Lemon Dietary Fibre-Based Powder as a Promising Ingredient for the Food Industry: Enhancing Mortadella Nutritional Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
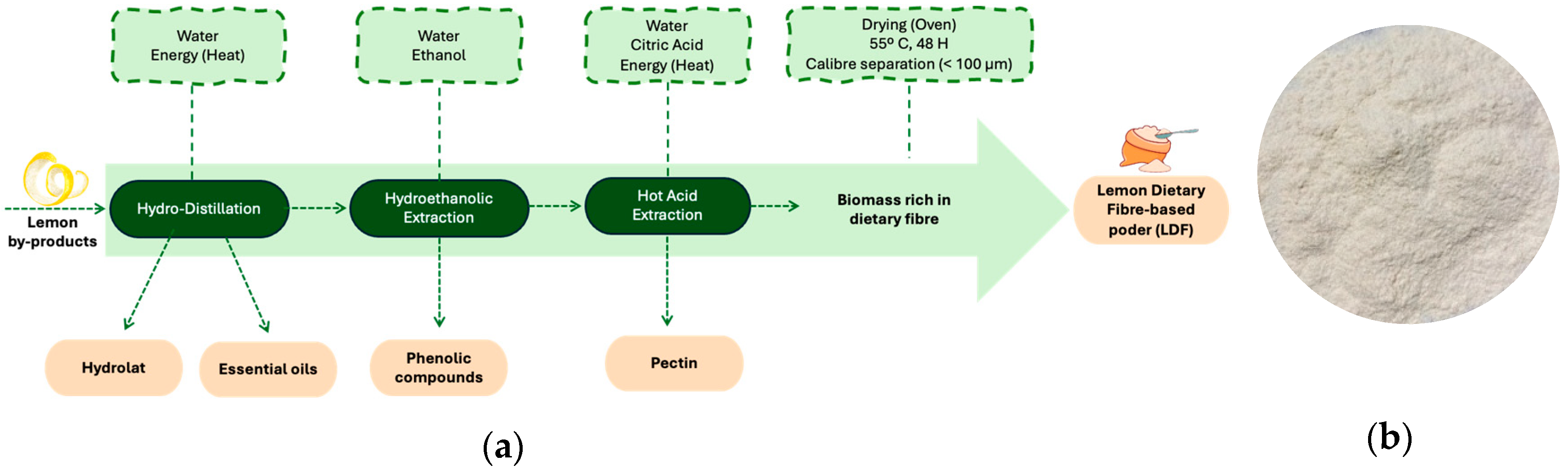
2.2. Lemon Dietary Fibre (LDF)-Based Powder Preparation
2.3. Chemical Composition Analysis
2.3.1. Proximate Composition
2.3.2. Mineral Composition
2.3.3. Cellulose, Hemicellulose, and Lignin
2.4. Physicochemical Properties
2.4.1. pH and Water Activity (aw)
2.4.2. Colour
2.5. Techno-Functional Properties
2.5.1. Gelling Capacity and Precipitate in the Oily Phase
2.5.2. Emulsifying Activity and Emulsion Stability
2.6. Structural Characterisation
2.6.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.6.2. Scanning Electron Microscopy (SEM)
2.7. Bioactive Characterisation
2.7.1. Free and Bound Phenolic Compound Extraction
2.7.2. Total Phenolic Content (TPC)
2.7.3. Phenolic Compound Quantification by HPLC-DAD
2.7.4. Antioxidant Capacity
ABTS Assay
DPPH Assay
Oxygen Radical Absorbance Capacity Assay (ORAC)
2.7.5. Antibacterial Activity
Bacterial Culture
Determination of Bacterial Inhibition
2.8. Sausage Elaboration Process
2.9. Emulsion Stability of Meat
2.10. Chemical Analysis
2.10.1. Proximate Composition
2.10.2. Minerals Composition
2.11. Technological Properties
2.11.1. pH and Water Activity (aw)
2.11.2. Colour
2.11.3. Textural Properties
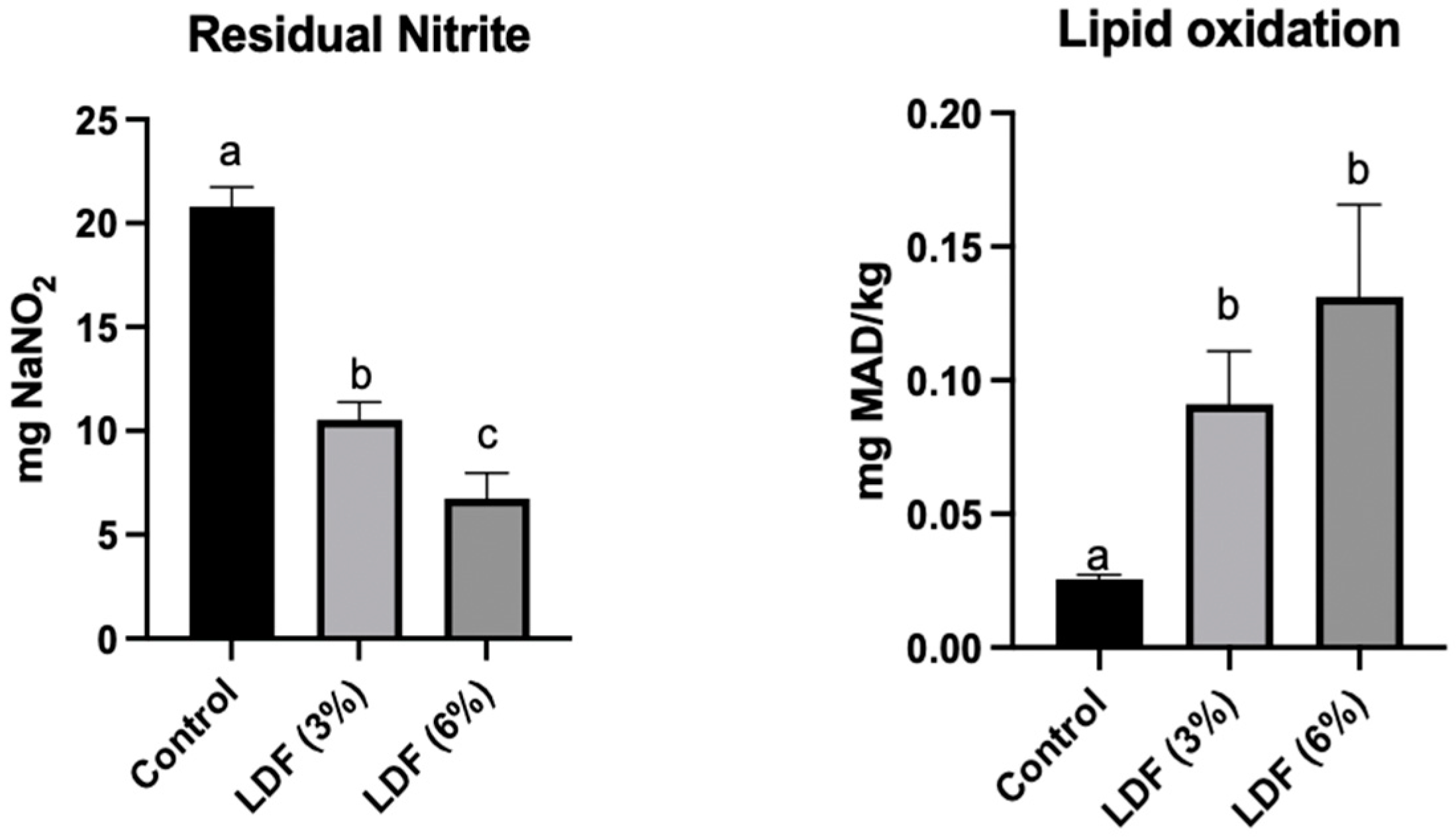
2.12. Residual Nitrite Level
2.13. Lipid Oxidation
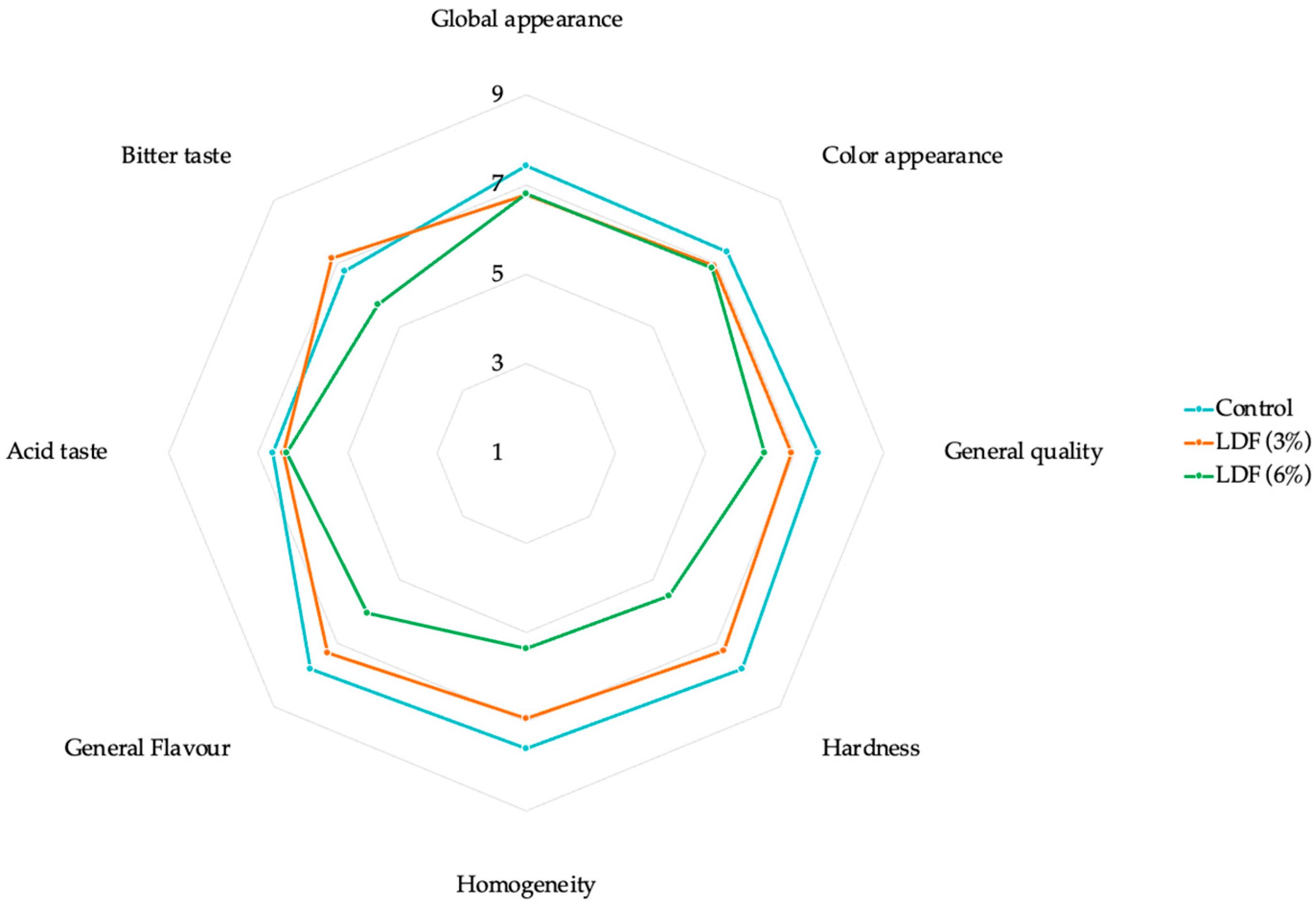
2.14. Sensory Evaluation
2.15. Statistical Analysis
3. Results and Discussion
3.1. LDF Analysis
3.1.1. Proximate Composition
3.1.2. Mineral Composition
3.2. Physicochemical Properties
pH, aw, and Colour
3.3. Structural Characterisation Analysis
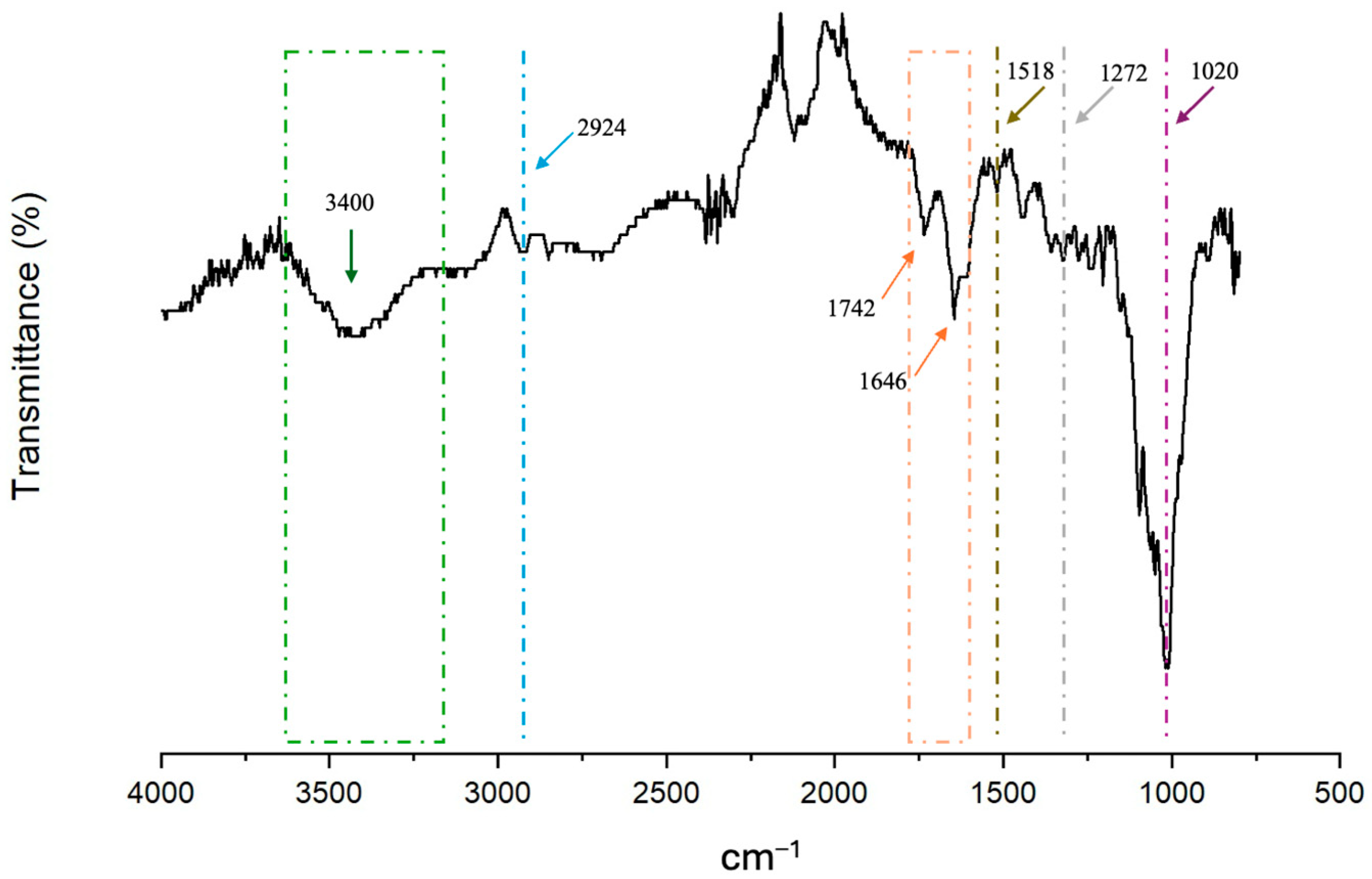
3.3.1. FT-IR Spectra
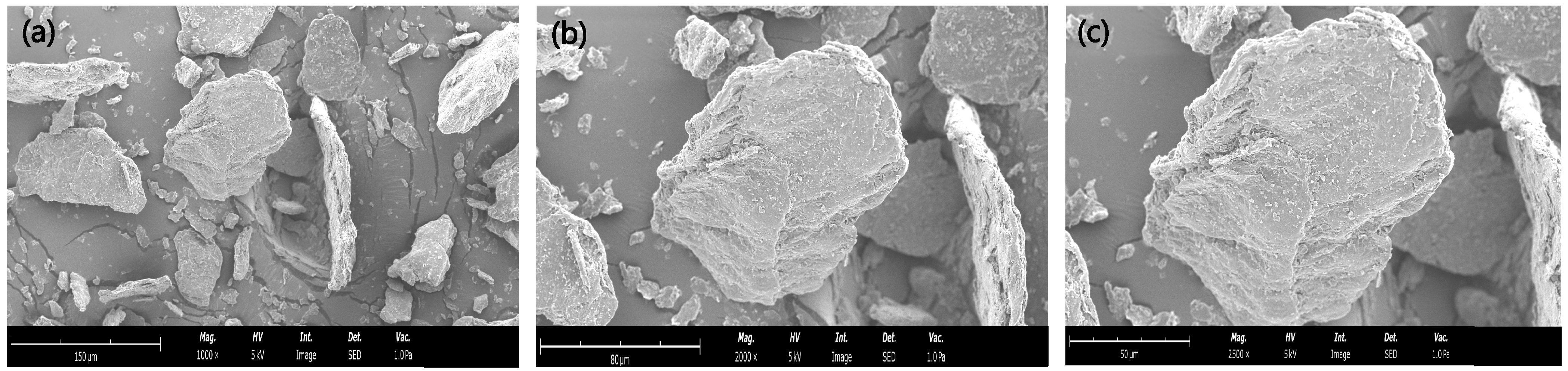
3.3.2. SEM
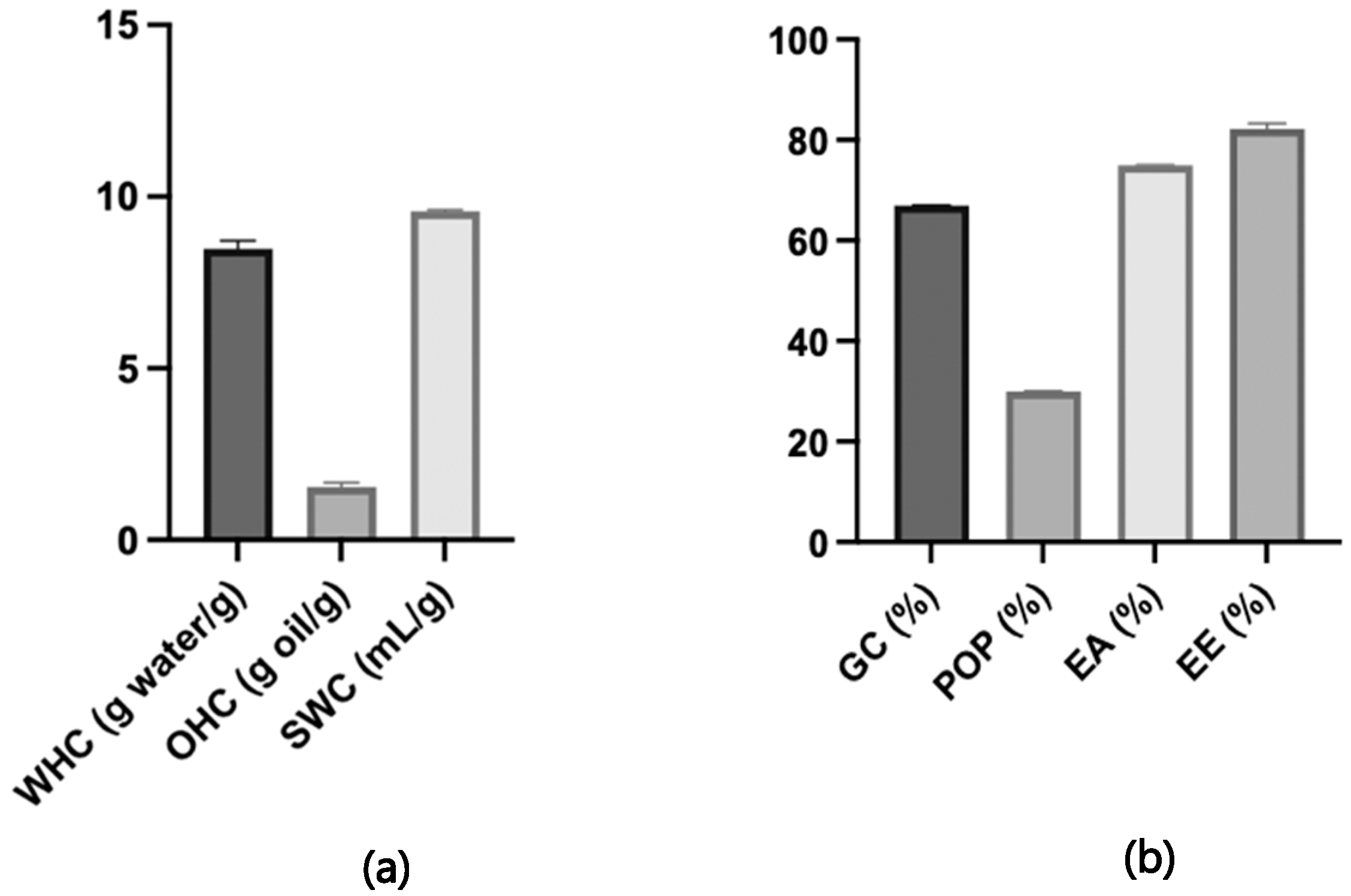
3.4. Techno-Functional Properties
3.5. Bioactive Properties
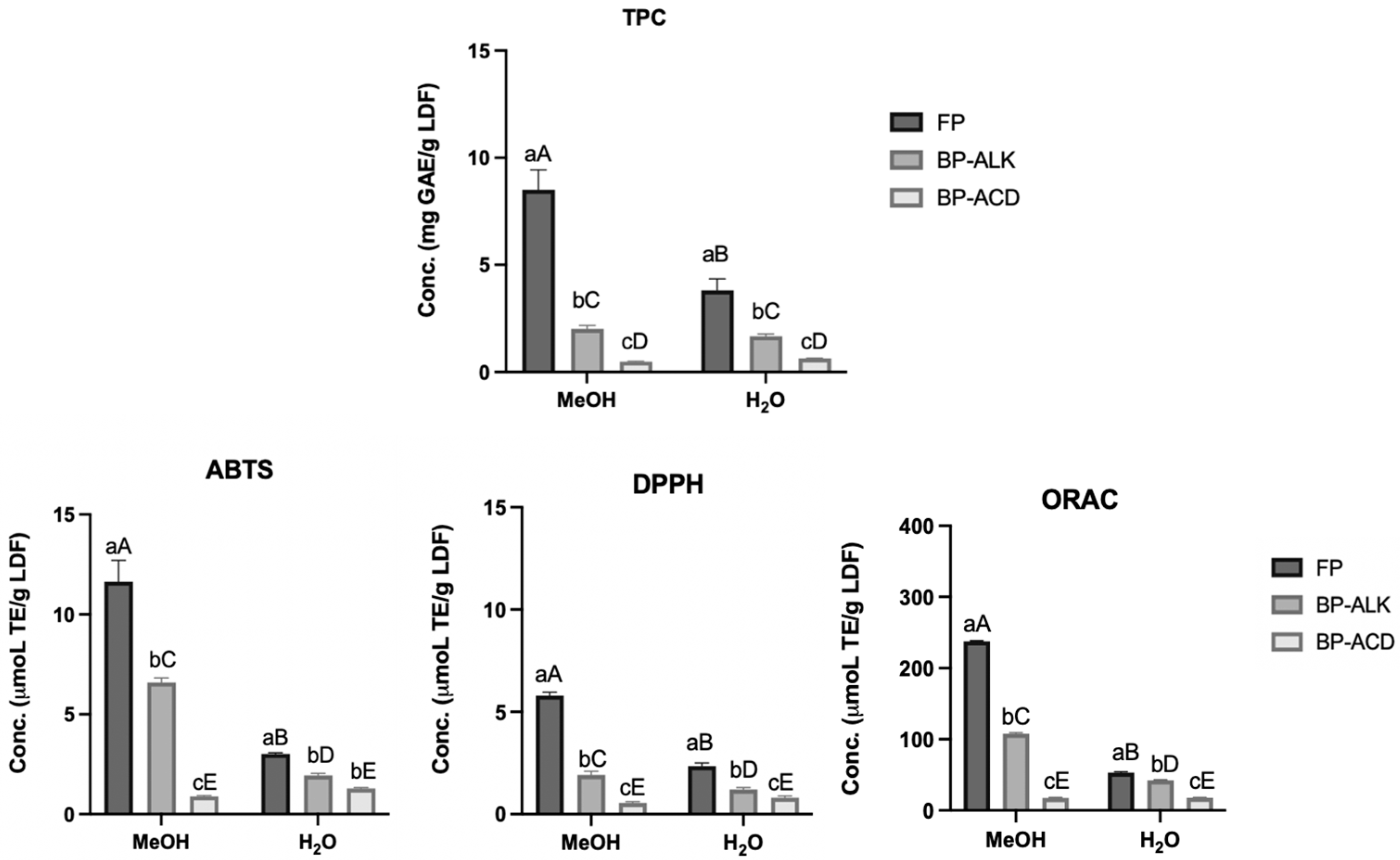
3.5.1. Total Phenolic Content
3.5.2. Phenolic Compound Quantification by HPLC-DAD
