Effect of Pre-Heating on Enhancing the Anti-Digestive and Antioxidant Properties of Curcumin Rice by Self-Emulsifying Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of the SEDS
2.3. Evaluation of SEDS Formulations
2.3.1. Calculation of HLB and SOR
- HLBmix = The final HLB value of the mixed surfactants.
- HLBi = The individual HLB values of surfactants.
- Wi = The respective amounts of surfactants (expressed as weight or molar ratio).
- msurfactant_mix = The total mass of surfactants.
- moil = The total mass of oil.
2.3.2. Emulsification Assessment
2.3.3. Curcumin Analysis of SEDS Formulation
2.3.4. Turbidity
2.4. Preparation of CIR
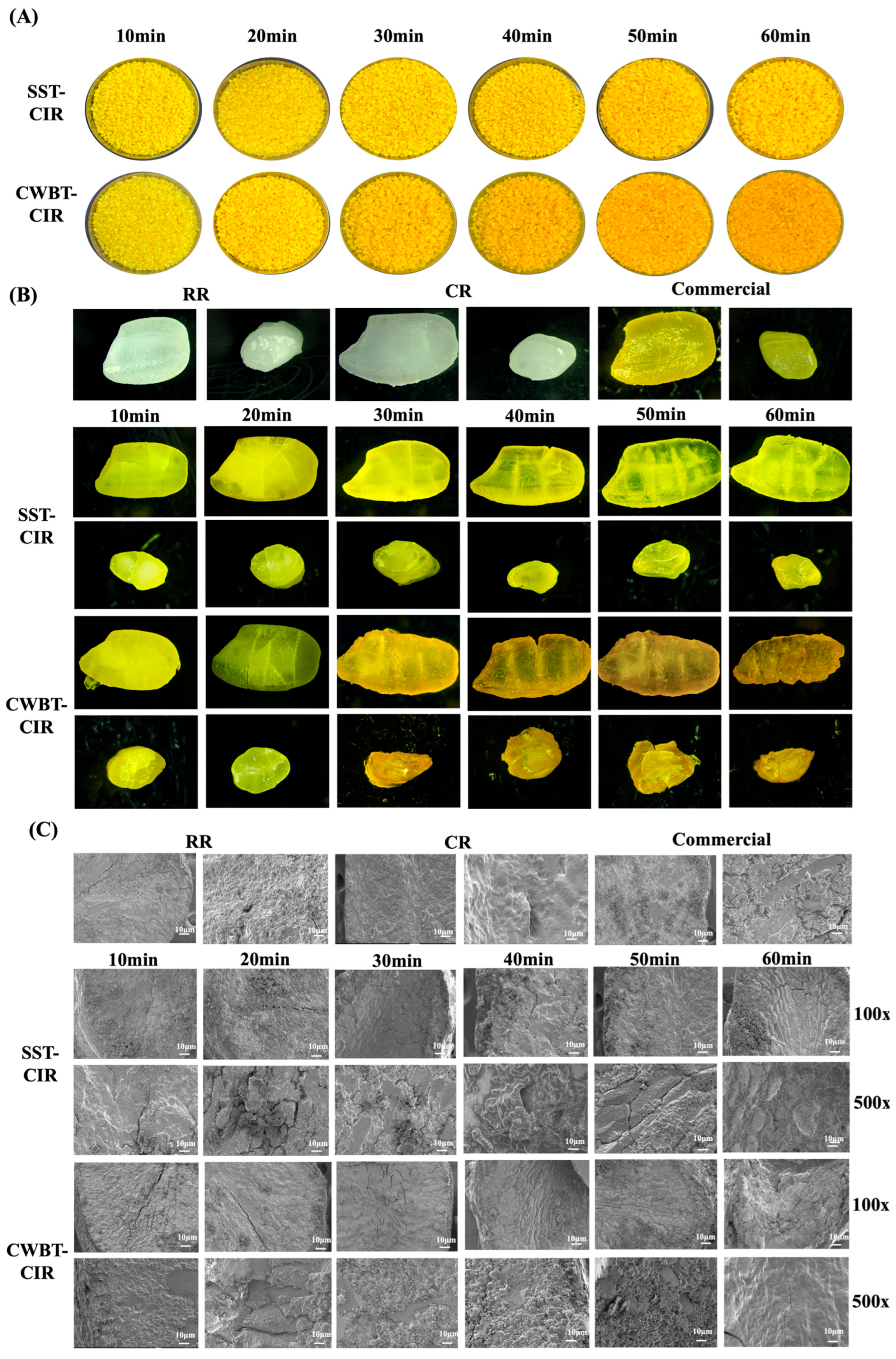
2.5. Physical Characteristics of CIR
2.5.1. Appearance
2.5.2. Scanning Electron Microscopy (FE-SEM)
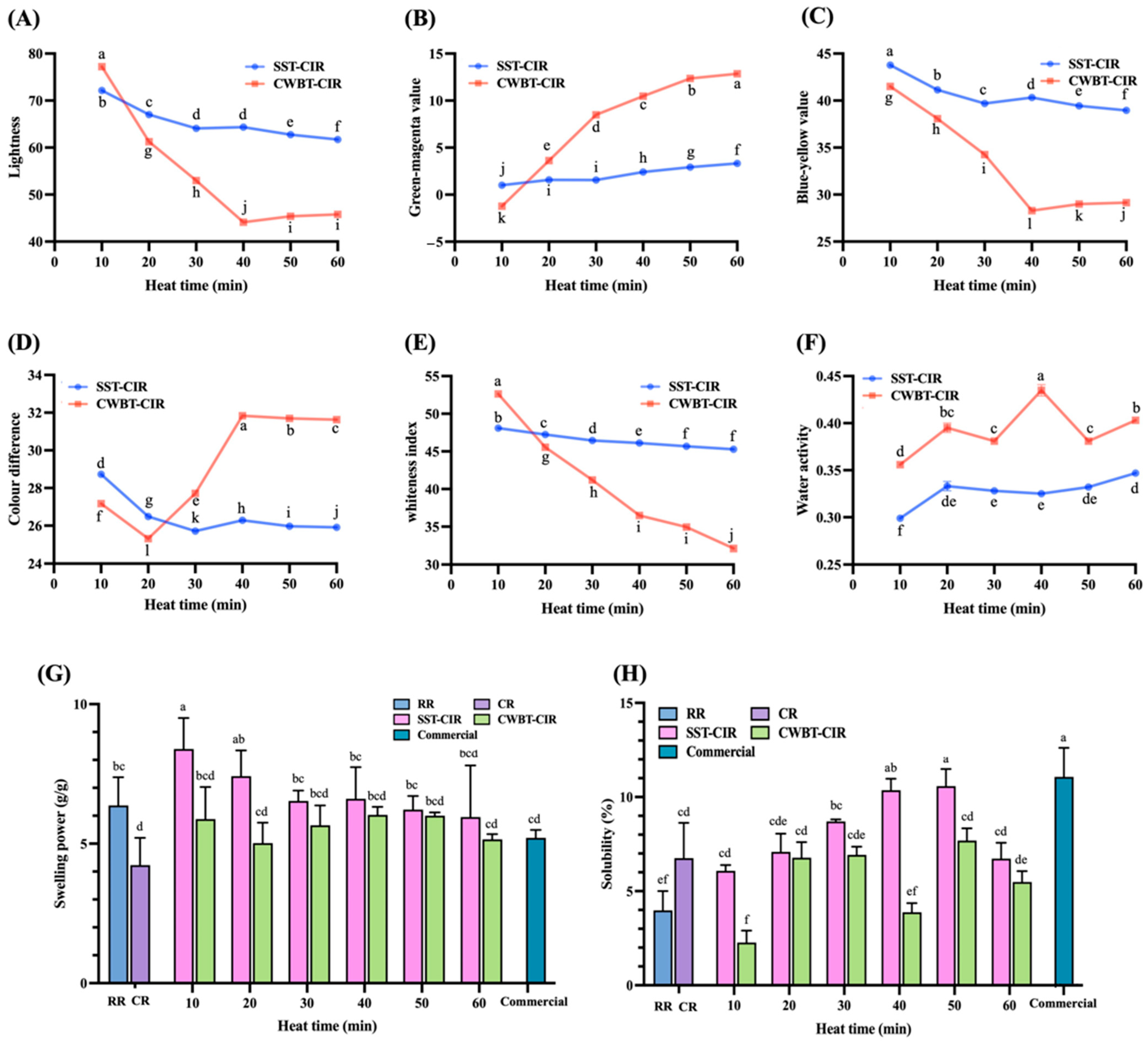
2.5.3. Color Analysis
2.5.4. Water Activity
2.5.5. Swelling Power and Solubility
2.6. Starch Structural of CIR
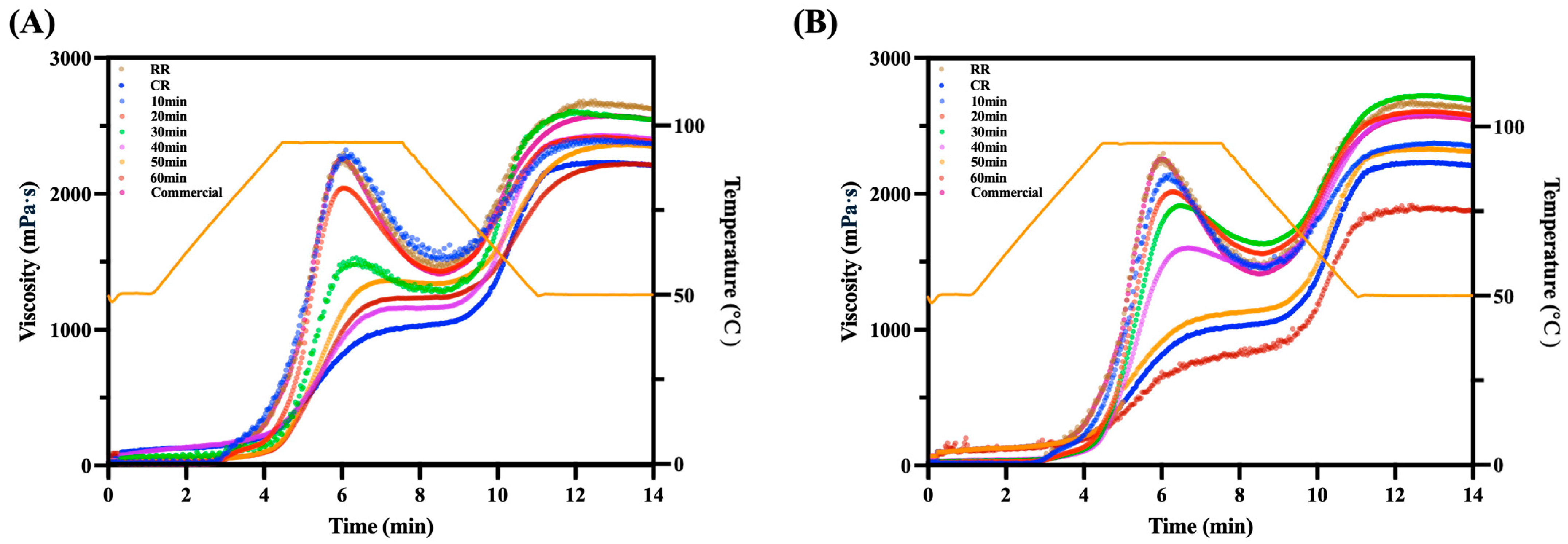
2.6.1. Rapid Visco Analyzer (RVA)
2.6.2. Differential Scanning Calorimetry (DSC)
2.6.3. In Vitro Digestibility
2.6.4. Complexing Index
2.7. Short-Range and Long-Range Ordered Molecular Sructure Analysis
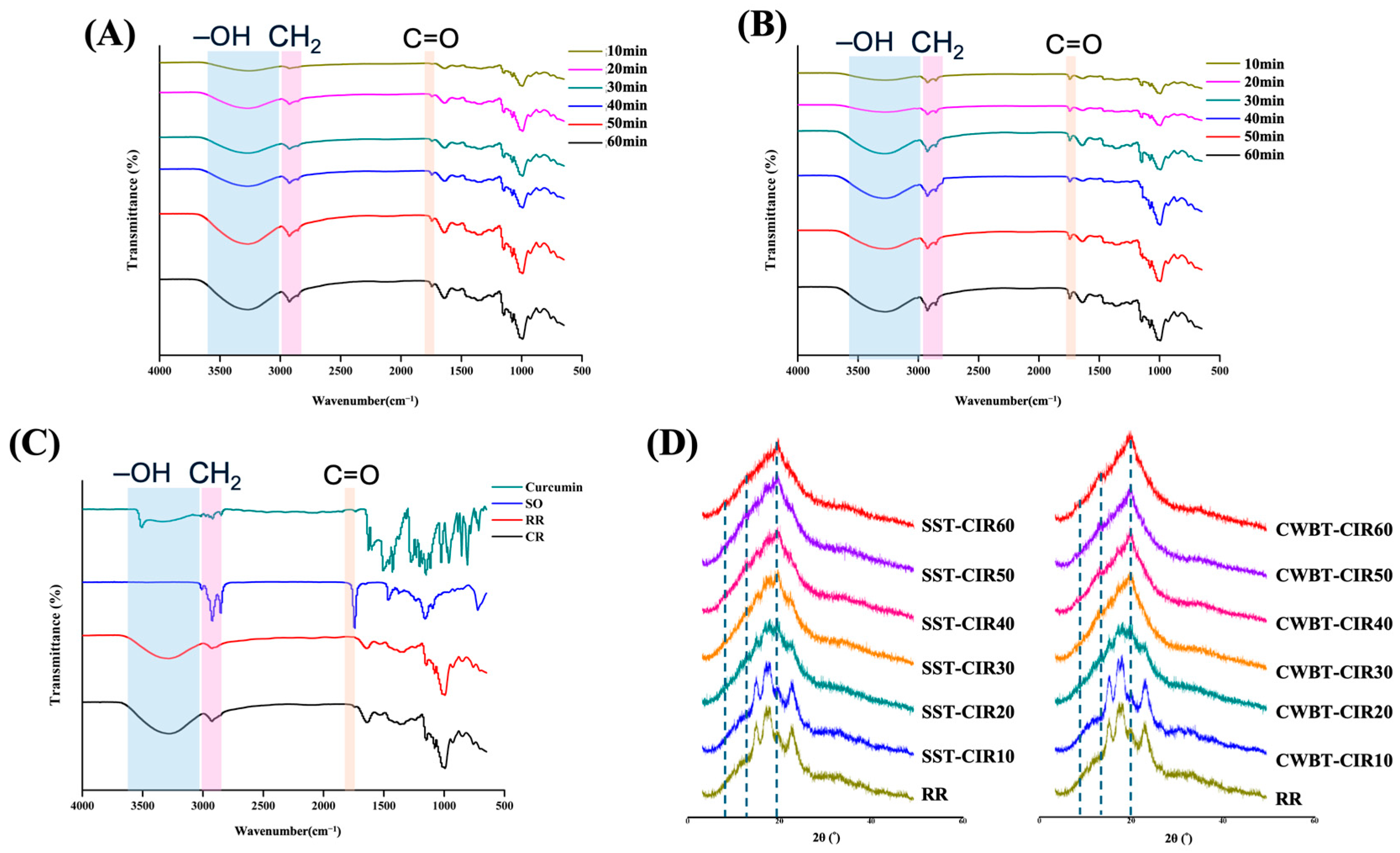
2.7.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.7.2. X-Ray Diffraction (XRD)
2.8. Antioxidant Components and Activities of CIR
2.8.1. Curcumin Content of SEDS Formulation
2.8.2. Total Phenolic Content (TPC)
2.8.3. DPPH Radical Scavenging Activity
2.8.4. ABTS+ Radical Scavenging Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Miscibility of SEDS Formulations
3.2. Physicochemical Properties of Curcumin Instant Rice
3.3. Rapid Visco Analysis of Curcumin Instant Rice
3.4. Thermal Properties of Curcumin Instant Rice
3.5. In Vitro Digestibility and Complexation Index of Curcumin Instant Rice
3.6. FTIR Spectra and X-Ray Diffraction of Curcumin Instant Rice
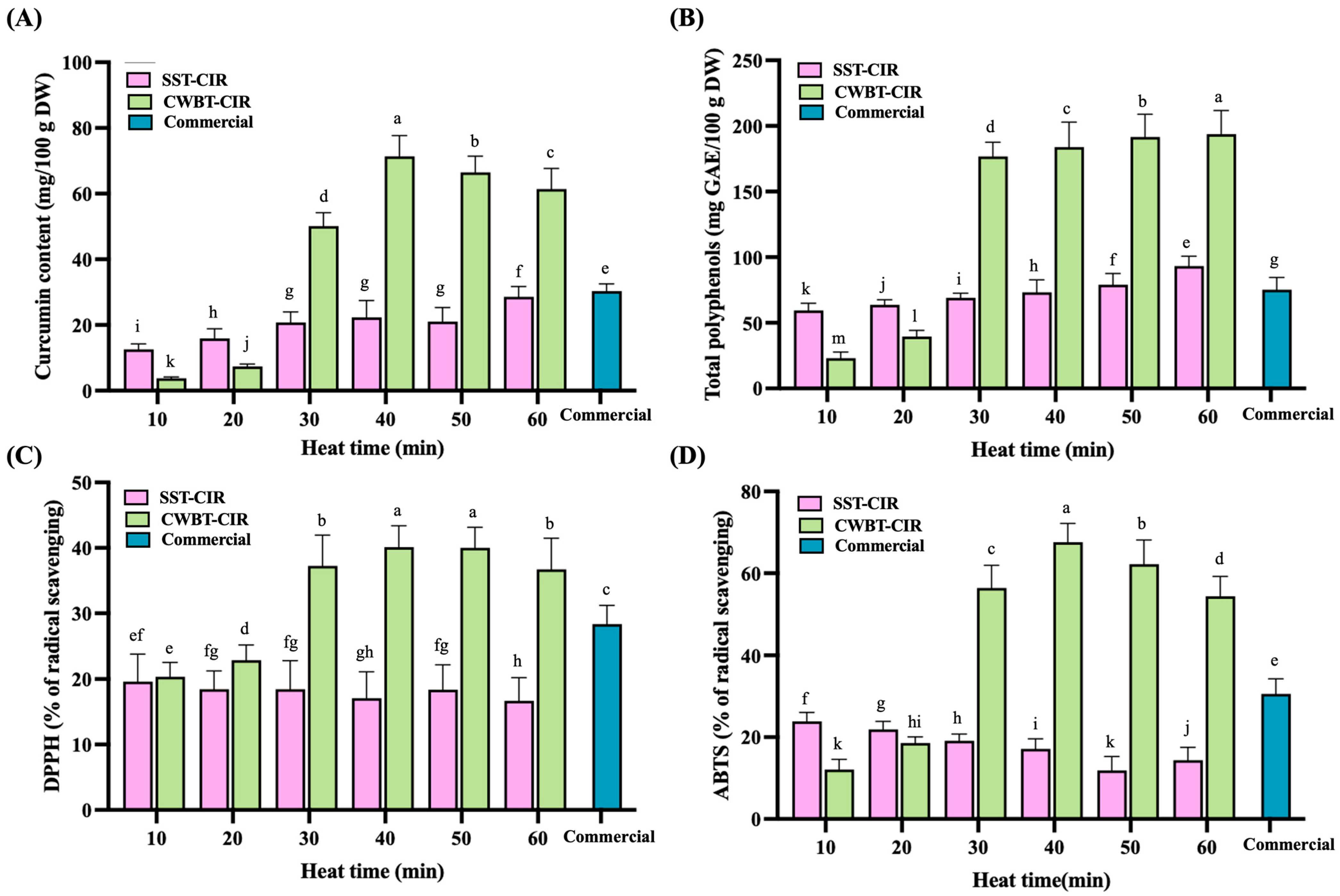
3.7. Antioxidants and Free Radical Scavenging Activity of Curcumin Instant Rice
3.8. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef]
- Tappiban, P.; Sraphet, S.; Srisawad, N.; Ahmed, S.; Bao, J.; Triwitayakorn, K. Cutting-edge progress in green technologies for resistant starch type 3 and type 5 preparation: An updated review. Food Chem. X 2024, 23, 101669. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Zhang, W.; Xue, B.; Luo, Z. Production and applications of amylose-lipid complexes as resistant starch: Recent approaches. Starch-Stärke 2021, 73, 2000249. [Google Scholar] [CrossRef]
- Li, C.; Dhital, S.; Gidley, M.J. High-amylose wheat bread with reduced in vitro digestion rate and enhanced resistant starch content. Food Hydrocoll. 2022, 123, 107181. [Google Scholar] [CrossRef]
- Shen, L.; Li, J.; Li, Y. Resistant starch formation in rice: Genetic regulation and beyond. Plant Commun. 2022, 3, 100329. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, T.; Wang, H.; Chen, L.; Zhou, Z. Studies on nutritional intervention of rice starch-oleic acid complex (resistant starch type V) in rats fed by high-fat diet. Carbohydr. Polym. 2020, 246, 116637. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Xu, Q.; Kong, Q.; Li, F.; Lu, L.; Xu, Y.; Wei, Y. Synthesis and functions of resistant starch. Adv. Nutr. 2023, 14, 1131–1144. [Google Scholar] [CrossRef]
- Yadav, G.P.; Kumar, D.; Dalbhagat, C.G.; Mishra, H.N. A comprehensive review on instant rice: Preparation methodology, characterization, and quality attributes. Food Chem. Adv. 2024, 4, 100581. [Google Scholar] [CrossRef]
