Elucidating the Nutritional Profile and Biochemical Characterization of High-Energy Nutritional Bar Formulated with Sukkari Date Paste and Mixed Nuts
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Ingredients
2.2. Formulation of High-Energy DNB
2.3. Nutritional Composition and Mineral Content of Different DNBs
2.4. Phytochemical Analysis of Different DNBs
2.5. Quantification of Phenolic Compounds of Different DNBs
2.6. Determination of AA Profile in Different DNBs
2.7. Determination of the FA Profile in Different DNBs
2.8. Quantification of Volatile Components in Different DNBs
Methylation Method
2.9. Statistical Analysis
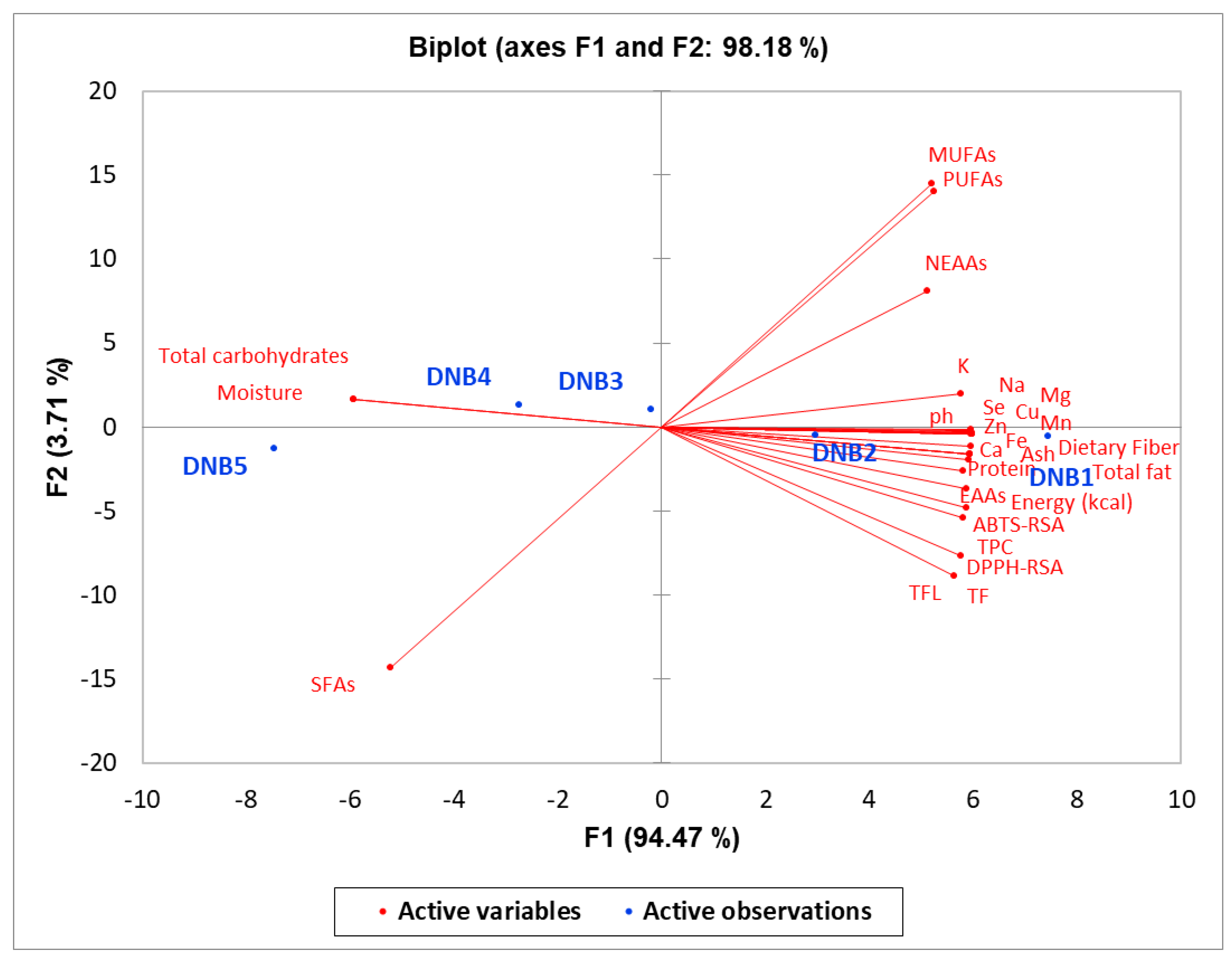
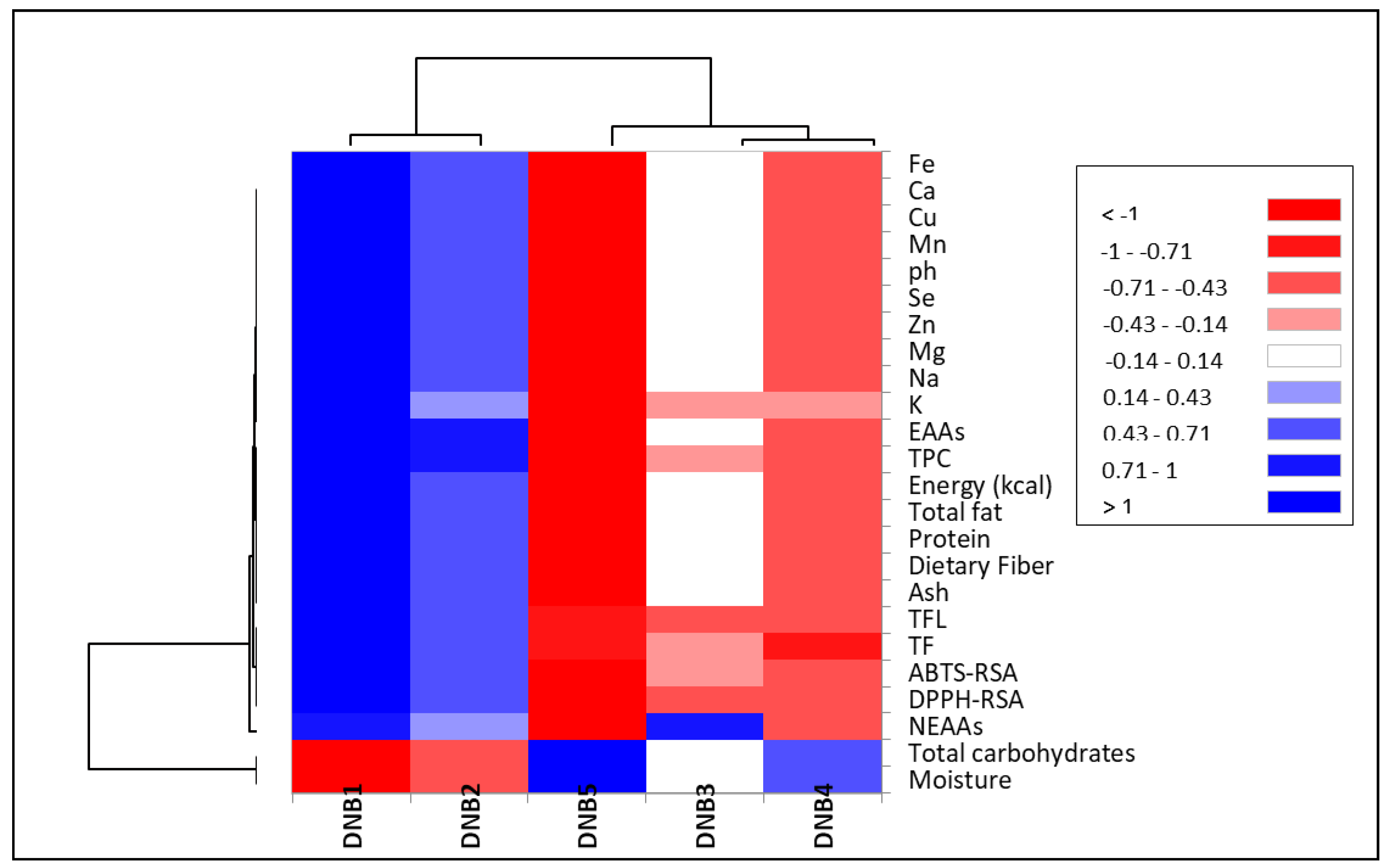
3. Results
3.1. Nutritional Composition of Formulated High-Energy DNBs
3.2. Mineral Content of Formulated High-Energy DNBs
3.3. Antioxidant Activities of Formulated High-Energy DNBs
3.4. Phenolic Profile of Formulated High-Energy DNBs
3.5. AA Composition of Different DNBs
3.6. FA Composition of Formulated High-Energy DNBs
3.7. GC-MS Volatile Profile Analysis of Formulated High-Energy DNBs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AlJaloudi, R.; Al-Dabbas, M.M.; Hamad, H.J.; Amara, R.A.; Al-Bashabsheh, Z.; Abughoush, M.; Choudhury, I.H.; Al-Nawasrah, B.a.A.; Iqbal, S. Development and Characterization of High-Energy Protein Bars with Enhanced Antioxidant, Chemical, Nutritional, Physical, and Sensory Properties. Foods 2024, 13, 259. [Google Scholar] [CrossRef]
- Carreiro, A.L.; Dhillon, J.; Gordon, S.; Higgins, K.A.; Jacobs, A.G.; McArthur, B.M.; Redan, B.W.; Rivera, R.L.; Schmidt, L.R.; Mattes, R.D. The Macronutrients, Appetite, and Energy Intake. Annu. Rev. Nutr. 2016, 36, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Lehoczki, A.; Kryczyk-Poprawa, A.; Zábó, V.; Varga, J.T.; Bálint, M.; Fazekas-Pongor, V.; Csípő, T.; Rząsa-Duran, E.; Varga, P. Functional Foods in Modern Nutrition Science: Mechanisms, Evidence, and Public Health Implications. Nutrients 2025, 17, 2153. [Google Scholar] [CrossRef] [PubMed]
- Pulidindi, K. Nutritional Bars Market—By Product, by Category, by Distribution Channel—Global Forecast, 2025–2034 (Report Id: Gmi5644). 2025. Available online: https://www.gminsights.com/industry-analysis/nutritional-bars-market (accessed on 13 September 2025).
- Barakat, H.; Alfheeaid, H.A. Date Palm Fruit (Phoenix dactylifera) and Its Promising Potential in Developing Functional Energy Bars: Review of Chemical, Nutritional, Functional, and Sensory Attributes. Nutrients 2023, 15, 2134. [Google Scholar] [CrossRef]
- Nazzaro, C.; Uliano, A.; Lerro, M.; Stanco, M. From Claims to Choices: How Health Information Shapes Consumer Decisions in the Functional Food Market. Foods 2025, 14, 699. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of Antioxidant Activity, Anthocyanins, Carotenoids, and Phenolics of Three Native Fresh and Sun-Dried Date (Phoenix dactylifera L.) Varieties Grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Al-Abid, M.; Al-Shoaily, K.; Al-Amry, M.; Al-Rawahy, F. Compositional and Functional Characteristics of Dates, Syrups, and Their by-Products. Food Chem. 2007, 104, 943–947. [Google Scholar] [CrossRef]
