Boiling-Resistant Single-Chain Sweet Protein Monellin as a Safe and Effective Sugar Alternative for Metabolic and Glycemic Management in Mice
Abstract
1. Introduction
2. Method
2.1. Plasmid Construction and Pichia pastoris Transformation
2.2. Expression and Purification of the Protein
2.2.1. Expression in P. pastoris
2.2.2. Protein Purification
2.3. Thermal Shift Assay
2.4. Cell Cytotoxicity Assay
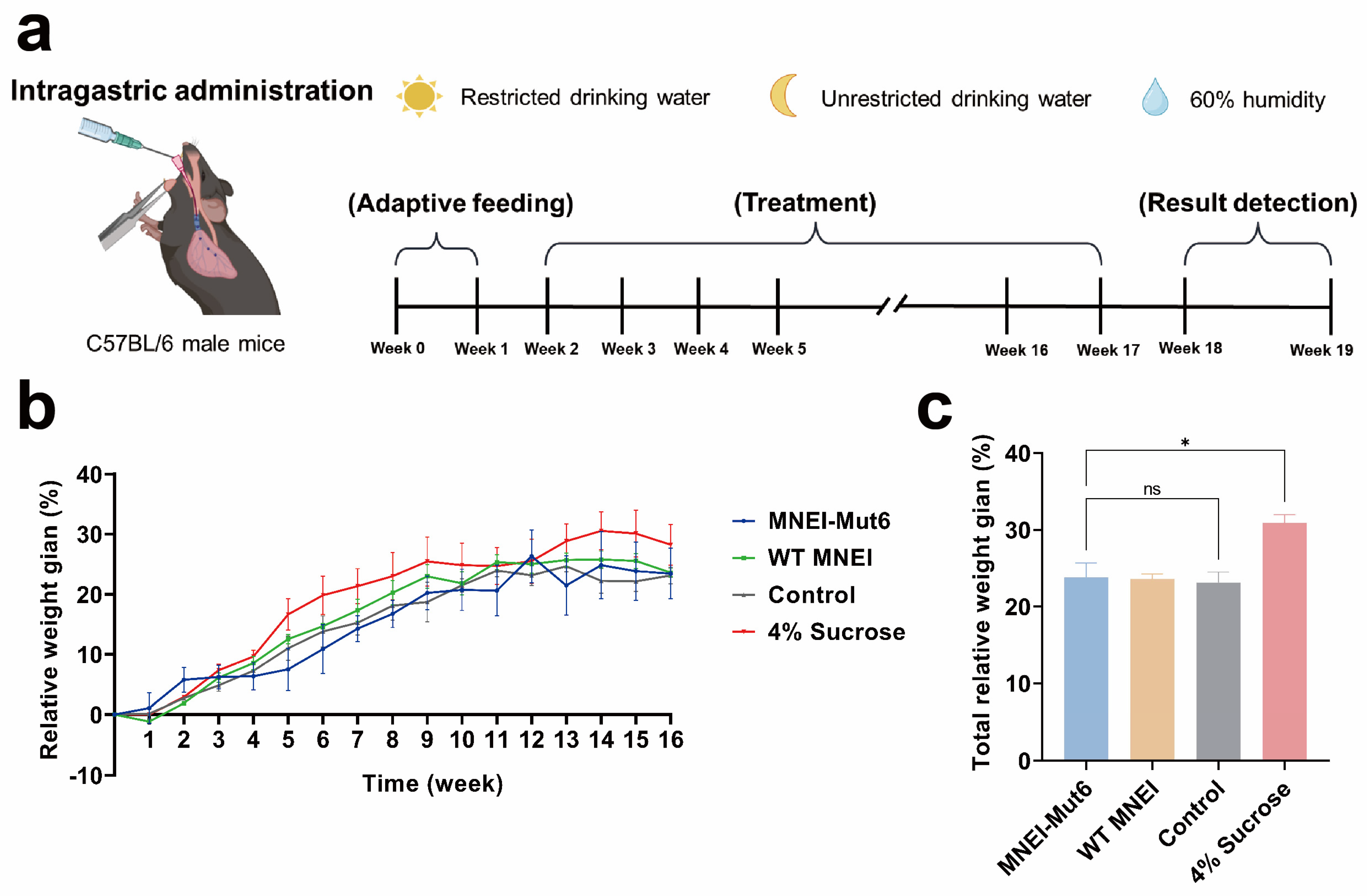
2.5. Mice and Diet
2.5.1. Animals and Diet
- -
- Control (n = 5, biological replicates; water, oral gavage, 10 mL/kg);
- -
- 4% sucrose solution (n = 5, biological replicates; Sigma-Aldrich, USA);
- -
- WT MNEI (n = 7, biological replicates; 0.0015% solution, equivalent sweetness to 4% sucrose);
- -
- MNEI-Mut6 (n = 7, biological replicates; 0.0015% solution, equivalent sweetness to 4% sucrose).
