Abstract
This study deciphered the aroma differences in three Kadsura coccinea cultivars (F023, F054, F055) through integrated volatile-omics and sensory analysis. HS-SPME-GC-MS identified 49 volatiles dominated by sesquiterpenes (65.2–78.4%). ROAV prioritization revealed cultivar-specific drivers: γ-dodecalactone (ROAV = 73.0) defined F054’s fruity–floral character; humulene (ROAV = 100) and β-caryophyllene shaped F023’s woody–pungent profile; and β-pinene (ROAV = 100) characterized F055’s herbaceous freshness. Molecular docking confirmed high-affinity binding of γ-dodecalactone to OR2W1 (ΔG = −6.42 kcal/mol via ASN155 H-bonding). Sensory PCA explained 83.48% of the variance, segregating cultivars along distinct axes (F054: sweet-aromatic; F023: woody-spicy; F055: herbaceous-fresh). Joint PCA validated γ-dodecalactone–coconut milk spatial co-localization (θ < 10°) and β-caryophyllene–woody note correlations (r > 0.9), establishing γ-dodecalactone as a breeding biomarker for aroma-driven cultivar improvement.
1. Introduction
Kadsura coccinea (Lem.) A. C. Smith (KC), a rare woody liana of the family Schisandraceae, is primarily distributed in southern China with sparse populations documented in Vietnam. In traditional ethnic medicine systems of China, its roots and vines have been utilized to treat gastrointestinal inflammation, dysmenorrhea, and arthralgia [1,2,3], with lignans and triterpenoids identified as the principal bioactive constituents [4]. The mature fruit presents as an aggregate structure exhibiting pink to dark red coloration, characterized by a distinctive aroma described as a “sweet, apple-like fragrance with cooling undertones” [5]. While historically consumed primarily by indigenous communities within its native range, the fruit has garnered broader consumer interest due to its striking morphological appearance (colloquially termed “devil fruit”). The species demonstrates remarkable environmental resilience, thriving in acidic sandy loam soils with tolerance to thermal extremes (−15 °C to >40 °C). Minimal cultivation management is required beyond pollination and irrigation, facilitating the recent expansion of commercial cultivation. The edible pulp possesses a sweet–sour sensory profile and is nutritionally enriched with vitamins, carbohydrates, and essential amino acids [6], earning the designation “fruit of longevity and beauty” among Hmong people. Contemporary phytochemical analyses confirm high concentrations of polysaccharides and polyphenols in the fruit, correlating with significant antioxidant capacity [7,8,9].
As an emerging specialty fruit, research on KC fruits has predominantly focused on nutritional profiles and health-promoting properties. However, with escalating consumer demand for fruit products, olfactory characteristics have emerged as a primary driver of consumer preference alongside taste attributes [10,11]. Concurrently, aroma compounds serve as critical quality indicators in KC’s traditional medicinal applications, where more intense aromas correlate with enhanced therapeutic efficacy—a key parameter in ethnopharmacological evaluations. Volatile aroma compounds represent essential metabolites in fruits, comprising a diverse array of chemical classes such as esters, aldehydes, alcohols, terpenoids, and ketones. The distinctive flavour of fruit is largely governed by the precise composition and abundance of these volatile substances, which collectively contribute to the unique aromatic characteristics that differentiate various fruit cultivars [12,13]. A single ethnopharmacological analysis identified 85 compounds in hydro distilled essential oils, revealing dominant sesquiterpenes and oxygenated sesquiterpenoids (e.g., β-caryophyllene, γ-amorphene) in peel/pulp, while seeds contained primarily aliphatic fatty acids (e.g., n-hexadecanoic acid, linoleic acid) [14]. Hydro distillation of KC vegetative tissues highlightedα/β-pinene as the major constituents in roots, stems, and leaves [15]. However, these studies have focused on biological activities. Some key flavour volatiles exhibit significant divergence across cultivars and ripening stages [16,17]. A prior work on related omija fruits (e.g., Schisandra chinensis) identified α-pinene, α-terpinene, and (E)-β-ocimene as primary discriminators of inter-freezing treatments aroma differences [18]. However, no systematic analysis exists correlating volatile composition with sensory attributes across KC cultivars.
Traditional techniques for extracting volatile compounds, primarily steam distillation and solvent extraction, have been historically employed for the isolation of aroma substances [19,20]. However, these conventional approaches are hampered by several intrinsic limitations, including the thermal degradation or alteration of heat-sensitive compounds, substantial sample requirements leading to low efficiency, and a reliance on organic solvents that raise environmental concerns. In recent years, Headspace Solid-Phase Microextraction (HS-SPME) has gained widespread adoption for the extraction of fruit aroma compounds. Compared to conventional methods, HS-SPME offers significant advantages, including high sensitivity, low costs, solvent-free operation, and procedural simplicity [21]. When coupled with Gas Chromatography–Mass Spectrometry (GC-MS), HS-SPME has become a powerful and routine tool for the identification and characterization of aroma profiles. This technique’s efficacy is demonstrated in diverse studies [22,23]. It is critical to emphasize the fundamental distinction between HS-SPME and exhaustive extraction techniques such as hydrodistillation. HS-SPME is a non-exhaustive, equilibrium-based sampling method designed to capture the volatile compounds readily released into the headspace under ambient or mild heating conditions, thereby providing a highly accurate representation of the aroma profile perceived by the human olfactory system [24]. This makes it the ideal choice for sensory-driven studies aiming to characterize the authentic, fresh aroma of biological samples without inducing thermal degradation or artifactual formation of compounds. In contrast, hydrodistillation is an exhaustive preparative technique designed to isolate the complete volatile fraction, including high-boiling-point compounds, through prolonged heating and co-distillation with water, resulting in an essential oil (EO) [25]. While hydrodistillation is indispensable for obtaining substantial quantities of material for downstream commercial applications (e.g., flavorant production, bioactivity testing, or product standardization), the aggressive thermal process can alter the native volatile profile through degradation, isomerization, or hydrolysis of heat-labile constituents [26]. Consequently, the VOC profiles generated by these two methods are inherently divergent and serve different purposes; HS-SPME reflects the sensorially relevant aroma, while hydrodistillation provides a complete extract for material production. Furthermore, HS-SPME transcends mere compound identification by enabling the dynamic monitoring of volatile compound behaviour under various conditions, including storage, processing, or environmental stress [27,28]. For instance, a recent study by López et al. effectively employed GC-MS to track the oxidative stability and molecular modifications of volatile compounds from oregano and hop essential oils in sunflower oil subjected to prolonged high-temperature heating (150 °C for 8 h) [29]. Their work demonstrated how key terpenes, such as terpinen-4-ol and β-myrcene, were monitored throughout the thermal process, revealing their persistence and contribution to antioxidant protection. This aligns perfectly with the capability of HS-SPME to monitor temporal changes, degradation kinetics, and transformation pathways of aroma-active molecules, making it a powerful tool for investigating postharvest physiology, processing impacts, and shelf-life stability.
While HS-SPME-GC-MS enables precise characterization of volatile profiles, determining the sensory significance of identified compounds requires evaluating their perceptual impact. The Odor Activity Value (OAV), defined as the ratio of a compound’s concentration to its ortho nasal detection threshold [30], provides a quantitative metric to prioritize aroma-active compounds. The strategic selection and breeding of cultivars represent a critical endeavour in agricultural science, enabling the systematic enhancement of commercially and nutritionally significant traits. This approach is paramount for developing tailored varieties that meet evolving consumer preferences, environmental challenges, and market opportunities.
However, a comprehensive and sensory-driven characterization of the aroma-active compounds responsible for the distinct flavour profiles across different KC cultivars remains unexplored. Specifically, the key odorants defining cultivar uniqueness and their potential interactions with olfactory receptors have not been elucidated, creating a knowledge gap that significantly hinders targeted quality improvement through breeding programs. To address this, the present study was designed with three interconnected objectives: first, to characterize and compare the key aroma-active compounds in three distinct cultivars of KC fruits using an integrated approach combining HS-SPME/GC–MS with ROAV analysis; second, to elucidate the interaction mechanisms between identified key aroma compounds and human olfactory receptors through molecular docking simulations, thereby providing a molecular-level understanding of perceived aroma attributes; and third, to correlate instrumental aroma analysis with sensory evaluation results, establishing holistic aroma profiles for each cultivar and identifying potential biomarkers for sensory-driven quality breeding. We hypothesize that significant differences exist in the type and concentration of key aroma-active compounds among cultivars, and that these differences can be explained at the molecular level through specific ligand-receptor interactions. Ultimately, this research aims to establish a scientific foundation for the quality assessment and genetic improvement of KC as an emerging fruit crop with significant commercial potential.
2. Materials and Methods
2.1. Plant Materials
The plant materials utilized in this study were selected from germplasm resources collected and maintained by our research group. Mature fruits of three KC accessions (F023, F054, F055) were harvested from their respective production regions during the October-November period of the 2023 growing season. To ensure representativeness, fruits were collected from at least 8 healthy adult plants per accession. The average fruit weight ranged from 0.6 to 0.8 kg across accessions, with F023, F054, and F055 exhibiting comparable sizes within this range. Morphologically, the fruits are aggregate structures with an irregular spherical shape and a rough skin texture. The diameter varies from 10 to 15 cm. The skin coloration is accession-specific: F023 exhibits bright red, F054 displays purplish red, and F055 features dark red hues. The flesh is white and juicy, containing multiple embedded seeds. All plant materials were taxonomically authenticated as Kadsura coccinea (Lem.) A.C. Smith, a species within the genus Kadsuraof the family Schisandraceae, by Associate Research Fellow Xujun Wang of the Hunan Academy of Forestry Sciences.