- Alfheeaid, H.A.; Barakat, H.; Althwab, S.A.; Musa, K.H.; Malkova, D. Nutritional and Physicochemical Characteristics of Innovative High Energy and Protein Fruit- and Date-Based Bars. Foods 2023, 12, 2777. [Google Scholar] [CrossRef]
- Barakat, H.; Almutairi, A.S. The Organoleptic and Nutritional Characteristics of Innovative High-Fiber Khalas Date-Based Bar. Ital. J. Food Sci. 2024, 36, 13. [Google Scholar] [CrossRef]
- Majid, A.; Naz, F.; Bhatti, S.; Phull, A.-R. Phenolic Profile and Antioxidant Activities of Three Date Seeds Varieties (Phoenix dactylifera L.) of Pakistan. Explor. Res. Hypothesis Med. 2023, 8, 195–201. [Google Scholar] [CrossRef]
- Tassoult, M.; Kati, D.E.; Fernández-Prior, M.Á.; Bermúdez-Oria, A.; Fernandez-Bolanos, J.; Rodríguez-Gutiérrez, G. Antioxidant Capacity and Phenolic and Sugar Profiles of Date Fruits Extracts from Six Different Algerian Cultivars as Influenced by Ripening Stages and Extraction Systems. Foods 2021, 10, 503. [Google Scholar] [CrossRef]
- Nawaz, A.; Li, E.; Khalifa, I.; Walayat, N.; Liu, J.; Ahsan, H.M.; Irshad, S.; Barakat, H.; Lorenzo, J.M.; Pateiro, M.; et al. Effect of Structurally Different Pectin on Dough Rheology, Structure, Pasting and Water Distribution Properties of Partially Meat-Based Sugar Snap Cookies. Foods 2021, 10, 2692. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. The Cardioprotective Properties of Selected Nuts: Their Functional Ingredients and Molecular Mechanisms. Foods 2024, 13, 242. [Google Scholar] [CrossRef]
- Singar, S.; Kadyan, S.; Patoine, C.; Park, G.; Arjmandi, B.; Nagpal, R. The Effects of Almond Consumption on Cardiovascular Health and Gut Microbiome: A Comprehensive Review. Nutrients 2024, 16, 1964. [Google Scholar] [CrossRef] [PubMed]
- Aslam, N.; Hassan, S.A.; Mehak, F.; Zia, S.; Bhat, Z.F.; Yıkmış, S.; Aadil, R.M. Exploring the Potential of Cashew Waste for Food and Health Applications- a Review. Future Foods 2024, 9, 100319. [Google Scholar] [CrossRef]
- Parn, O.J.; Bhat, R.; Yeoh, T.; Al-Hassan, A. Development of Novel Fruit Bars by Utilizing Date Paste. Food Biosci. 2015, 9, 20–27. [Google Scholar] [CrossRef]
- Snelson, M.; Biesiekierski, J.R.; Chen, S.; Sultan, N.; Cardoso, B.R. The Effects of Nut Intake on Gut Microbiome Composition and Gut Function in Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. 2025, 16, 100465. [Google Scholar] [CrossRef]
- Mederle, A.L.; Dima, M.; Stoicescu, E.R.; Căpăstraru, B.F.; Levai, C.M.; Hațegan, O.A.; Maghiari, A.L. Impact of Gut Microbiome Interventions on Glucose and Lipid Metabolism in Metabolic Diseases: A Systematic Review and Meta-Analysis. Life 2024, 14, 1485. [Google Scholar] [CrossRef]
- Hong, M.Y.; Groven, S.; Marx, A.; Rasmussen, C.; Beidler, J. Anti-Inflammatory, Antioxidant, and Hypolipidemic Effects of Mixed Nuts in Atherogenic Diet-Fed Rats. Molecules 2018, 23, 3126. [Google Scholar] [CrossRef]
