Structural and Functional Properties of Underutilised Cowpea and Moth Bean Starches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Isolation
2.3. Microscopy
2.4. Particle Size Distribution
2.5. Apparent Amylose Content (ACC)
2.6. X-Ray Diffraction (XRD)
2.7. Gelatinisation and Retrogradation
2.8. Pasting Properties
2.9. Texture Analysis
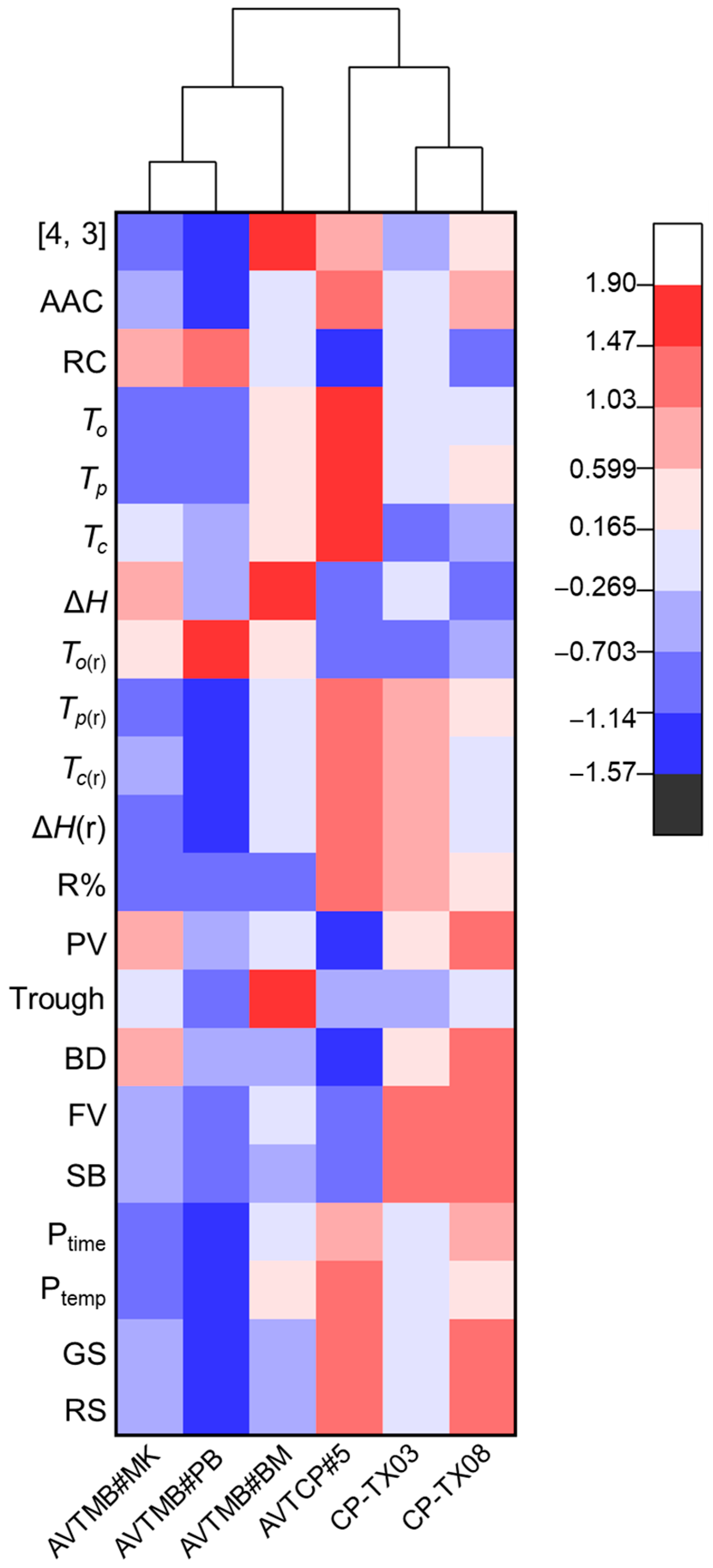
2.10. Cluster Heatmap Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Particle Size of Starch
3.2. Apparent Amylose Content (AAC) and Crystallinity
3.3. Thermal and Retrogradation Properties
3.4. Pasting Properties
3.5. Texture Properties
3.6. Cluster Heatmap Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horn, L.N.; Nghituwamata, S.N.; Isabella, U. Cowpea Production Challenges and Contribution to Livelihood in Sub-Saharan Region. Agric. Sci. 2022, 13, 25–32. [Google Scholar] [CrossRef]
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O.; et al. Cowpea (Vigna unguiculata): Genetics, genomics and breeding. Plant Breed. 2018, 138, 415–424. [Google Scholar] [CrossRef]
- Kim, D.K.; Ochar, K.; Iwar, K.; Ha, B.K.; Kim, S.H. Cowpea (Vigna unguiculata L.) production, genetic resources and strategic breeding priorities for sustainable food security: A review. Front. Plant Sci. 2025, 16, 1562142. [Google Scholar] [CrossRef]
- Rani, J.; Dhull, S.B.; Kinabo, J.; Kidwai, M.K.; Sangwan, A. A narrative review on nutritional and health benefits of underutilized summer crop to address agriculture challenges: Moth bean (Vigna aconitifolia L.). Legume Science 2023, 5, e204. [Google Scholar] [CrossRef]
- Suranjika, S.; Pradhan, S.; Nayak, S.S.; Parida, A. De novo transcriptome assembly and analysis of gene expression in different tissues of moth bean (Vigna aconitifolia) (Jacq.) Marechal. BMC Plant Biol. 2022, 22, 198. [Google Scholar] [CrossRef] [PubMed]