2.5.2. Sample Collection
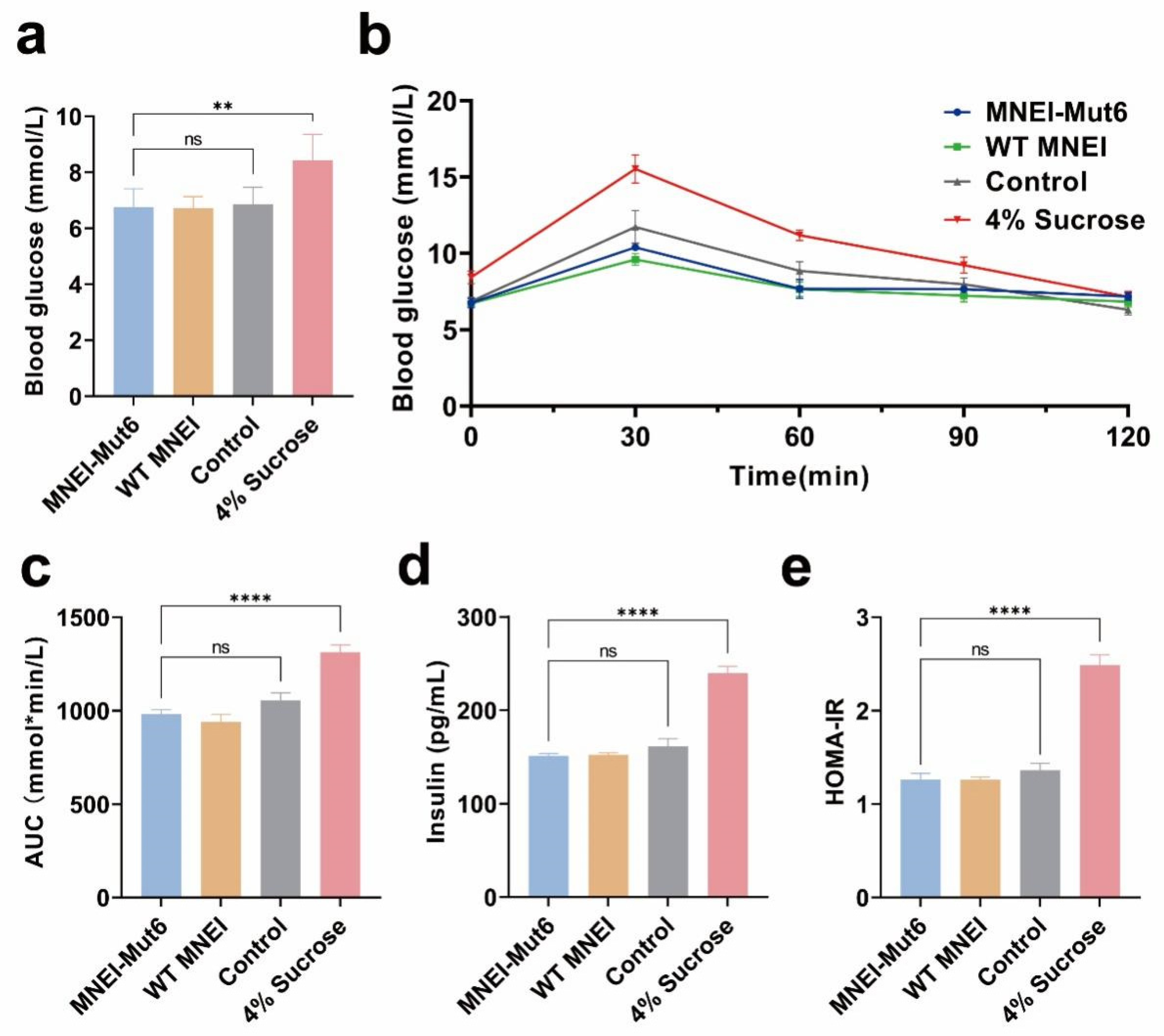
2.6. Glucose Tolerance Test and Serum Insulin Analysis
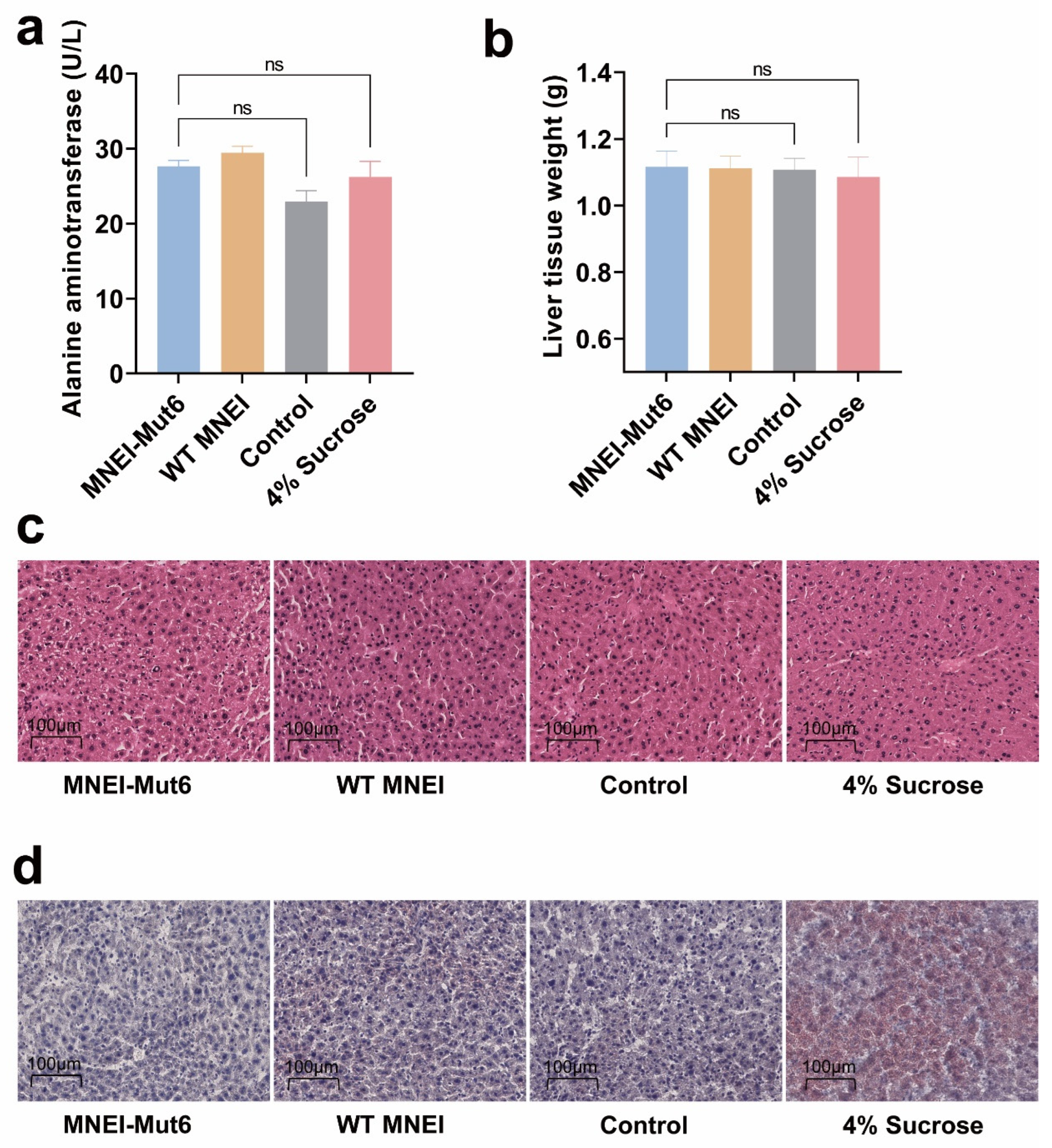
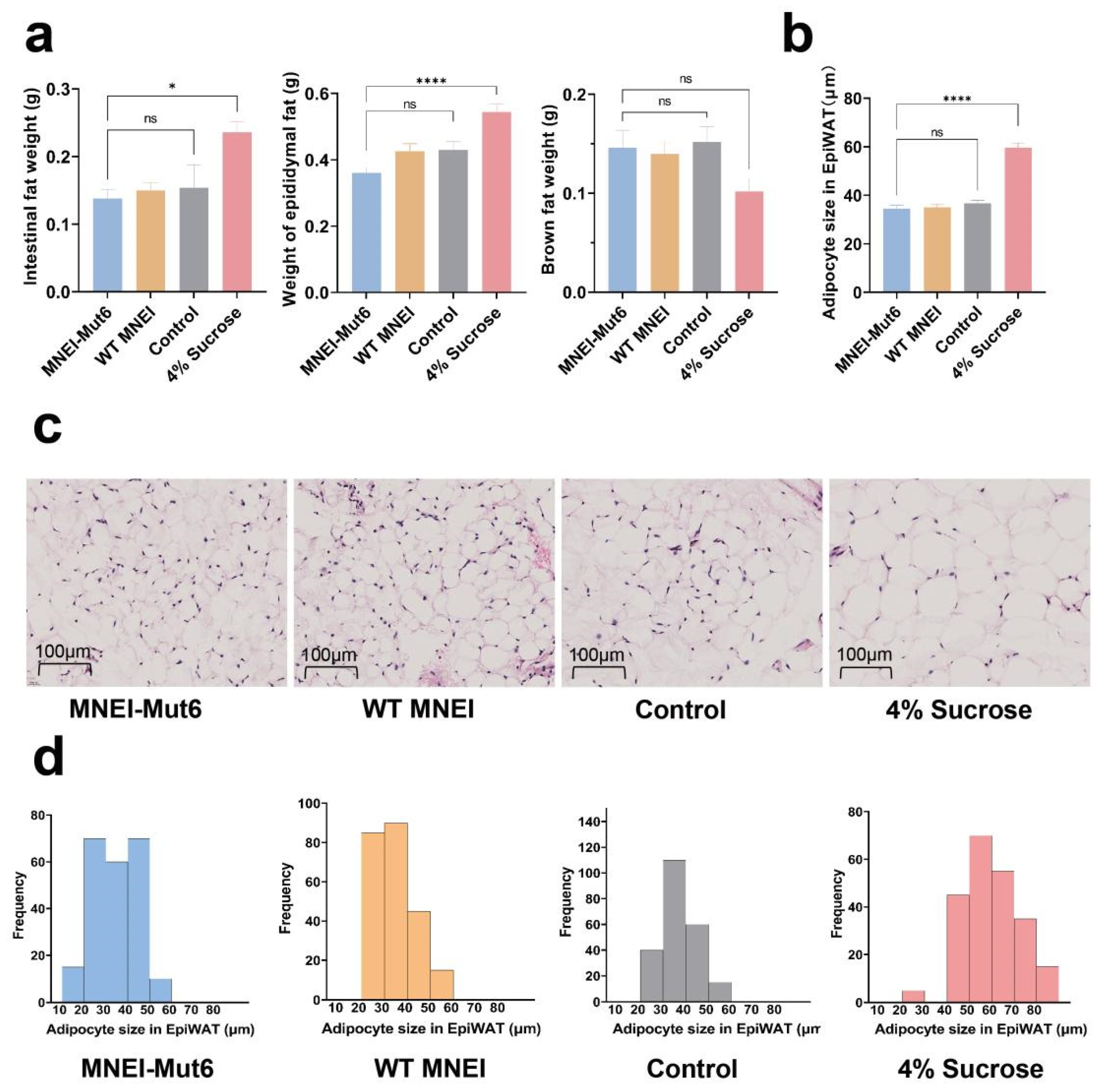
2.7. Histology
2.8. Statistics
3. Results
3.1. Production and Biochemical Characterization of Mnei-Mut6
3.2. Long-Term Mnei-Mut6 Supplementation Exhibits Neutral Effects on Body Weight Compared to Sucrose
3.3. Long-Term Mnei-Mut6 Supplementation Maintains Glucose Homeostasis
3.4. Long-Term Mnei-Mut6 Supplementation Does Not Induce Liver Toxicity or Fat Accumulation
3.5. Long-Term Mnei-Mut6 Supplementation Mitigates Fat Accumulation
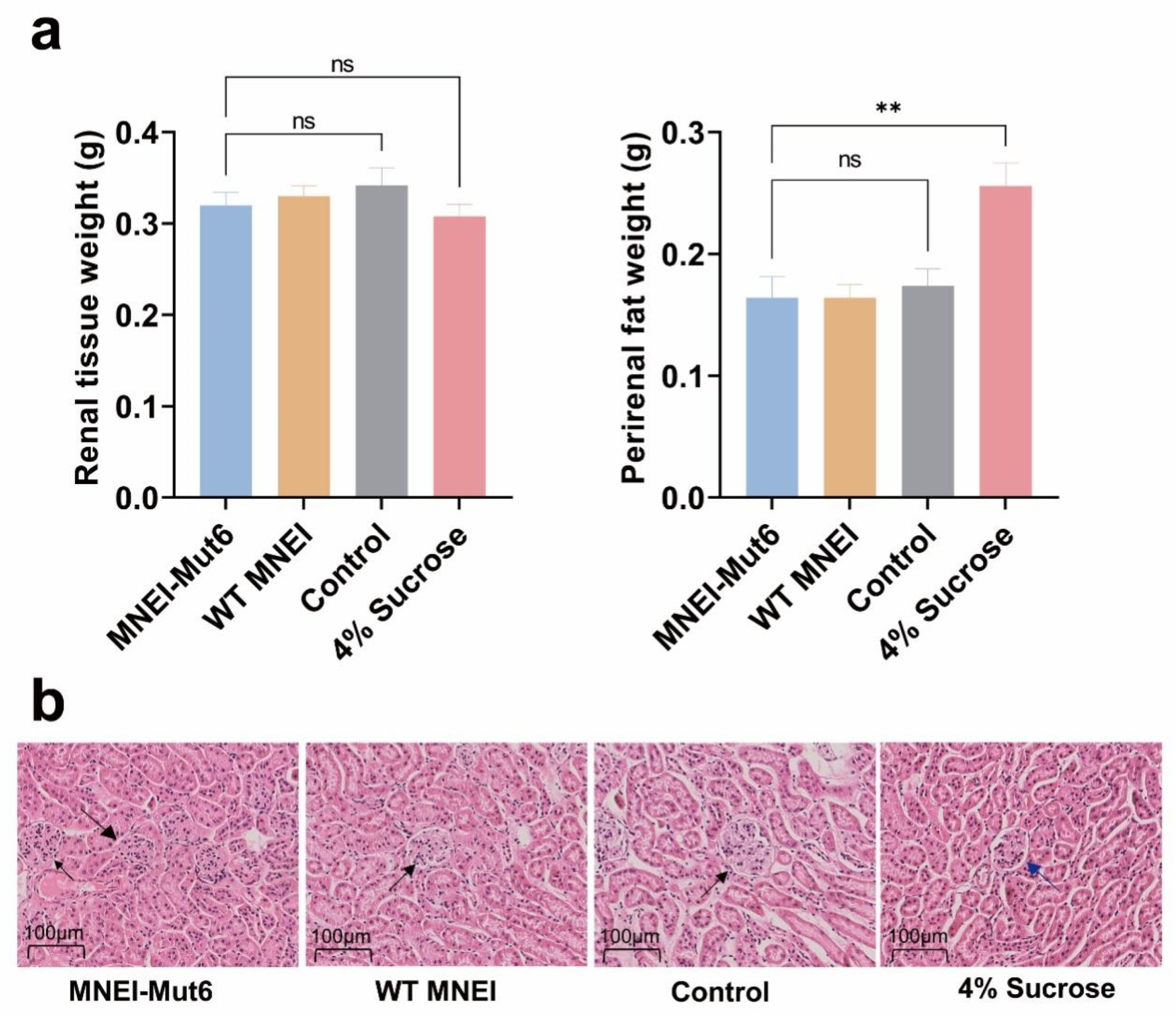
3.6. Long-Term Mnei-Mut6 Supplementation Does Not Impair Renal Structure or Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCaughey, S.A. The taste of sugars. Neurosci. Biobehav. Rev. 2008, 32, 1024–1043. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Despres, J.P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Bremer, A.A.; Medici, V.; Nakajima, K.; Ito, Y.; Nakano, T.; Chen, G.; Fong, T.H.; Lee, V.; Menorca, R.I.; et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J. Clin. Endocrinol. Metab. 2011, 96, E1596–E1605. [Google Scholar] [CrossRef]
