yarrowia lipolytica: Sustainable Production of High-Value Metabolites and Biocompounds—A Review. Fermentation 2025, 11, 326. [Google Scholar] [CrossRef]
- Colacicco, M.; Ciliberti, C.; Biundo, A.; Agrimi, G.; Pisano, I. Study of Lipase Production by Yarrowia lipolytica Grown in High Concentration of Hydrophobic Carbon Sources. Chem. Eng. Trans. 2022, 93, 247–252. [Google Scholar] [CrossRef]
- Colacicco, M.; Ciliberti, C.; Agrimi, G.; Biundo, A.; Pisano, I. Towards the Physiological Understanding of Yarrowia lipolytica Growth and Lipase Production Using Waste Cooking Oils. Energies 2022, 15, 5217. [Google Scholar] [CrossRef]
- Abdelaziz, A.A.; Abo-Kamar, A.M.; Elkotb, E.S.; Al-Madboly, L.A. Microbial Lipases: Advances in Production, Purification, Biochemical Characterization, and Multifaceted Applications in Industry and Medicine. Microb. Cell Factories 2025, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- al Mualad, W.; Bouchedja, N.; Selmania, A.; Maadadi, R.; Ikhlef, A.; Kabouche, Z.; Elmechta, L.; Boudjellal, A. Recycling Pollutants and Used Oils as Substrates for Producing Useful Lipids in the Form of Single-Cell Oil by the Aerobic Yeast Yarrowia lipolytica. Int. J. Environ. Res. 2022, 16, 97. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Nicaud, J.-M. Yarrowia lipolytica as a Biotechnological Chassis to Produce Usual and Unusual Fatty Acids. Prog. Lipid Res. 2016, 61, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Biryukova, E.N.; Arinbasarova, A.Y.; Medentsev, A.G. Induction of Alcohol Dehydrogenase in Yarrowia Lipolitica Yeast under Stress Conditions. Microbiology 2024, 93, 985–988. [Google Scholar] [CrossRef]
- Polez, S.; Origi, D.; Zahariev, S.; Guarnaccia, C.; Tisminetzky, S.G.; Skoko, N.; Baralle, M. A Simplified and Efficient Process for Insulin Production in Pichia pastoris. PLoS ONE 2016, 11, e0167207. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Ohashi, T.; Misaki, R.; Limtong, S.; Fujiyama, K. Ethanol and H2O2 Stresses Enhance Lipid Production in an Oleaginous Rhodotorula Toruloides Thermotolerant Mutant L1-1. FEMS Yeast Res. 2020, 20, foaa030. [Google Scholar] [CrossRef]
- Bouchedja, D.N.; Danthine, S.; Kar, T.; Fickers, P.; Sassi, H.; Boudjellal, A.; Blecker, C.; Delvigne, F. pH Level Has a Strong Impact on Population Dynamics of the Yeast Yarrowia lipolytica and Oil Micro-Droplets in Multiphasic Bioreactor. FEMS Microbiol. Lett. 2018, 365, fny173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wan, Y.; Cai, W.; Liu, N.; Zeng, J.; Liu, C.; Peng, H.; Fu, G. Effects on Cell Membrane Integrity of Pichia Anomala by the Accumulating Excessive Reactive Oxygen Species under Ethanol Stress. Foods 2022, 11, 3744. [Google Scholar] [CrossRef]
- Chandler, M.; Stanley, G.A.; Rogers, P.; Chambers, P. A Genomic Approach to Defining the Ethanol Stress Response in the Yeast Saccharomyces cerevisiae. Ann. Microbiol. 2004, 54, 427–454. [Google Scholar]
- Liu, K.; Liu, Y.; Yang, Z.; Yu, J.; Yao, G. Regulation of Cultivation Temperature on Biomass and Activity of Bifidobacterium breve B2798. Fermentation 2024, 10, 553. [Google Scholar] [CrossRef]
- Fabiszewska, A.; Zieniuk, B.; Jasińska, K.; Nowak, D.; Sasal, K.; Kobus, J.; Jankiewicz, U. Extracellular Lipases of Yarrowia lipolytica Yeast in Media Containing Plant Oils—Studies Supported by the Design of Experiment Methodology. Appl. Sci. 2024, 14, 11449. [Google Scholar] [CrossRef]
- BS EN ISO 5509:2001; Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. British Standards Institution: London, UK, 2001; ISBN 0-580-34823-7.