3.5.3. Antioxidant Activity
3.5.4. Antibacterial Activity
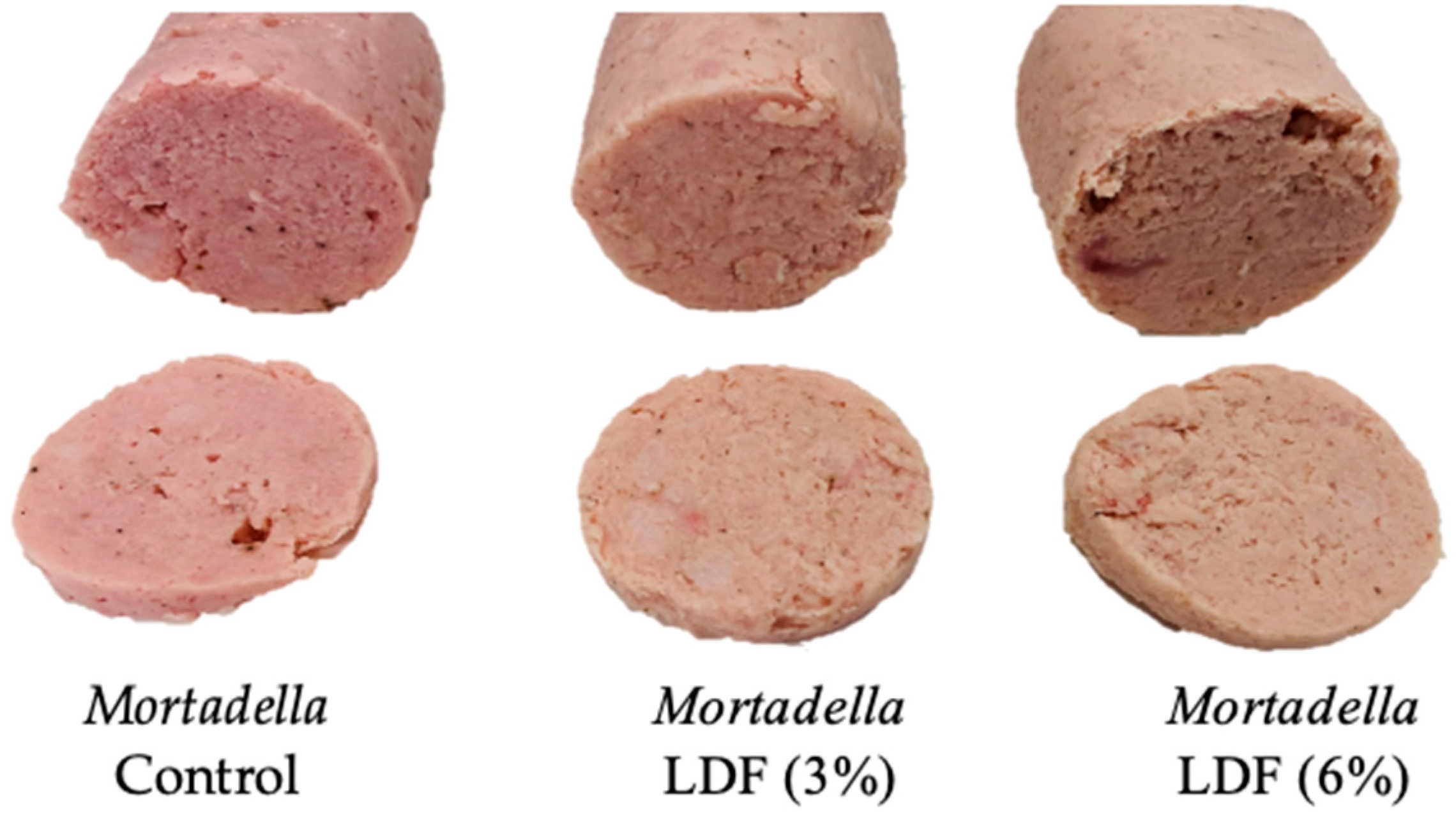
3.6. Sausage Analysis
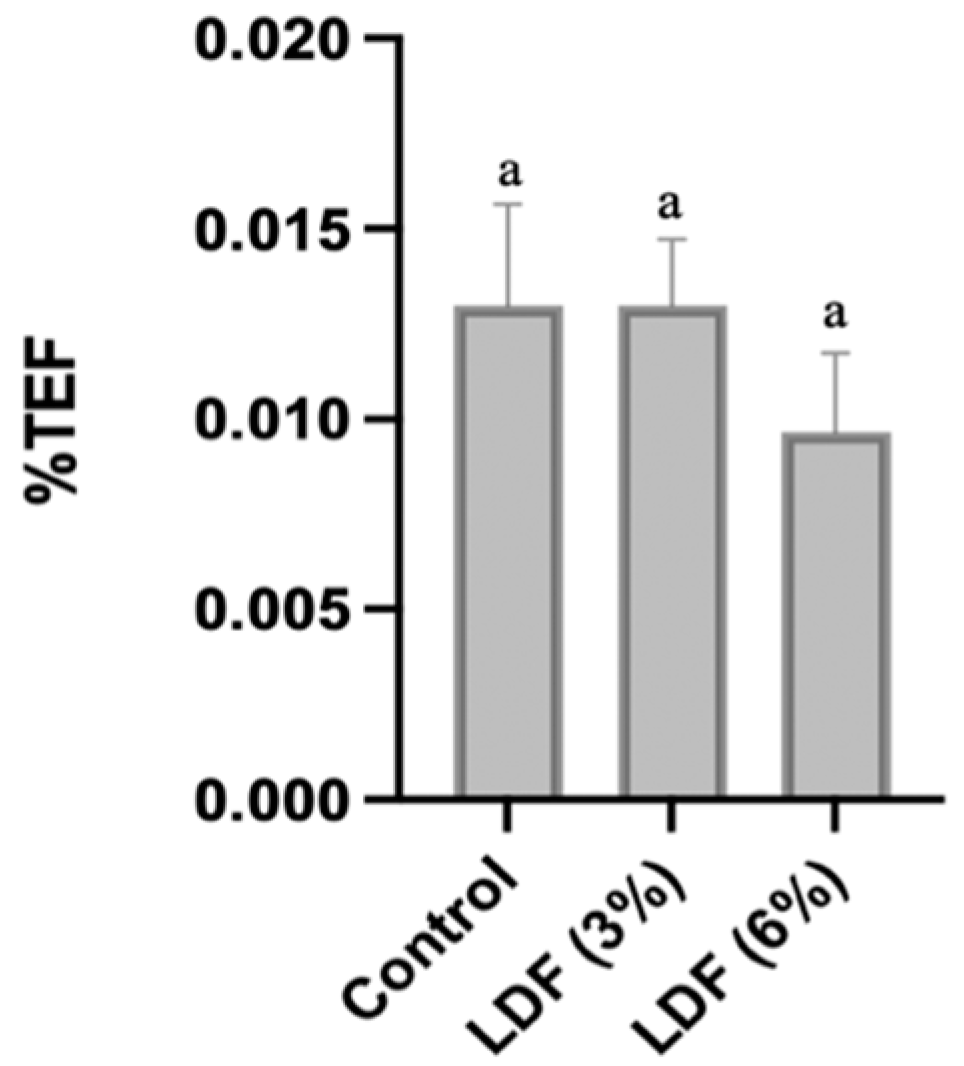
3.6.1. Emulsion Stability
3.6.2. Chemical Composition
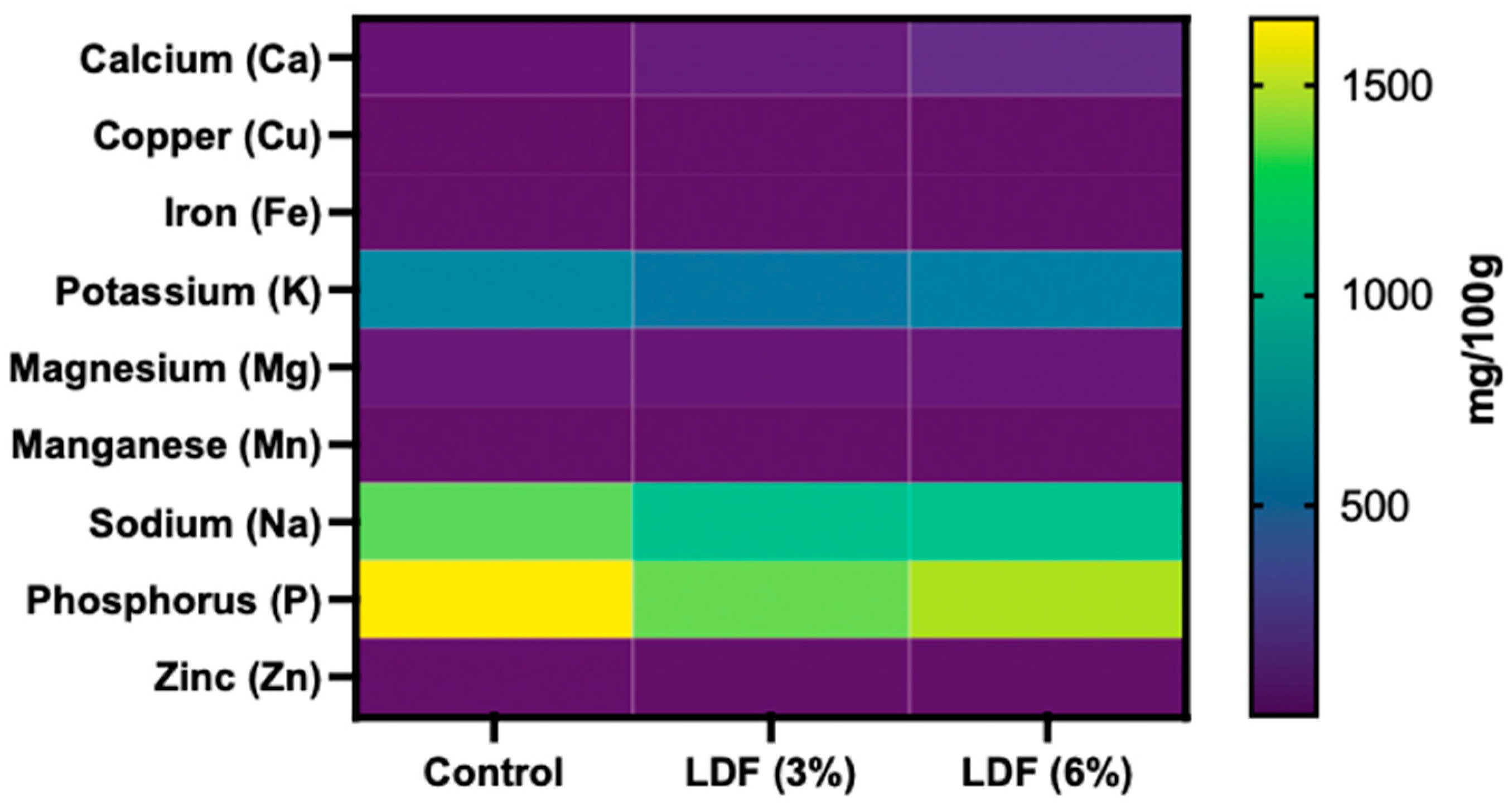
3.6.3. Mineral Composition
3.6.4. Physicochemical Parameters
pH and Water Activity
Colour
3.6.5. Textural Properties
3.6.6. Residual Nitrite Level and Lipid Oxidation
3.7. Sensory Evaluation
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashwan, A.K.; Bai, H.; Osman, A.I.; Eltohamy, K.M.; Chen, Z.; Younis, H.A.; Al-Fatesh, A.; Rooney, D.W.; Yap, P.S. Recycling Food and Agriculture By-Products to Mitigate Climate Change: A Review. Environ. Chem. Lett. 2023, 21, 3351–3375. [Google Scholar] [CrossRef]
- Magalhães, D.; Vilas-Boas, A.A.; Teixeira, P.; Pintado, M. Functional Ingredients and Additives from Lemon By-Products and Their Applications in Food Preservation: A Review. Foods 2023, 12, 1095. [Google Scholar] [CrossRef] [PubMed]
- Vilas-boas, A.A.; Magalhães, D.; Campos, D.A.; Porretta, S.; Dellapina, G.; Poli, G.; Istanbullu, Y.; Demir, S.; San Martín, Á.M.; García-Gómez, P.; et al. Innovative Processing Technologies to Develop a New Segment of Functional Citrus-Based Beverages: Current and Future Trends. Foods 2022, 11, 3859. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.; Sharma, M.; Sharma, A. Development of Product Rich in Dietary Fiber and Antioxidant Prepared from Lemon Peel. Glob. J. Biol. Agric. Health Sci. 2016, 5, 120–123. [Google Scholar]
- Yang, Y.Y.; Ma, S.; Wang, X.X.; Zheng, X.L. Modification and Application of Dietary Fiber in Foods. J. Chem. 2017, 2017, 9340427. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F. Dietary Fiber: An Opportunity for a Global Control of Hyperlipidemia. Oxidative Med. Cell. Longev. 2021, 2021, 5542342. [Google Scholar] [CrossRef]
- Terpstra, A.H.M.; Beynen, A.C.; Lapré, J.A.; De Vries, H.T.; De Vries, H.T. The Hypocholesterolemic effect of Lemon Peels, Lemon Pectin, and the Waste Stream material of Lemon Peels in Hybrid F1B Hamsters. Eur. J. Nutr. 2002, 41, 19–26. [Google Scholar] [CrossRef]