- Huynh, K.; Partch, C.L. Analysis of protein stability and ligand interactions by thermal shift assay. Curr. Protoc. Protein Sci. 2015, 79, 28.9.1–28.9.14. [Google Scholar] [CrossRef]
- Kant, R. Sweet proteins--potential replacement for artificial low calorie sweeteners. Nutr. J. 2005, 4, 5. [Google Scholar] [CrossRef]
- Kim, H.; Kang, J.; Hong, S.; Jo, S.; Noh, H.; Kang, B.H.; Park, S.; Seo, Y.J.; Kong, K.H.; Hong, S. 3M-Brazzein as a Natural Sugar Substitute Attenuates Obesity, Metabolic Disorder, and Inflammation. J. Agric. Food Chem. 2020, 68, 2183–2192. [Google Scholar] [CrossRef]
- WHO. Use of Non-Sugar Sweeteners: WHO Guideline; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Xue, H.; Kang, X.; Hong, K.; Gao, Y.; Tang, Y.; Lin, Y.; Liu, X.; Huang, W.; Zhan, J.; You, Y. Effects of artificial and natural sweeteners on host metabolic health: A double-edged sword. Food Res. Int. 2025, 220, 117158. [Google Scholar] [CrossRef]
- Ming, D.; Hellekant, G. Brazzein, a new high-potency thermostable sweet protein from Pentadiplandra brazzeana B. FEBS Lett. 1994, 355, 106–108. [Google Scholar] [CrossRef]
- Masuda, T.; Ueno, Y.; Kitabatake, N. Sweetness and enzymatic activity of lysozyme. J. Agric. Food Chem. 2001, 49, 4937–4941. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Maeda, S.; Hu, Z.; Aiuchi, T.; Nakaya, K.; Kurihara, Y. Purification, complete amino acid sequence and structural characterization of the heat-stable sweet protein, mabinlin II. Eur. J. Biochem. 1993, 211, 281–287. [Google Scholar] [CrossRef]