- Keskin, S.O.; Ali, T.M.; Ahmed, J.; Shaikh, M.; Siddiq, M.; Uebersax, M.A. Physico-chemical and functional properties of legume protein, starch, and dietary fiber—A review. Legume Sci. 2021, 4, e117. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, X.; Wang, P.; Jiang, H.; Li, W. Compositional, morphological, and physicochemical properties of starches from red adzuki bean, chickpea, faba bean, and baiyue bean grown in China. Food Sci. Nutr. 2019, 7, 2485–2494. [Google Scholar] [CrossRef]
- Dou, Z.; Hu, B.; Kang, Y.; Zhu, Y.; Chen, X.; Niu, H.; Zeng, S.; Zhang, W.; Duan, Q.; Huang, Q.; et al. Unveiling multifaceted effects of Lactobacillus fermentation on red pitaya (Hylocereus polyrhizus) Pulp: An integrated in silico and in vitro-vivo study. Food Chem. X 2025, 31, 103057. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Kayitesi, E.; Adebo, O.A.; Oyedeji, A.B.; Ogundele, O.M.; Obilana, A.O.; Njobeh, P.B. A review on the physicochemical properties and potential food applications of cowpea (Vigna unguiculata) starch. Int. J. Food Sci. Technol. 2020, 56, 52–60. [Google Scholar] [CrossRef]
- Kumar, R.; Ghoshal, G.; Goyal, M. Moth bean starch (Vigna aconitifolia): Isolation, characterization, and development of edible/biodegradable films. J. Food Sci. Technol. 2019, 56, 4891–4900. [Google Scholar] [CrossRef]
- Kanishka, R.C.; Gayacharan; Basavaraja, T.; Rahul, C.; Jai, C.R. Moth bean (Vigna aconitifolia): A minor legume with major potential to address global agricultural challenges. Front. Plant Sci. 2023, 14, 1179547. [Google Scholar] [CrossRef]