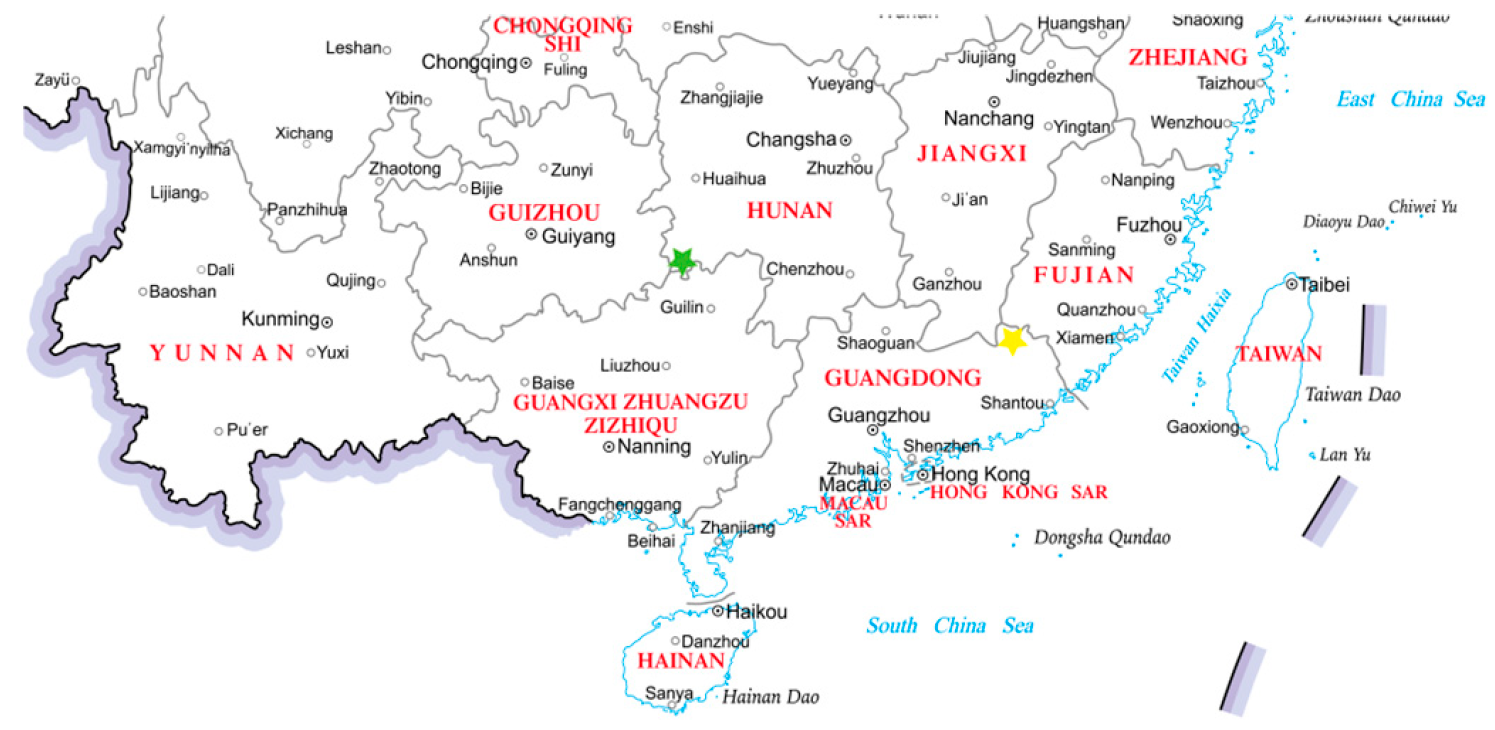
The two production regions are situated in distinct agro-climatic zones, which may contribute to phenotypic and phytochemical variations among the accessions. Tongdao County, Hunan (F023, F054) is characterized by a mid-subtropical monsoon climate with an average annual temperature of 16.3 °C and abundant rainfall (≈1300 mm). Its acidic red soil (pH 4.5–5.5) is typical of the hilly terrain of southern China. In contrast, Pingyuan County, Guangdong (F055) experiences a southern subtropical climate with higher average annual temperatures (20.8 °C) and significantly greater precipitation (≈1600 mm). The region features lateritic red soils with slightly higher pH (5.0–6.0) and distinct mineral profiles. Geographical distribution of sampling locations was shown in Figure 1. While a comprehensive soil and climate analysis across all cultivation sites was beyond the scope of this study, these known environmental differences provide an important context for interpreting the observed chemotypic variations in volatile profiles among the accessions. Consequently, the findings of this study reflect the combined influence of genetic background and potential environmental effects, offering a realistic representation of the fruit’s aroma composition under diverse growing conditions.
Figure 1.
Regional map of sample collection sites. Yellow star denotes Pingyuan County, while green star represents Tongdao County.
Based on systematic sensory evaluation conducted by our research team, the three accessions exhibited significant divergence in aroma intensity: F023 was characterized by a relatively weak aroma, F054 presented a moderate aromatic profile, while F055 demonstrated the most intense and complex fragrance. Notably, F054 is the predominant cultivar in current commercial production and constitutes the major genotype circulating in the marketplace.
2.2. Sample Preparation
Fresh KC tissues were harvested from remote production sites and transported to the laboratory under refrigerated conditions (4 °C with ice packs) to minimize metabolic activity during transit. The transport duration was approximately 7 h due to geographical distances. Upon arrival, samples were immediately subjected to cryogenic grinding with a liquid nitrogen-cooled cryogenic planetary ball mill (QM-2L, JieChenLab, Shaoxing, China). This approach preserves thermo-labile aroma constituents by instantaneously halting enzymatic activity and suppressing molecular mobility. Powders were freeze-dried at −50 °C/0.1 mbar for 72 h to preserve thermo-labile volatiles, referencing stability benchmarks for lychee matrices [31]. Dried samples were immediately transferred to gas-impermeable laminated bags with oxygen scavengers (Ageless® ZP series, Mitsubishi Gas Chemical, Japan), flushed with nitrogen, and sealed under negative pressure. Each bag was assigned a unique alphanumeric code to ensure traceability. A homogenized QC blend was prepared by combining equal masses of powdered materials from fruits of three accessions (F023, F054, F055). The mixture underwent vortex blending (3000 rpm, 10 min) followed by sonication-assisted homogenization (40 kHz, 15 min) to achieve particle size uniformity (D90 < 50 μm). For headspace analysis, 0.5 g (±0.001 g) of each sample and the QC blend were precisely weighed into silanized glass vials.
2.3. HS-SPME-GC-MS Analysis
The SPME fibre assembly (50/30 μm DVB/CAR/PDMS, Anpel, Shanghai, China) was conditioned by inserting it into the GC injector port (240 °C) for 40 min prior to initial use to remove contaminants. For extraction, 0.5 g of cryogenically ground sample was placed in a headspace vial. The vial was incubated at 50 °C for 40 min to achieve matrix-volatile equilibrium. Chromatographic separation was performed using GC-MS spectrometer (QP2010, Shimadzu, Tokyo, Japan) equipped with an HP-88 capillary column (100 m × 0.25 mm, 0.20 μm; Agilent, Santa Clara, CA, USA) with helium (99.999% purity) as carrier gas at a constant flow of 1.37 mL/min. The injector temperature was maintained at 240 °C in split mode (split ratio 5:1). The oven temperature program was as follows: 60 °C (hold 5 min), ramp to 140 °C at 3 °C/min (hold 5 min), then to 210 °C at 5 °C/min (hold 10 min), and finally to 240 °C at 5 °C/min (hold 10 min). The transfer line temperature was 220 °C. Mass spectrometric detection employed electron ionization (70 eV) at 200 °C source temperature. Full-scan data acquisition (m/z 50–600) at 0.50 scans/s was performed with solvent delay of 9.5 min. Compound identification was achieved by comparing mass spectra with NIST 14, 14s, 17, and 17s libraries (match factor > 800). All experimental procedures were performed in triplicate to ensure statistical robustness and reproducibility. The relative abundance of each volatile compound was quantified using the peak area normalization method, whereby the integrated chromatographic peak area of individual compounds was expressed as a percentage of the total peak area across all detected volatiles.
2.4. Relative Odour Activity Value Assessment
Odor threshold values (OTs) were obtained from the VCF Online 16.10 database (https://www.vcf-online.nl/VcfHome.cfm, accessed on 15 September 2025). Thresholds in air (OA) were prioritized for use in this study. When unavailable, odour thresholds in water (OW) were applied. For compounds with multiple reported values, the arithmetic mean was used as the final representative threshold. The contribution of individual volatile compounds to the overall aroma profile was quantified using the Relative Odor Activity Value (ROAV) approach [32]. This method evaluates the perceptual significance of volatiles by integrating concentration data with olfactory thresholds, defined as
where C%i = the relative concentration of compound i (peak area % via normalization); Ti = the odor threshold of compound i (mg/kg in water); C%max = the highest relative concentration among all volatiles; and Tmax = the odor threshold of the dominant compound. ROAV ≥ 1.0 indicates key aroma compounds (decisive impact on overall flavour); 0.1 ≤ ROAV < 1.0 indicates modifier compounds (nuance-enhancing effects).
2.5. Molecular Docking
Based on the target odorant molecules, ten potential human olfactory receptors (ORs) were selected, namely OR1A1 [33], OR1G1 [34], OR2W1 [35], OR5AN1 [36], OR2AG1 [37], OR10G4 [38], OR51E2 (PDB: 8F76) [39], OR5M3 [40], OR7D4 [41], OR8D1 [34], OR5P3 [42]. Due to the lack of experimentally determined structures for target ORs (except OR51E2), high-confidence AlphaFold2 prediction models were utilized for molecular docking studies [43]. Structural templates for the target proteins were acquired from the UniProt knowledgebase (https://www.uniprot.org/, accessed on 5 September 2025) and the RCSB Protein Data Bank (https://www.rcsb.org/, accessed on 5 September 2025). All ligand structures, in the form of SDF format, were sourced from the PubChem chemical compound database (https://pubchem.ncbi.nlm.nih.gov/, accessed on 5 September 2025). The molecular docking studies were performed using the Schrödinger computational chemistry software suite (release 2021-2). The protein structures were prepared with the Protein Preparation Wizard module. This process involved the assignment of bond orders, addition of hydrogens, creation of disulfide bonds, and optimization of hydrogen bonding networks. The protonation states of ionizable residues were predicted at physiological pH (7.0 ± 2.0) using the integrated PROPKA tool. Ligand structures were prepared using the LigPrep module, which generated possible tautomers, protonation states, and stereoisomers at pH 7.0 ± 2.0. Energy minimization was performed with the OPLS4 force field to ensure proper geometry. The receptor grid generation was conducted using the Receptor Grid Generation tool. The centroid of the co-crystallized ligand (when available) or the predicted binding site residues was used to define the grid box (size: 36 × 36 × 36 Å). Default van der Waals scaling (1.0) and partial charge cutoff (0.25) were applied. Molecular docking was executed using the Glide module with Standard Precision (SP) mode for screening. The docking poses were evaluated based on the docking score. The top-ranked poses were selected for subsequent analysis and visualization using Maestro 12.8 and Protein Ligand Interaction Profiler (PLIP) (https://plip-tool.biotec.tu-dresden.de/plip-web/plip/index, accessed on 10 September 2025) [44].