- Kurihara, K.; Beidler, L.M. Taste-modifying protein from miracle fruit. Science 1968, 161, 1241–1243. [Google Scholar] [CrossRef]
- Morris, J.A.; Cagan, R.H. Purification of monellin, the sweet principle of Dioscoreophyllum cumminsii. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1972, 261, 114–122. [Google Scholar] [CrossRef]
- Shirasuka, Y.; Nakajima, K.; Asakura, T.; Yamashita, H.; Yamamoto, A.; Hata, S.; Nagata, S.; Abo, M.; Sorimachi, H.; Abe, K. Neoculin as a new taste-modifying protein occurring in the fruit of Curculigo latifolia. Biosci. Biotechnol. Biochem. 2004, 68, 1403–1407. [Google Scholar] [CrossRef]
- der Wel, H.v.; Larson, G.; Hladik, A.; Hladik, C.M.; Hellekant, G.; Glaser, D. Isolation and characterization of pentadin, the sweet principle of Pentadiplandra brazzeana Baillon. Chem. Senses 1989, 14, 75–79. [Google Scholar] [CrossRef]
- van der Wel, H.; Loeve, K. Isolation and characterization of thaumatin I and II, the sweet-tasting proteins from Thaumatococcus daniellii Benth. Eur. J. Biochem. 