- Hall, A.E. Phenotyping cowpeas for adaptation to drought. Front. Physiol. 2012, 3, 155. [Google Scholar] [CrossRef] [PubMed]
- Dhull, S.B.; Rani, J.; Rohilla, S.; Rose, P.K.; Chawla, P.; Kidwai, M.K.; Chandak, A.; Bamel, P.; Kumar, M.; Kirrolia, A.S. Moth Bean (Vigna aconitifolia) Starch: Properties, Modifications and Applications—A Review. Legume Sci. 2024, 6, e237. [Google Scholar] [CrossRef]
- Fiscus, C.J.; Herniter, I.A.; Tchamba, M.; Paliwal, R.; Munoz-Amatriain, M.; Roberts, P.A.; Abberton, M.; Alaba, O.; Close, T.J.; Oyatomi, O.; et al. The pattern of genetic variability in a core collection of 2,021 cowpea accessions. G3 (Bethesda) 2024, 14, jkae071. [Google Scholar] [CrossRef]
- Nkhoma, N.; Shimelis, H.; Laing, M.D.; Shayanowako, A.; Mathew, I. Assessing the genetic diversity of cowpea [Vigna unguiculata (L.) Walp.] germplasm collections using phenotypic traits and SNP markers. BMC Genet 2020, 21, 110. [Google Scholar] [CrossRef]
- Huong, N.T.M.; Hoa, P.N.; Hung, P.V. Varying amylose contents affect the structural and physicochemical characteristics of starch in mung bean. Int. J. Food Prop. 2021, 24, 737–748. [Google Scholar] [CrossRef]
- Seung, D. Amylose in starch: Towards an understanding of biosynthesis, structure and function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef]
- Kumar, G.; Hooper, J.F.; Li, E.; Devkota, L.; Dhital, S. Ultrasound-assisted hydration offers a novel approach to anneal in-situ pinto bean starch. Carbohydr. Polym. 2025, 360, 123602. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, B.; Huang, Q.; Li, C.; Pletsch, E.A.; Fu, X. Variation in the rate and extent of starch digestion is not determined by the starch structural features of cooked whole pulses. Food Hydrocoll. 2018, 83, 340–347. [Google Scholar] [CrossRef]