- Thienhirun, S.; Chung, S. Consumer attitudes and preferences toward cross-cultural ready-to-eat (RTE) food. J. Food Prot. 2018, 24, 56–79. [Google Scholar] [CrossRef]
- Lin, J.; Li, C. Influence of instant rice characteristics and processing conditions on starch digestibility—A review. J. Food Sci. 2023, 88, 3143–3154. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Niu, L.; Tu, J.; Xiao, J. The effect of different tea products on flavor, texture, antioxidant and in vitro digestion properties of fresh instant rice after commercial sterilization at 121 C. Food Chem. 2021, 360, 130004. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving curcumin bioavailability: Current strategies and future perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Opustilová, K.; Lapčíková, B.; Lapčík, L.; Gautam, S.; Valenta, T.; Li, P. Physico-chemical study of curcumin and its application in O/W/O multiple emulsion. Foods 2023, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gong, J.; Deng, X.; Shen, L.; Wu, S.; Fan, H.; Liu, L. Curcumin and nanodelivery systems: New directions for targeted therapy and diagnosis of breast cancer. Biomed. Pharmacother. 2024, 180, 117404. [Google Scholar] [CrossRef]
- Zhang, S.; Asghar, S.; Yu, F.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, Z.; Xiao, Y. The enhancement of N-acetylcysteine on intestinal absorption and oral bioavailability of hydrophobic curcumin. Eur. J. Pharm. Sci. 2020, 154, 105506. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of turmeric and its principal bioactive curcumin on human health: Pharmaceutical, medicinal, and food applications: A comprehensive review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammal, A.; Jothi, R.; Pandian, S. Biomedical applications and bioavailability of curcumin—An updated overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Liang, Y.X.; Li, P.H.; Chiang, Y.C.; Song, H.Y.; Lai, Y.J.; Chiang, P.Y. Assessment of curcumin self-emulsion containing high methoxyl pectin-whey protein complex: Quality stability by thermal, freeze-thaw treatment, and release characteristics. LWT 2023, 188, 115398. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Alipour, M.; Dalir Abdolahinia, E.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, H.; Rahbar Saadat, Y.; Sharifi, S.; Zununi Vahed, S. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytother. Res. 2022, 36, 1156–1181. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Kucinska, M.; Tomczak, S.; Mlynarczyk, D.T.; Piskorz, J.; Goslinski, T.; Murias, M.; Jelinska, A. Liposomal nanoformulation as a carrier for curcumin and pEGCG—Study on stability and anticancer potential. Nanomaterials 2022, 12, 1274. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.M.; Chiang, P.Y. Preparation and evaluation of release formulation of γ-oryzanol/algae oil self-emulsified with alginate beads. Mar. Drugs. 2019, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Ameta, R.K.; Soni, K.; Bhattarai, A. Recent advances in improving the bioavailability of hydrophobic/lipophilic drugs and their delivery via self-emulsifying formulations. Colloids Interfaces 2023, 7, 16. [Google Scholar] [CrossRef]
