Enhancing the Anti-Aging Potential of Green Tea Extracts Through Liquid-State Fermentation with Aspergillus niger RAF106
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Unfermented and Fermented Tea Extracts
2.3. Assay for C. elegans Lifespan Under Normal and Stressful Conditions
2.4. Analysis of Body Length and Body Bends of C. elegans
2.5. Assay for Lipid Accumulation, Mitochondrial Membrane Potential, and the Production of Reactive Oxygen Species (ROS), Glutathione (GSH), Malondialdehyde (MDA), Superoxide Dismutases (SODs) and Catalases (CATs)
2.6. Non-Targeted Metabolomic Analysis
2.7. Statistical Analysis
3. Results
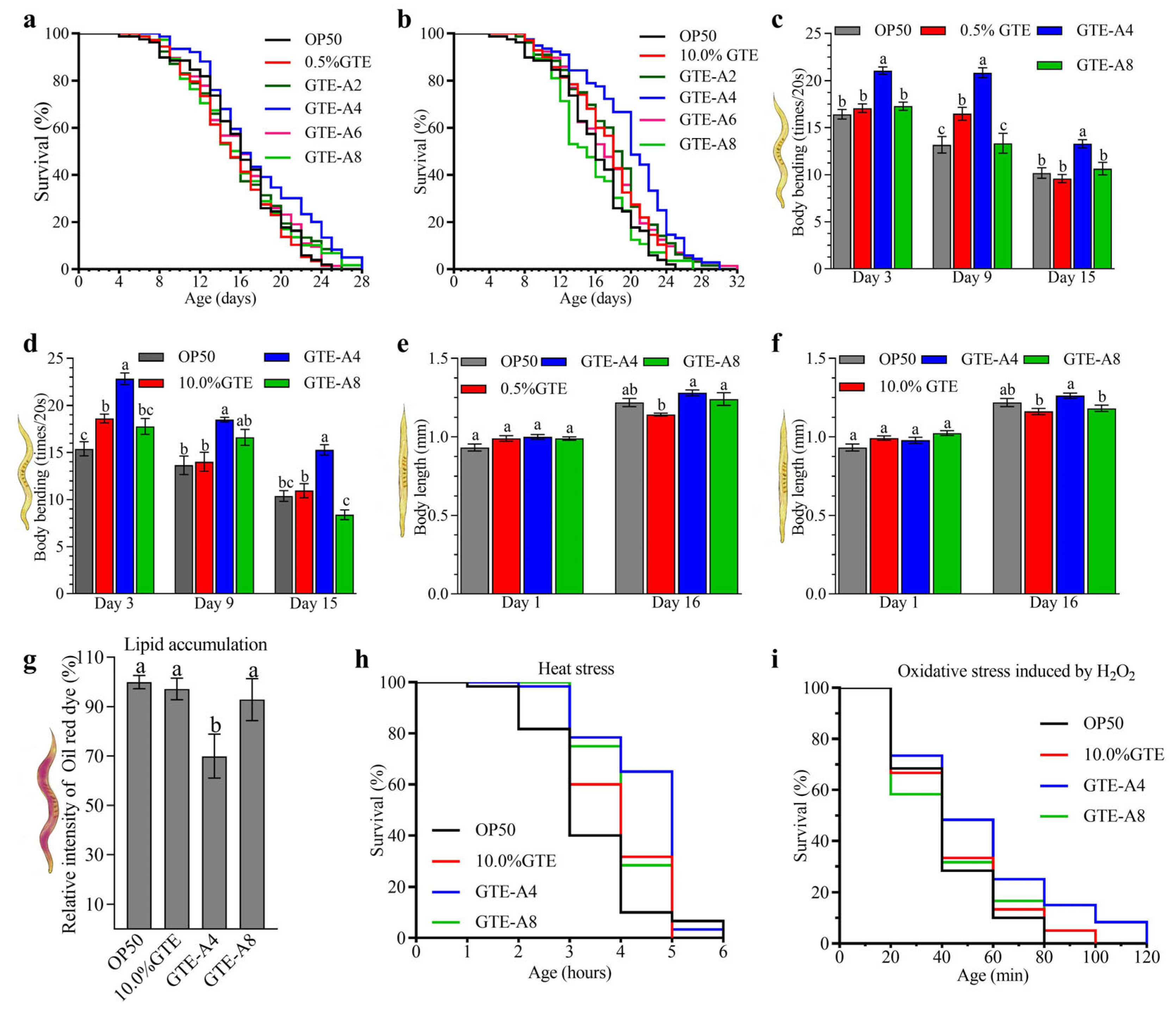
3.1. Dynamic Changes in Anti-Aging Effects of Tea Extracts During the Liquid-State Fermentation of Green Tea Extracts (GTE) with A. niger RAF106
3.2. RAF106 Fermentation Modified the Impact of Tea Extracts on Mitochondrial Function and the Antioxidant Status of C. elegans
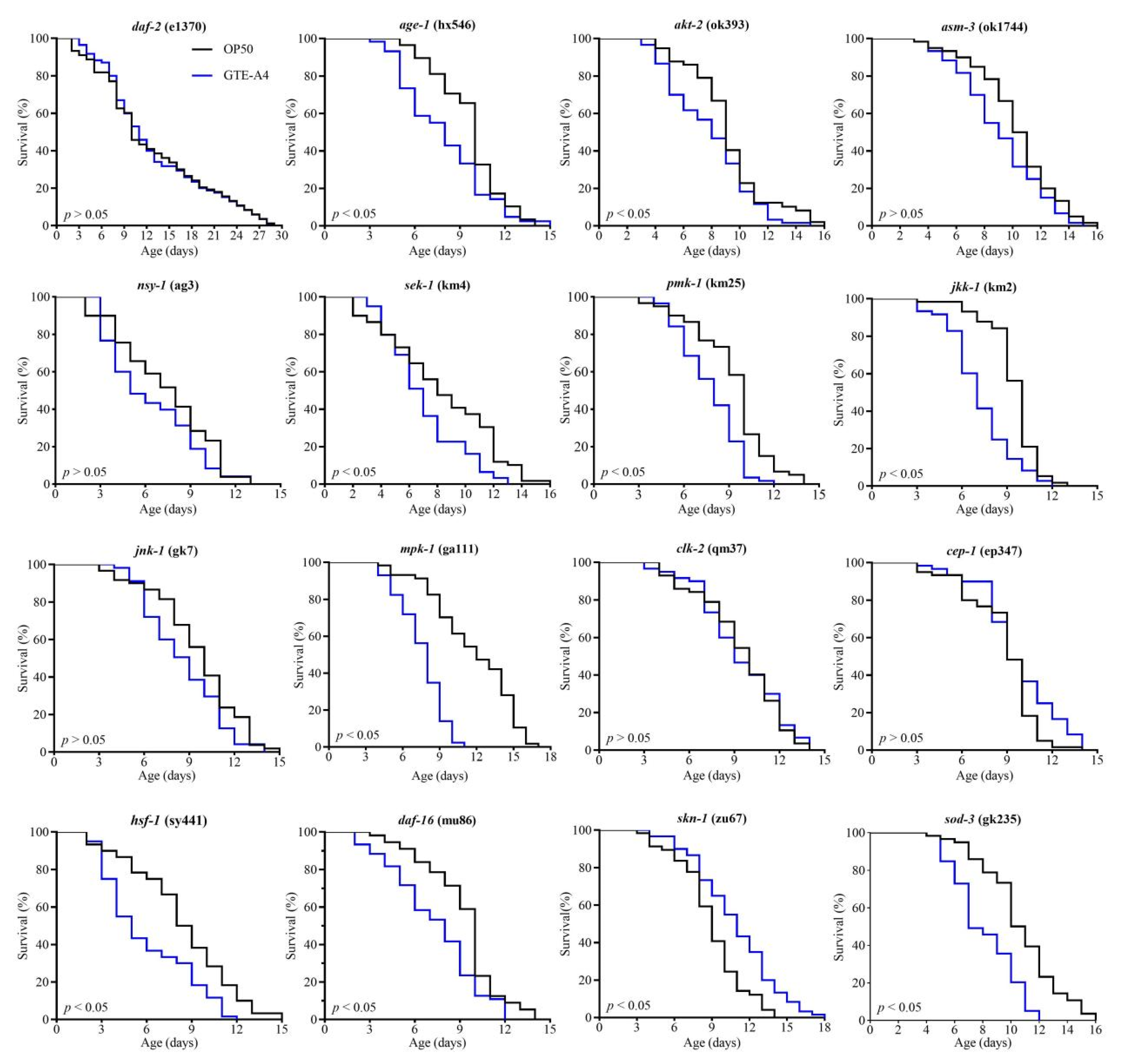
3.3. RAF106-Fermented GTE (GTE-A4) Promoted Longevity via Multiple Pathways
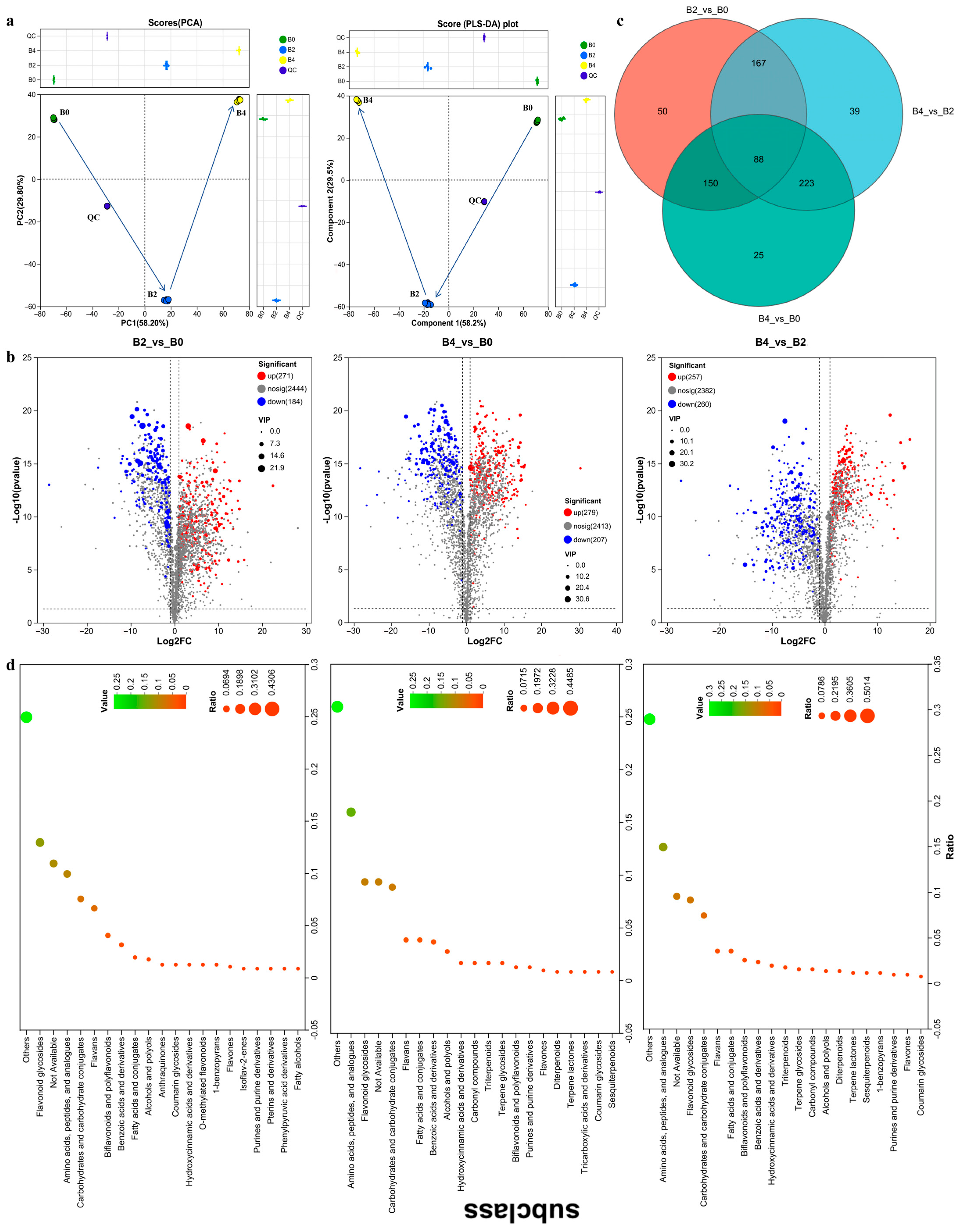
3.4. RAF106 Fermentation Modified the Chemical Profile of GTE, Leading to the Enrichment of Numerous Longevity-Promoting Metabolites in GTE-A4
4. Discussion
4.1. Fermentation with RAF106 for 4 Days Improved Anti-Aging Effects of GTE
4.2. RAF106-Fermented GTE-A4 Promoted Longevity via Multi-Pathways
4.3. Longevity-Promoting Metabolites in RAF106-Fermented GTE-A4
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Huang, X.Q.; Dou, L.; Yan, M.J.; Shen, T.; Tang, W.Q.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Chen, J.; Yu, R.F. Dose the increasing burden of social endowment affect sustainable development economy. PLoS ONE 2024, 19, e0296512. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Sun, Z.; Sun, T. Lacticaseibacillus rhamnosus Probio-M9 extends the lifespan of Caenorhabditis elegans. Community Biol. 2022, 5, 1139. [Google Scholar] [CrossRef]
- Xu, Y.; Song, J.; Huang, Q.; Wei, X.; Deng, Z.; Song, Z.; Huang, H.; Luo, C.; Zhang, D.; Han, L. Functional foods and nutraceuticals with anti-aging effects: Focus on modifying the enteral microbiome. J. Funct. Foods 2025, 128, 106786. [Google Scholar] [CrossRef]