- Bandara, R.R.; Louis-Gavet, C.; Bryś, J.; Mańko-Jurkowska, D.; Górska, A.; Brzezińska, R.; Siol, M.; Makouie, S.; Palani, B.K.; Obranović, M.; et al. Enzymatic Interesterification of Coconut and Hemp Oil Mixtures to Obtain Modified Structured Lipids. Foods 2024, 13, 2722. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, I.; Ostrowska-Ligęza, E.; Wiktor, A.; Górska, A. Ultrasound and Pulsed Electric Field Treatment Effect on the Thermal Properties, Oxidative Stability and Fatty Acid Profile of Oils Extracted from Berry Seeds. J. Therm. Anal. Calorim. 2024, 150, 1311–1325. [Google Scholar] [CrossRef]
- Lairón-Peris, M.; Routledge, S.J.; Linney, J.A.; Alonso-Del-Real, J.; Spickett, C.M.; Pitt, A.R.; Guillamón, J.M.; Barrio, E.; Goddard, A.D.; Querol, A. Lipid Composition Analysis Reveals Mechanisms of Ethanol Tolerance in the Model Yeast Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2021, 87, e0044021. [Google Scholar] [CrossRef]
- Ji, X.-X.; Zhang, Q.; Yang, B.-X.; Song, Q.-R.; Sun, Z.-Y.; Xie, C.-Y.; Tang, Y.-Q. Response Mechanism of Ethanol-Tolerant Saccharomyces cerevisiae Strain ES-42 to Increased Ethanol during Continuous Ethanol Fermentation. Microb. Cell Factories 2025, 24, 33. [Google Scholar] [CrossRef]
- Gatter, M.; Ottlik, S.; Kövesi, Z.; Bauer, B.; Matthäus, F.; Barth, G. Three Alcohol Dehydrogenase Genes and One Acetyl-CoA Synthetase Gene Are Responsible for Ethanol Utilization in Yarrowia lipolytica. Fungal Genet. Biol. 2016, 95, 30–38. [Google Scholar] [CrossRef]
- Navarro-Tapia, E.; Querol, A.; Pérez-Torrado, R. Membrane Fluidification by Ethanol Stress Activates Unfolded Protein Response in Yeasts. Microb. Biotechnol. 2018, 11, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Jiang, S.; Lu, S.; Jiang, S.; Jiang, S.; Deng, Y.; Lu, J.; Wang, H.; Zhou, Y. Ethanol Yield Improvement in Saccharomyces cerevisiae GPD2 Delta FPS1 Delta ADH2 Delta DLD3 Delta Mutant and Molecular Mechanism Exploration Based on the Metabolic Flux and Transcriptomics Approaches. Microb. Cell Factories 2022, 21, 160. [Google Scholar] [CrossRef]
- Timoumi, A.; Guillouet, S.E.; Molina-Jouve, C.; Fillaudeau, L.; Gorret, N. Impacts of Environmental Conditions on Product Formation and Morphology of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2018, 102, 3831–3848. [Google Scholar] [CrossRef]
- Lopes, M.; Araújo, C.; Aguedo, M.; Gomes, N.; Gonçalves, C.; Teixeira, J.A.; Belo, I. The Use of Olive Mill Wastewater by Wild Type Yarrowia lipolytica Strains: Medium Supplementation and Surfactant Presence Effect. J. Chem. Technol. Biotechnol. 2009, 84, 533–537. [Google Scholar] [CrossRef]
- Lopes, M.; Gomes, N.; Mota, M.; Belo, I. Yarrowia lipolytica Growth Under Increased Air Pressure: Influence on Enzyme Production. Appl. Biochem. Biotechnol. 2009, 159, 46–53. [Google Scholar] [CrossRef]