2.6. Descriptive Sensory Analysis
Written informed consent was obtained from all participants prior to the commencement of the study. The consent form explicitly outlined the study procedures and guaranteed the right to withdraw at any time without penalty. All participant data were anonymized and handled confidentially.
A panel of twelve assessors (6 males, 6 females; aged 22–35 years) was recruited and rigorously screened in accordance with ISO 8586:2012 standards. Screening included basic taste identification and odour detection threshold tests using a series of aqueous solutions and odorants relevant to the KC fruit profile (e.g., citric acid for sourness, linalool for floral notes). Following successful screening, the panel underwent 40 h of standardized training over four weeks to develop a consensus lexicon and scale the intensity of attributes. Seven key sensory attributes were selected to describe the odour intensity of KC fruits (Table 1). Training sessions involved repeated exposure to these reference standards to calibrate the panel’s understanding of each attribute and the full 0–10 intensity scale (0 = absent; 5 = moderate; 10 = extremely strong).

Table 1.
Sensory lexicon generated for descriptive sensory analysis of KC fruit odour.
Individual KC fruits of uniform size were peeled and homogenized. A 10 g aliquot of fruit pulp was placed into 150 mL odourless disposable plastic cups and sealed with a lid. Each cup was labelled with a unique random three-digit code. The coded samples were presented to the assessors in a dedicated sensory laboratory conforming to ISO 8589:2007 standards. The environment was strictly maintained at 25 ± 0.5 °C and 50 ± 5% relative humidity, with positive air pressure and ambient lighting to standardize testing conditions and prevent olfactory fatigue. The QDA protocol required assessors to evaluate each sample and score the perceived intensity of each of the seven attributes on the 0–10 scale. Each assessor evaluated all three cultivars (F023, F054, F055) in a randomized, balanced order across three independent sessions (replications), with a 24-h interval between sessions to prevent carry-over effects. Between samples, evaluators were asked to cleanse their palate with salt-free biscuits and deionized water (20 °C). Paper forms were used to collect data.
2.7. Statistical Analysis
Experimental data represent the mean ± SD of triplicate determinations (n = 3). Significant differences (p < 0.05) among groups were evaluated by one-way ANOVA followed by Duncan’s or Turkey’s post hoc test using SPSS 26.0.
3. Results and Discussion
3.1. VOCs Analysis in Fruits
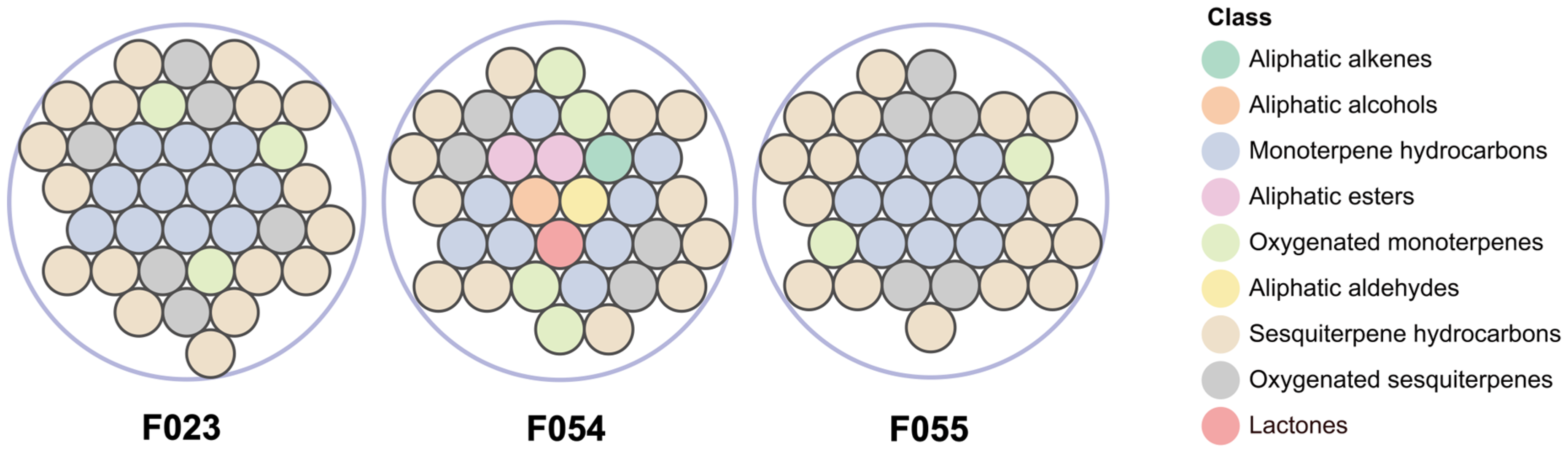
HS-SPME-GC-MS analysis identified 49 VOCs across three KC accessions (F023, F054, F055), categorized into 9 chemical classes based on structural characteristics (Figure 2): 12 monoterpene hydrocarbons, 20 sesquiterpene hydrocarbons, 11 oxygenated monoterpenes, 2 aliphatic esters, 1 aliphatic alkene, 1 aliphatic alcohol, 1 aliphatic aldehyde, and 1 lactone. A total of 19 volatile compounds were shared among the three accessions. Accession F054 contained 7 unique constituents, including 6 non-terpenoid compounds. Accessions F055 and F023 exhibited 4 and 1 accession-specific terpenoids, respectively (Table 2). The current investigation identified 49 volatile compounds, with 20 demonstrating substantial overlap with prior reports [14], including characteristic terpenoids such as α-pinene, β-pinene, α-terpinene, and β-caryophyllene. Divergently, two high-abundance compounds previously detected in fruit pulp—γ-curcumene (12.55%) and α-bisabolol (6.17%)—were not observed. This discrepancy arises from: (i) the whole-fruit homogenization approach diluting pulp-specific volatiles, thereby reducing headspace concentrations below detection thresholds; and (ii) the limited volatility of high-boiling-point compounds (e.g., γ-curcumene; b.p. 265–268 °C) under HS-SPME conditions, contrasting with hydro-distillation’s efficacy in recovering such thermostable terpenes. Although cross-study comparisons remain constrained by methodological disparities (e.g., hydro-distillation vs. HS-SPME) and potential genotypic differences, the consistent detection of fruit-like volatiles—specifically aliphatic esters and lactones—exclusively in F054 across biological replicates confirms its unique chemotype. This accession-specific signature suggests a genetically encoded metabolic predisposition potentially amplified by post-harvest enzymatic activity, independent of extraction artifacts [45].
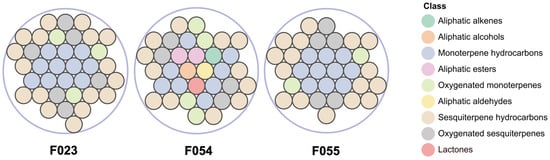
Figure 2.
Comparative analysis of VOC categories among three KC accessions. Total compounds identified: F023 (37), F054 (34), F055 (33).

Table 2.
Volatile organic compound composition in fruits of different KC accessions.
Our results position F054 as a sensorially distinct cultivar characterized by six signature volatiles. The sensory impact of these compounds follows a synergistic model: butyl butanoate and butyl caproate establish the primary fruity frame (pineapple and apple) [46,47], while γ-dodecalactone introduces lactone-derived sweetness (milky, creamy and coconut) that exceeds the additive effects of individual components [48]. The C9 aldehyde (nonanal) operates sub-threshold as a flavour modifier, amplifying perceived complexity through waxy-citrus interactions [49]. Meanwhile, the C6 alcohol contribute green-herbaceous accents that complete the aroma profile [50,51]. Although 1,3,5-heptatriene has not been definitively linked to specific flavour attributes in any study, its methylated derivatives are recurrently identified in volatile profiles of herbal species and are postulated to impart herbaceous and floral nuances to complex aroma matrices [52,53]. The dominance of γ-dodecalactone in F054 presents a striking parallel to its role in strawberry [54], yet its occurrence in Schisandraceae fruits is unprecedented. Prior studies on related Schisandra chinensis identified α-pinene and limonene as key volatiles [18], but lactones were conspicuously absent—highlighting a unique biosynthetic pathway activation in KC. This divergence may arise from differential expression of enzymes related to lactone synthesis, suggesting KC-specific genetic regulation. Experimental evidence confirms that 9-lipoxygenase and epoxide hydrolase 2 genes directly regulate the biosynthetic flux of mango lactones through the oxylipin pathway, significantly altering their endogenous accumulation levels [55]. Transcriptional profiling confirms that FaFAD1modulates the flux of hydroxyl fatty acid precursors in the lactone biosynthesis pathway, directly governing γ-decalactone (C10) and γ-dodecalactone (C12) accumulation in strawberry [56,57,58].