- Bansal, S.; Choudhary, S.; Sharma, M.; Kumar, S.S.; Lohan, S.; Bhardwaj, V.; Syan, N.; Jyoti, S. Tea: A native source of antimicrobial agents. Food Res. Int. 2013, 53, 568–584. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.M.; Zhong, Y.Z.; Duan, Y.H.; Chen, Q.H.; Li, F.N. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Xiang, Y.; Hu, H.; Chen, H.; Tang, D.; Huang, Z.; Zhang, Y.; Wang, Z.; Wang, Z.; Yangla; Han, M.; et al. Tea consumption and attenuation of bilogical aging: A longitudinal analysis from two cohort studies. Lancet Reg. Health West Pac. 2024, 42, 100955. [Google Scholar]
- Fei, T.; Fei, J.; Huang, F.; Xie, T.; Xu, J.; Zhou, Y.; Yang, P. The anti-aging and anti-oxidation effects of tea water extract in Caenorhabditis elegans. Exp. Gerontol. 2017, 97, 89–96. [Google Scholar] [CrossRef]
- Ke, J.; Li, J.; Yang, Z.; Wu, H.; Yu, J.; Yang, Y.; Chen, C.; Zhou, P.; Hua, F.; Wang, W.; et al. Unraveling anti-aging mystery of green tea in C. elegans: Chemical truth and multiple mechanisms. Food Chem. 2024, 460, 140510. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Portocarrero, A.C.M.; López, J.M.M.; Lombardo, M.; Koch, W.; Raposo, A.; EI-Seedi, H.R.; de Brito Alves, J.L.; Esatbeyoglu, T.; Karav, S.; et al. The impact of fermentation on the antioxidant activity of food products. Molecules 2024, 29, 3941. [Google Scholar] [CrossRef]
- Zhao, D.; Shah, N.P. Lactic acid bacterial fermentation modified phenolic composition in tea extracts and enhanced their antioxidant activity and cellular uptake of phenolic compounds following in vitro digestion. J. Funct. Food. 2016, 20, 182–194. [Google Scholar] [CrossRef]
- Kim, M.J.; John, K.M.M.; Choi, J.N.; Lee, S.; Kim, A.J.; Kim, Y.M.; Lee, C.H. Changes in secondary metabolites of green tea during fermentation by Aspergillus oryzae and its effect on antioxidant potential. Food Res. Int. 2013, 53, 670–677. [Google Scholar] [CrossRef]
- Kritsadaruangchai, U.; Chaiwut, P.; Chomnunti, P.; Thaochan, N.; Saikeur, A.; Pintathong, P. Effect of solid state fermentation with Trichoderma spp. on phenolic content and antioxidant capacities of mature Assam tea leaves. J. Food Sci. Agric. Technol. 2019, 5, 106–113. [Google Scholar]