- Karaman, E.; Yılmaz, E.; Tuncel, N.B. Physicochemical, Microstructural and Functional Characterization of Dietary Fibers Extracted from Lemon, Orange and Grapefruit Seeds Press Meals. Bioact. Carbohydr. Diet. Fibre 2017, 11, 9–17. [Google Scholar] [CrossRef]
- Borghi, S.; Pavanelli, W.R.; Kumar Saini, R.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Girinur Mallikarjuna Gowda, K.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.J.; Tomas, M.; et al. Functional Implications of Bound Phenolic Compounds and Phenolics–Food Interaction: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef]
- Angulo-López, J.E.; Flores-Gallegos, A.C.; Ascacio-Valdes, J.A.; Contreras Esquivel, J.C.; Torres-León, C.; Rúelas-Chácon, X.; Aguilar, C.N. Antioxidant Dietary Fiber Sourced from Agroindustrial Byproducts and Its Applications. Foods 2023, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Lario, Y.; Sendra, E.; García-Pérez, J.; Fuentes, C.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Alvarez, J.A. Preparation of High Dietary Fiber Powder from Lemon Juice By-Products. Innov. Food Sci. Emerg. Technol. 2004, 5, 113–117. [Google Scholar] [CrossRef]
- Pathania, S.; Kaur, N. Utilization of Fruits and Vegetable By-Products for Isolation of Dietary Fibres and Its Potential Application as Functional Ingredients. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfei, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Thomson, C.; Garcia, A.L.; Edwards, C.A. Interactions between Dietary Fibre and the Gut Microbiota. Proc. Nutr. Soc. 2021, 80, 398–408. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on Dietary Reference Values for Carbohydrates and Dietary Fibre. EFSA J. 2016, 8, 1462. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline Carbohydrate Intake for Adults and Children; World Health Organization: New Delhi, India, 2023. [Google Scholar]