Quantitative results show that sesquiterpene hydrocarbons constituted the most abundant chemical class across all accessions, ranging from 65.2% to 78.4% of total VOC abundance. F023 showed the highest sesquiterpene accumulation (78.4%), primarily driven by exceptionally high β-caryophyllene (34.02%) and humulene (0.19%) levels. These two compounds exhibited a progressively decreasing trend across the accessions (F023→F054→F055), demonstrating a statistically significant negative correlation with the aromatic evolution pattern of KC fruits (p < 0.001). F054 exhibited dual terpenoid/non-terpenoid profile with elevated α-pinene (9.24%) and non-terpenoid compounds (totalling 1.03%). F055 showed distinct sesquiterpene pattern with high γ-maaliene (12.50%) and β-elemene (9.73%), and they showed an increasing trend and were positively correlated with odour intensity (p < 0.001). In F055, six quantitatively dominant VOCs (β-selinene: 10.87%, γ-maaliene: 12.50%, β-pinene: 10.32%, γ-muurolene: 10.37%, β-elemene: 9.73%, and β-Caryophyllene: 12.80%) collectively constitute 66.59% of total volatiles. Their synergistic interactions, particularly between sesquiterpenes, likely underlie the complex, intense fragrance (Section 2.1). The antagonism between β-caryophyllene (F023-dominant) and β-pinene (F055-dominant) suggests competitive inhibition in terpene synthase pathways. While such metabolite trade-offs are documented in grape, tomato and freesia [59,60,61], our report provides the first evidence in Schisandraceae. β-Caryophyllene and β-pinene engage in metabolic antagonism through competition for the shared IPP/DMAPP precursor pool and membrane-bound catalytic sites. Their flux partitioning is co-determined by the FDPS/GPPS enzymatic activity ratio and cultivar-specific environmental adaptations [62,63]. This biochemical divergence likely reflects adaptive responses to native environments. F055’s Guangdong provenance (high humidity and temperature) may favour the expression and release of monoterpene oxidation products [64], whereas F023’s Hunan highland origin selects for sesquiterpene-based cold resistance [65]. A critical question arising from these findings is whether the distinct chemotypic profiles of the three cultivars are driven primarily by genetic differences or by environmental influences (e.g., climate, soil) of their respective growing regions. While the potential contribution of environmental factors cannot be entirely ruled out, the evidence suggests that genetic background is the predominant driver. The most compelling support for this conclusion comes from the comparison between F023 and F054, which were grown in the same geographical location (Tongdao County, Hunan) and were therefore subjected to identical climatic and edaphic conditions. Despite this environmental uniformity, their volatile compositions were fundamentally different: F023 exhibited a sesquiterpene-dominant (e.g., β-caryophyllene) and woody–pungent profile, whereas F054 was characterized by a fruity–floral signature rich in esters and lactones (e.g., γ-dodecalactone). This stark contrast under a shared environment strongly indicates that the divergence is under robust genetic control, likely involving differential expression or activity of key biosynthetic enzymes.
The role of environment may be more modulatory, potentially explaining certain quantitative variations, such as the elevated levels of monoterpene oxidation products in F055, which was grown in the warmer and more humid climate of Guangdong Province. To definitively apportion the variance between genetic and environmental effects, future studies should employ a common garden experimental design, cultivating all genotypes in a controlled environment or across multiple locations.
3.2. ROAV-Driven Comparative Analysis of Odour Activity Characteristics Among Varieties
ROAV analysis was employed to decipher the contribution of individual volatile compounds to the overall aroma profile of each KC accession. Compounds with ROAV ≥ 1 were defined as key aroma contributors, while those with ROAV = 0 were sensorially negligible (either absent or below perceptual thresholds). The contribution of VOCs in KC fruit to overall odour is shown in Table 3. F023 exhibited a warm, resinous-woody character dominated by humulene (ROAV = 100.0; odour threshold: 0.16 mg/m3), which imparts balsamic and earthy notes, alongside β-myrcene (ROAV = 97.6; threshold: 0.061 mg/m3) contributing herbal-fruity nuances. This dominance suggests high terpenoid synthase activity in F023 [66]. The high ROAV of D-limonene (31.1) further added a subtle citrus undertone, absent in other accessions. F054 demonstrated a fruity–floral signature, primarily driven by β-myrcene (ROAV = 100.0) and γ-dodecalactone (ROAV = 73.0; threshold: 0.0048 mg/m3), whose ultra-low threshold explains its outsized sensory impact despite moderate concentration (0.24%). Butyl butanoate (ROAV = 18.8) and linalool (ROAV = 23.6) amplified the fruity and floral layers, synergistically reinforcing the “fruity-sweet” character. Notably, γ-dodecalactone—undetected in F023/F055—may serve as a chemotaxonomic marker for F054. F055 was characterized by a fresh herbaceous-pine profile, with β-pinene (ROAV = 100.0; threshold: 0.14 mg/m3) as the dominant contributor, delivering pine and resinous notes. β-Myrcene (ROAV = 88.5) provided supporting green-herbal accents, while β-phellandrene (ROAV = 4.2; detected only in F055) added minty nuances. This correlates with the “fresh-green” sensory evaluation. Cross-variety patterns revealed β-Myrcene was a universal high-impact compound across all accessions (ROAV > 88), attributed to its exceptionally low threshold (0.061 mg/m3), enabling dominance even at sub-percentage concentrations; Humulene showed accession-dependent effects, via dominant in F023 (ROAV = 100) but moderate in F054/F055 (ROAV = 51.9/31.0), potentially linked to varietal terpene synthase isoforms; Synergistic interactions were evident in F055, where β-pinene and β-myrcene collectively accounted for 188.5% of the top ROAV, suggesting additive potentiation of herbaceous notes. ROAV analysis resolved discrepancies between compound abundance and sensory impact. For instance, β-Caryophyllene showed high concentration in F023 (34.02%) but moderate ROAV (35.6), indicating limited perceptual influence despite quantitative dominance; Nonanal (ROAV = 0.8 in F054) and 1-hexanol (ROAV = 0.3) acted as background modifiers but were non-decisive individually. Their collective role in aroma rounding warrants further study. Accession F054 is identified as a premium candidate for targeted breeding and commercialization, owing to its genetically encoded synthesis of fruity-scented volatiles (e.g., γ-dodecalactone, butyl esters) and well-balanced terpenoid backbone.

Table 3.
Odour thresholds, ROAVs across varieties, and sensory profiles of volatile compounds.
3.3. Molecular Docking Elucidates Binding Patterns of Key Odorants
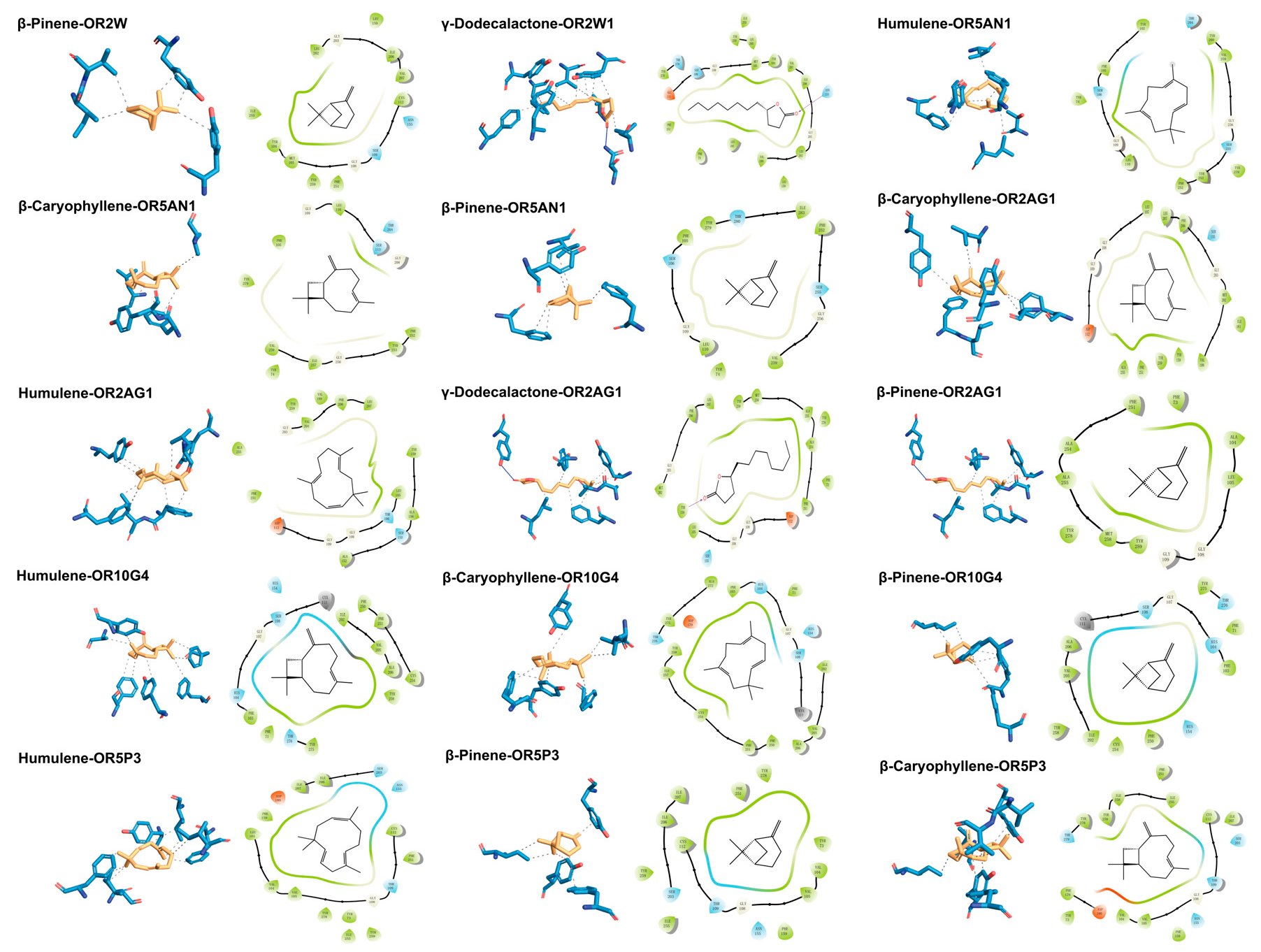
To mechanistically decipher the odour contribution of ROAV-identified key compounds, molecular docking simulations were performed between eight target odorants—five shared across cultivars (β-Pinene, β-Myrcene, β-Ocimene, β-Caryophyllene, and Humulene) and three unique to F054 (Butyl butanoate, Linalool, γ-Dodecalactone)—and 11 human ORs. The docking protocol followed Section 2.5, with binding energies < −5 kcal/mol indicating stable complexes (Wu et al., 2025) [67]. Shared terpenoids exhibited superior binding affinity. β-Pinene, Humulene, and β-Caryophyllene showed the lowest binding energies (range: −7.439 to −5.875 kcal/mol), forming stable complexes with multiple ORs (e.g., OR2AG1, OR5AN1, OR10G4) (Table 4). This multi-receptor engagement suggests their role as universal chemical cues for KC’s core flavour profile. OR5AN1 is currently the only olfactory receptor exclusively reported to recognize musky odorants, with Tyr102−Tyr279 identified as its key recognition residues. Humulene, β-caryophyllene, and β-pinene—structurally analogous to musky compounds due to their macrocyclic/polycyclic scaffolds—demonstrate binding to OR5AN1’s ligand pocket, engaging critical residues including Phe105 and Tyr279 in molecular docking simulations [36]. γ-Dodecalactone uniquely formed hydrogen bonds. As the sole F054-specific compound with significant ROAV (73.0), it established H-bonds with ASN155 of OR2W1 (binding energy: −6.422 kcal/mol), potentially underpinning its cultivar-distinct sensory impact. OR2W1 is recognized as the olfactory receptor with the broadest ligand recognition spectrum reported to date. Our molecular docking results demonstrate that γ-dodecalactone forms a high-affinity hydrogen bond with ASN155 of OR2W1, providing mechanistic validation beyond empirical correlation. Complementary in vitro functional assays confirm OR2W1’s specificity for lactone compounds, with cell-based agonist screening identifying γ-lactones as potent activators (75% hit rate) [35]. Hydrophobic interactions dominated binding mechanisms. Across all 15 stable complexes, hydrophobic packing accounted for 86.7% of interactions (13 complexes), involving residues such as TYR104, PHE251, and VAL207. This aligns with the hydrophobic nature of both odorants and OR binding pockets [36,68,69]. Visualization of high-affinity complexes confirmed these patterns (Figure 3). For instance, β-Pinene occupied a hydrophobic sub-pocket of OR2W1 via hydrophobic Interaction contacts with TYR104, ILE206, VAL207 and TYR259; γ-Dodecalactone adopted a pose where its lactone ring formed H-bonds with ASN155 while its alkyl chain engaged in hydrophobic stacking with PHE73, TYR104, LEU159, VAL207, PHE251, ILE255, TYR259 and TYR278. These structural insights provide a molecular basis for the ROAV-driven odorant prioritization, highlighting how shared terpenoids stabilize KC’s foundational aroma, while γ-dodecalactone drives F054’s uniqueness through dual interaction modes.