- Ke, J.; Zhang, Y.; Li, J.; Wu, H.; Yu, J.; Chen, C.; Yang, Y.; Wang, W.; Hu, F.; Bao, G. Cordyceps militaris fermentation changes the flavor and chemical profiles of Lu’an GuaPian green tea with fat-lowering and anti-aging activities. Microchem. J. 2024, 201, 110676. [Google Scholar] [CrossRef]
- Cai, M.; Huang, L.; Dong, S.; Diao, N.; Ye, W.; Peng, Z.; Fang, X. Enhancing the flavor profile of summer green tea via fermentation with Aspergillus niger RAF106. Foods 2023, 12, 3420. [Google Scholar] [CrossRef]
- Chen, S.; Fu, Y.; Bian, X.; Zhao, M.; Zuo, Y.; Ge, Y.; Xiao, Y.; Xiao, J.; Li, N.; Wu, J. Investigation and dynamic profiling of oligopeptides, free amino acids and derivatives during Pu-erh tea fermentation by ultra-high performance liquid chromatography tandem mass spectrometry. Food Chem. 2022, 371, 131176. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Zhao, Y.; Jia, W.; Niu, L.; Bouphun, T.; Li, P.; Chen, S.; Chen, W.; Tang, D.; Zhao, Y.; et al. Untargeted metabolomics and quantification analysis reveal the shift of chemical constituents between instant dark teas individually liquid-state fermented by Aspergillus cristatus, Aspergillus niger, and Aspergillus tubingensis. Front. Microbiol. 2023, 14, 1124546. [Google Scholar] [CrossRef]
- Sun, H.; Fan, R.; Fang, R.; Shen, S.; Wang, Y.; Fu, J.; Hou, R.; Sun, R.; Bao, S.; Chen, Q.; et al. Dynamics changes in metabolites and pancreatic lipase inhibitory ability of instant dark tea during liquid-state fermentation by Aspergillus niger. Food Chem. 2024, 448, 139136. [Google Scholar] [CrossRef]
- Fang, X.; Du, M.; Liu, T.; Fang, Q.; Liao, Z.; Zhong, Q.; Chen, J.; Meng, X.; Zhou, S.; Wang, J. Changes in the biotransformation of green tea catechins induced by different carbon and nitrogen sources in Aspergillus niger RAF106. Front. Microbiol. 2019, 10, 2521. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Du, M.; Chen, J.; Liu, T.; Zheng, Y.; Liao, Z.; Zhong, Q.; Wang, L.; Fang, X.; Wang, J. Degradation and detoxification of aflatoxin B1 by tea-derived Aspergillus niger RAF106. Toxins 2020, 12, 777. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Du, M.; Wang, Y.; Fang, X.; Peng, H.; Shi, Q.; Xie, X.; Zhou, G. The interplays between epigallocatechin-3-gallate (EGCG) and Aspergillus niger RAF106 based on metabolism. Fungal Biol. 2022, 126, 727–737. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, G.; Du, M.; Zhang, X.; Zhou, S.; Chen, G.; Liao, Z.; Zhong, Q.; Wang, L.; Xu, X.; et al. The interplay between (-)-epigallocatechin-3-gallate (EGCG) and Aspergillus niger RAF106, an EGCG-biotransforming fungus derived from Pu-erh tea. LWT-Food Sci. Technol. 2023, 180, 114678. [Google Scholar] [CrossRef]
- Cai, M.; Peng, Z.; Xu, P.; Yu, M.; Diao, N.; Cao, Y.; Dong, S.; Fang, X. Comprehensive analysis of the flavor and color characteristics of light-fermented sour tea mediated by Aspergillus niger RAF106. Food Chem. 2025, 481, 143866. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Zhang, Z.; Jiang, J.; Gao, X.; Yue, P. Multiple responses optimization of instant dark tea production by submerged fermentation using response surface methodology. J. Food Sci. Technol. 2018, 55, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Kirchweger, B.; Zwirchmayr, J.; Grienke, U.; Rollinger, J.M. The role of Caenorhabditis elegans in the discovery of natural products for healthy aging. Nat. Prod. Rep. 2023, 40, 1849. [Google Scholar] [CrossRef]
- Stenvall, J.; Fierro-Gonzalez, J.C.; Swoboda, P.; Saamarthy, K.; Cheng, Q.; Cacho-Valadez, B.; Arnér, E.S.J.; Persson, O.P.; Miranda-Vizuete, A.; Tuck, S. Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2011, 108, 1064–1069. [Google Scholar] [CrossRef]
- Wang, W.Q.; Li, S.P.; Heng, X.; Chu, W.H. Weissella confusa CGMCC 19,308 Strain protects against oxidative stress, increases lifespan, and bacterial disease resistance in Caenorhabditis elegans. Probiotics Antimicrob. Proteins 2022, 14, 121–129. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Liao, Z.; Xia, Z.; Huang, H.; Huang, J.; Chen, L.; Fang, X.; Gao, C.; Wang, J. Lactobacillus fermentum WC2020 increased the longevity of Caenorhabditis elegans via JNK-mediated antioxidant pathway. J. Food Sci. 2024, 89, 3713–3728. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, X.; Huang, Z.; Lyu, F.; Hu, X.; Han, S.; Ren, H.; Zhang, A. Effect of Eurotium cristatum fermentation on chemincal composition and hypoglycemic and sedative activities of Anji Baicha (Camellia sinensis). J. Food Sci. 2025, 90, e70042. [Google Scholar] [CrossRef]