- Venter, C.; Meyer, R.W.; Greenhawt, M.; Pali-Schöll, I.; Nwaru, B.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; et al. Role of Dietary Fiber in Promoting Immune Health—An EAACI Position Paper. Eur. J. Allergy Clin. Immunol. 2022, 77, 3185–3198. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The Health Benefits of Dietary Fiber: Beyond the Usual Suspects of Type 2 Diabetes Mellitus, Cardiovascular Disease and Colon Cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef]
- Fernández-López, J.; Cruz López-Marcos, M.; Bailina, C.; Viuda-Martos, M.; Angel Pérez-Alvarez, J. Properties of Dietary Fibers from Agroindustrial Coproducts as Source for Fiber-Enriched Foods. Food Bioprocess Technol. 2015, 8, 2400–2408. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Effect of Added Citrus Fibre and Spice Essential Oils on Quality Characteristics and Shelf-Life of Mortadella. Meat Sci. 2010, 85, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Effect of Packaging Conditions on Shelf-Life of Mortadella Made with Citrus Fibre Washing Water and Thyme or Rosemary Essential Oil. Food Nutr. Sci. 2011, 2, 1–10. [Google Scholar] [CrossRef][Green Version]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Antioxidant Dietary Fibre Enriched Meat-Based Functional Foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- FAO. Urbanization Is Transforming Agrifood Systems across the Rural–Urban Continuum Creating Challenges and Opportunities to Access Affordable Healthy Diets; FAO: Rome, Italy, 2023; ISBN 978-92-5-138222-6. [Google Scholar]
- Michel, M.; Eldridge, A.L.; Hartmann, C.; Klassen, P.; Ingram, J.; Meijer, G.W. Benefits and Challenges of Food Processing in the Context of Food Systems, Value Chains and Sustainable Development Goals. Trends Food Sci. Technol. 2024, 153, 104703. [Google Scholar] [CrossRef]
- López-Vargas, J.H.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Viuda-Martos, M. Quality Characteristics of Pork Burger Added with Albedo-Fiber Powder Obtained from Yellow Passion Fruit (Passiflora edulis Var. Flavicarpa) Co-Products. Meat Sci. 2014, 97, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Petridis, D.; Raizi, P.; Ritzoulis, C. Influence of Citrus Fiber, Rice Bran and Collagen on the Texture and Organoleptic Properties of Low-Fat Frankfurters. J. Food Process Preserv. 2014, 38, 1759–1771. [Google Scholar] [CrossRef]