- Chen, L.; Lin, X.; Fan, X.; Lv, Q.; Fang, H.; Chenchen, Y.; Teng, H. A self-emulsifying formulation of Sonchus oleraceus Linn for an improved anti-diabetic effect in vivo. Food Funct. 2020, 11, 1225–1229. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, J.; Cheng, Y.; Huang, Q. Recent advances in pickering double emulsions and potential applications in functional foods: A perspective paper. Foods 2023, 12, 992. [Google Scholar] [CrossRef]
- Wu, D.Y.; Chiang, P.Y. The evaluation of self-emulsifying delivery system (SEDS) on curcumin loading and its vitro simulated releasing contents. Taiwan. J. Agric. Chem. Food Sci. 2019, 57, 154–163. [Google Scholar] [CrossRef]
- Shahba, A.A.W.; Mohsin, K.; Alanazi, F.K. The studies of phase equilibria and efficiency assessment for self-emulsifying lipid-based formulations. AAPS PharmSciTech. 2012, 13, 522–533. [Google Scholar] [CrossRef][Green Version]
- Shirsath, S.R.; Sable, S.S.; Gaikwad, S.G.; Sonawane, S.H.; Saini, D.R.; Gogate, P.R. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: Effect of different operating parameters. Ultrason. Sonochem. 2017, 38, 437–445. [Google Scholar] [CrossRef]
- Zhu, S.M.; Hu, F.F.; Ramaswamy, H.S.; Yu, Y.; Yu, L.; Zhang, Q.T. Effect of high pressure treatment and degree of milling on gelatinization and structural properties of brown rice. Food Bioproc. Technol. 2016, 9, 1844–1853. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Cai, C.; Tian, Y.; Sun, C.; Jin, Z. Resistant structure of extruded starch: Effects of fatty acids with different chain lengths and degree of unsaturation. Food Chem. 2022, 374, 131510. [Google Scholar] [CrossRef]
- Chumsri, P.; Panpipat, W.; Cheong, L.Z.; Chaijan, M. Formation of intermediate amylose rice starch–lipid complex assisted by ultrasonication. Foods 2022, 11, 2430. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.C.; Chiang, P.Y. Accentuation of the browning characteristics and functional properties of aged tomatoes (Solanum lycopersicum cv.). Food Chem. X 2024, 22, 101499. [Google Scholar] [CrossRef] [PubMed]
- Chuang, K.C.; Chiang, Y.C.; Chang, Y.J.; Lee, Y.C.; Chiang, P.Y. Evaluation of Antioxidant and Anti-Glycemic Characteristics of Aged Lemon Peel Induced by Three Thermal Browning Models: Hot-Air Drying, High Temperature and Humidity, and Steam-Drying Cycle. Foods 2024, 13, 3053. [Google Scholar] [CrossRef] [PubMed]
- Bhoopathy, S.; Inbakandan, D.; Rajendran, T.; Chandrasekaran, K.; Kasilingam, R.; Gopal, D. Curcumin loaded chitosan nanoparticles fortify shrimp feed pellets with enhanced antioxidant activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111737. [Google Scholar] [CrossRef]
- Colón-Quintana, G.S.; Clarke, T.B.; Dick, J.E. Interfacial solute flux promotes emulsification at the water| oil interface. Nat. Commun. 2023, 14, 705. [Google Scholar] [CrossRef]
- Uttreja, P.; Karnik, I.; Adel Ali Youssef, A.; Narala, N.; Elkanayati, R.M.; Baisa, S.; Alshammari, N.D.; Banda, S.; Vemula, S.K.; Repka, M.A. Self-emulsifying drug delivery systems (SEDDS): Transition from liquid to solid—A comprehensive review of formulation, characterization, applications, and future trends. Pharmaceutics 2025, 17, 63. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Q.; Li, Z.; Chen, W.; Gu, W.; Zhang, W. Microstructure and emulsifying properties of rice starch-fatty acid complexes prepared by ultra-high-pressure treatment. Food Hydrocoll. 2025, 158, 110486. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, W.; Pan, Y.; Ali, B.; Xu, D.; Xu, X. Physicochemical, crystalline characterization and digestibility of wheat starch under superheated steam treatment. Food Hydrocoll. 2021, 118, 106720. [Google Scholar] [CrossRef]