- Wang, G.; Fei, W.; Zhi, L.; Bai, X.; You, B. Fermented tea leave extract against oxidative stress and ageing of skin in vitro and in vivo. Int. J. Cosmet. Sci. 2024, 47, 1–17. [Google Scholar] [CrossRef]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Liu, X.; Ju, Y.; Zeng, H.; Wen, S.; Wang, C.; Jiang, M.; Tian, B.; Huang, J.; Liu, Z. Green tea fermented by Ganoderma lucidum presented anti-obesity properties via enhanced thermogenesis in vitro and on C57BL/6J mice. Food Res. Int. 2025, 207, 116092. [Google Scholar] [CrossRef]
- Li, W.; Gao, L.; Huang, W.; Ma, Y.; Muhammad, I.; Hanif, A.; Ding, Z.; Guo, X. Antioxidant properties of lactic acid bacteria isolated from traditional fermented yak milk and their probiotic effects on the oxidative senescence of Caenorhabditis elegans. Food Funct. 2022, 13, 3690–3703. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Siems, W.; Mazurek, B.; Gross, J.; Schroeder, P.; Voss, P.; Grune, T. Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic. Res. 2006, 40, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Joishy, T.; Das, S.; Kalita, M.C.; Mukherjee, A.K.; Khan, M.R. A potential probiotic Lactobacillus plantarum JBC5 improves longevity and healthy aging by modulating antioxidative, innate immunity and serotonin-signaling pathways in Caenorhabditis elegans. Antioxidants 2022, 11, 268. [Google Scholar] [CrossRef]
- Nakagawa, H.; Shiozaki, T.; Kobatake, E.; Hosoya, T.; Moriya, T.; Sakai, F.; Taru, H.; Miyazaki, T. Effects and mechanisms of prolongevity induced by Lactobacillus gasseri SBT2055 in Caenorhabditis elegans. Aging Cell 2015, 15, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Poupet, C.; Chassard, C.; Nivoliez, A.; Bornes, S. Caenorhabditis elegans, a host to investigate the probiotic properties of beneficial microorganisms. Front. Nutr. 2020, 7, 135. [Google Scholar] [CrossRef]
- Yoon, D.S.; Cha, D.S.; Choi, Y.; Lee, J.W.; Lee, M.H. MPK-1/ERK is required for the full activity of resveratrol in extended lifespan and reproduction. Aging Cell 2019, 18, e12867. [Google Scholar] [CrossRef]