- Hoover, R.; Ratnayake, W.S. Determination of total amylose content of starch. In Current Protocols in Food Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2001; pp. E2–E3. [Google Scholar] [CrossRef]
- Xiong, W.; Kumar, G.; Zhang, B.; Dhital, S. Sonication-mediated modulation of macronutrient structure and digestibility in chickpea. Ultrason. Sonochemistry 2024, 106, 106904. [Google Scholar] [CrossRef]
- Xiong, W.; Devkota, L.; Dhital, S. Substitution of wheat semolina with intact chickpea cells: A study on extruded pasta quality. Food Res. Int. 2025, 202, 115687. [Google Scholar] [CrossRef] [PubMed]
- Atuobi, C.; Sakyi-Dawson, E. Microstructural and Physico-Functional Characterization of Starches from Selected Cowpea (Vigna unguiculata L. Walp.) Varieties Developed for Pest and Disease Resistance. J. Nutr. Food Sci. 2011, 1, 104. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Woo, K.S.; Chung, H.J. Starch characteristics of cowpea and mungbean cultivars grown in Korea. Food Chem. 2018, 263, 104–111. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Hoover, R.; Donner, E.; Liu, Q.; Jaiswal, S.; Chibbar, R.; Nantanga, K.K.M.; Seetharaman, K. Structure of faba bean, black bean and pinto bean starches at different levels of granule organization and their physicochemical properties. Food Res. Int. 2011, 44, 2962–2974. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Lim, S.-T. Digestibility of legume starches as influenced by their physical and structural properties. Carbohydr. Polym. 2008, 71, 245–252. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, W.; Li, T.; Wang, L. Understanding the Relationship between the Molecular Structure and Physicochemical Properties of Soft Rice Starch. Foods 2023, 12, 3611. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, P.; Sui, Z.; Bao, J. Physicochemical properties of starches from diverse rice cultivars varying in apparent amylose content and gelatinisation temperature combinations. Food Chem. 2015, 172, 433–440. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Punia, R.; Sharma, M.M.; Kalita, D.; Mukhrjee, J.; Nayak, T.; Singh, H. Physicochemical, morphological, thermal and pasting characteristics of starches from moth bean (Vigna aconitifolia) cultivars grown in India: An underutilized crop. J. Food Sci. Technol. 2017, 54, 4484–4492. [Google Scholar] [CrossRef] [PubMed]
- Ratnaningsih, N.; Suparmo; Harmayani, E.; Marsono, Y. Composition, microstructure, and physicochemical properties of starches from Indonesian cowpea (Vigna unguiculata) varieties. Int. Food Res. J. 2016, 23, 2041–2049. [Google Scholar]
- Li, H.; Dhital, S.; Flanagan, B.M.; Mata, J.; Gilbert, E.P.; Gidley, M.J. High-amylose wheat and maize starches have distinctly different granule organization and annealing behaviour: A key role for chain mobility. Food Hydrocoll. 2020, 105, 105820. [Google Scholar] [CrossRef]
- Pang, Y.; Ali, J.; Wang, X.; Franje, N.J.; Revilleza, J.E.; Xu, J.; Li, Z. Relationship of rice grain amylose, gelatinization temperature and pasting properties for breeding better eating and cooking quality of rice varieties. PLoS ONE 2016, 11, e0168483. [Google Scholar] [CrossRef]
- Li, C. Recent progress in understanding starch gelatinization—An important property determining food quality. Carbohydr. Polym. 2022, 293, 119735. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Jackson, D.S. Gelatinization and solubility of corn starch during heating in excess water: New insights. J. Agric. Food Chem. 2006, 54, 3712–3716. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, J.H.; Chung, H.J. Effect of molecular structure on phase transition behavior of rice starch with different amylose contents. Carbohydr. Polym. 2021, 259, 117712. [Google Scholar] [CrossRef]
- Yu, S.; Ma, Y.; Sun, D.-W. Impact of amylose content on starch retrogradation and texture of cooked milled rice during storage. J. Cereal Sci. 2009, 50, 139–144. [Google Scholar] [CrossRef]
- Miles, M.J.; Morris, V.J.; Orford, P.D.; Ring, S.G. The roles of amylose and amylopectin in the gelation and retrogradation of starch. Carbohydr. Res. 1985, 135, 271–281. [Google Scholar] [CrossRef]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid visco analyser (RVA) as a tool for measuring starch-related physiochemical properties in cereals: A review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
- Li, C.; Dhital, S.; Gilbert, R.G.; Gidley, M.J. High-amylose wheat starch: Structural basis for water absorption and pasting properties. Carbohydr. Polym. 2020, 245, 116557. [Google Scholar] [CrossRef]
- Jane, J.; Chen, Y.Y.; Lee, L.F.; McPherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
- Yu, S.; Ma, Y.; Menager, L.; Sun, D.-W. Physicochemical properties of starch and flour from different rice cultivars. Food Bioprocess Technol. 2010, 5, 626–637. [Google Scholar] [CrossRef]
- Wang, L.; Xie, B.; Shi, J.; Xue, S.; Deng, Q.; Wei, Y.; Tian, B. Physicochemical properties and structure of starches from Chinese rice cultivars. Food Hydrocoll. 2010, 24, 208–216. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
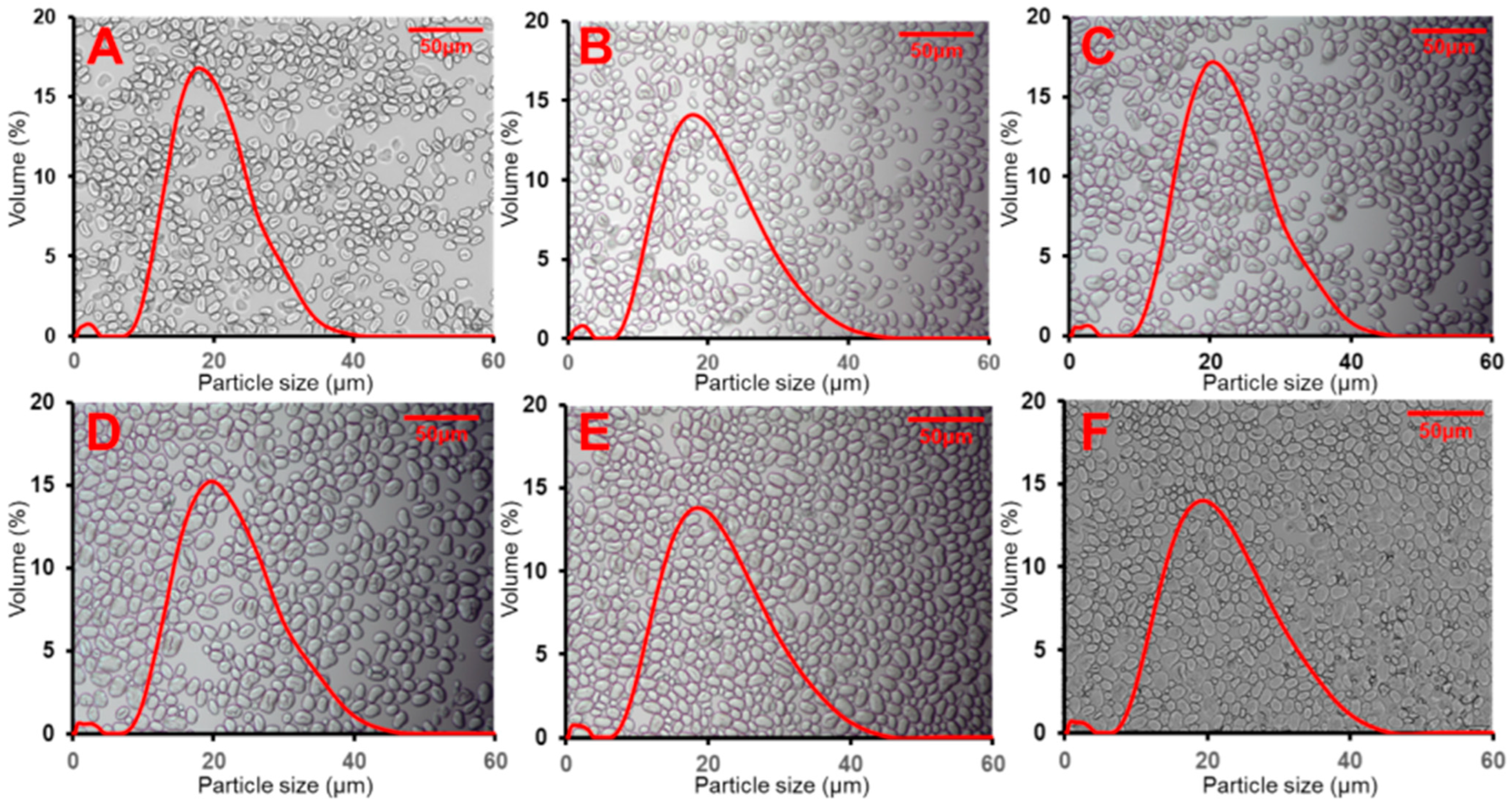
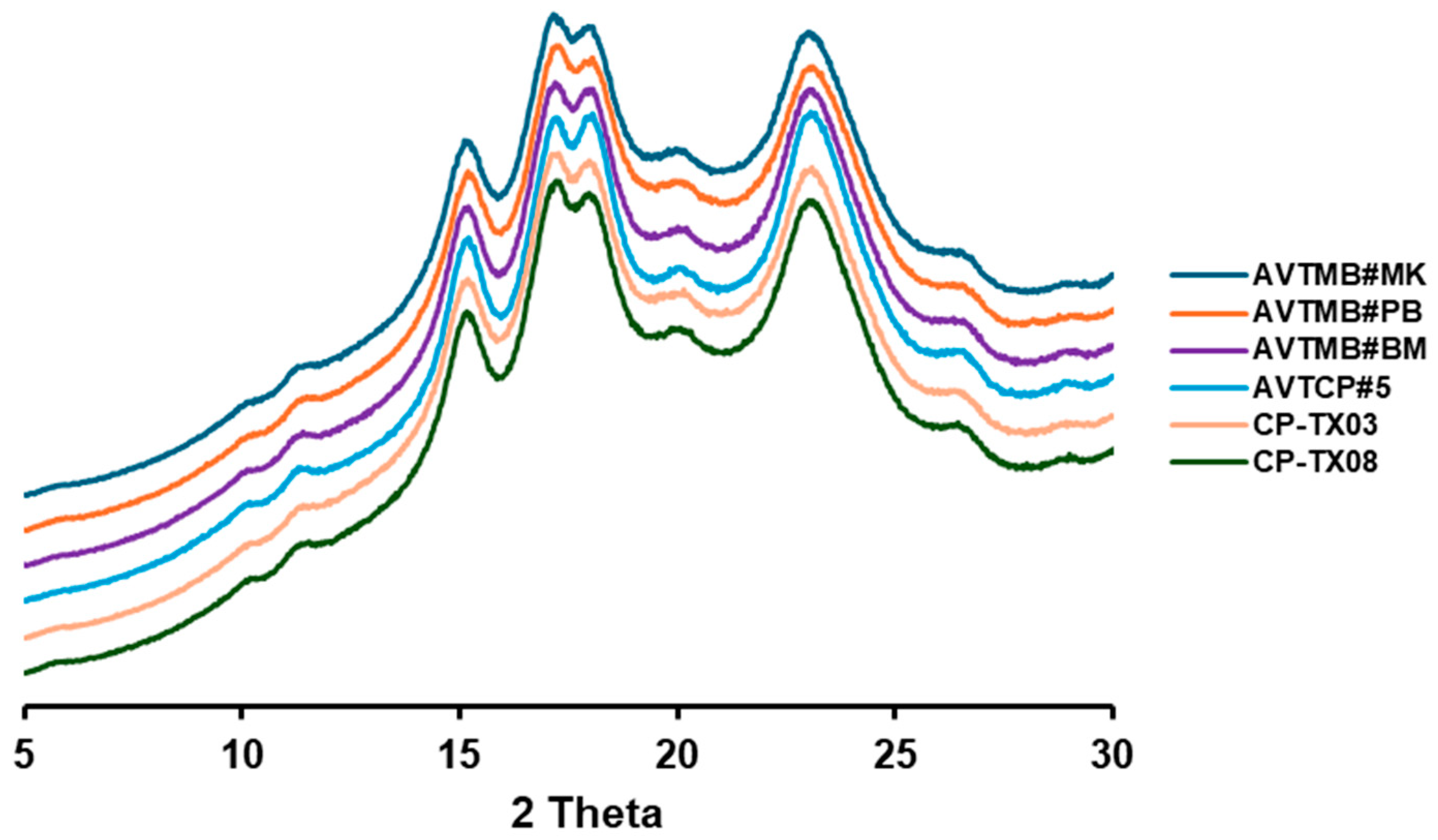
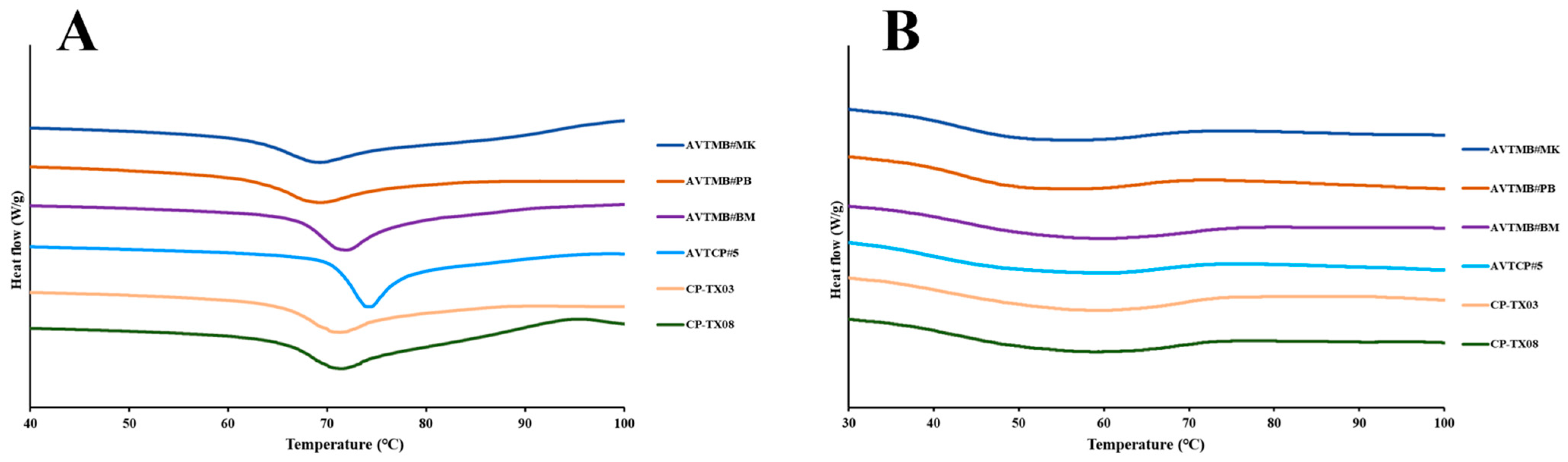