- Powell, M.J.; Sebranek, J.G.; Prusa, K.J.; Tarté, R. Evaluation of Citrus Fiber as a Natural Replacer of Sodium Phosphate in Alternatively-Cured All-Pork Bologna Sausage. Meat Sci. 2019, 157, 107883. [Google Scholar] [CrossRef]
- Soncu, E.D.; Kolsarıcı, N.; Cicek, N.; Öztürk, G.S.; Arıcı, Y.K. The Comparative Effect of Carrot and Lemon Fiber as a Fat Replacer on Physico-Chemical, Textural, and Organoleptic Quality of Low-Fat Beef Hamburger. Korean J. Food Sci. Anim. Resour. 2015, 35, 370–381. [Google Scholar] [CrossRef]
- Song, J.; Pan, T.; Wu, J.; Ren, F. The Improvement Effect and Mechanism of Citrus Fiber on the Water-Binding Ability of Low-Fat Frankfurters. J. Food Sci. Technol. 2016, 53, 4197–4204. [Google Scholar] [CrossRef]
- Fernández-Ginés, J.M.; Fernández-López, J.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.A. Lemon Albedo as a New Source of Dietary Fiber: Application to Bologna Sausages. Meat Sci. 2004, 67, 7–13. [Google Scholar] [CrossRef]
- Haque, A.; Ahmad, S.; Azad, Z.R.A.A.; Adnan, M.; Ashraf, S.A. Incorporating Dietary Fiber from Fruit and Vegetable Waste in Meat Products: A Systematic Approach for Sustainable Meat Processing and Improving the Functional, Nutritional and Health Attributes. PeerJ 2023, 11, e14977. [Google Scholar] [CrossRef]
- Koishybayeva, A.; Korzeniowska, M. Utilization and Effect of Apple Pomace Powder on Quality Characteristics of Turkey Sausages. Foods 2024, 13, 2807. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Guo, C.; Liu, X.; Xiao, C. Effects of Insoluble Dietary Fiber from Kiwi Fruit Pomace on the Physicochemical Properties and Sensory Characteristics of Low-Fat Pork Meatballs. J. Food Sci. Technol. 2021, 58, 1524–1537. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC: Rockville, MD, USA, 1990. [Google Scholar]
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure (LAP) (Revised July 2011); NREL: Golden, CO, USA, 2008. [Google Scholar]