1972, 31, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Ogata, C.; Hatada, M.; Tomlinson, G.; Shin, W.C.; Kim, S.H. Crystal structure of the intensely sweet protein monellin. Nature 1987, 328, 739–742. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA FOIA Log—December 2024. Available online: https://www.fda.gov/media/184910/download (accessed on 3 December 2024).
- Kim, S.-H.; Kang, C.-H.; Kim, R.; Cho, J.M.; Lee, Y.-B.; Lee, T.-K. Redesigning a sweet protein: Increased stability and renaturability. Protein Eng. Des. Sel. 1989, 2, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Tancredi, T.; Iijima, H.; Saviano, G.; Amodeo, P.; Temussi, P.A. Structural determination of the active site of a sweet protein A1H NMR investigation of pMNEI. FEBS Lett. 1992, 310, 27–30. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Ma, M.; You, T.; Ye, S.; Liu, S. Computational design towards a boiling-resistant single-chain sweet protein monellin. Food Chem. 2024, 440, 138279. [Google Scholar] [CrossRef] [PubMed]
- Novik, T.S.; Koveshnikova, E.I.; Kotlobay, A.A.; Sycheva, L.P.; Kurochkina, K.G.; Averina, O.A.; Belopolskaya, M.V.; Sergiev, P.V.; Dontsova, O.A.; Lazarev, V.N.; et al. Sweet-Tasting Natural Proteins Brazzein and Monellin: Safe Sugar Substitutes for the Food Industry. Foods 2023, 12, 4065. [Google Scholar] [CrossRef]