- Park, G.; Johnson, K.; Miller, K.; Kadyan, S.; Singar, S.; Patoine, C.; Hao, F.; Lee, Y.; Patterson, A.D.; Arjmandi, B. Almond Snacking Modulates Gut Microbiome and Metabolome in Association with Improved Cardiometabolic and Inflammatory Markers. NPJ Sci. Food 2025, 9, 35. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Tranchant, C.C.; Al-u’datt, D.; Gammoh, S.; Alrosan, M.; Bani-Melhem, K.; Aldughpassi, A.; Alkandari, D.; AbuJalban, D. Date Palm (Phoenix dactylifera) Bioactive Constituents and Their Applications as Natural Multifunctional Ingredients in Health—Promoting Foods and Nutraceuticals: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70084. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, A.; Amal, T.C.; Sarvalingam, A.; Vasanth, K. A Review on the Influence of Nutraceuticals and Functional Foods on Health. Food Chem. Adv. 2024, 5, 100749. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Aoac, 17th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2000. [Google Scholar]
- Borah, S.; Baruah, A.; Das, A.; Borah, J. Determination of Mineral Content in Commonly Consumed Leafy Vegetables. Food Anal. Methods 2009, 2, 226–230. [Google Scholar] [CrossRef]
- Bettaieb, I.; Bourgou, S.; Wannes, W.A.; Hamrouni, I.; Limam, F.; Marzouk, B. Essential Oils, Phenolics, and Antioxidant Activities of Different Parts of Cumin (Cuminum cyminum L.). J. Agric. Food Chem. 2010, 58, 10410–10418. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, I.; Barakat, H.; El-Mansy, H.; Soliman, S. Optimizing Bioactive Substances Extraction Procedures from Guava, Olive and Potato Processing Wastes and Evaluating Their Antioxidant Capacity. J. Food Chem. Nanotechnol. 2016, 2, 170–177. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolics, Ascorbic Acid, Carotenoids and Antioxidant Activity of Broccoli and Their Changes During Conventional and Microwave Cooking. Food Chem. 2004, 88, 503–509. [Google Scholar] [CrossRef]
- Almundarij, T.I.; Alharbi, Y.M.; Abdel-Rahman, H.A.; Barakat, H. Antioxidant Activity, Phenolic Profile, and Nephroprotective Potential of Anastatica Hierochuntica Ethanolic and Aqueous Extracts against Ccl4-Induced Nephrotoxicity in Rats. Nutrients 2021, 13, 2973. [Google Scholar] [CrossRef]
- Barakat, H.; Almundarij, T.I. Phenolic Compounds and Hepatoprotective Potential of Anastatica Hierochuntica Ethanolic and Aqueous Extracts against Ccl4-Induced Hepatotoxicity in Rats. Tradit. Chin. Med. 2020, 40, 947. [Google Scholar]
- Kumaran, A.; Karunakaran, R.J. In Vitro Antioxidant Activities of Methanol Extracts of Five Phyllanthus Species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Schneider, S. Quality Analysis of Extra Virgin Olive Oils—Part 6 Nutritive Benefi Ts—Phenolic Compounds in Virgin Olive Oil. Agilent Technology Application Note 2016. Available online: https://www.agilent.com/cs/library/applications/5991-3801EN.pdf?srsltid=AfmBOooDCe5tYCzUiGVHbzNcfo0MsWZ3sCxKzyl8T-9CSY9Y8swms0Xp (accessed on 9 April 2024).