Table 4.
Molecular docking results of key odorants (ROAV > 1) from cultivar F054 with human odorant receptors: shared with and unique to the other two cultivars.
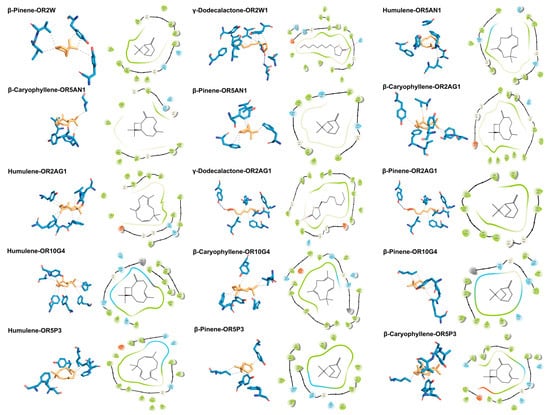
Figure 3.
Two-dimensional and 3D binding poses of olfactory receptors with key odorant molecules; 3D poses generated by PLIP: hydrophobic interactions (black dashed lines), hydrogen bonds (dark blue solid lines); 2D poses generated by Maestro: hydrogen bonds (rose red arrows).
3.4. Multivariate Analysis of Sensory Profiles and Volatile Compounds in KC Cultivars
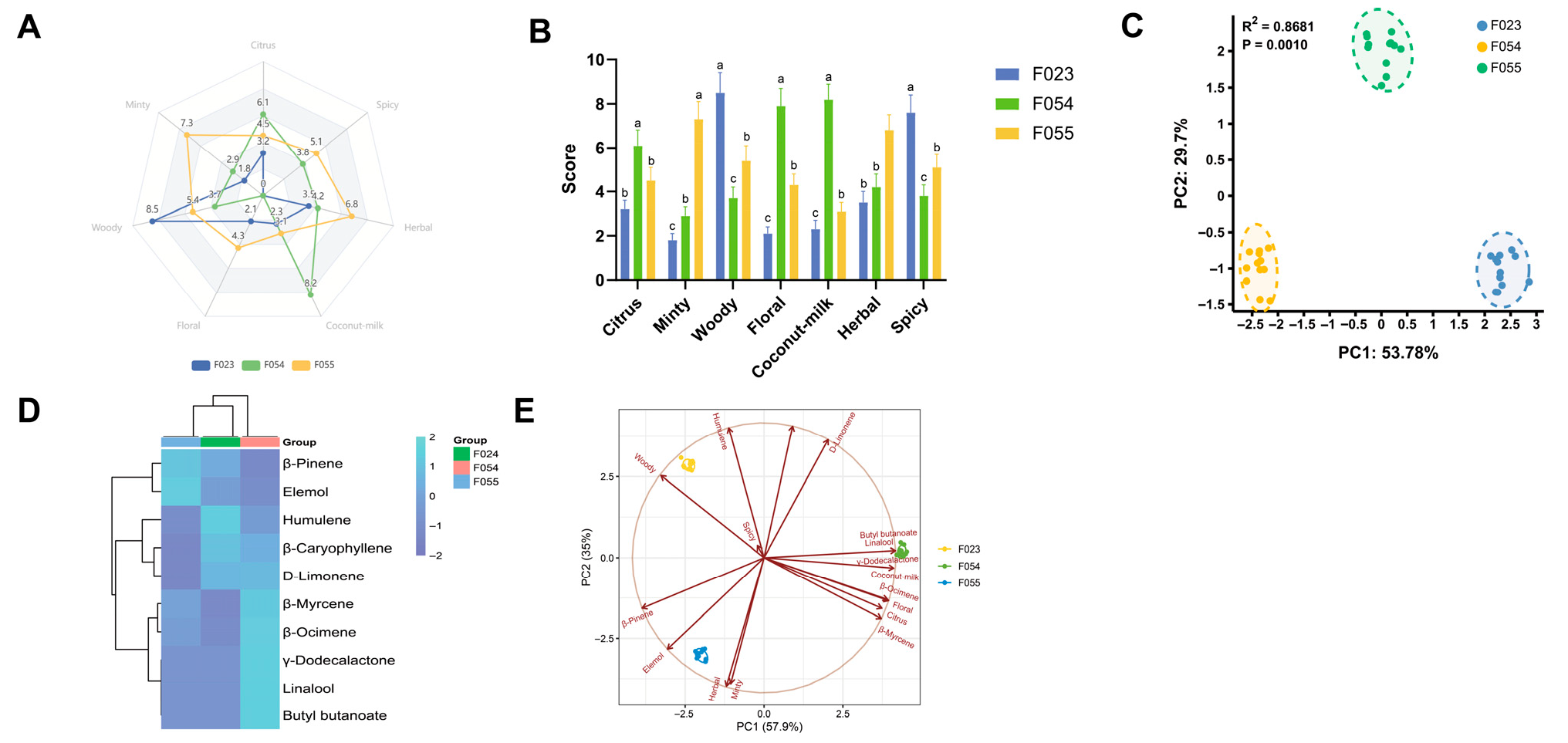
The flavour characteristics of three KC cultivars (F023, F054, F055) were systematically evaluated through descriptive sensory analysis and principal component analysis (PCA), revealing distinct flavour profiles and their underlying chemical drivers. Sensory attribute intensity patterns visualized through radar plot (Figure 4A) demonstrated cultivar-specific dominance: F054 exhibited pronounced coconut-milk and floral attributes (scores 8.2 ± 0.7 and 7.9 ± 0.8, respectively), establishing its “sweet-aromatic” signature. F055 showed dominance in citrus and minty dimensions (7.3 ± 0.8 and 6.8 ± 0.7), characterizing its “fresh-fruity” profile. F023 displayed elevated woody and spicy intensities (8.5 ± 0.9 and 7.6 ± 0.8) with minimal fruity notes, defining its “woody-pungent” character. Statistical validation through ANOVA with Tukey’s post hoc test (Figure 4B) confirmed significant inter-cultivar differences (p < 0.001) across all attributes. Coconut-milk intensity followed F054 > F055 ≈ F023 (a, b, c grouping), while Woody intensity showed F023 > F055 > F054 (a, b, c grouping). This clear segregation indicates genetic regulation of flavour compound biosynthesis pathways.
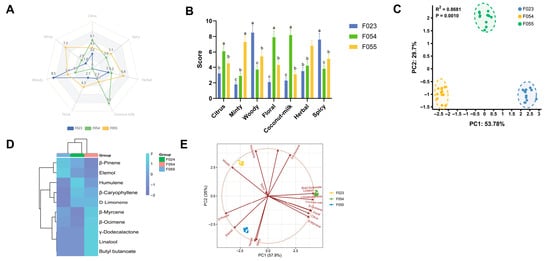
Figure 4.
Sensory attribute analysis of three kc varieties. (A) Radar map of sensory attribute descriptors; (B) Difference analysis of sensory attributes; (C) PCA of sensory attributes of different KC varieties; (D) Cluster Heatmap of Key Aroma Compounds in Three KC Cultivars; (E) PCA loading plot for sensory attributes combined with key odour molecules.