- Lopes, M.; Mota, M.; Belo, I. Comparison of Yarrowia lipolytica and Pichia pastoris Cellular Response to Different Agents of Oxidative Stress. Appl. Biochem. Biotechnol. 2013, 170, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Gurramkonda, C.; Polez, S.; Skoko, N.; Adnan, A.; Gäbel, T.; Chugh, D.; Swaminathan, S.; Khanna, N.; Tisminetzky, S.; Rinas, U. Application of Simple Fed-Batch Technique to High-Level Secretory Production of Insulin Precursor Using Pichia pastoris with Subsequent Purification and Conversion to Human Insulin. Microb. Cell Factories 2010, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Singh, V. Economic and Environmental Bottlenecks in the Industrial-scale Production of Lipid-derived Biofuels from Oleaginous Yeasts: A Review of the Current Trends and Future Prospects. Glob. Change Biol. Bioenergy 2024, 16, e13173. [Google Scholar] [CrossRef]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.-L.; Molina-Jouve, C.; Nicaud, J.-M. Yarrowia lipolytica as a Model for Bio-Oil Production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.K.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of Added-value Metabolites by Yarrowia lipolytica Growing in Olive Mill Wastewater-based Media under Aseptic and Non-aseptic Conditions. Eng. Life Sci. 2017, 17, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Aggelis, G. Lipids of Oleaginous Yeasts. Part I: Biochemistry of Single Cell Oil Production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Saygün, A.; Şahin-Yeşilçubuk, N.; Aran, N. Effects of Different Oil Sources and Residues on Biomass and Metabolite Production by Yarrowia lipolytica YB 423-12. J. Am. Oil Chem. Soc. 2014, 91, 1521–1530. [Google Scholar] [CrossRef]
- Portelli, B. Biologie Systémique et Intégrative Pour l’Amélioration de l’Accumulation et de La Sélectivité Des Acides Gras Accumulés dans les Espèces Levuriennes. Ph.D. Thesis, INSA Toulouse, Toulouse, France, 2011. Available online: https://theses.fr/2011ISAT0031 (accessed on 25 July 2025).
- Sarris, D.; Tsouko, E.; Kothri, M.; Anagnostou, M.; Karageorgiou, E.; Papanikolaou, S. Upgrading Major Waste Streams Derived from the Biodiesel Industry and Olive Mills via Microbial Bioprocessing with Non-Conventional Yarrowia lipolytica Strains. Fermentation 2023, 9, 251. [Google Scholar] [CrossRef]
- Zaghen, S.; Konzock, O.; Fu, J.; Kerkhoven, E.J. Abolishing Storage Lipids Induces Protein Misfolding and Stress Responses in Yarrowia lipolytica. J. Ind. Microbiol. Biotechnol. 2023, 50, kuad031. [Google Scholar] [CrossRef]
- Su, H.; Shi, P.; Shen, Z.; Meng, H.; Meng, Z.; Han, X.; Chen, Y.; Fan, W.; Fa, Y.; Yang, C.; et al. High-Level Production of Nervonic Acid in the Oleaginous Yeast Yarrowia lipolytica by Systematic Metabolic Engineering. Commun. Biol. 2023, 6, 1125. [Google Scholar] [CrossRef]