- Ha, M.; Jeong, H.Y.; Lim, S.T.; Chung, H.J. The cooking method features controlling eating quality of cooked rice: An explanation from the view of starch structure in leachate and morphological characteristics. Food Res. Int. 2022, 162, 111980. [Google Scholar] [CrossRef]
- Tien, N.N.T.; Phi, N.T.L.; Thu, N.N.A.; Oanh, T.T.H.; Van Hung, P. Cooking quality, textural characteristics and sensory evaluation of heat-moisture treated unpolished red rice under different cooking conditions. Int. J. Food Sci. Technol. 2024, 59, 7776–7785. [Google Scholar] [CrossRef]
- Govindaraju, I.; Chakraborty, I.; Baruah, V.J.; Sarmah, B.; Mahato, K.K.; Mazumder, N. Structure and morphological properties of starch macromolecule using biophysical techniques. Starch-Stärke 2021, 73, 2000030. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, J.; Gao, M.; Liu, Y.; Dou, B.; Zhang, N. Effect of different heat-moisture treatment times on the structure, physicochemical properties and in vitro digestibility of japonica starch. Int. J. Biol. Macromol. 2024, 259, 129173. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Chu, Y.H.; Farahnaky, A. Effects of ohmic and microwave cooking on textural softening and physical properties of rice. J. Food Eng. 2019, 243, 114–124. [Google Scholar] [CrossRef]
- Park, C.Y.; Lee, K.-Y.; Gul, K.; Rahman, M.S.; Kim, A.-N.; Chun, J.; Kim, H.-J.; Choi, S.-G. Phenolics and antioxidant activity of aqueous turmeric extracts as affected by heating temperature and time. LWT 2019, 105, 149–155. [Google Scholar] [CrossRef]
- Musakhanian, J.; Rodier, J.D.; Dave, M. Oxidative stability in lipid formulations: A review of the mechanisms, drivers, and inhibitors of oxidation. AAPS PharmSciTech. 2022, 23, 151. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Al-Maqtari, Q.A.; Mohammed, J.K.; Al-Ansi, W.; Cui, H.; Lin, L. Enhancement of antioxidant activity, antifungal activity, and oxidation stability of Citrus reticulata essential oil nanocapsules by clove and cinnamon essential oils. Food Biosci. 2021, 43, 101226. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Yang, Y.; Yu, X.; Xu, L.; Jiao, A.; Jin, Z. Structure, physicochemical properties and in vitro digestibility of extruded starch-lauric acid complexes with different amylose contents. Food Hydrocoll. 2023, 136, 108239. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, Y.; Cao, H.; Li, S.; Zhang, Y.; Huang, K.; Song, H.; Guan, X. Exploring the remarkable effects of microwave treatment on starch modification: From structural evolution to changed physicochemical and digestive properties. Carbohydr. Polym. 2024, 343, 122412. [Google Scholar] [CrossRef]
- Xiang, G.; Li, J.; Lin, Q.; Zhang, Y.; Ding, Y.; Guo, X.; Pan, Q.; Liu, Q.; Fu, X.; Yang, Y.; et al. The effect of heat-moisture treatment changed the binding of starch, protein and lipid in rice flour to affect its hierarchical structure and physicochemical properties. Food Chem. X 2023, 19, 100785. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, S.; Chi, G.; Li, H.; Chen, K.; Wang, Z.; Kan, J. Preparation and functional characteristics of starch-lipid complexes with different oleic acid-rich glycerolipids. Food Chem. 2025, 476, 143450. [Google Scholar] [CrossRef]