- King, D.A. AMSA Meat Color Measurement Guidelines; American Meat Science Association: Kearney, MO, USA, 2012. [Google Scholar]
- Chau, C.F.; Huang, Y.L. Comparison of the Chemical Composition and Physicochemical Properties of Different Fibers Prepared from the Peel of Citrus sinensis L. Cv. Liucheng. J. Agric. Food Chem. 2003, 51, 2615–2618. [Google Scholar] [CrossRef] [PubMed]
- Tinh, N.T.T.; Sitolo, G.C.; Yamamoto, Y.; Suzuki, T. Citrus limon Peel Powder Reduces Intestinal Barrier Defects and Inflammation in a Colitic Murine Experimental Model. Foods 2021, 10, 240. [Google Scholar] [CrossRef]
- Vilas-boas, A.A.; Campos, D.A.; Nunes, C.; Ribeiro, S. Polyphenol Extraction by Different Techniques for Valorisation of Non-Compliant Portuguese Sweet Cherries towards a Novel Antioxidant Extract. Sustainability 2020, 12, 5556. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Barros, A.S.; Silva Ferreira, A.C.; Silva, A.M.S. Influence of the Temperature and Oxygen Exposure in Red Port Wine: A Kinetic Approach. Food Res. Int. 2015, 75, 337–347. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH·Free Radical Method. LWT—Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Ubeda, C.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Evaluation of Antioxidant Activity and Total Phenols Index in Persimmon Vinegars Produced by Different Processes. LWT 2011, 44, 1591–1596. [Google Scholar] [CrossRef]
- Botella-Martínez, C.; Viuda-Martos, M.; Angel Pérez-Alvarez, J.; Fernández-López, J. Total and Partial Fat Replacement by Gelled Emulsion (Hemp Oil and Buckwheat Flour) and Its Impact on the Chemical, Technological and Sensory Properties of Frankfurters. Foods 2021, 10, 1681. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Bourne, M.C. Texture Profile Analysis. Food Technol. 1978, 32, 62–72. [Google Scholar]
- ISO 2918:1975; ISO International Standard 2918. Meat and Meat Products: Determination of Nitrite Content. International Organization for Standardization: Genevè, Switzerland, 1975.