- Chiang, W.; Ching, T.; Lee, H.C.; Mousigian, C.; Hsu, A. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell 2012, 148, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Kaletsky, R.; Lakhina, V.; Arey, R.; Williams, A.; Landis, J.; Ashraf, J.; Murphy, C.T. The C. elegans adult neuronal IIS/FOXO transcriptome reveals adult phenotype regulators. Nature 2016, 529, 92. [Google Scholar] [CrossRef]
- Keshet, A.; Mertenskötter, A.; Winter, S.A.; Brinkmann, V.; Dölling, R.; Paul, R.J. PMK-1 p38 MAPK promotes cadmium stress resistance, the expression of SKN-1/Nrf and DAF-16 target genes, and protein biosynthesis in Caenorhabditis elegans. Mol. Genet. Genom. 2017, 292, 1341–1361.10. [Google Scholar] [CrossRef]
- Mertenskötter, A.; Keshet, A.; Gerke, P.; Paul, R.J. The p38 MAPK PMK-1 shows heat-induced nuclear translocation, supports chaperone expression, and affects the heat tolerance of Caenorhabditis elegans. Cell Stress Chaperones 2013, 18, 293–306. [Google Scholar] [CrossRef]
- Tullet, J.M.A.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct inhibition of the longevity-promoting factor SKN-1 by Insulin-like signaling in C. elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef]
- Bianco, J.N.; Schumacher, B. MPK-1/ERK pathway regulates DNA damage response during development through DAF-16/FOXO. Nucleic Acids Res. 2018, 46, 6129–6139. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Mukhopadhyay, A.; Svrzikapa, N.; Jiang, F.; Davis, R.J.; Tissenbaum, H.A. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 2005, 102, 4494–4499. [Google Scholar] [CrossRef]
- Tambara, A.L.; de Los, S.M.L.; Dal Forno, A.H. Purple pitanga fruit (Eugenia uniflora, L) protects against oxidative stress and increase the lifespan in Caenorhabditis elegans via the DAF-16/FOXO pathway. Food Chem. Toxicol. 2018, 120, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, X.; Shi, Y.; Gao, J.; He, Y.; Li, F.; Shi, J.; Gong, Q. Trilobatin, a component from Lithocarpus polystachyrus Rehd., increased longevity in C. elegans through activating SKN1/SIRT3/DAF16 signaling pathway. Front. Pharmacol. 2021, 12, 655045. [Google Scholar]
- Arum, O.; Johnson, T.E. Reduced expression of the Caenorhabditis elegans p53 ortholog cep-1 results in increased longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 951–959. [Google Scholar] [CrossRef]