- Chao, C.; Yu, J.; Wang, S.; Copeland, L.; Wang, S. Mechanisms underlying the formation of complexes between maize starch and lipids. J. Agric. Food Chem. 2018, 66, 272–278. [Google Scholar] [CrossRef]
- Zheng, M.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Effects of chain length and degree of unsaturation of fatty acids on structure and in vitro digestibility of starch–protein–fatty acid complexes. J. Agric. Food Chem. 2018, 66, 1872–1880. [Google Scholar] [CrossRef]
- Kang, X.; Jia, S.; Gao, W.; Wang, B.; Zhang, X.; Tian, Y.; Sun, Q.; Atef, M.; Cui, B.; Abd El-Aty, A.M. The formation of starch-lipid complexes by microwave heating. Food Chem. 2022, 382, 132319. [Google Scholar] [CrossRef]
- Gutierrez, T.J.; Tovar, J. Update of the concept of type 5 resistant starch (RS5): Self-assembled starch V-type complexes. Trends Food Sci. Technol. 2021, 109, 711–724. [Google Scholar] [CrossRef]
- Chen, J.; Cai, H.; Yang, S.; Zhang, M.; Wang, J.; Chen, Z. The formation of starch-lipid complexes in instant rice noodles incorporated with different fatty acids: Effect on the structure, in vitro enzymatic digestibility and retrogradation properties during storage. Food Res. Int. 2022, 162, 111933. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Karaca, A.C.; Deng, L.; Colussi, R.; Narita, I.M.P.; Kaur, K.; Aaliya, B.; Sunooj, V.; Falsafi, S.R. Oat starch-How physical and chemical modifications affect the physicochemical attributes and digestibility. Carbohydr. Polym. 2022, 296, 119931. [Google Scholar] [CrossRef]
- Cui, J.; Zheng, B.; Liu, Y.; Chen, L.; Li, B.; Li, L. Insights into the effect of structural alternations on the digestibility of rice starch-fatty acid complexes prepared by high-pressure homogenization. LWT 2021, 136, 110294. [Google Scholar] [CrossRef]
- Guo, T.; Hou, H.; Liu, Y.; Chen, L.; Zheng, B. In vitro digestibility and structural control of rice starch-unsaturated fatty acid complexes by high-pressure homogenization. Carbohydr. Polym. 2021, 256, 117607. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Wang, N.; Yang, L.; Zhou, Y. Effect of water content of high-amylose corn starch and glutinous rice starch combined with lipids on formation of starch–lipid complexes during deep-fat frying. Food Chem. 2019, 278, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.W.; Lai, L.S. Impact of Ultrasonic-Assisted Preparation of Water Caltrop Starch–Lipid Complex: Structural and Physicochemical Properties. Foods 2025, 14, 240. [Google Scholar] [CrossRef]
- Khatun, A.; Waters, D.L.; Liu, L. A review of rice starch digestibility: Effect of composition and heat-moisture processing. Starch-Stärke 2019, 71, 1900090. [Google Scholar] [CrossRef]
- Tamura, M.; Kumagai, C.; Kaur, L.; Ogawa, Y.; Singh, J. Cooking of short, medium and long-grain rice in limited and excess water: Effects on microstructural characteristics and gastro-small intestinal starch digestion in vitro. LWT 2021, 146, 111379. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Z.; Yu, M.; Ji, N.; Dai, L.; Dong, X.; Xiong, L.; Sun, Q. Characterization of complexes formed between debranched starch and fatty acids having different carbon chain lengths. Int. J. Biol. Macromol. 2021, 167, 595–604. [Google Scholar] [CrossRef]
- Aussanasuwannakul, A.; Singkammo, S. Multiscale Characterization of Rice Starch Gelation and Retrogradation Modified by Soybean Residue (Okara) and Extracted Dietary Fiber Using Rheology, Synchrotron Wide-Angle X-Ray Scattering (WAXS), and Fourier Transform Infrared (FTIR) Spectroscopy. Foods 2025, 14, 1862. [Google Scholar] [CrossRef]