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Nempeque, L.V.J.; Cabrera, A.P.G.; Moncayo, J.Y.C. Evaluation of Tahiti Lemon Shell Flour (Citrus latifolia Tanaka) as a Fat Mimetic. J. Food Sci. Technol. 2021, 58, 720–730. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Yuan, F.; Pan, Q.; Fan, R.; Gao, Y. Physicochemical Characterization of Five Types of Citrus Dietary Fibers. Biocatal. Agric. Biotechnol. 2015, 4, 250–258. [Google Scholar] [CrossRef]
- Fu, J.T.; Chang, Y.H.; Shiau, S.Y. Rheological, Antioxidative and Sensory Properties of Dough and Mantou (Steamed Bread) Enriched with Lemon Fiber. LWT 2015, 61, 56–62. [Google Scholar] [CrossRef]
- Xiao, R.; Qi, J.; Liao, J.; Wei, H.; Zhuo, T. Preparation of Citrus Fiber from Lemon Peel Residue: Effects of Structure and Endogenous Pectin Components on Emulsifying Properties of Citrus Fiber. Int. J. Biol. Macromol. 2024, 283, 137679. [Google Scholar] [CrossRef]
- Parra, M.; Hortelano, D.; García-Sánchez, F.; Intrigliolo, D.S.; Rubio-Asensio, J.S. Effects of Drip Irrigation Design on a Lemon and a Young Persimmon Orchard in Semi-Arid Conditions. Water 2021, 13, 1795. [Google Scholar] [CrossRef]
- Sánchez-Bravo, P.; Noguera-Artiaga, L.; Martínez-Tomé, J.; Hernández, F.; Sendra, E. Effect of Organic and Conventional Production on the Quality of Lemon “Fino 49”. Agronomy 2022, 12, 980. [Google Scholar] [CrossRef]
- Idamokoro, E.M.; Hosu, Y.S.; Oyedeji, O.O.; Miya, G.M.; Kuria, S.K.; Oyedeji, A.O. A Comparative Analysis of the Proximate and Mineral Composition of Whole Citrus limon and Citrus clementina as a Prospective Alternative Feed Resource for Livestock Farming in South Africa. Front. Sustain. Food Syst. 2022, 6, 1021175. [Google Scholar] [CrossRef]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural Bioactive Compounds of Citrus limon for Food and Health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef]
- Shkembi, B.; Huppertz, T. Calcium Absorption from Food Products: Food Matrix Effects. Nutrients 2022, 14, 180. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific Opinion on Dietary Reference Values for Phosphorus. EFSA J. 2015, 13, 4185. [Google Scholar] [CrossRef]
- Czech, A.; Zarycka, E.; Yanovych, D.; Zasadna, Z.; Grzegorczyk, I.; Kłys, S. Mineral Content of the Pulp and Peel of Various Citrus Fruit Cultivars. Biol. Trace Elem. Res. 2020, 193, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (Aw) on Microbial Stability as a Hurdle in Food Preservation. In Water Activity in Foods: Fundamentals and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 323–355. [Google Scholar]
- Bakshi, G.; Ananthanarayan, L. Characterization of Lemon Peel Powder and Its Application as a Source of Pectin Degrading Enzyme in Clarification of Cloudy Apple Juice. J. Food Sci. Technol. 2022, 59, 2535–2544. [Google Scholar] [CrossRef]
- Peng, F.; Ren, X.; Du, B.; Chen, L.; Yu, Z.; Yang, Y. Structure, Physicochemical Property, and Functional Activity of Dietary Fiber Obtained from Pear Fruit Pomace (Pyrus Ussuriensis Maxim) via Different Extraction Methods. Foods 2022, 11, 2161. [Google Scholar] [CrossRef]
- Canteri, M.H.G.; Renard, C.M.G.C.; Le Bourvellec, C.; Bureau, S. ATR-FTIR Spectroscopy to Determine Cell Wall Composition: Application on a Large Diversity of Fruits and Vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, M.; Huang, Y.; Ma, C.; Mu, S.; Li, H.; Liu, X.; Ma, Y.; Liu, Y.; Hou, J. Comparison and Characterization of the Structure and Physicochemical Properties of Three Citrus Fibers: Effect of Ball Milling Treatment. Foods 2022, 11, 2665. [Google Scholar] [CrossRef]
- Núñez-Gómez, V.; San Mateo, M.; González-Barrio, R.; Periago, M.J. Chemical Composition, Functional and Antioxidant Properties of Dietary Fibre Extracted from Lemon Peel after Enzymatic Treatment. Molecules 2024, 29, 269. [Google Scholar] [CrossRef]
- Huang, J.; Liao, J.; Qi, J.; Jiang, W.; Yang, X.Q. Structural and Physicochemical Properties of Pectin-Rich Dietary Fiber Prepared from Citrus Peel. Food Hydrocoll. 2021, 110, 106140. [Google Scholar] [CrossRef]
- Al-Qassabi, J.S.A.; Weli, A.M.; Hossain, M.A. Comparison of Total Phenols Content and Antioxidant Potential of Peel Extracts of Local and Imported Lemons Samples. Sustain. Chem. Pharm. 2018, 8, 71–75. [Google Scholar] [CrossRef]
- Carboni Martins, C.; Rodrigues, R.C.; Domeneghini Mercali, G.; Rodrigues, E. New Insights into Non-Extractable Phenolic Compounds Analysis. Food Res. Int. 2022, 157, 111487. [Google Scholar] [CrossRef] [PubMed]
- Durmus, N.; Kilic-Akyilmaz, M. Bioactivity of Non-Extractable Phenolics from Lemon Peel Obtained by Enzyme and Ultrasound Assisted Extractions. Food Biosci. 2023, 53, 102571. [Google Scholar] [CrossRef]
- Zappia, A.; Spanti, A.; Princi, R.; Imeneo, V.; Piscopo, A. Evaluation of the Efficacy of Antioxidant Extract from Lemon By-Products on Preservation of Quality Attributes of Minimally Processed Radish (Raphanus sativus L.). Antioxidants 2023, 12, 235. [Google Scholar] [CrossRef]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior Antibacterial Activity of Integral Lemon Pectin Extracted via Hydrodynamic Cavitation. ChemistryOpen 2020, 9, 628–630. [Google Scholar] [CrossRef]
- Jeong, Y.; Han, Y. Effect on the Emulsification Stability and Quality of Emulsified Sausages Added with Wanggasi-Chunnyuncho (Opuntia humifusa f. Jeollaensis) Fruit Powders. Food Sci. Anim. Resour. 2019, 39, 953–965. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Q.; Zhuang, X.; Wang, Y.; Zhou, G.; Zhang, W. Effect of Regenerated Cellulose Fiber on the Physicochemical Properties and Sensory Characteristics of Fat-Reduced Emulsified Sausage. LWT 2018, 97, 157–163. [Google Scholar] [CrossRef]
- Diao, X.; Zhu, J.; Huang, L.; Li, S.; Mao, X.; Li, C.; Ke, W. Effect of Citrus Fiber on Physicochemical Quality of Frankfurter Sausages: A Study on Lipid Oxidation and Protein Gel Characteristics. LWT 2024, 209, 116778. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.H.; Zhang, Y.Y.; Li, S.; Dong, X.; Qin, L. Effect of Sodium Salt on Meat Products and Reduction Sodium Strategies—A Review. Meat Sci. 2023, 205, 109296. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline Sodium Intake for Adults and Children; World Health Organization: New Delhi, India, 2012. [Google Scholar]