- Derry, W.B.; Bierings, R.; van Iersel, M.; Satkunendran, T.; Reinke, V.; Rothman, J.H. Regulation of developmental rate and germ cell proliferation in Caenorhabditis elegans by the p53 gene network. Cell Death Differ. 2007, 14, 662–670. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Li, X.; Shen, Q.; Wu, Q.; Zhuang, J.; Zhang, X.; Xia, E.; Zhang, Z.; Qian, Y.; et al. Comparative analysis of phenolic compound metabolism among tea plants in the section Thea of the genus Camellia. Food Res. Int. 2020, 135, 109276. [Google Scholar] [CrossRef]
- Sanlier, N.; Gokcen, B.B.; Altuğ, M. Tea consumption and disease correlations. Trends Food Sci. Technol. 2018, 78, 95–106. [Google Scholar] [CrossRef]
- An, T.; Chen, M.; Zu, Z.; Chen, Q.; Lu, H.; Yue, P.; Gao, X. Untargeted and targeted metabolomics reveal changes in the chemical constituents of instant dark tea during liquid-state fermentation by Eurotium cristatum. Food Res. Int. 2021, 148, 110623. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Nanjo, F. Metabolism of (-)-epigallocatechin gallate by rat intestinal flora. J. Agric. Food Chem. 2010, 58, 1313–1321. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.; Xu, W.; Li, J.; Lin, H.; Zhang, Z.; Xiao, J.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Huang, J.; Zhang, S.; Wu, B.; Kapahi, P.; Zhang, X.; Shen, Z. Icariin and its derivative icariside II extend healthspan via Insulin/IGF-1 pathway in C. elegans. PLoS ONE 2011, 6, e28835. [Google Scholar] [CrossRef]
- Malar, D.S.; Prasanth, M.I.; Jeyakumar, M.; Balamurugan, K.; Devi, K.P. Vitexin prevents Aβ proteotoxicity in transgenic Caenorhabditis elegans model of Alzheimer’s disease by modulating unfolded protein response. J. Biochem. Mol. Toxicol. 2020, 35, e22632. [Google Scholar] [CrossRef]
- Nas, J.S.B.; Manalo, R.V.M.; Medina, P.M.B. Peonidin-3-glucoside extends the lifespan of Caenorhabditis elegans and enhances its tolerance to heat, UV, and oxidative stresses. ScienceAsia 2021, 47, 457–468. [Google Scholar] [CrossRef]
- Okoro, N.O.; Odiba, A.S.; Osadebe, P.O.; Omeje, E.O.; Liao, G.; Fang, W.; Jin, C.; Wang, B. Bioactive phytochemicals with anti-aging and lifespan Extending potentials in Caenorhabditis elegans. Molecules 2021, 26, 7323. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Cabello, J.; Gómez-Orte, E.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Dueñas, M. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011, 2, 445–456. [Google Scholar] [CrossRef]