- Fabiszewska, A.U.; Zieniuk, B.; Kozłowska, M.; Mazurczak-Zieniuk, P.M.; Wołoszynowska, M.; Misiukiewicz-Stępień, P.; Nowak, D. Studies on Upgradation of Waste Fish Oil to Lipid-Rich Yeast Biomass in Yarrowia lipolytica Batch Cultures. Foods 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Fabiszewska, A.U.; Kobus, J.; Górnicka, M.; Piotrowicz, A.; Piasecka, I.; Nowak, D. Valorisation of Waste Oils Through Oleaginous Yarrowia lipolytica Yeast: Insights into Lipid Stability and Nutritive Properties of Lipid-Rich Biomass. Appl. Sci. 2025, 15, 6796. [Google Scholar] [CrossRef]
- Malvis, A.; Šimon, P.; Dubaj, T.; Sládková, A.; Ház, A.; Jablonský, M.; Sekretár, S.; Schmidt, Š.; Kreps, F.; Burčová, Z.; et al. Determination of the Thermal Oxidation Stability and the Kinetic Parameters of Commercial Extra Virgin Olive Oils from Different Varieties. J. Chem. 2019, 2019, 4567973. [Google Scholar] [CrossRef]
- Tengku-Rozaina, T.M.; Birch, E.J. Thermal Oxidative Stability Analysis of Hoki and Tuna Oils by Differential Scanning Calorimetry and Thermogravimetry: Oxidative Stability of Hoki and Tuna Oils by DSC and TGA. Eur. J. Lipid Sci. Technol. 2016, 118, 1053–1061. [Google Scholar] [CrossRef]
| Fatty Acid | WFO | OMW |
|---|---|---|
| C16:0 (Palmitic acid) | 11.71 ± 0.54 | 13.83 ± 0.71 |
| C16:1 (Palmitoleic acid) | 00.00 ± 0.00 | 0.97 ± 0.05 |
| C18:0 (Stearic acid) | 14.44 ± 1.61 | 3.95 ± 0.06 |
| C18:1 (Oleic acid) | 50.45 ± 2.43 | 43.70 ± 0.25 |
| C18:2 (Linoleic acid) | 19.99 ± 4.04 | 33.35 ± 0.37 |
| C18:3 (α-Linolenic acid) | 3.42 ± 0.54 | 4.22 ± 0.10 |
| ΣSFA | 26.15 ± 1.07 | 17.78 ± 0.66 |
| ΣMUFA | 50.45 ± 2.43 | 44.66 ± 0.20 |
| ΣPUFA | 23.41 ± 3.50 | 37.57 ± 0.47 |
| Fatty Acid | Ethanol in Medium (% v/v) | |||
|---|---|---|---|---|
| 0 | 3 | 5 | 7 | |
| C16:0 (Palmitic acid) | 5.54 ± 1.21 A | 6.16 ± 0.09 A | 5.85 ± 0.03 A | 6.00 ± 0.05 A |
| C16:1 (Palmitoleic acid) | 1.95 ± 0.46 A | 0.76 ± 0.01 B | 0.60 ± 0.00 B | 0.42 ± 0.00 B |
| C18:0 (Stearic acid) | 2.20 ± 0.01 C | 2.62 ± 0.06 A | 2.39 ± 0.00 B | 2.45 ± 0.08 B |
| C18:1 (Oleic acid) | 38.67 ± 1.59 D | 52.58 ± 1.04 C | 56.16 ± 0.53 B | 61.49 ± 0.19 A |
| C18:2 (Linoleic acid) | 19.44 ± 0.89 C | 23.42 ± 0.42 B | 26.27 ± 0.05 A | 23.69 ± 0.01 B |
| C18:3 (α-Linolenic acid) | 4.62 ± 0.19 D | 6.71 ± 0.13 B | 7.01 ± 0.02 A | 5.12 ± 0.07 C |
| C20:1 (Eicosenoic acid) | 16.25 ± 2.50 A | 4.25 ± 0.11 B | 0.86 ± 0.01 C | 0.03 ± 0.03 C |
| Other | 11.35 ± 1.87 A | 3.53 ± 0.21 B | 0.87 ± 0.58 C | 0.83 ± 0.09 C |
| ΣSFA | 7.74 ± 1.22 A | 8.77 ± 0.16 A | 8.24 ± 0.03 A | 8.44 ± 0.03 A |
| ΣMUFA | 56.86 ± 0.45 B | 57.58 ± 0.92 B | 57.61 ± 0.54 B | 61.94 ± 0.16 A |
| ΣPUFA | 24.06 ± 1.08 D | 30.13 ± 0.54 B | 33.27 ± 0.03 A | 28.81 ± 0.06 C |