- Ratnaningsih, N.; Harmayani, E.; Marsono, Y. Physicochemical properties, in vitro starch digestibility, and estimated glycemic index of resistant starch from cowpea (Vigna unguiculata) starch by autoclaving-cooling cycles. Int. J. Biol. Macromol. 2020, 142, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Lai, S.; Ouyang, K.; Hu, H.; Hu, X.; Xiong, H.; Zhao, Q. Impact of encapsulation conditions on V-type granular starch-curcumin complexes. J. Food Eng. 2025, 389, 112402. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Cheng, L.; Li, Z.; Li, C.; Hong, Y.; Gu, Z. Effects of heat moisture treatment on the structure and digestibility of high amylose starch-lauric acid complexes. Food Hydrocoll. 2024, 151, 109803. [Google Scholar] [CrossRef]
- Gao, Q.; Feng, R.; Yu, M.J.; Tao, H.; Zhang, B. Oleic acid treatment of rice grains reduces the starch digestibility: Formation, binding state and fine structure of starch-lipid complexes. Food Chem. 2024, 457, 140191. [Google Scholar] [CrossRef]
- He, H.; Zheng, B.; Wang, H.; Li, X.; Chen, L. Insights into the multi-scale structure and in vitro digestibility changes of rice starch-oleic acid/linoleic acid complex induced by heat-moisture treatment. Food Res. Int. 2020, 137, 109612. [Google Scholar] [CrossRef]
- Le-Bail, P.; Houinsou-Houssou, B.; Kosta, M.; Pontoire, B.; Gore, E.; Le-Bail, A. Molecular encapsulation of linoleic and linolenic acids by amylose using hydrothermal and high-pressure treatments. Food Res. Int. 2015, 67, 223–229. [Google Scholar] [CrossRef]
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. Changes in curcuminoids and chemical components of turmeric (Curcuma longa L.) under freeze-drying and low-temperature drying methods. Food Chem. 2021, 339, 128121. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Baez, J.E.; Martínez, L.M.; Welti-Chanes, J.; Buitimea-Cantúa, G.V.; Escobedo-Avellaneda, Z. High hydrostatic pressure to increase the biosynthesis and extraction of phenolic compounds in food: A review. Molecules 2022, 27, 1502. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, W.; Gilbert, R.G. The effects of starch molecular fine structure on thermal and digestion properties of rice starch. Foods 2022, 11, 4012. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Cai, C.; Wang, F.; Zhan, J.; Tian, Y. Improvement of resistant starch content and thermal-stability of starch-linoleic acid complex: An attempt application in extruded recombinant rice. Food Chem. 2024, 445, 138768. [Google Scholar] [CrossRef]
- Chiodetti, M.; Tuccio, M.G.; Carini, E. Effect of water content on gelatinization functionality of flour from sprouted sorghum. Curr. Res. Food Sci. 2024, 8, 100780. [Google Scholar] [CrossRef]


| Oil:Surfactant | Tween80:Span80 | |||||
|---|---|---|---|---|---|---|
| 4:1 | 3:2 | 1:1 | 2:3 | 1:4 | ||
| Sunflower oil | 2:1 | ● | ● | ● | ● | ○ |
| 3:2 | ● | ● | ○ | ○ | ○ | |
| 1:1 | ● | ○ | ○ | ○ | ○ | |
| Sunflower Oil | Oil:Tween 80:Span 80 | |||||||
|---|---|---|---|---|---|---|---|---|
| 10:1:4 | 3:1:1 | 15:4:6 | 15:2:8 | 5:3:2 | 2:1:1 | 5:2:3 | 5:1:4 | |
| HLB | 6.44 | 9.65 | 8.58 | 6.44 | 10.72 | 9.65 | 8.58 | 6.44 |
| SOR | 0.50 | 0.67 | 0.67 | 0.67 | 1.00 | 1.00 | 1.00 | 1.00 |
| Spontaneity | poor | moderate | poor | poor | good | poor | poor | poor |
| Homogeneity | poor | poor | moderate | poor | good | poor | poor | poor |