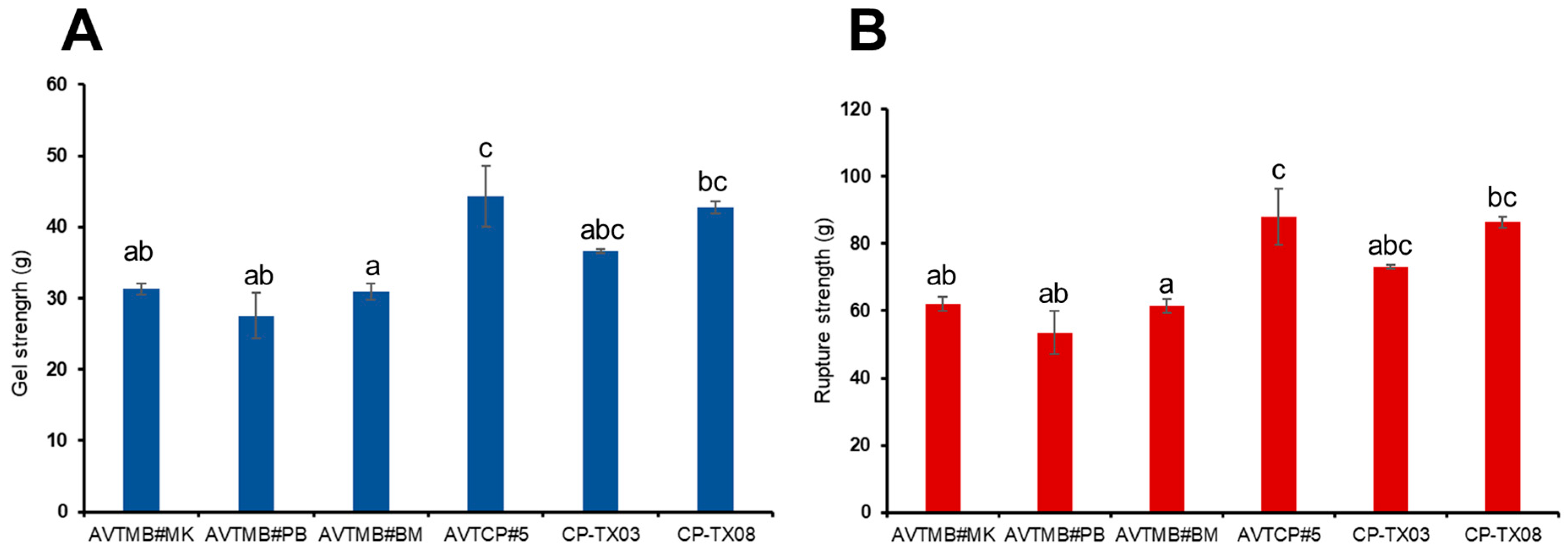
| Samples | D [4, 3] (μm) 2 | AAC (%) | RC (%) |
|---|---|---|---|
| AVTMB#MK | 16.17 ± 0.01 b | 27.40 ± 0.71 b | 35.27 ± 0.32 ab |
| AVTMB#PB | 15.86 ± 0.14 a | 22.23 ± 1.65 a | 35.53 ± 0.12 b |
| AVTMB#BM | 18.35 ± 0.01 f | 27.57 ± 1.70 bc | 34.68 ± 0.24 ab |
| AVTCP#5 | 17.54 ± 0.02 e | 33.90 ± 1.47 d | 33.75 ± 0.41 a |
| CP-TX03 | 16.52 ± 0.02 c | 28.07 ± 1.43 bc | 34.49 ± 0.21 ab |
| CP-TX08 | 17.16 ± 0.03 d | 32.23 ± 1.31 cd | 33.93 ± 0.32 a |
| Samples | To (°C) 2 | Tp (°C) | Tc (°C) | ΔH (J/g) | To(r) (°C) | Tp(r) (°C) | Tc(r) (°C) | ΔH(r) (J/g) | R% |
|---|---|---|---|---|---|---|---|---|---|
| AVTMB#MK | 61.27 ± 0.14 a | 69.21 ± 0.08 a | 93.78 ± 0.22 a | 17.43 ± 0.35 bc | 37.11 ± 0.12 ab | 51.90 ± 0.18 a | 72.19 ± 0.52 ab | 8.46 ± 0.29 ab | 48.56 |
| AVTMB#PB | 61.88 ± 0.19 a | 69.21 ± 0.08 a | 93.40 ± 0.47 a | 15.31 ± 0.47 ab | 37.81 ± 0.19 b | 51.56 ± 0.59 a | 69.19 ± 1.46 a | 7.67 ± 0.35 a | 50.12 |
| AVTMB#BM | 66.22 ± 0.12 c | 72.07 ± 0.03 c | 93.92 ± 1.05 a | 18.88 ± 0.36 c | 36.90 ± 0.43 ab | 54.74 ± 1.79 ab | 74.13 ± 1.06 bc | 9.09 ± 0.13 abc | 48.12 |
| AVTCP#5 | 70.43 ± 0.08 d | 74.15 ± 0.10 d | 94.83 ± 1.53 a | 15.05 ± 0.45 a | 36.11 ± 0.49 a | 58.22 ± 0.76 b | 79.00 ± 1.63 d | 11.34 ± 1.61 c | 75.37 |
| CP-TX03 | 64.79 ± 0.25 b | 71.35 ± 0.20 b | 93.01 ± 1.65 a | 15.85 ± 1.08 ab | 36.22 ± 0.78 a | 57.13 ± 1.07 b | 77.24 ± 1.89 c | 10.59 ± 0.68 bc | 66.83 |
| CP-TX08 | 64.60 ± 0.51 b | 71.72 ± 0.16 bc | 93.38 ± 1.03 a | 14.72 ± 0.44 a | 36.60 ± 0.04 a | 56.49 ± 0.09 b | 73.81 ± 0.24 abc | 9.44 ± 0.12 abc | 64.17 |
| Samples | Peak Viscosity (mPa·s) | Trough (mPa⋅s) | Breakdown (mPa⋅s) | Final Viscosity (mPa⋅s) | Setback (mPa⋅s) | Peak Time (min) | Pasting Temperature (°C) |