- Cohen, S.A.; Meys, M.; Travin, T.L. The Pico Tag Method a Manual of Advanced Techniques for Amino Acid Analysis; Waters Chromatography Division: Milford, MA, USA, 1989. [Google Scholar]
- WHO/FAO/UNU. Protein and Amino Acid Requirements in Human Nutrition; 0512-3054 (Print); World Health Organization: Geneva, Switzerland, 2007; pp. 1–265. [Google Scholar]
- Chavan, U.D.; McKenzie, D.B.; Shahidi, F. Protein Classification of Beach Pea (Lathyrus maritimus L.). Food Chem. 2001, 75, 145–153. [Google Scholar] [CrossRef]
- Aldai, N.; Osoro, K.; Barron, L.; Nájera, A. Gas–Liquid Chromatographic Method for Analysing Complex Mixtures of Fatty Acids Including Conjugated Linoleic Acids (Cis9trans11 and Trans10cis12 Isomers) and Long-Chain (N-3 or N-6) Polyunsaturated Fatty Acids: Application to the Intramuscular Fat of Beef Meat. J. Chromatogr. A 2006, 1110, 133–139. [Google Scholar] [PubMed]
- Hewavitharana, G.G.; Perera, D.N.; Navaratne, S.B.; Wickramasinghe, I. Extraction Methods of Fat from Food Samples and Preparation of Fatty Acid Methyl Esters for Gas Chromatography: A Review. Arab. J. Chem. 2020, 13, 6865–6875. [Google Scholar] [CrossRef]
- Steel, R.G. Pinciples and Procedures of Statistics a Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- Ayad, A.A.; Williams, L.L.; Gad El-Rab, D.A.; Ayivi, R.; Colleran, H.L.; Aljaloud, S.; Ibrahim, S.A. A Review of the Chemical Composition, Nutritional and Health Benefits of Dates for Their Potential Use in Energy Nutrition Bars for Athletes. Cogent Food Agric. 2020, 6, 1809309. [Google Scholar] [CrossRef]
- Al-Dashti, Y.A.; Holt, R.R.; Keen, C.L.; Hackman, R.M. Date Palm Fruit (Phoenix dactylifera): Effects on Vascular Health and Future Research Directions. Int. J. Mol. Sci 2021, 22, 4665. [Google Scholar] [CrossRef]
- Al-Sayyed, H.F.; Abu-Qatouseh, L.F.; Malkawy, M.; Al-Wawi, S.; Al Kafaween, M. Extracts of Jordanian Date Palm Fruit (Phoenix dactylifera L.) Inhibit Human Mammary Adenocarcinoma (Mcf-7) Cells in Vitro by Inducing Cell Viability. Curr. Res. Nutr. Food Sci. 2021, 9, 423–430. [Google Scholar] [CrossRef]
- Al-Zeiny, S.S.M.; Alyaqubi, K.J.; Abbas, D.A.H. In Vitro: Anticancer Effect of Oily and Methanolic Extracts of Al-Zahdi (Phoenix dactylifera L.) from Dry Dates and Leaves on Amn3, Hela and Ref Cancer Cell Cultures. Kufa J. Vet. Med. Sci. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Gonçalves, B.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Cosme, F. Composition of Nuts and Their Potential Health Benefits—An Overview. Foods 2023, 12, 942. [Google Scholar] [CrossRef]
- Assirey, E.A.R. Nutritional Composition of Fruit of 10 Date Palm (Phoenix dactylifera L.) Cultivars Grown in Saudi Arabia. J. Taibah Univ. Sci. 2015, 9, 75–79. [Google Scholar] [CrossRef]
- Eid, W.A.M.; Azab, D.E.-S.H.; Negm, S.H. Characterization of a Novel Date Energy Bar Fortified with Moringa Oleifera Leaves Powder. J. Future Foods 2025, 5, 266–275. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of Phenolics and Dietary Fibre Extraction from Date Seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef]
- Bouaziz, M.A.; Besbes, S.; Blecker, C.; Wathelet, B.; Deroanne, C.; Attia, H. Protein and Amino Acid Profiles of Tunisian Deglet Nour and Allig Date Palm Fruit Seeds. Fruits 2008, 63, 37–43. [Google Scholar] [CrossRef]
- Vayalil, P.K. Antioxidant and Antimutagenic Properties of Aqueous Extract of Date Fruit (Phoenix dactylifera L. Arecaceae). J. Agric. Food Chem. 2002, 50, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Vayalil, P.K. Date Fruits (Phoenix dactylifera Linn): An Emerging Medicinal Food. Crit. Rev. Food Sci. Nutr. 2012, 52, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Żukowska, R.; Puścion-Jakubik, A.; Grabia, M.; Perkowski, J.; Nowakowski, P.; Bielecka, J.; Soroczyńska, J.; Kańgowski, G.; Bołtryk, J.M.; Socha, K. Nuts as a Dietary Enrichment with Selected Minerals—Content Assessment Supported by Chemometric Analysis. Foods 2022, 11, 3152. [Google Scholar] [CrossRef] [PubMed]
- Dghaim, R.; Hammami, Z.; Al Ghali, R.; Smail, L.; Haroun, D. The Mineral Composition of Date Palm Fruits (Phoenix dactylifera L.) under Low to High Salinity Irrigation. Molecules 2021, 26, 7361. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.; Sathe, S.K. Chemical Composition of Selected Edible Nut Seeds. J. Agric. Food Chem. 2006, 54, 4705–4714. [Google Scholar] [CrossRef]