PCA of sensory attributes (Figure 4C) explained 83.48% of the total variance (PC1: 53.78%, PC2: 29.7%) with high statistical significance (p = 0.0010). The score plot revealed F054 clustering along the PC1-positive axis versus F023 along the negative axis, indicating orthogonal flavour profiles. F055 separated along PC2, suggesting that its unique flavour architecture is distinct from the PC1-driven “sweet-woody” continuum. The cluster heatmap analysis, based on Z-score normalized values, provided a comprehensive visualization of the relative abundance patterns of key aroma-active compounds across three distinct KC cultivars (Figure 4D). The analysis revealed pronounced differences in the expression profiles of terpenoid compounds, which emerged as the primary discriminators among cultivars. β-Pinene exhibited markedly elevated expression in cultivar F055, characterizing it as a signature compound for this genotype, whereas it showed suppressed levels in F023 and F054. Conversely, eucalyptol demonstrated highest abundance in F023, suggesting its role as a potential biomarker for this cultivar, with minimal expression in F055. Humulene displayed relatively uniform expression across all cultivars but with a slight elevation in F054, indicating moderate contribution to its aroma profile. In contrast, ester compounds, such as γ-dodecalactone and butyl butanoate, displayed more conserved expression patterns; γ-dodecalactone was consistently low across all cultivars, forming a distinct cluster separate from terpenoids, which implies divergent regulatory mechanisms or biosynthetic pathways. Butyl butanoate showed a subtle increase in F054 but remained near mean levels in others. Additional compounds, including aldehydes and alcohols like hexanal and phenethyl alcohol, exhibited minimal variation, indicating their secondary role in cultivar differentiation. Hierarchical clustering of compounds (row-wise) yielded two primary clusters: one encompassing most terpenoids (e.g., β-pinene, eucalyptol, humulene) with similar expression profiles, suggesting co-regulation under shared biosynthetic pathways, and another comprising esters (e.g., γ-dodecalactone) with distinct behaviour, reinforcing the metabolic divergence between these compound classes. Cultivar clustering (column-wise) showed that F054 and F055 grouped together first, subsequently clustering with F024, indicating that F054 and F055 share more similar aroma compound compositions, while F024 possesses a unique chemotype. This clustering pattern aligns with prior PCA results, corroborating the phenotypic divergence observed in sensory and chemical analyses. Joint PCA loading plot (Figure 4E) integrating sensory and chemical data revealed compound-attribute correlations driving cultivar differentiation. γ-Dodecalactone showed vector alignment with coconut-milk attribute (θ < 10°), confirming its role as key odorant for F054’s signature flavour, consistent with prior ROAV analysis (ROAV = 73.0) and molecular docking results (−6.422 kcal/mol binding energy). Similarly, β-Caryophyllene spatially correlated with woody attribute (r > 0.9), explaining F023’s woody character. The combined model explained 83.5% variance (PC1: 57.9% + PC2: 25.6%), demonstrating enhanced explanatory power through chemical data integration. The covariance patterns in loading plots suggest modular organization of flavour traits: “coconut-floral” (F054), “citrus-minty” (F055), and “woody-spicy” (F023) modules likely reflect coordinated regulation of biosynthetic pathways. PC1 appears governed by genetic background regulating terpene synthase expression, while PC2 may involve environment-responsive pathways (e.g., monoterpene oxidation in F055’s growing region). Practical implications include using PC1 > 0.5 with γ-dodecalactone dominance as selection criterion for sweet-aromatic cultivars. Future research should employ GC-O-MS to identify odour-active compounds corresponding to each sensory vector and conduct consumer acceptance tests to validate preference patterns. In conclusion, multivariate analysis revealed three distinct flavour phenotypes in KC cultivars governed by coordinated expression of flavour compound modules. The strong sensory–chemical correlations provide a framework for targeted flavour breeding and product development.
It is important to contextualize the analytical approach taken in this study. The cryogenic grinding and subsequent storage under a nitrogen atmosphere in gas-impermeable packaging were employed to effectively ‘freeze’ the metabolic state of the fruit at the moment of sampling. This stringent preservation method was essential to establish a definitive baseline volatile profile for each cultivar, minimizing post-harvest biochemical changes and oxidative degradation that would otherwise confound the assessment of genotypic differences [70]. Consequently, the aroma profiles reported herein represent the innate potential of each cultivar under ideal preservation conditions.
However, as astutely noted, this controlled environment does not reflect the practical realities of a commercial supply chain, where fruits are typically stored in permeable packaging at ambient or refrigerated temperatures. Under such conditions, the key aroma-active compounds identified—particularly the highly volatile esters (e.g., ethyl butanoate) and oxidation-sensitive terpenes (e.g., β-pinene)—are susceptible to rapid degradation through enzymatic activity, oxidation, and evaporation [71]. Therefore, the sensory experience of the consumer, which is the ultimate determinant of commercial success, will be governed by the stability of this aroma portfolio during postharvest handling. This presents a critical avenue for future research. Our study provides the essential reference point for such investigations. Subsequent work should focus on monitoring the depletion of these key odorants (tracked via OAV) under realistic storage scenarios. Evaluating the efficacy of practical preservation technologies, such as modified atmosphere packaging, edible coatings, or cold chain logistics, in maintaining the desirable aroma attributes of cultivars like F054 will be vital. Ultimately, integrating genotypic selection with optimized postharvest protocols will be key to delivering the unique sensory promise of KC to the consumer.
4. Conclusions
This study establishes that the distinct aroma profiles of KC cultivars arise from genetically encoded volatile signatures, which were validated through integrated chemosensory analysis. F054 exhibits a premium fruity–floral character driven by γ-dodecalactone, which forms high-affinity hydrogen bonds with ASN155 of the olfactory receptor OR2W1, as revealed by molecular docking. F023’s woody–pungent profile correlates with sesquiterpene dominance (humulene), while F055’s herbaceous freshness stems from β-pinene and monoterpene oxides. PCA of sensory and chemical data cumulatively explained 83.5% of the variance. Sensory quantification confirmed F054’s superiority in coconut-milk (8.2 ± 0.7) and floral (7.9 ± 0.8) intensities, contrasting with F023’s peak woody (8.5 ± 0.9) and spicy (7.6 ± 0.8) attributes. Joint PCA loadings spatially co-localized γ-dodecalactone with coconut milk (θ < 10°) and β-caryophyllene with woody notes (r > 0.9), mechanistically linking chemistry to perception. These findings provide actionable targets for flavour breeding: γ-dodecalactone synthase upregulation enhances fruity complexity, while OR2W1-binding affinity screening (ΔG < −6.0 kcal/mol) enables precise selection of high-aroma genotypes, positioning KC as a novel flavour-focused fruit crop.
Author Contributions
Conceptualization, L.W., Q.T. and Z.D.; methodology, R.Z.; software, R.Z. and Z.D.; validation, R.Z., H.W., J.X. and Z.D.; formal analysis, L.W., R.Z. and Z.D.; investigation, L.W., R.Z. and J.X.; resources, Q.T.; data curation, H.W.; writing—original draft, L.W., R.Z. and Z.D.; writing—review & editing, Q.T. and Z.D.; visualization, Z.D.; supervision, H.W., J.X. and Q.T.; project administration, Q.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The primary activity of this study involved descriptive sensory analysis of food products and did not constitute invasive research or medical trials affecting human physiological or psychological functions. All test samples were edible fruits with safety equivalent to conventional foods. The experimental procedure involved only normal sensory tasting and evaluation, presenting minimal risk. Therefore, in accordance with institutional policies and the relevant provisions of the Ethical Review Measures for Life Sciences and Medical Research Involving Humans, this study was granted an exemption from ethics review.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, H.; Wang, L.; Yang, Z.; Kitanaka, S. Kadsuralignans H-K from Kadsura coccinea and Their Nitric Oxide Production Inhibitory Effects. J. Nat. Prod. 2007, 70, 1999–2002. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, L.; Gong, Y.; Zhang, C.; Yang, Y.; Wang, W.; Qin, L. Isovaleroylbinankadsurin A Ameliorates Atherosclerosis and Restenosis by Promoting LXRα Signaling Pathway and Inhibiting TGF-Β1 and FHL1 Signaling Pathways. Phytomedicine 2025, 139, 156451. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Yu, H.; Xie, Q.; Wang, B.; Jiang, S.; Su, W.; Mao, Y.; Li, B.; Peng, C.; et al. Sesquiterpenes from Kadsura coccinea Attenuate Rheumatoid Arthritis-Related Inflammation by Inhibiting the NF-κB and JAK2/STAT3 Signal Pathways. Phytochemistry 2022, 194, 113018. [Google Scholar] [CrossRef]