|---|---|---|---|---|---|---|---|
| AVTMB#MK | 5559.5 ± 112.5 ab | 871.5 ± 6.5 a | 4688.0 ± 119.0 b | 2434.5 ± 36.5 a | 1563.0 ± 30.0 a | 4.5 ± 0.1 ab | 76.0 ± 0.4 a |
| AVTMB#PB | 5223.5 ± 163.5 ab | 843.5 ± 17.5 a | 4380.0 ± 146.0 ab | 2380.0 ± 65.0 a | 1536.5 ± 47.5 a | 4.4 ± 0.0 a | 74.8 ± 0.0 a |
| AVTMB#BM | 5321.5 ± 44.5 ab | 929.0 ± 5.0 a | 4392.5 ± 49.5 ab | 2488.0 ± 43.0 a | 1559.0 ± 38.0 a | 4.6 ± 0.0 bc | 78.0 ± 0.1 b |
| AVTCP#5 | 5060.5 ± 61.5 a | 850.0 ± 6.0 a | 4210.5 ± 55.5 a | 2388.5 ± 15.5 a | 1538.5 ± 21.5 a | 4.7 ± 0.0 c | 80.0 ± 0.5 c |
| CP-TX03 | 5446.0 ± 50.0 ab | 847.0 ± 39.0 a | 4599.0 ± 11.0 ab | 2592.5 ± 47.5 a | 1745.5 ± 8.5 a | 4.6 ± 0.0 bc | 77.6 ± 0.4 b |
| CP-TX08 | 5597.0 ± 28.0 b | 869.5 ± 23.5 a | 4727.5 ± 4.5 b | 2588.0 ± 66.0 a | 1718.5 ± 89.5 a | 4.7 ± 0.0 c | 78.0 ± 0.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, W.; Zhu, M.; Bhattarai, S.P.; Dhital, S. Structural and Functional Properties of Underutilised Cowpea and Moth Bean Starches. Foods 2025, 14, 3647. https://doi.org/10.3390/foods14213647
Xiong W, Zhu M, Bhattarai SP, Dhital S. Structural and Functional Properties of Underutilised Cowpea and Moth Bean Starches. Foods. 2025; 14(21):3647. https://doi.org/10.3390/foods14213647
Chicago/Turabian StyleXiong, Weiyan, Minqian Zhu, Surya P. Bhattarai, and Sushil Dhital. 2025. "Structural and Functional Properties of Underutilised Cowpea and Moth Bean Starches" Foods 14, no. 21: 3647. https://doi.org/10.3390/foods14213647
APA StyleXiong, W., Zhu, M., Bhattarai, S. P., & Dhital, S. (2025). Structural and Functional Properties of Underutilised Cowpea and Moth Bean Starches. Foods, 14(21), 3647. https://doi.org/10.3390/foods14213647