| Fatty Acid | Ethanol in Medium (% v/v) | |||
|---|---|---|---|---|
| 0 | 3 | 5 | 7 | |
| C16:0 (Palmitic acid) | 16.00 ± 2.50 A | 9.77 ± 0.21 B | 11.74 ± 0.11 B | 10.94 ± 0.04 B |
| C16:1 (Palmitoleic acid) | 3.07 ± 0.33 A | 0.00 ± 0.00 B | 0.00 ± 0.00 B | 0.00 ± 0.00 B |
| C18:0 (Stearic acid) | 4.05 ± 0.39 A | 3.31 ± 0.01 C | 3.79 ± 0.01 AB | 3.47 ± 0.01 BC |
| C18:1 (Oleic acid) | 40.56 ± 1.44 B | 41.70 ± 0.18 B | 47.33 ± 0.07 A | 48.61 ± 0.20 A |
| C18:2 (Linoleic acid) | 32.55 ± 0.78 A | 26.77 ± 0.34 C | 32.30 ± 0.06 A | 31.06 ± 0.01 B |
| C18:3 (α-Linolenic acid) | 3.80 ± 0.22 B | 3.79 ± 0.04 B | 3.98 ± 0.03 B | 4.35 ± 0.13 A |
| C20:1 (Eicosenoic acid) | 0.00 ± 0.00 C | 8.36 ± 0.42 A | 1.58 ± 0.12 B | 1.58 ± 0.12 B |
| C20:2 (Eicosadienoic acid) | 0.00 ± 0.00 B | 6.31 ± 0.34 A | 0.00 ± 0.00 B | 0.00 ± 0.00 B |
| ΣSFA | 20.04 ± 2.11 A | 13.08 ± 0.21 C | 15.52 ± 0.11 B | 14.41 ± 0.05 BC |
| ΣMUFA | 43.62 ± 1.10 C | 50.06 ± 0.24 A | 48.21 ± 0.08 B | 50.19 ± 0.08 A |
| ΣPUFA | 36.34 ± 1.00 AB | 36.87 ± 0.04 A | 36.28 ± 0.04 AB | 35.41 ± 0.13 B |
| Ethanol Addition (v/v %) | τon (min) | τmax (min) |
|---|---|---|
| 0 | 1.02 ± 0.18 A | 3.45 ± 0.13 B |
| 3 | 0.86 ± 0.45 AB | 5.02 ± 0.65 A |
| 5 | 0.50 ± 0.18 B | 4.51 ± 0.37 A |
| 7 | 0.97 ± 0.23 AB | 3.35 ± 0.67 B |
| Ethanol Addition (v/v %) | τon (min) | τmax (min) |
|---|---|---|
| 0 | 30.48 ± 0.80 B | 35.73 ± 0.62 B |
| 3 | 47.07 ± 3.92 A | 54.04 ± 1.99 A |
| 5 | 12.15 ± 0.12 C | 16.92 ± 0.16 C |
| 7 | 4.99 ± 0.21 D | 9.10 ± 0.37 D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laribi, A.; Bryś, J.; Selmania, A.; Ikhlef, A.; Btaïche, I.; Mouzai, A.; Zieniuk, B.; Bouchedja, D.N. Impact of Ethanol Stress on Yarrowia lipolytica for Sustainable Bioconversion of Agro-Food Oil Wastes into Lipases and Lipids. Foods 2025, 14, 3696. https://doi.org/10.3390/foods14213696
Laribi A, Bryś J, Selmania A, Ikhlef A, Btaïche I, Mouzai A, Zieniuk B, Bouchedja DN. Impact of Ethanol Stress on Yarrowia lipolytica for Sustainable Bioconversion of Agro-Food Oil Wastes into Lipases and Lipids. Foods. 2025; 14(21):3696. https://doi.org/10.3390/foods14213696
Chicago/Turabian StyleLaribi, Amina, Joanna Bryś, Abderrahmane Selmania, Assia Ikhlef, Insaf Btaïche, Abdelghani Mouzai, Bartłomiej Zieniuk, and Doria Naila Bouchedja. 2025. "Impact of Ethanol Stress on Yarrowia lipolytica for Sustainable Bioconversion of Agro-Food Oil Wastes into Lipases and Lipids" Foods 14, no. 21: 3696. https://doi.org/10.3390/foods14213696
APA StyleLaribi, A., Bryś, J., Selmania, A., Ikhlef, A., Btaïche, I., Mouzai, A., Zieniuk, B., & Bouchedja, D. N. (2025). Impact of Ethanol Stress on Yarrowia lipolytica for Sustainable Bioconversion of Agro-Food Oil Wastes into Lipases and Lipids. Foods, 14(21), 3696. https://doi.org/10.3390/foods14213696