| Dispersibility | poor | moderate | poor | poor | good | poor | poor | poor |
| Appearance | milky | semi- transparent | semi-transparent | transparent | transparent | semi-transparent | milky | milky |
| Curcumin (mg/g) | 2.70 ± 0.03 h | 5.57 ± 0.09 d | 4.78 ± 0.03 e | 3.19 ± 0.01 g | 7.92 ± 0.04 a | 6.90 ± 0.03 b | 5.90 ± 0.02 c | 3.92 ± 0.01 f |
| Pasting Temperature (°C) | Peak Viscosity (mPa·s) | Trough Viscosity (mPa·s) | Breakdown Viscosity (mPa·s) | Final Viscosity (mPa·s) | Setback Viscosity (mPa·s) | |
|---|---|---|---|---|---|---|
| RR | 76.55 ± 0.61 g | 2256.49 ± 35.11 a | 1602.10 ± 41.37 a | 656.81 ± 8.64 b | 2546.84 ± 32.52 e | 946.02 ± 11.82 i |
| CR | 51.70 ± 0.77 j | 996.02 ± 10.11 n | 946.08 ± 14.47 k | 49.94 ± 9.47 n | 2124.88 ± 18.98 m | 1178.80 ± 19.02 h |
| SST-CIR10 | 87.35 ± 0.97 f | 2123.94 ± 36.98 d | 1522.39 ± 28.57 d | 633.55 ± 15.63 c | 2368.72 ± 45.81 h | 868.44 ± 5.11 j |
| SST-CIR20 | 90.85 ± 0.42 de | 2043.28 ± 27.91 e | 1458.85 ± 29.94 e | 593.58 ± 11.72 e | 2384.10 ± 48.61 g | 934.58 ± 17.01 i |
| SST-CIR30 | 91.75 ± 0.81 cd | 1529.38 ± 20.94 i | 1103.72 ± 39.02 i | 492.84 ± 10.82 h | 2551.34 ± 35.67 d | 1447.62 ± 25.17 c |
| SST-CIR40 | 92.25 ± 0.48 bcd | 1163.49 ± 19.10 l | 827.03 ± 19.82 l | 343.30 ± 9.77 j | 2403.84 ± 21.17 f | 1583.65 ± 27.72 b |
| SST-CIR50 | 93.85 ± 0.33 ab | 1363.59 ± 22.47 j | 1037.04 ± 28.36 j | 333.10 ± 16.47 k | 2351.59 ± 35.64 j | 1321.02 ± 13.88 e |
| SST-CIR60 | 92.55 ± 0.45 abcd | 1234.04 ± 23.04 k | 951.95 ± 36.37 k | 284.38 ± 13.83 l | 2214.05 ± 33.83 l | 1264.94 ± 20.14 g |
| CWBT-CIR10 | 72.30 ± 0.48 h | 2142.43 ± 35.37 c | 1540.47 ± 25.39 b | 602.45 ± 9.39 d | 2353.65 ± 39.72 i | 813.57 ± 10.97 k |
| CWBT-CIR20 | 89.95 ± 0.43 e | 2016.59 ± 13.98 f | 1450.48 ± 19.23 f | 566.03 ± 10.40 f | 2577.38 ± 36.35 b | 1127.68 ± 14.21 h |
| CWBT-CIR30 | 94.29 ± 0.26 a | 1912.44 ± 25.11 g | 1400.37 ± 38.28 g | 512.04 ± 8.08 g | 2693.48 ± 40.06 a | 1293.77 ± 19.59 f |
| CWBT-CIR40 | 93.95 ± 0.67 ab | 1603.45 ± 17.43 h | 1239.95 ± 18.28 h | 403.92 ± 10.73 i | 2569.59 ± 42.06 c | 1369.92 ± 22.47 d |
| CWBT-CIR50 | 93.00 ± 0.71 abc | 1085.28 ± 16.01 m | 1039.84 ± 18.04 j | 45.44 ± 10.62 n | 2214.02 ± 37.94 l | 1174.18 ± 21.73 h |
| CWBT-CIR60 | 54.65 ± 0.55 i | 838.38 ± 9.11 o | 603.38 ± 20.73 m | 238.72 ± 9.36 m | 2323.07 ± 25.67 k | 1719.69 ± 24.43 a |
| Commercial | 76.89 ± 0.19 g | 2193.84 ± 36.11 b | 1538.45 ± 37.39 c | 693.84 ± 12.47 a | 1880.93 ± 23.17 n | 342.48 ± 9.38 l |
| Peak I | Peak II | Peak III | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | To (°C) | Tp (°C) | Te (°C) | ΔH (J/g) | To (°C) | Tp (°C) | Te (°C) | ΔH (J/g) | To (°C) | Tp (°C) | Te (°C) | ΔH (J/g) |
| RR | 64.91 ± 0.13 a | 71.14 ± 0.01 abc | 79.23 ± 0.45 bc | 1.72 ± 0.24 a | – | – | – | – | – | – | – | – |
| CR | – | – | – | – | – | – | – | – | – | – | – | – |
| SCIR10 | 63.32 ± 0.07 ab | 69.02 ± 0.01 bcdef | 76.84 ± 0.17 bcde | 1.36 ± 0.07 ab | – | – | – | – | 106.95 ± 0.27 a | 116.14 ± 0.01 ab | 127.02 ± 0.16 abc | 0.24 ± 0.04 l |
| SCIR20 | 61.09 ± 0.04 abc | 72.65 ± 0.01 a | 80.46 ± 0.44 ab | 0.19 ± 0.01 c | – | – | – | – | 105.03 ± 0.27 ab | 116.15 ± 0.62 ab | 126.41 ± 4.36 bc | 0.26 ± 0.02 k |
| SCIR30 | – | – | – | – | – | – | – | – | 101.40 ± 0.01 b | 110.74 ± 4.14 b | 124.01 ± 2.36 c | 0.42 ± 0.05 j |
| SCIR40 | – | – | – | – | – | – | – | – | 105.41 ± 5.51 ab | 115.89 ± 5.32 ab | 131.05 ± 1.47 a | 0.60 ± 0.05 g |