- Weaver, C.M.; Dwyer, J.; Fulgoni, V.L., III; King, J.C.; Leveille, G.A.; MacDonald, R.S.; Ordovas, J.; Schnakenberg, D. Processed Foods: Contributions to Nutrition. Am. J. Clin. Nutr. 2014, 99, 1525–1542. [Google Scholar] [CrossRef]
- Al-Shahib, W.; Marshall, R.J. The Fruit of the Date Palm: Its Possible Use as the Best Food for the Future? Int. J. Food Sci. Nutr. 2003, 54, 247–259. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in Disease Prevention and Overall Health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium Deficiency and Oxidative Stress: An Update. BioMedicine 2016, 6, 20. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron Bioavailability and Dietary Reference Values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Fageer, A.S.; Eltayeb, M.M.; Mohamed Ahmed, I.A. Chemical Composition, Antioxidant Capacity, and Mineral Extractability of Sudanese Date Palm (Phoenix dactylifera L.) Fruits. Food Sci. Nutr. 2014, 2, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Al-Shwyeh, H.A. Date Palm (Phoenix dactylifera L.) Fruit as Potential Antioxidant and Antimicrobial Agents. J. Pharm. Bioallied Sci. 2019, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Youn, U.-Y.; Kim, R.-H.; Kim, G.-N.; Lee, S.-C. Antioxidant and Anti-Adipogenic Activities of the Nuts of Castanopsis cuspidata Var. Thunbergii. Food Sci. Biotechnol. 2017, 26, 1407–1414. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Tammam, M.A.; Ali, M.Y.; Sharaky, M.; Selim, K.; Semida, W.M.; Abd El-Mageed, T.A.; Ramadan, M.F.; Oraby, H.F.; Diab, Y.M. Antioxidant and Anticancer Assessment and Phytochemical Investigation of Three Varieties of Date Fruits. Metabolites 2023, 13, 816. [Google Scholar] [CrossRef] [PubMed]
- Abuelgassim, A.O.; Eltayeb, M.A.; Ataya, F.S. Palm Date (Phoenix dactylifera) Seeds: A Rich Source of Antioxidant and Antibacterial Activities. Czech J. Food Sci. 2020, 38, 171–178. [Google Scholar] [CrossRef]
- Staveckienė, J.; Kulaitienė, J.; Levickienė, D.; Vaitkevičienė, N.; Vaštakaitė-Kairienė, V. The Effect of Ripening Stages on the Accumulation of Polyphenols and Antioxidant Activity of the Fruit Extracts of Solanum Species. Plants 2023, 12, 2672. [Google Scholar] [CrossRef]
- Mrabet, A.; Jiménez-Araujo, A.; Fernández-Bolaños, J.; Rubio-Senent, F.; Lama-Muñoz, A.; Sindic, M.; Rodríguez-Gutiérrez, G. Antioxidant Phenolic Extracts Obtained from Secondary Tunisian Date Varieties (Phoenix dactylifera L.) by Hydrothermal Treatments. Food Chem. 2016, 196, 917–924. [Google Scholar] [CrossRef]
- Chaari, A.; Abdellatif, B.; Nabi, F.; Khan, R.H. Date Palm (Phoenix dactylifera L.) Fruit’s Polyphenols as Potential Inhibitors for Human Amylin Fibril Formation and Toxicity in Type 2 Diabetes. Int. J. Biol. Macromol. 2020, 164, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.D.; Vu, D.C.; Alvarez, S.; Nguyen, K.D.; Nguyen, T.L.T.; Tuan, N.N.; Minh-Nguyet, N.T.; Tam, L.N.; Ho, T.L.; Vo, X.T. Comparative Examination of Phytonutrients and Antioxidant Activity of Commonly Consumed Nuts and Seeds Grown in Vietnam. Horticulturae 2022, 8, 521. [Google Scholar] [CrossRef]
- Ghazzawi, H.A.; Al-Ismail, K. A Comprehensive Study on the Effect of Roasting and Frying on Fatty Acids Profiles and Antioxidant Capacity of Almonds, Pine, Cashew, and Pistachio. J. Food Qual. 2017, 2017, 9038257. [Google Scholar] [CrossRef]
- Otles, S.; Selek, I. Phenolic Compounds and Antioxidant Activities of Chestnut (Castanea sativa Mill.) Fruits. Qual. Assur. Saf. Crops Foods 2012, 4, 199–205. [Google Scholar] [CrossRef]
- Saafi, E.B.; El Arem, A.; Chahdoura, H.; Flamini, G.; Lachheb, B.; Ferchichi, A.; Hammami, M.; Achour, L. Nutritional Properties, Aromatic Compounds and in Vitro Antioxidant Activity of Ten Date Palm Fruit (Phoenix dactylifera L.) Varieties Grown in Tunisia. Braz. J. Pharm. Sci. 2022, 58, e18871. [Google Scholar] [CrossRef]
- Kong, C.-S.; Jeong, C.-H.; Choi, J.-S.; Kim, K.-J.; Jeong, J.-W. Antiangiogenic Effects of P-Coumaric Acid in Human Endothelial Cells. Phytother. Res. 2013, 27, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, I.; Kilani, A.; Ben Othman, K.; Benabderrahim, M.A.; Elfalleh, W. Phenolic Profile, Sugar Composition, and Antioxidant Capacities of Some Common Date Palm (Phoenix dactylifera L.) Cultivars as a Potential Nutraceutical and Functional Food Ingredients. J. Food Qual. 2023, 2023, 2474900. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Church, D.D.; Ferrando, A.A.; Moughan, P.J. Consideration of the Role of Protein Quality in Determining Dietary Protein Recommendations. Front. Nutr. 2024, 11, 1389664. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J. Amino Acid Scoring Patterns for Protein Quality Assessment. Br. J. Nutr. 2012, 108, S31–S43. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Sukor, R.; Anwar, F.; Murugesu, S.; Selamat, J.; Raseetha, S. Nutritional, Nutraceutical Attributes, Microbiological and Chemical Safety of Different Varieties of Dates—A Review. Future Foods 2024, 10, 100421. [Google Scholar] [CrossRef]