- Lifshitz, Y.; Paz, S.; Saban, R.; Zuker, I.; Shmuely, H.; Gorshkov, K.; Meetro, J.; Tafazoli, S.; Vo, T.; Amiram, G.; et al. Safety Evaluation of Serendipity Berry Sweet Protein from Komagataella phaffii. J. Appl. Toxicol. 2025, 45, 1455–1475. [Google Scholar] [CrossRef] [PubMed]
- Veselovsky, V.A.; Boldyreva, D.I.; Olekhnovich, E.I.; Klimina, K.M.; Babenko, V.V.; Zakharevich, N.V.; Larin, A.K.; Morozov, M.D.; Zoruk, P.Y.; Sergiev, P.V.; et al. Effect of the consumption of brazzein and monellin, two recombinant sweet-tasting proteins, on rat gut microbiota. Front. Nutr. 2024, 11, 1362529. [Google Scholar] [CrossRef]
- Lu, R.; Li, X.; Hu, J.; Wang, Y.; Jin, L. Expression of a single-chain monellin (MNEI) mutant with enhanced stability in transgenic mice milk. Transgenic Res. 2024, 33, 211–218. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, D.M.; Travlos, G.S. (Eds.) The Clinical Chemistry of Laboratory Animals; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Chung, J.H.; Kong, J.N.; Choi, H.E.; Kong, K.H. Antioxidant, anti-inflammatory, and anti-allergic activities of the sweet-tasting protein brazzein. Food Chem. 2018, 267, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chang, S.; Wang, Y.; Liu, B. Advances in genetic engineering and molecular modification of sweet-tasting proteins. Chin. J. Biotechnol. 2025, 41, 559–573. [Google Scholar] [CrossRef]