- Yang, Y.; Hussain, N.; Zhang, L.; Jia, Y.; Jian, Y.; Li, B.; Iqbal Choudhary, M.; Rahman, A.; Wang, W. Kadsura coccinea: A Rich Source of Structurally Diverse and Biologically Important Compounds. Chin. Herbal Med. 2020, 12, 214–223. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Fang, L.; Yang, A.; Luo, F.; Lu, J.; Ren, J. Physicochemical Properties, Profile of Volatiles, Fatty Acids, Lipids and Concomitants from Four Kadsura coccinea Seed Oils. Food Chem. X 2024, 23, 101765. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, W.; Xiong, K.; Li, W. Profiling of nutrient metabolites in Kadsura coccinea fruitand their enrichment patterns in pulp, peel, and seed tissues. Food Ferment. Ind. 2022, 48, 268–275. [Google Scholar] [CrossRef]
- Long, H.; Xia, X.; Liao, S.; Wu, T.; Wang, L.; Chen, Q.; Wei, S.; Gu, X.; Zhu, Z. Physicochemical Characterization and Antioxidant and Hypolipidaemic Activities of a Polysaccharide from the Fruit of Kadsura coccinea (Lem.) A. C. Smith. Front. Nutr. 2022, 9, 903218. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tian, M.; Cheng, Y.; Ji, C.; Hu, S.; Liu, H.; Lu, J.; Ren, J. Effects of Simulated in Vitro Gastrointestinal Digestion on Antioxidant Activities and Potential Bioaccessibility of Phenolic Compounds from K. coccinea Fruits. Front. Nutr. 2022, 9, 1024651. [Google Scholar] [CrossRef] [PubMed]
- Sritalahareuthai, V.; Temviriyanukul, P.; On-Nom, N.; Charoenkiatkul, S.; Suttisansanee, U. Phenolic Profiles, Antioxidant, and Inhibitory Activities of Kadsura heteroclita (Roxb.) Craib and Kadsura coccinea (Lem.) A.C. Sm. Foods 2020, 9, 1222. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Dong, H.; Zhuang, L.; Wu, X.; Wu, B.; Lan, W.; Su, M.; Tao, G.; Xu, B.; Xu, C.; et al. Multi-Omics Approach Reveals Differences in Aroma and Metabolic Characteristics of Two Types of Jackfruits. Food Chem. X 2025, 29, 102806. [Google Scholar] [CrossRef]
- Guan, S.; Yang, F.; Yao, J.; Liu, C.; Wang, R.; Ruan, M.; Yao, Z.; Liu, C.; Wan, H.; Li, Z.; et al. Dynamic Changes in Volatile Organic Compounds of Cherry Tomato Fruits during Storage at Different Temperatures Using HS-GC-IMS. Food Res. Int. 2025, 218, 116790. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhang, X.; Wang, J.; Wu, C.; Wang, W.; Yan, G.; Zhou, Y.; Zhang, K.; Duan, X. Characterization of Volatile Profiles in Cherry Fruits: Integration of E-Nose and HS-SPME-GC–MS. Food Chem. X 2025, 28, 102632. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R. Volatile Flavor Constituents of Acerola (Malpighia Emarginata DC.) Fruit. J. Agric. Food Chem. 2001, 49, 5880–5882. [Google Scholar] [CrossRef]
- Dong, J.; Ma, J.; Yang, W.; Cai, W.; Wu, W. Characterization of the Volatile Profile and Its Estrogenic Activity in Kadsura coccinea Fruit. J. Ethnopharmacol. 2023, 309, 116341. [Google Scholar] [CrossRef]
- Zhao, T.; Ma, C.; Zhu, G. Chemical Composition and Biological Activities of Essential Oils from the Leaves, Stems, and Roots of Kadsura coccinea. Molecules 2021, 26, 6259. [Google Scholar] [CrossRef]
- Jo, S.M.; Moon, H.S.; Hong, S.J.; Yoon, S.; Jeong, H.; Park, H.; Ban, Y.; Youn, M.Y.; Lee, Y.; Park, S.-S.; et al. Exploring Flavor Patterns in the Peel of Tangor: A New Citrus Variety Based on Electronic Sensors and GC-MS/O. Food Chem. 2025, 485, 144415. [Google Scholar] [CrossRef]
- Zheng, X.-D.; Wang, Z.; Tan, M.-N.; Dong, M.; Pan, S.-X.; Cao, N.; Qin, L. A Comprehensive Study on the Mechanism of Apple Flavor Quality Formation: Comparison of Different Apple Cultivars in the Weihai Region. Food Chem. 2025, 489, 144894. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, Y.-Y.; Lee, K.; Jang, H.W. Instrumental Volatile Flavor Analysis of Omija (Schisandra Chinesis Baillon) Using Headspace Stir-Bar Sorptive Extraction-Gas Chromatography-Mass Spectrometry and Its Relationship to Human Sensory Perceptions. Food Res. Int. 2019, 120, 650–655. [Google Scholar] [CrossRef]
- Barroso, A.S.; Massing, L.T.; Suemitsu, C.; Mourão, R.H.V.; Figueiredo, P.L.B.; Maia, J.G.S. Volatile Constituents of Some Myrtaceous Edible and Medicinal Fruits from the Brazilian Amazon. Foods 2024, 13, 1490. [Google Scholar] [CrossRef]
- Hanousek Čiča, K.; Mrvčić, J.; Srečec, S.; Filipan, K.; Blažić, M.; Stanzer, D. Physicochemical and Aromatic Characterization of Carob Macerates Produced by Different Maceration Conditions. Food Sci. Nutr. 2020, 8, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Martínez, J.R. Sampling Volatile Compounds from Natural Products with Headspace/Solid-Phase Micro-Extraction. J. Biochem. Biophys. Methods 2007, 70, 235–242. [Google Scholar] [CrossRef]
- Arango, J.P.B.; Ospina, A.P.; Ladino, J.A.F.; Ocampo, G.T. Volatilomic Analysis in Peel, Pulp and Seed of Hass Avocado (Persea americana Mill.) from the Northern Subregion of Caldas by Gas Chromatography with Mass Spectrometry. Food Sci. Nutr. 2025, 13, e70489. [Google Scholar] [CrossRef]
- Hu, Y.-Y.; Liao, J.-L.; Qian, W.-Z.; Fan, S.-J.; Xiao, X.-Y.; Yang, Y.; Guo, J.-L.; Gao, S. Metabolomics, E-Tongue and HS-SPME-GC-MS Reveal the Smoking Process of Prunus mume: Changes in Flavor and Chemical Compositions. Food Chem. 2025, 484, 144401. [Google Scholar] [CrossRef]
- Burzynski-Chang, E.A.; Ryona, I.; Reisch, B.I.; Gonda, I.; Foolad, M.R.; Giovannoni, J.J.; Sacks, G.L. HS-SPME-GC-MS Analyses of Volatiles in Plant Populations-Quantitating Compound × Individual Matrix Effects. Molecules 2018, 23, 2436. [Google Scholar] [CrossRef]
- Planonth, S.; Chantarasiri, A.; Maitip, J.; Wongkattiya, N.; Noyraksa, S.; Luangkamin, S.; Tanruean, K.; Suttiarporn, P. Green NADES-Based Pretreatment Combined with Microwave-Assisted Hydrodistillation for Enhanced Fennel Essential Oil Production. Molecules 2025, 30, 3734. [Google Scholar] [CrossRef]
- Chang, H.-T.; Lin, C.-Y.; Hsu, L.-S.; Chang, S.-T. Thermal Degradation of Linalool-Chemotype Cinnamomum Osmophloeum Leaf Essential Oil and Its Stabilization by Microencapsulation with β-Cyclodextrin. Molecules 2021, 26, 409. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Chou, C.-Y.; Yang, K.-M.; Wu, C.-S.; Chu, L.-P.; Hsu, Y.-L.; Chen, H.-C. Effects of Storage Time and Temperature on the Aroma Quality and Color of Vanilla Beans (Vanilla planifolia) from Taiwan. Food Chem. X 2024, 24, 101761. [Google Scholar] [CrossRef]
- Zhao, T.; Cao, Z.; Yu, J.; Weng, X.; Benjakul, S.; Guidi, A.; Ying, X.; Ma, L.; Xiao, G.; Deng, S. Gas-Phase Ion Migration Spectrum Analysis of the Volatile Flavors of Large Yellow Croaker Oil after Different Storage Periods. Curr. Res. Food Sci. 2022, 5, 813–822. [Google Scholar] [CrossRef] [PubMed]
- López, P.L.; Enemark, G.K.G.; Grosso, N.R.; Olmedo, R.H. Oxidative Protection of Sunflower Oil Used in Industrial Process at High Temperature by Volatile Components from Origanum vulgare and Humulus lupulus Essential Oils. Food Bioprocess. Technol. 2023, 16, 2813–2824. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, Y.; Qiu, J.; Cao, J.; Sun, Y.; Li, H.; Kong, F. Coupled Multidimensional GC and Odor Activity Value Calculation to Identify Off-Odors in Thermally Processed Muskmelon Juice. Food Chem. 2019, 301, 125307. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Tang, J.; Shi, W.; Wang, Z.; Xiang, Y.; Deng, T.; Gao, X.; Li, W.; Shi, S. Effects of Three Drying Methods on Polyphenol Composition and Antioxidant Activities of Litchi Chinensis Sonn. Food Sci. Biotechnol. 2019, 29, 351–358. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, X.; Hu, S.; He, R.; Ju, X.; Wang, Z.; Aluko, R.E. Effects of Phytase/Ethanol Treatment on Aroma Characteristics of Rapeseed Protein Isolates. Food Chem. 2024, 431, 137119. [Google Scholar] [CrossRef]
- Ben Khemis, I.; Bouzid, M.; Mechi, N.; Ben Lamine, A. Statistical Physics Modeling and Interpretation of the Adsorption of Enantiomeric Terpenes onto the Human Olfactory Receptor OR1A1. Int. J. Biol. Macromol. 2021, 171, 428–434. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, L.; Li, P.; Pu, D.; Fu, Y.; Zheng, R.; Xi, H.; Qiao, K.; Wang, D.; Sun, B.; et al. Molecular Mechanisms of Caramel-like Odorant-Olfactory Receptor Interactions Based on a Computational Chemistry Approach. Food Res. Int. 2023, 171, 113063. [Google Scholar] [CrossRef]
- Haag, F.; Di Pizio, A.; Krautwurst, D. The Key Food Odorant Receptive Range of Broadly Tuned Receptor OR2W1. Food Chem. 2022, 375, 131680. [Google Scholar] [CrossRef]
- Ahmed, L.; Zhang, Y.; Block, E.; Buehl, M.; Corr, M.J.; Cormanich, R.A.; Gundala, S.; Matsunami, H.; O’Hagan, D.; Ozbil, M.; et al. Molecular Mechanism of Activation of Human Musk Receptors OR5AN1 and OR1A1 by (R)-Muscone and Diverse Other Musk-Smelling Compounds. Proc. Natl. Acad. Sci. USA 2018, 115, E3950–E3958. [Google Scholar] [CrossRef]