- Adhikari, S.; Schop, M.; de Boer, I.J.M.; Huppertz, T. Protein Quality in Perspective: A Review of Protein Quality Metrics and Their Applications. Nutrients 2022, 14, 947. [Google Scholar] [CrossRef]
- Chapman, D.G.; Castillo, R.; Campbell, J.A. Evaluation of Protein in Foods. I. A Method for the Determination of Protein Efficiency Ratios. Can. J. Biochem. Physiol. 1959, 37, 679–686. [Google Scholar] [CrossRef]
- Yajima, K.; Chiba, S.; Park, I.; Ogata, H.; Kayaba, M.; Ishihara, A.; Tanaka, Y.; Simeng, Z.; Jaehoon, S.; Katakura, M.; et al. Dietary Palmitic Acid to Oleic Acid Ratio Modulates Energy Metabolism and Biological Rhythms in Young Healthy Japanese Males. Br. J. Nutr. 2024, 131, 447–460. [Google Scholar] [CrossRef]
- Alsuhebani, H.; Sakr, S.S.; Elkashef, H.; Algheshairy, R.M.; Alfheeaid, H.A.; Algeffari, M.; Alharbi, H.F. Novel High Protein-Energy Balls Formulated with Date Paste Enriched with Samh Seeds Powder and/or Different Milk Protein Origins: Effect on Protein Digestibility in Vitro and Glycemic Response in Young Adults. Front. Nutr. 2025, 12, 1538441. [Google Scholar] [CrossRef]
- Valdés García, A.; Sánchez Romero, R.; Juan Polo, A.; Prats Moya, S.; Maestre Pérez, S.E.; Beltrán Sanahuja, A. Volatile Profile of Nuts, Key Odorants and Analytical Methods for Quantification. Foods 2021, 10, 1611. [Google Scholar] [CrossRef]
- Flowers, J.M.; Hazzouri, K.M.; Lemansour, A.; Capote, T.; Gros-Balthazard, M.; Ferrand, S.; Lebrun, M.; Amiri, K.M.A.; Purugganan, M.D. Patterns of Volatile Diversity Yield Insights into the Genetics and Biochemistry of the Date Palm Fruit Volatilome. Front. Plant Sci. 2022, 13, 853651. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.-R.; Wei, C.-K.; Liu, D.-H.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Gc-Ms and Gc×Gc-Tof-Ms Analysis of Roasted/Broth Flavors Produced by Maillard Reaction System of Cysteine-Xylose-Glutamate. Curr. Res. Food Sci. 2023, 6, 100445. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Salim-ur-Rehman; Muhammad Anjum, F.; Murtaza, M.A.; Mueen-ud-Din, G. Development, Characterization, and Optimization of Protein Level in Date Bars Using Response Surface Methodology. Sci. World J. 2012, 2012, 518702. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | DNB1 | DNB2 | DNB3 | DNB4 | DNB5 |
|---|---|---|---|---|---|
| Sukkari date paste | 40.00 | 50.00 | 60.00 | 70.00 | 80.00 |
| dried Sukkari date powder | 12.00 | 10.00 | 8.00 | 6.00 | 4.00 |
| Roasted almond | 9.00 | 7.50 | 6.00 | 4.50 | 3.00 |
| Pistachio | 6.00 | 5.00 | 4.00 | 3.00 | 2.00 |
| Whole oats | 6.00 | 5.00 | 4.00 | 3.00 | 2.00 |
| Cow’s milk powder | 6.00 | 5.00 | 4.00 | 3.00 | 2.00 |
| Roasted cashew | 4.50 | 3.75 | 3.00 | 2.25 | 1.50 |
| Roasted sesame seeds | 4.50 | 3.75 | 3.00 | 2.25 | 1.50 |
| Walnuts | 3.00 | 2.50 | 2.00 | 1.50 | 1.00 |
| Hulled pumpkin seeds | 3.00 | 2.50 | 2.00 | 1.50 | 1.00 |
| Hulled sunflower seeds | 3.00 | 2.50 | 2.00 | 1.50 | 1.00 |
| Chia seeds | 1.80 | 1.50 | 1.20 | 0.90 | 0.60 |
| Dried cinnamon powder | 0.60 | 0.50 | 0.40 | 0.30 | 0.20 |
| Dried ginger powder | 0.60 | 0.50 | 0.40 | 0.30 | 0.20 |
| Composition * | High-Energy DNB Formulas | ||||
|---|---|---|---|---|---|
| DNB1 | DNB2 | DNB3 | DNB4 | DNB5 | |
| Moisture | 9.68 ± 0.14 a | 10.61 ± 0.16 b | 11.55 ± 0.15 c | 12.49 ± 0.21 d | 13.43 ± 0.18 e |
| Protein | 10.89 ± 0.16 a | 9.56 ± 0.15 b | 8.23 ± 0.11 c | 6.90 ± 0.11 d | 5.56 ± 0.07 e |
| Total fat | 18.66 ± 0.27 a | 15.65 ± 0.24 b | 12.64 ± 0.17 c | 9.63 ± 0.16 d | 6.62 ± 0.09 e |
| Ash | 3.16 ± 0.05 a | 3.11 ± 0.05 ab | 3.04 ± 0.04 b | 2.98 ± 0.05 bc | 2.92 ± 0.04 c |
| Dietary Fiber | 6.23 ± 0.09 a | 6.11 ± 0.09 a | 5.99 ± 0.08 a | 5.87 ± 0.1 a | 5.74 ± 0.08 a |
| Total carbohydrates | 57.62 ± 0.82 a | 61.08 ± 0.94 b | 64.54 ± 0.86 c | 68.01 ± 1.13 d | 71.47 ± 0.95 e |
| Energy (kcal) | 417.01 ± 5.96 a | 398.95 ± 5.70 b | 380.88 ± 5.44 c | 362.81 ± 5.18 d | 344.74 ± 4.92 e |