| SCIR50 | – | – | – | – | 89.50 ± 2.87 de | 97.44 ± 0.31 f | 103.40 ± 2.54 c | 0.17 ± 0.01 cd | 107.17 ± 3.16 a | 116.57 ± 6.18 ab | 129.36 ± 0.04 ab | 0.56 ± 0.12 h |
| SCIR60 | – | – | – | – | 94.55 ± 4.83 abcd | 101.45 ± 2.58 abcd | 107.36 ± 1.51 c | 0.13 ± 0.03 cd | 106.88 ± 0.59 a | 120.69 ± 0.01 a | 127.88 ± 1.90 abc | 0.50 ± 0.03 i |
| CCIR10 | 61.98 ± 0.29 abc | 67.58 ± 0.01 ef | 74.21 ± 0.67 cde | 0.95 ± 0.67 b | 88.66 ± 1.03 e | 97.70 ± 0.08 f | 106.03 ± 0.30 c | 0.15 ± 0.02 cd | 105.22 ± 0.55 ab | 115.58 ± 0.08 ab | 130.31 ± 1.67 ab | 0.70 ± 0.14 f |
| CCIR20 | 56.74 ± 4.70 cd | 70.24 ± 0.70 abcde | 74.32 ± 3.16 cde | 0.26 ± 0.13 c | 95.17 ± 1.28 abc | 101.95 ± 2.01 abcd | 108.29 ± 1.82 bc | 0.12 ± 0.01 d | 105.62 ± 0.26 ab | 113.29 ± 0.01 b | 130.27 ± 0.16 ab | 1.11 ± 0.18 e |
| CCIR30 | – | – | – | – | 90.01 ± 1.64 de | 99.24 ± 0.08 def | 116.04 ± 1.02 ab | 0.24 ± 002 c | 105.55 ± 4.94 ab | 112.95 ± 1.58 b | 129.60 ± 0.10 ab | 1.18 ± 0.85 d |
| CCIR40 | – | – | – | – | 95.32 ± 0.71 abc | 101.13 ± 0.01 abcde | 110.37 ± 0.47 bc | 0.23 ± 0.05 cd | 106.17 ± 0.88 ab | 120.73 ± 3.13 a | 127.13 ± 3.14 abc | 1.37 ± 0.08 c |
| CCIR50 | – | – | – | – | 99.44 ± 0.37 a | 103.33 ± 1.61 a | 108.74 ± 1.05 bc | 0.35 ± 0.07 b | 105.95 ± 0.28 ab | 116.38 ± 0.01 ab | 129.65 ± 1.82 ab | 1.64 ± 0.26 b |
| CCIR60 | – | – | – | – | 99.73 ± 0.85 a | 101.14 ± 0.08 abcde | 109.33 ± 0.55 bc | 0.51 ± 0.15 a | 105.83 ± 0.18 ab | 115.60 ± 0.08 ab | 130.64 ± 0.67 ab | 3.04 ± 0.25 a |
| Commercial | 58.96 ± 1.15 bc | 67.80 ± 0.01 def | 72.55 ± 4.10 de | 1.03 ± 0.12 b | – | – | – | – | 104.70 ± 0.59 ab | 113.75 ± 0.15 b | 129.18 ± 0.27 ab | 0.16 ± 0.01 m |
| R1047/1022 | R995/1022 | |
|---|---|---|
| RR | 1.536 ± 0.002 a | 0.956 ± 0.005 bc |
| CR | 1.263 ± 0.003 cd | 0.894 ± 0.006 e |
| SST-CIR10 | 1.102 ± 0.005 d | 0.985 ± 0.004 a |
| SST-CIR20 | 1.112 ± 0.004 d | 0.952 ± 0.004 bc |
| SST-CIR30 | 1.209 ± 0.005 cd | 0.935 ± 0.006 d |
| SST-CIR40 | 1.223 ± 0.003 cd | 0.906 ± 0.006 e |
| SST-CIR50 | 1.335 ± 0.001 bc | 0.903 ± 0.002 e |
| SST-CIR60 | 1.613 ± 0.006 a | 0.852 ± 0.002 f |
| CWBT-CIR10 | 1.075 ± 0.007 d | 0.981 ± 0.006 a |
| CWBT-CIR20 | 1.094 ± 0.003 d | 0.961 ± 0.007 b |
| CWBT-CIR30 | 1.225 ± 0.006 cd | 0.943 ± 0.001 cd |
| CWBT-CIR40 | 1.456 ± 0.008 ab | 0.963 ± 0.006 b |
| CWBT-CIR50 | 1.514 ± 0.004 a | 0.933 ± 0.004 d |
| CWBT-CIR60 | 1.613 ± 0.008 a | 0.905 ± 0.003 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.-Y.; Chiang, Y.-C.; Chiang, P.-Y. Effect of Pre-Heating on Enhancing the Anti-Digestive and Antioxidant Properties of Curcumin Rice by Self-Emulsifying Technology. Foods 2025, 14, 3668. https://doi.org/10.3390/foods14213668
Ma C-Y, Chiang Y-C, Chiang P-Y. Effect of Pre-Heating on Enhancing the Anti-Digestive and Antioxidant Properties of Curcumin Rice by Self-Emulsifying Technology. Foods. 2025; 14(21):3668. https://doi.org/10.3390/foods14213668
Chicago/Turabian StyleMa, Chien-Yu, Yi-Chan Chiang, and Po-Yuan Chiang. 2025. "Effect of Pre-Heating on Enhancing the Anti-Digestive and Antioxidant Properties of Curcumin Rice by Self-Emulsifying Technology" Foods 14, no. 21: 3668. https://doi.org/10.3390/foods14213668
APA StyleMa, C.-Y., Chiang, Y.-C., & Chiang, P.-Y. (2025). Effect of Pre-Heating on Enhancing the Anti-Digestive and Antioxidant Properties of Curcumin Rice by Self-Emulsifying Technology. Foods, 14(21), 3668. https://doi.org/10.3390/foods14213668