- Park, S.J.; Kwon, O.S.; Lee, S.H.; Song, H.S.; Park, T.H.; Jang, J. Ultrasensitive Flexible Graphene Based Field-Effect Transistor (FET)-Type Bioelectronic Nose. Nano Lett. 2012, 12, 5082–5090. [Google Scholar] [CrossRef]
- Mainland, J.D.; Keller, A.; Li, Y.R.; Zhou, T.; Trimmer, C.; Snyder, L.L.; Moberly, A.H.; Adipietro, K.A.; Liu, W.L.L.; Zhuang, H.; et al. The Missense of Smell: Functional Variability in the Human Odorant Receptor Repertoire. Nat. Neurosci. 2014, 17, 114–120. [Google Scholar] [CrossRef]
- Billesbølle, C.B.; de March, C.A.; van der Velden, W.J.C.; Ma, N.; Tewari, J.; Del Torrent, C.L.; Li, L.; Faust, B.; Vaidehi, N.; Matsunami, H.; et al. Structural Basis of Odorant Recognition by a Human Odorant Receptor. Nature 2023, 615, 742–749. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, X.; Tian, H.; Li, Z. Integrated Characterization of Arabica Coffee Husk Tea Using Flavoromics, Targeted Screening, and in Silico Approaches. Food Chem. X 2024, 23, 101556. [Google Scholar] [CrossRef]
- de March, C.A.; Topin, J.; Bruguera, E.; Novikov, G.; Ikegami, K.; Matsunami, H.; Golebiowski, J. Odorant Receptor 7D4 Activation Dynamics. Angew. Chem. Int. Ed. Engl. 2018, 57, 4554–4558. [Google Scholar] [CrossRef]
- Audouze, K.; Tromelin, A.; Le Bon, A.M.; Belloir, C.; Petersen, R.K.; Kristiansen, K.; Brunak, S.; Taboureau, O. Identification of Odorant-Receptor Interactions by Global Mapping of the Human Odorome. PLoS ONE 2014, 9, e93037. [Google Scholar] [CrossRef]
- He, X.-H.; You, C.-Z.; Jiang, H.-L.; Jiang, Y.; Xu, H.E.; Cheng, X. AlphaFold2 versus Experimental Structures: Evaluation on G Protein-Coupled Receptors. Acta Pharmacol. Sin. 2023, 44, 1–7. [Google Scholar] [CrossRef]
- Schake, P.; Bolz, S.N.; Linnemann, K.; Schroeder, M. PLIP 2025: Introducing Protein–Protein Interactions to the Protein–Ligand Interaction Profiler. Nucleic Acids Res. 2025, 53, W463–W465. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Zacarías, L. Biosynthesis and Esterification of Carotenoids During Fruit Ripening. In Carotenoid Esters in Foods: Physical, Chemical and Biological Properties; Mercadante, A.Z., Ed.; Food Chemistry, Function and Analysis; The Royal Society of Chemistry: London, UK, 2019; pp. 137–159. ISBN 978-1-78801-242-3. [Google Scholar]
- George, J.; Nguyen, T.; Williams, D.; Hardner, C.; Sanewski, G.; Smyth, H.E. Review of the Aroma Chemistry of Pineapple (Ananas comosus). J. Agric. Food Chem. 2023, 71, 4069–4082. [Google Scholar] [CrossRef]
- Ma, N.; Zhu, J.; Wang, H.; Qian, M.C.; Xiao, Z. Comparative Investigation of Aroma-Active Volatiles in (“Ruixue”, “Liangzhi”, “Crystal Fuji,” and “Guifei”) Apples by Application of Gas Chromatography-Mass Spectrometry-Olfactometry (GC-MS-O) and Two-Dimensional Gas Chromatography-Quadrupole Mass Spectrometry (GC × GC-qMS) Coupled with Sensory Molecular Science. J. Agric. Food Chem. 2024, 72, 25229–25250. [Google Scholar] [CrossRef]
- Xing, R.; Wang, Y.; Dai, J.; Zhang, C.; Hua, Y.; Chen, Y.; Kong, X. Volatile Flavor Characterization of Raw Coconut Milk and the Impact of Sterilization: A Comparative Analysis for Coconut Flavor Preservation. Food Chem. 2025, 490, 145123. [Google Scholar] [CrossRef]
- Motooka, R.; Usami, A.; Nakahashi, H.; Koutari, S.; Nakaya, S.; Shimizu, R.; Tsuji, K.; Marumoto, S.; Miyazawa, M. Characteristic Odor Components of Essential Oils from Eurya japonica. J. Oleo Sci. 2015, 64, 577–584. [Google Scholar] [CrossRef][Green Version]
- Liu, G.; Chen, Q.; Gou, M.; Bi, J. Formation of Key Aroma-Active and off-Flavor Components in Concentrated Peach Puree. Food Chem. 2024, 439, 138105. [Google Scholar] [CrossRef]
- Wei, Y.; Yin, X.; Wu, H.; Zhao, M.; Huang, J.; Zhang, J.; Li, T.; Ning, J. Improving the Flavor of Summer Green Tea (Camellia sinensis L.) Using the Yellowing Process. Food Chem. 2022, 388, 132982. [Google Scholar] [CrossRef]
- Chen, S.; Rui, R.; Wang, S.; He, X. Comparative Analysis of the Floral Fragrance Compounds of Panax Notoginseng Flowers under the Panax Notoginseng-Pinus Agroforestry System Using SPME-GC-MS. Molecules 2022, 27, 3565. [Google Scholar] [CrossRef]
- Liu, M.; Su, Y.; Guo, Y. Headspace-Low Water Absorption Trap Technique: Analysis of Low-Abundance Volatile Compounds from Fresh Artemisia annua L. with GC-MS. J. Chromatogr. Sci. 2022, 60, 907–915. [Google Scholar] [CrossRef]
- Fan, Z.; Hasing, T.; Johnson, T.S.; Garner, D.M.; Schwieterman, M.L.; Barbey, C.R.; Colquhoun, T.A.; Sims, C.A.; Resende, M.F.R.; Whitaker, V.M. Strawberry Sweetness and Consumer Preference Are Enhanced by Specific Volatile Compounds. Hortic. Res. 2021, 8, 66. [Google Scholar] [CrossRef]
- Deshpande, A.B.; Chidley, H.G.; Oak, P.S.; Pujari, K.H.; Giri, A.P.; Gupta, V.S. Isolation and Characterization of 9-Lipoxygenase and Epoxide Hydrolase 2 Genes: Insight into Lactone Biosynthesis in Mango Fruit (Mangifera Indica L.). Phytochemistry 2017, 138, 65–75. [Google Scholar] [CrossRef]
- Leonardou, V.K.; Doudoumis, E.; Tsormpatsidis, E.; Vysini, E.; Papanikolopoulos, T.; Papasotiropoulos, V.; Lamari, F.N. Quality Traits, Volatile Organic Compounds, and Expression of Key Flavor Genes in Strawberry Genotypes over Harvest Period. Int. J. Mol. Sci. 2021, 22, 13499. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sevilla, J.F.; Cruz-Rus, E.; Valpuesta, V.; Botella, M.A.; Amaya, I. Deciphering Gamma-Decalactone Biosynthesis in Strawberry Fruit Using a Combination of Genetic Mapping, RNA-Seq and eQTL Analyses. BMC Genom. 2014, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Zeliou, K.; Papasotiropoulos, V.; Manoussopoulos, Y.; Lamari, F.N. Physical and Chemical Quality Characteristics and Antioxidant Properties of Strawberry Cultivars (Fragaria × ananassa Duch.) in Greece: Assessment of Their Sensory Impact. J. Sci. Food Agric. 2018, 98, 4065–4073. [Google Scholar] [CrossRef] [PubMed]
- Bosman, R.N.; Lashbrooke, J.G. Grapevine Mono- and Sesquiterpenes: Genetics, Metabolism, and Ecophysiology. Front. Plant Sci. 2023, 14, 1111392. [Google Scholar] [CrossRef]
- Gao, F.; Liu, B.; Li, M.; Gao, X.; Fang, Q.; Liu, C.; Ding, H.; Wang, L.; Gao, X. Identification and Characterization of Terpene Synthase Genes Accounting for Volatile Terpene Emissions in Flowers of Freesia x hybrida. J. Exp. Bot. 2018, 69, 4249–4265. [Google Scholar] [CrossRef]
- Wang, F.; Park, Y.-L.; Gutensohn, M. Epidermis-Specific Metabolic Engineering of Sesquiterpene Formation in Tomato Affects the Performance of Potato Aphid Macrosiphum euphorbiae. Front. Plant Sci. 2021, 12, 793313. [Google Scholar] [CrossRef]
- Gutensohn, M.; Henry, L.K.; Gentry, S.A.; Lynch, J.H.; Nguyen, T.T.H.; Pichersky, E.; Dudareva, N. Overcoming Bottlenecks for Metabolic Engineering of Sesquiterpene Production in Tomato Fruits. Front. Plant Sci. 2021, 12, 691754. [Google Scholar] [CrossRef]
- Ma, Y.; Zu, Y.; Huang, S.; Stephanopoulos, G. Engineering a Universal and Efficient Platform for Terpenoid Synthesis in Yeast. Proc. Natl. Acad. Sci. USA 2023, 120, e2207680120. [Google Scholar] [CrossRef]
- Khruengsai, S.; Phalangrit, K.-A.; Sripahco, T.; Kaewfoo, M.; Sittikarn, N.; Poshyachinda, S.; Pongpiachan, S.; Pripdeevech, P. Diurnal Variations of Biogenic Volatile Organic Compounds and Their Role in Secondary Pollutant Formation in the Huai Hong Khrai Subtropical Forest, Thailand. Environ. Pollut. 2025, 372, 126044. [Google Scholar] [CrossRef]
- Qin, L.; Ma, D.; Lin, G.; Sun, W.; Li, C. Low Temperature Promotes the Production and Efflux of Terpenoids in Yeast. Bioresour. Technol. 2024, 395, 130376. [Google Scholar] [CrossRef]
- Nie, S.; Wang, S.; Chen, R.; Ge, M.; Yan, X.; Qiao, J. Catalytic Mechanism and Heterologous Biosynthesis Application of Sesquiterpene Synthases. J. Agric. Food Chem. 2024, 72, 6871–6888. [Google Scholar] [CrossRef]
- Wu, H.; Yang, B.; Zhang, R.; Dong, Z.; Xie, H. Optimization of Harvest Time for Medicinal Use of Acorus tatarinowii Schott: A Comprehensive Analytical Approach Using HS-SPME-GC-MS, Network Pharmacology, and Molecular Simulation Techniques. Ind. Crops Prod. 2025, 233, 121424. [Google Scholar] [CrossRef]
- Del Mármol, J.; Yedlin, M.A.; Ruta, V. The Structural Basis of Odorant Recognition in Insect Olfactory Receptors. Nature 2021, 597, 126–131. [Google Scholar] [CrossRef]
- Mayhew, E.J.; Arayata, C.J.; Gerkin, R.C.; Lee, B.K.; Magill, J.M.; Snyder, L.L.; Little, K.A.; Yu, C.W.; Mainland, J.D. Transport Features Predict If a Molecule Is Odorous. Proc. Natl. Acad. Sci. USA 2022, 119, e2116576119. [Google Scholar] [CrossRef]
- Liang, J.; Shalaby, N.; Rigling, M.; Wagner, T.; Heimbach, J.; Fries, A.; Kohlus, R.; Zhang, Y. Characterization of Hay-like off-Odor in Basil Samples after Various Processing and Strategies for Reducing the off-Odor. Food Res. Int. 2022, 162, 112080. [Google Scholar] [CrossRef]
- Brizzolara, S.; Manganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary Metabolism in Fresh Fruits During Storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).