| Minerals and Trace Elements (mg kg−1) | High-Energy DNB Formulas | ||||
|---|---|---|---|---|---|
| DNB1 | DNB2 | DNB3 | DNB4 | DNB5 | |
| Calcium | 422.12 ± 7.04 a | 360.1 ± 6.55 b | 324.88 ± 6.5 c | 296.37 ± 5.39 d | 241.06 ± 4.02 e |
| Sodium | 57.54 ± 0.96 a | 49.95 ± 0.91 b | 45.87 ± 0.92 c | 42.68 ± 0.78 d | 35.97 ± 0.6 e |
| Potassium | 1247.0 ± 20.78 a | 1165.9 ± 21.2 b | 1147.59 ± 22.95 b | 1145.02 ± 20.82 b | 1079.54 ± 17.99 c |
| Phosphorus | 785.95 ± 13.1 a | 665.46 ± 12.1 b | 595.68 ± 11.91 c | 538.58 ± 9.79 d | 430.77 ± 7.18 e |
| Magnesium | 326.52 ± 5.44 a | 281.1 ± 5.11 b | 256.01 ± 5.12 c | 235.99 ± 4.29 d | 195.66 ± 3.26 e |
| Manganese | 3.57 ± 0.06 a | 3.02 ± 0.05 b | 2.70 ± 0.09 c | 2.44 ± 0.11 d | 1.95 ± 0.03 e |
| Copper | 1.67 ± 0.07 a | 1.41 ± 0.03 ab | 1.26 ± 0.11 b | 1.14 ± 0.12 bc | 0.91 ± 0.08 c |
| Iron | 6.97 ± 0.12 a | 5.96 ± 0.11 b | 5.38 ± 0.11 c | 4.92 ± 0.09 d | 4.02 ± 0.07 e |
| Zinc | 5.46 ± 0.09 a | 4.60 ± 0.08 b | 4.09 ± 0.08 c | 3.68 ± 0.07 d | 2.91 ± 0.05 e |
| Selenium (µg kg−1) | 21.89 ± 0.36 a | 18.49 ± 0.34 b | 16.51 ± 0.33 c | 14.88 ± 0.27 d | 11.84 ± 0.21 e |
| Phytochemical Analysis * | High-Energy DNB Formulas | ||||
|---|---|---|---|---|---|
| DNB1 | DNB2 | DNB3 | DNB4 | DNB5 | |
| TPC [mg GAE g−1] | 37.98 ± 2.37 a | 33.77 ± 3.52 ab | 25.98 ± 1.35 bc | 22.08 ± 3.37 bc | 18.17 ± 2.24 cd |
| DPPH-RSA [µmol of TE g−1] | 48.29 ± 6.69 a | 40.53 ± 5.62 ab | 29.88 ± 3.40 c | 29.26 ± 2.49 cd | 23.09 ± 4.31 d |
| ABTS-RSA [µmol of TE g−1] | 62.76 ± 8.91 a | 48.65 ± 5.75 ab | 38.84 ± 6.52 bc | 36.57 ± 4.61 c | 27.71 ± 2.37 d |
| TF [mg QE g−1] | 26.01 ± 3.37 a | 20.26 ± 3.31 ab | 15.33 ± 2.2 b | 12.14 ± 1.2 c | 10.91 ± 0.91 d |
| TFL [mg QE g−1] | 20.80 ± 1.73 a | 15.19 ± 2.33 b | 9.96 ± 1.53 c | 9.11 ± 2.15 c | 7.75 ± 2.21 c |
| RT | Name | DNB1 | DNB3 | DNB5 |
|---|---|---|---|---|
| Phenolic acids (mg kg−1) | ||||
| 2.97 | Gallic acid | 10.44 | 15.56 | 10.73 |
| 3.43 | Catechol | 4183.11 | 4640.26 | 3676.47 |
| 3.89 | p-hydroxybenzoic acid | 76.03 | 89.09 | 75.66 |
| 5.33 | Vanillic acid | 495.79 | 528.95 | 418.57 |
| 6.73 | Syringic acid | 0 | 5.31 | 0 |
| 6.92 | p-coumaric | 2.38 | 0 | 2.87 |
| 9.22 | O-cumaric | 3.92 | 3.22 | 0 |
| Flavonoids (mg kg−1) | ||||
| 9.64 | Rutin | 2.11 | 1.35 | 0 |
| 10.85 | Myricetin | 0.59 | 0.25 | 0 |
| 12.1 | Quercetin | 2.79 | 0.66 | 5.82 |
| 14.04 | Kaempferol | 0.14 | 0.39 | 0 |
| 14.55 | Apigenin | 1.48 | 0.69 | 0 |
| AAs | DNB1 | DNB2 | DNB3 | DNB4 | DNB5 |
|---|---|---|---|---|---|
| Essential AAs (EAAs) | |||||
| Leucine | 0.57 | 0.55 | 0.50 | 0.42 | 0.33 |
| Lysine | 0.33 | 0.32 | 0.28 | 0.24 | 0.19 |
| Valine | 0.43 | 0.42 | 0.35 | 0.32 | 0.30 |
| Methionine | 0.14 | 0.13 | 0.10 | 0.09 | 0.11 |
| Histidine | 0.35 | 0.32 | 0.28 | 0.26 | 0.22 |
| Threonine | 0.29 | 0.27 | 0.24 | 0.22 | 0.17 |
| Phenylalanine | 0.45 | 0.46 | 0.396 | 0.36 | 0.31 |
| Isoleucine | 0.25 | 0.25 | 0.21 | 0.18 | 0.13 |
| Cystine | 0.22 | 0.23 | 0.1769 | 0.16 | 0.20 |
| Non-Essential AAs (NEAAs) | |||||
| Aspartic Acid | 0.84 | 0.79 | 0.72 | 0.64 | 0.50 |
| Glutamic Acid | 1.92 | 1.80 | 1.64 | 1.46 | 1.08 |
| Serine | 0.43 | 0.41 | 0.37 | 0.33 | 0.26 |
| Glycine | 0.43 | 0.40 | 0.37 | 0.33 | 0.25 |
| Arginine | 0.64 | 0.71 | 0.62 | 0.46 | 0.34 |
| Alanine | 0.41 | 0.38 | 0.36 | 0.32 | 0.25 |
| Tyrosine | 0.08 | 0.12 | 0.87 | 0.07 | 0.09 |
| Proline | 0.69 | 0.43 | 0.56 | 0.42 | 0.56 |
| Essential AAs | 3.03 | 2.95 | 2.52 | 2.24 | 1.97 |
| Non-Essential AAs | 5.45 | 5.04 | 5.50 | 4.03 | 3.32 |
| EAAs/NEAAs ratio | 0.56 | 0.59 | 0.46 | 0.56 | 0.59 |
| Total AAs | 8.47 | 7.99 | 8.02 | 6.26 | 5.29 |
| Parameters | DNB1 | DNB2 | DNB3 | DNB4 | DNB5 |
|---|---|---|---|---|---|
| Total BCAAs (mg g−1 protein) | 148.07 | 152.71 | 131.33 | 145.75 | 144.80 |
| Total BAAs (mg g−1 protein) | 155.50 | 168.73 | 147.42 | 154.05 | 141.78 |
| Total Aromatic AA (mg g−1 protein) | 62.29 | 73.48 | 157.89 | 67.53 | 75.05 |
| Total uncharged polar AAs (mg g−1 protein) | 445.61 | 452.50 | 500.00 | 458.49 | 433.08 |
| Total Conditional AA (mg g−1 protein) | 469.21 | 462.39 | 526.57 | 462.32 | 475.80 |
| BV | 43.06 | 50.44 | 45.84 | 45.09 | 52.05 |
| EAAI | 54.09 | 56.27 | 53.58 | 53.90 | 55.92 |
| Requirement index (infants) | 115.89 | 120.55 | 114.79 | 115.47 | 119.80 |
| Requirement index (preschool child) | 125.86 | 130.93 | 124.67 | 125.41 | 130.11 |