- Tao, M.; Li, R.; Xu, T.; Zhang, Z.; Wu, T.; Pan, S.; Xu, X. Flavonoids from the mung bean coat promote longevity and fitness in Caenorhabditis elegans. Food Funct. 2021, 12, 8196–8207. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Amrouche, A.T.; Huang, W.; Lu, B. Asperuloside, the bioactive compound in the edible Eucommia ulmoides male flower, delays muscle aging by daf-16 mediated improvement in mitochondrial dysfunction. Food Funct. 2023, 14, 5562–5575. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, G.; Gai, T.; Zhou, X.; Zhu, J.; Wang, R.; Wang, X.; Guo, Y.; Wang, Y.; Xie, Z. L-Theanine prolongs the lifespan by activating multiple molecular pathways in ultraviolet C-exposed Caenorhabditis elegans. Molecules 2024, 29, 2691. [Google Scholar] [CrossRef] [PubMed]
- Zarse, K.; Jabin, S.; Ristow, M. L-Theanine extends lifespan of adult Caenorhabditis elegans. Eur. J. Nutr. 2012, 51, 765–768. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, X.; Liu, X.; Li, R.; Yang, X.; Liao, Z.; Fang, X.; Wang, J. Enhancing the Anti-Aging Potential of Green Tea Extracts Through Liquid-State Fermentation with Aspergillus niger RAF106. Foods 2025, 14, 3548. https://doi.org/10.3390/foods14203548
Liu Y, Zhang X, Liu X, Li R, Yang X, Liao Z, Fang X, Wang J. Enhancing the Anti-Aging Potential of Green Tea Extracts Through Liquid-State Fermentation with Aspergillus niger RAF106. Foods. 2025; 14(20):3548. https://doi.org/10.3390/foods14203548
Chicago/Turabian StyleLiu, Yuju, Xiao Zhang, Xingbing Liu, Ruixuan Li, Ximiao Yang, Zhenlin Liao, Xiang Fang, and Jie Wang. 2025. "Enhancing the Anti-Aging Potential of Green Tea Extracts Through Liquid-State Fermentation with Aspergillus niger RAF106" Foods 14, no. 20: 3548. https://doi.org/10.3390/foods14203548
APA StyleLiu, Y., Zhang, X., Liu, X., Li, R., Yang, X., Liao, Z., Fang, X., & Wang, J. (2025). Enhancing the Anti-Aging Potential of Green Tea Extracts Through Liquid-State Fermentation with Aspergillus niger RAF106. Foods, 14(20), 3548. https://doi.org/10.3390/foods14203548