- Ibrahim, H.M.; Hassan, I.M.; Hamed, A.A. Application of Lemon and Orange Peels in Meat Products: Quality and Safety. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2703–2723. [Google Scholar] [CrossRef]
- Gedikoğlu, A.; Clarke, A.D. Quality Attributes of Citrus Fiber Added Ground Beef and Consumer Acceptance of Citrus Fiber Added Turkish Meat-Balls. Food Health 2019, 5, 205–214. [Google Scholar] [CrossRef]
- Sarıçoban, C.; Unal, K. Influence of Pre-Treated Bitter Orange Albedo on the Physicochemical, Textural and Sensory Properties of Fermented Sausages (Sucuk). J. Food Sci. Technol. 2022, 59, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, M.; Kononiuk, A.; Wójciak, K.M. Impact of Sodium Nitrite Reduction on Lipid Oxidation and Antioxidant Properties of Cooked Meat Products. Antioxidants 2020, 9, 9. [Google Scholar] [CrossRef]
- Čakar, U.; Čolović, M.; Milenković, D.; Pagnacco, M.; Maksimović, J.; Krstić, D.; Đorđević, B. Strawberry and Drupe Fruit Wines Antioxidant Activity and Protective Effect Against Induced Oxidative Stress in Rat Synaptosomes. Antioxidants 2025, 14, 155. [Google Scholar] [CrossRef]
| LDF | |
|---|---|
| Moisture (%) | 3.8 ± 0.0 |
| Protein (%) | 4.6 ± 0.0 |
| Lipids (%) | 0.0 ± 0.0 |
| Ash (%) | 1.2 ± 0.0 |
| Carbohydrates (%) | 90.5 ± 0.0 |
| Total Dietary Fibre (TDF) (%) | 85.8 ± 1.1 |
| Insoluble Dietary Fibre (IDF) (%) | 52.6 ± 0.7 |
| Soluble Dietary Fibre (SDF) (%) | 33.2 ± 0.8 |
| Cellulose (as glucose) | 9.6 ± 0.9 |
| Hemicellulose | 6.8 ± 0.4 |
| Soluble Lignin | 9.9 ± 0.4 |
| Insoluble Lignin | 5.6 ± 0.4 |
| LDF (mg/100 g) | |
|---|---|
| Calcium | 425.96 ± 4.54 |
| Phosphorus | 184.60 ± 7.27 |
| Potassium | 77.92 ± 6.53 |
| Magnesium | 15.31 ± 1.60 |
| Sodium | 9.92 ± 1.33 |
| Iron | 1.28 ± 0.46 |
| Copper | 0.62 ± 0.01 |
| Zinc | 0.58 ± 0.27 |
| Manganese | 0.17 ± 0.00 |
| LDF | |
|---|---|
| pH | 3.27 ± 0.01 |
| aw | 0.424 ± 0.02 |
| L* | 77.91 ± 1.35 |
| a* | 0.69 ± 0.08 |
| b* | 13.59 ± 0.38 |
| C* | 13.74 ± 0.37 |
| h° | 86.89 ± 0.43 |
| Phenolic Compound | RT (min) | Free Phenolics (FP) | Bound Phenolics (BP-ALK) | Bound Phenolics (BP-ACD) | ||||
|---|---|---|---|---|---|---|---|---|
| MeOH | H2O | MeOH | H2O | MeOH | H2O | |||
| (mg/100 g DW) | ||||||||
| Vanillin | 22.02 | 9.16 ± 2.14 a | 5.90 ± 1.80 b | |||||
| Eriocitrin | 23.42 | 146.35 ± 0.81 a | 68.75 ± 4.23 b | |||||
| p-coumaric acid | 23.99 | 7.06 ± 0.33 a | 5.47 ± 1.70 b | |||||
| Ferulic acid | 25.80 | 5.43 ± 0.55 a | 3.93 ± 0.19 b | |||||
| Apigenin-7-O-Glucoside | 29.02 | 8.82 ± 1.84 | ||||||
| Hesperidin | 29.24 | 894.44 ± 4.63 a | 89.97 ± 1.39 b | |||||
| Rutin | 29.55 | 3.41 ± 0.22 | ||||||
| Luteolin-7-O-Glucoside | 30.27 | 7.43 ± 1.93 | ||||||
| Hesperetin | 48.11 | 5.30 ± 0.21 a | 6.00 ± 0.19 a | |||||
| Log10 (CFU/mL) | Log Reduction | ||
|---|---|---|---|
| Gram-positive | S. aureus Control | 9.5 ± 0.0 a | - |
| S. aureus LDF (3%) | 9.2 ± 0.0 b | 0.3 ± 0.1 | |
| B. cereus Control | 9.8 ± 0.0 a | - | |
| B. cereus LDF (3%) | Total inhibition b | ||
| Gram-negative | E. coli Control | 9.6 ± 0.0 a | - |
| E. coli LDF (3%) | 9.5 ± 0.1 b | 0.1 ± 0.0 | |
| S. enterica Control | 9.9 ± 0.0 a | - | |
| S. enterica LDF (3%) | 9.9 ± 0.0 a | 0.0 ± 0.0 | |
| Formulation | Moisture (%) | Fat (%) | Protein (%) | Ash (%) |
|---|---|---|---|---|
| Control | 66.48 ± 0.00 a | 11.94 ± 0.05 a | 14.75 ± 0.44 a | 3.78 ± 0.00 a |
| LDF (3%) | 63.65 ± 0.02 ab | 11.75 ± 0.57 a | 14.88 ± 0.53 a | 3.46 ± 0.00 a |
| LDF (6%) | 62.15 ± 0.00 b | 11.32 ± 0.26 a | 15.15 ± 1.26 a | 3.53 ± 0.00 a |
| Formulation | pH | aw |
|---|---|---|
| Control | 6.02 ± 0.02 ª | 0.965 ± 0.00 a |
| LDF (3%) | 5.66 ± 0.03 b | 0.965 ± 0.00 a |
| LDF (6%) | 5.36 ± 0.03 c | 0.963 ± 0.00 a |
| Formulation | L* | a* | b* | C* | h° |
|---|---|---|---|---|---|
| Control | 70.93 ± 0.79 a | 6.96 ± 0.11 a | 10.79 ± 0.20 a | 12.84 ± 0.16 a | 57.17 ± 0.73 a |
| LDF (3%) | 73.41 ± 0.93 b | 5.79 ± 0.21 b | 12.45 ± 1.23 a | 13.59 ± 1.08 a | 66.29 ± 2.54 b |
| LDF (6%) | 73.96 ± 0.93 b | 5.43 ± 0.24 b | 14.33 ± 0.57 b | 15.46 ± 0.56 b | 68.00 ± 0.97 b |
| Formulation | Hardness (N) | Cohesiveness | Springiness (mm) | Chewiness (N mm) |
|---|---|---|---|---|
| Control | 33.79 ± 0.85 a | 0.27 ± 0.03 a | 0.39 ± 0.04 a | 3.48 ± 0.60 a |
| LDF (3%) | 41.40 ± 2.57 b | 0.23 ± 0.03 a | 0.33 ± 0.03 ab | 3.57 ± 0.21 a |
| LDF (6%) | 45.10 ± 2.65 b | 0.24 ± 0.02 a | 0.30 ± 0.01 b | 2.45 ± 0.34 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, D.; Rodrigues, C.V.; Botella-Martinez, C.; Muñoz-Tebar, N.; Pérez-Álvarez, J.A.; Viuda-Martos, M.; Teixeira, P.; Pintado, M. Lemon Dietary Fibre-Based Powder as a Promising Ingredient for the Food Industry: Enhancing Mortadella Nutritional Quality. Foods 2025, 14, 1693. https://doi.org/10.3390/foods14101693
Magalhães D, Rodrigues CV, Botella-Martinez C, Muñoz-Tebar N, Pérez-Álvarez JA, Viuda-Martos M, Teixeira P, Pintado M. Lemon Dietary Fibre-Based Powder as a Promising Ingredient for the Food Industry: Enhancing Mortadella Nutritional Quality. Foods. 2025; 14(10):1693. https://doi.org/10.3390/foods14101693
Chicago/Turabian StyleMagalhães, Daniela, Cristina V. Rodrigues, Carmen Botella-Martinez, Nuria Muñoz-Tebar, José Angel Pérez-Álvarez, Manuel Viuda-Martos, Paula Teixeira, and Manuela Pintado. 2025. "Lemon Dietary Fibre-Based Powder as a Promising Ingredient for the Food Industry: Enhancing Mortadella Nutritional Quality" Foods 14, no. 10: 1693. https://doi.org/10.3390/foods14101693
APA StyleMagalhães, D., Rodrigues, C. V., Botella-Martinez, C., Muñoz-Tebar, N., Pérez-Álvarez, J. A., Viuda-Martos, M., Teixeira, P., & Pintado, M. (2025). Lemon Dietary Fibre-Based Powder as a Promising Ingredient for the Food Industry: Enhancing Mortadella Nutritional Quality. Foods, 14(10), 1693. https://doi.org/10.3390/foods14101693