| Requirement index (schoolchild) | 137.73 | 143.27 | 136.42 | 137.23 | 142.38 |
| Requirement index (adult) | 144.84 | 150.67 | 143.47 | 144.32 | 149.73 |
| DNB1 | DNB3 | DNB5 | |
|---|---|---|---|
| Saturated FAs | % | % | % |
| Butyric (C4:0) | 0.19 | 0.24 | 0.32 |
| Caproic (C6:0) | 0.14 | 0.18 | 0.28 |
| Caprylic (C8:0) | 0.09 | 0.15 | 0.19 |
| Capric (C10:0) | 0.24 | 0.29 | 0.57 |
| Lauric (C12:0) | 0.39 | 0.53 | 0.77 |
| Myristic (C14:0) | 1.85 | 2.18 | 3.16 |
| Myristoleic (C14:0) | 0.06 | 0.21 | 0.11 |
| Pentadecanoic (C15:0) | 0.22 | 0.26 | 0.38 |
| Palmitic (C16:0) | 15.06 | 16.39 | 20.26 |
| Margaric acid (C17:0) | 0.17 | 0.19 | 0.24 |
| Stearic (C18:0) | 5.33 | 5.44 | 6.42 |
| Arachidic (C20:0) | 0.24 | 0.22 | 0.22 |
| Behenic (C22:0) | 0.13 | 0.12 | 0.11 |
| Monounsaturated FAs | |||
| cis-10-pentadecenoic acid (C15:1) | 0.03 | 0.04 | 0.06 |
| Palmitoleic (C16:1) | 0.51 | 0.62 | 0.62 |
| cis-10-Heptadecenoic acid (C17:1) | 0.09 | 0.08 | 0.08 |
| Oleic (C18:1 n9) | 44.94 | 43.76 | 40.51 |
| Polyunsaturated FAs | |||
| Arachidonic acid (20:4 n6) | 0.03 | 0.04 | 0.06 |
| Linoleic (C18:2 n6) | 27.38 | 26.12 | 23.12 |
| Linolenic (C18:3 n3) | 2.9 | 2.92 | 2.52 |
| Name | RT | DNB1 | DNB3 | DNB5 |
|---|---|---|---|---|
| Benzene, (1-butylhexyl)- | 18.06 | 1.68 | 1.13 | 1.71 |
| Benzene, (1-propylheptyl)- | 18.26 | 1.38 | 0.91 | 1.45 |
| Benzene, (1-ethyloctyl)- | 18.68 | 1.16 | 0.74 | 1.23 |
| Benzene, (1-methylnonyl)- | 19.52 | 1.05 | - | 1.08 |
| Benzene, (1-pentylhexyl)- | 20.24 | 1.67 | 0.99 | 1.82 |
| Benzene, (1-butylheptyl)- | 20.31 | 4.31 | 2.53 | 4.63 |
| Benzene, (1-propyloctyl)- | 20.53 | 3.1 | 1.82 | 3.4 |
| Benzene, (1-ethylnonyl)- | 20.98 | 2.59 | 1.51 | 2.76 |
| Benzene, (1-methyldecyl)- | 21.79 | 2.46 | 1.54 | 2.55 |
| Benzene, (1-pentylheptyl)- | 22.38 | 4.65 | 3.82 | 4.77 |
| Benzene, (1-butyloctyl)- | 22.47 | 4.95 | 3.15 | 5.37 |
| Benzene, (1-propylnonyl)- | 22.73 | 3.44 | 2.08 | 3.65 |
| Benzene, (1-ethyldecyl)- | 23.17 | 2.72 | 1.58 | 2.92 |
| Benzene, (1-methylundecyl)- | 23.97 | 2.39 | 1.39 | 2.78 |
| Benzene, (1-pentyloctyl)- | 24.44 | 3.74 | 2.29 | 4.01 |
| Benzene, (1-butylnonyl)- | 24.57 | 2.92 | 1.61 | 2.99 |
| Benzene, (1-propylheptadecyl)- | 24.83 | 2.18 | 1.06 | 2 |
| Benzene, (1-ethylundecyl)- | 25.29 | 1.78 | 0.85 | 1.56 |
| 7-Hexadecenoic acid, methyl ester, (Z)- | 25.88 | 0.00 | 0.48 | 0.00 |
| Benzene, (1-methyldodecyl)- | 26.06 | 1.73 | 1.07 | 1.68 |
| Hexadecanoic acid, methyl ester | 26.4 | 7.74 | 12.6 | 9.07 |
| 17-Octadecynoic acid | 27.63 | - | - | 0.38 |
| 9,12-Octadecadienoic acid (Z, Z)-, methyl ester | 29.42 | 12.8 | 16.06 | 6.65 |
| 9-Octadecenoic acid, methyl ester,(E)- | 29.59 | 25.31 | 30.08 | 19.21 |
| Methyl stearate | 30.15 | 3.29 | 6.52 | 4.97 |
| Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 32.08 | - | 0.62 | 0.72 |
| 9-Octadecenoic acid (Z)- | 32.19 | - | - | 1.08 |
| 9 12-octadecadienoic acid (Z)- 2-hydroxy-1-(hydroxymethyl)ethyl ester | 34.78 | - | 0.59 | 0.58 |
| 9,12-Octadecadienoyl chloride, (Z, Z)- | 34.94 | 0.84 | 2.99 | 3.59 |
| Mono(2-ethylhexyl) phthalate | 36.62 | 0.38 | - | 1 |
| 1,2-Benzenedicarboxylic acid | 39.81 | - | - | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, H.; Alfheeaid, H.A.; Aljutaily, T.; Alayouni, R.; Alharbi, H.F.; Alsanei, W.A. Elucidating the Nutritional Profile and Biochemical Characterization of High-Energy Nutritional Bar Formulated with Sukkari Date Paste and Mixed Nuts. Foods 2025, 14, 3661. https://doi.org/10.3390/foods14213661
Barakat H, Alfheeaid HA, Aljutaily T, Alayouni R, Alharbi HF, Alsanei WA. Elucidating the Nutritional Profile and Biochemical Characterization of High-Energy Nutritional Bar Formulated with Sukkari Date Paste and Mixed Nuts. Foods. 2025; 14(21):3661. https://doi.org/10.3390/foods14213661
Chicago/Turabian StyleBarakat, Hassan, Hani A. Alfheeaid, Thamer Aljutaily, Raed Alayouni, Hend F. Alharbi, and Woroud A. Alsanei. 2025. "Elucidating the Nutritional Profile and Biochemical Characterization of High-Energy Nutritional Bar Formulated with Sukkari Date Paste and Mixed Nuts" Foods 14, no. 21: 3661. https://doi.org/10.3390/foods14213661
APA StyleBarakat, H., Alfheeaid, H. A., Aljutaily, T., Alayouni, R., Alharbi, H. F., & Alsanei, W. A. (2025). Elucidating the Nutritional Profile and Biochemical Characterization of High-Energy Nutritional Bar Formulated with Sukkari Date Paste and Mixed Nuts. Foods, 14(21), 3661. https://doi.org/10.3390/foods14213661