- Richter, P.; Sebald, K.; Fischer, K.; Schnieke, A.; Jlilati, M.; Mittermeier-Klessinger, V.; Somoza, V. Gastric digestion of the sweet-tasting plant protein thaumatin releases bitter peptides that reduce H. pylori induced pro-inflammatory IL-17A release via the TAS2R16 bitter taste receptor. Food Chem. 2024, 448, 139157. [Google Scholar] [CrossRef]
- Hong, S.; Kim, H.; Kong, K.-H.; Hong, S. 3M-Brazzein as a natural sugar substitute rescued obesity in maternal and offspring mice. J. Funct. Foods 2024, 115, 106104. [Google Scholar] [CrossRef]
- Delfi, M.; Emendato, A.; Leone, S.; Lampitella, E.A.; Porcaro, P.; Cardinale, G.; Petraccone, L.; Picone, D. A Super Stable Mutant of the Plant Protein Monellin Endowed with Enhanced Sweetness. Life 2021, 11, 236. [Google Scholar] [CrossRef]
- Song, X.; Yi, Y.; Liu, L.; He, M.; Deng, S.; Tian, H.; Yao, W.; Gao, X. Design and development of a high temperature stable sweet protein base on monellin. Process Biochem. 2020, 89, 29–36. [Google Scholar] [CrossRef]
- Leone, S.; Pica, A.; Merlino, A.; Sannino, F.; Temussi, P.A.; Picone, D. Sweeter and stronger: Enhancing sweetness and stability of the single chain monellin MNEI through molecular design. Sci. Rep. 2016, 6, 34045. [Google Scholar] [CrossRef] [PubMed]
- Samuel Varman, T.; Shulman Gerald, I. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Ahima, R.S. Leptin signaling. F1000prime Rep. 2014, 6, 73. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, T.; Li, X.; Lai, L.; You, T.; Ma, M.; Ye, S.; Liu, S. Boiling-Resistant Single-Chain Sweet Protein Monellin as a Safe and Effective Sugar Alternative for Metabolic and Glycemic Management in Mice. Foods 2025, 14, 3667. https://doi.org/10.3390/foods14213667
Qi T, Li X, Lai L, You T, Ma M, Ye S, Liu S. Boiling-Resistant Single-Chain Sweet Protein Monellin as a Safe and Effective Sugar Alternative for Metabolic and Glycemic Management in Mice. Foods. 2025; 14(21):3667. https://doi.org/10.3390/foods14213667
Chicago/Turabian StyleQi, Tingting, Xiaoya Li, Lunmeng Lai, Tianjie You, Mingxue Ma, Sheng Ye, and Si Liu. 2025. "Boiling-Resistant Single-Chain Sweet Protein Monellin as a Safe and Effective Sugar Alternative for Metabolic and Glycemic Management in Mice" Foods 14, no. 21: 3667. https://doi.org/10.3390/foods14213667
APA StyleQi, T., Li, X., Lai, L., You, T., Ma, M., Ye, S., & Liu, S. (2025). Boiling-Resistant Single-Chain Sweet Protein Monellin as a Safe and Effective Sugar Alternative for Metabolic and Glycemic Management in Mice. Foods, 14(21), 3667. https://doi.org/10.3390/foods14213667




