Analysis of Volatile Organic Compounds in Wines from Vitis amurensis Varieties in Xinjiang, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Sampling and Winemaking
2.2. Chemical Reagents
2.3. Determination of the Chemical Indicators Parameters of Wines
2.4. Determination of Organic Acid Compounds
2.5. Determination of Aroma Compounds
2.5.1. HS-GC-IMS Analysis
2.5.2. E-Nose Analysis
2.6. Statistical Analysis and Data Visualization
3. Results and Discussion
3.1. Chemical Indicators of Wines
3.2. Analysis of Organic Acid Content in Four Wine Varieties
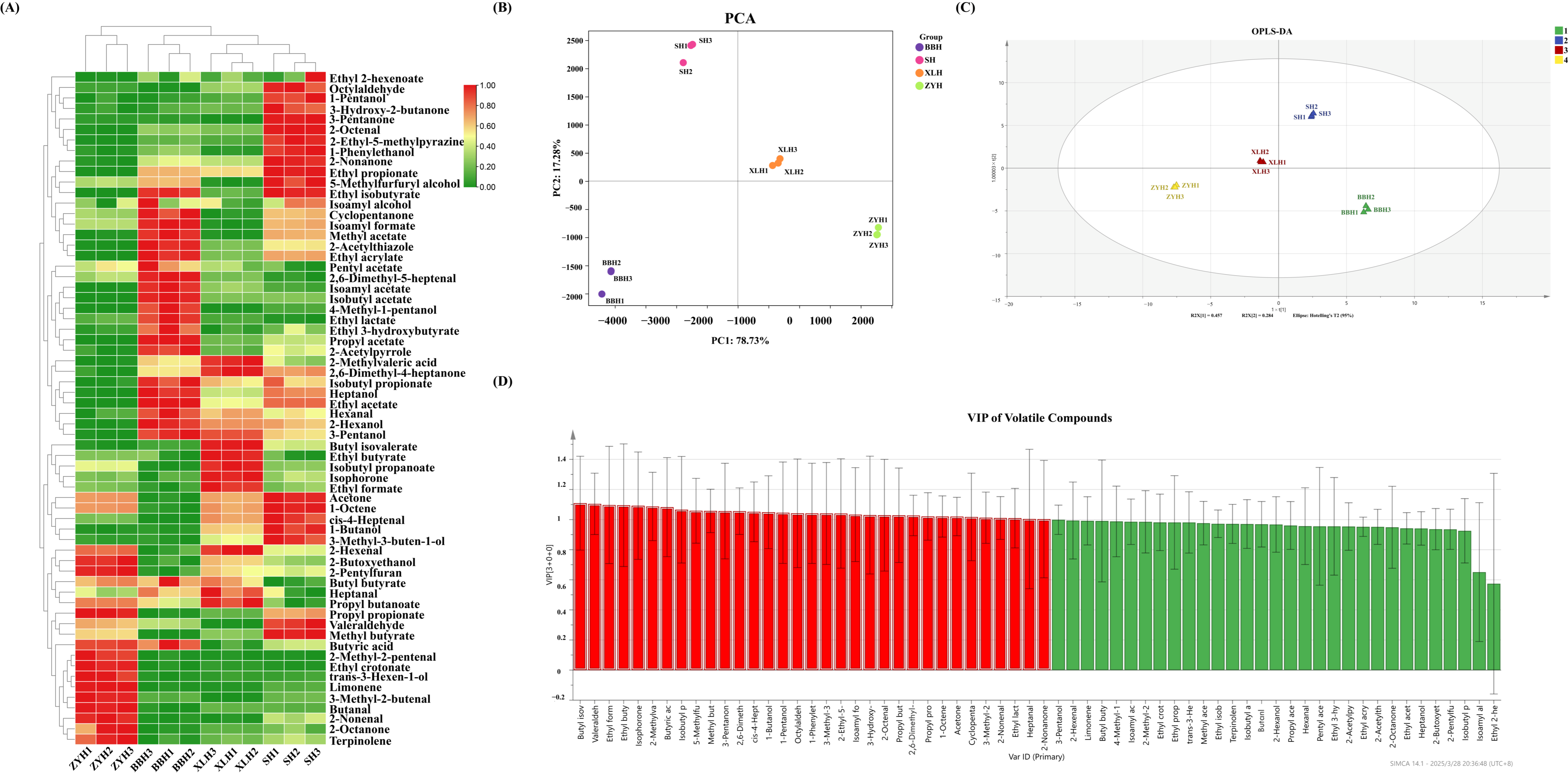
3.3. Analysis of Volatile Compounds in Four Wine Varieties
3.3.1. Volatile Compound Content Analysis
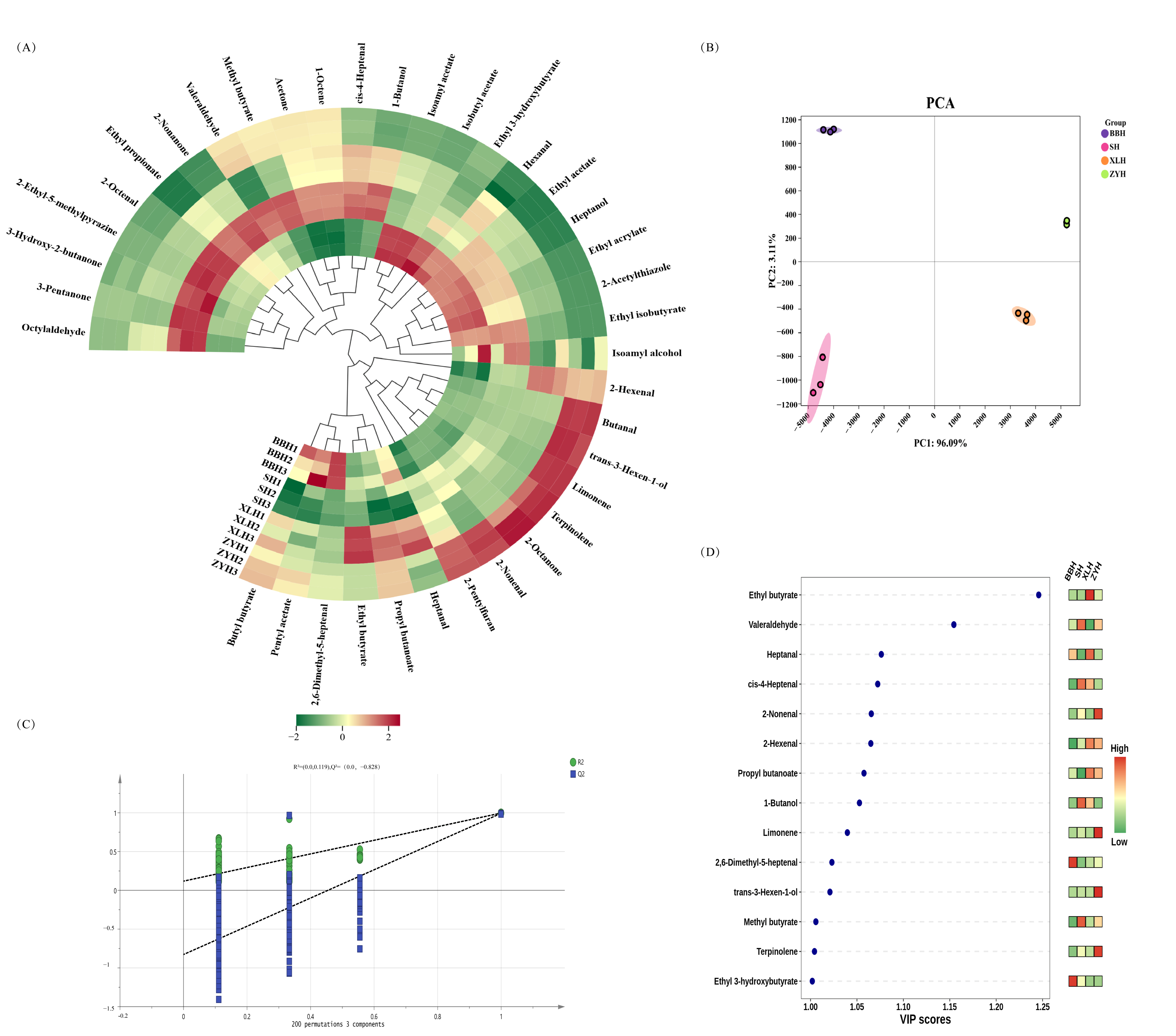
3.3.2. Multivariate Statistical Analysis
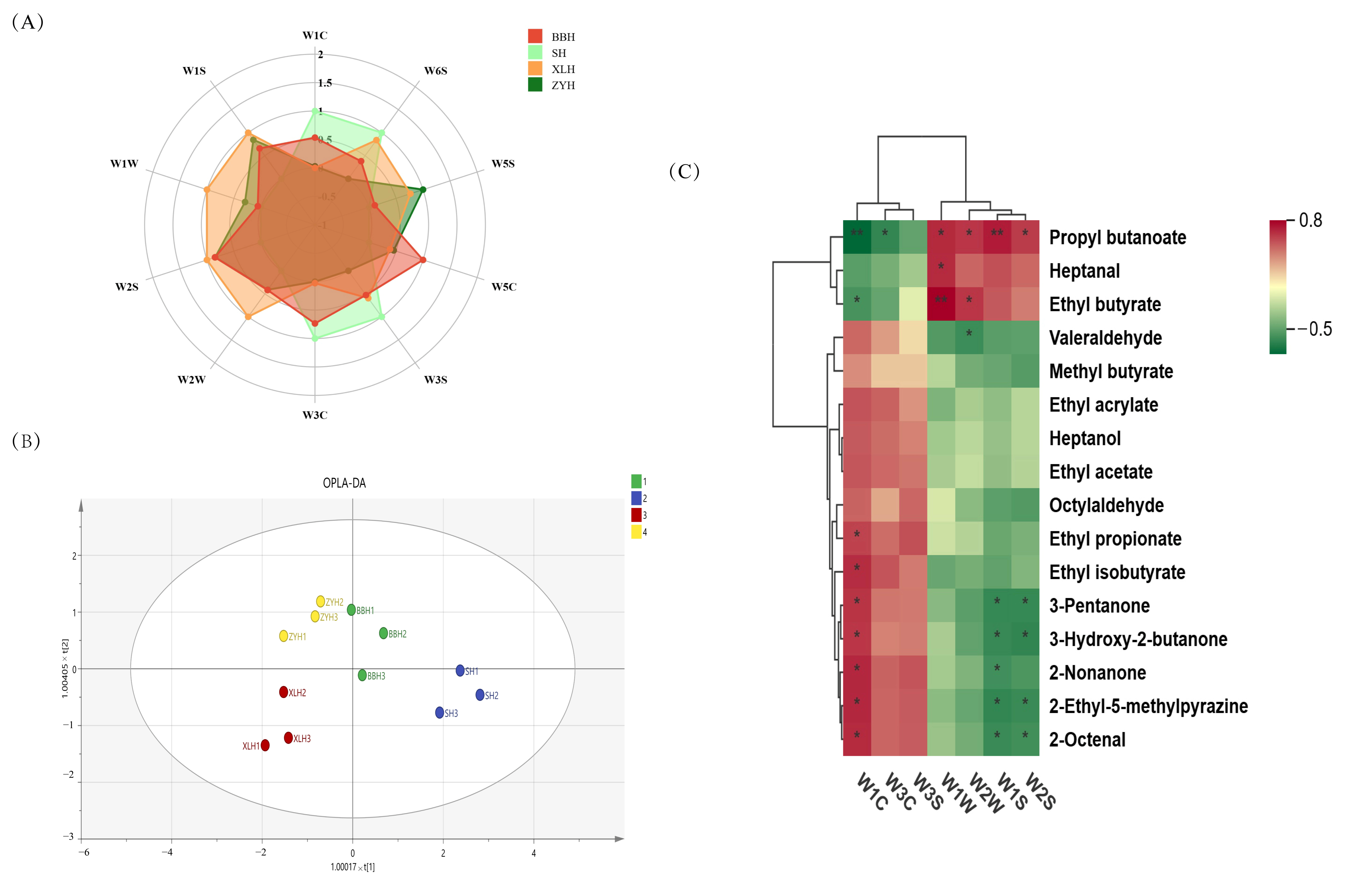
3.4. Analysis of Aroma Volatile Compounds in Four Wine Varieties
3.5. Comparative Analysis of Overall Aroma Profiles in Four Wine Varieties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GC-MS | Gas Chromatography–Mass Spectrometry |
| HCA | Hierarchical cluster analysis |
| HPLC | High-Performance Liquid Chromatography |
| HS-GC-IMS | Headspace-Gas Chromatography–Ion Mobility Spectrometry |
| MLF | Malolactic fermentation |
| OAV | Odor Activity Value |
| OPLS-DA | Orthogonal Partial Least Squares-Discriminant Analysis |
| PCA | Principal component analysis |
| QDA | Quantitative descriptive analysis |
| VIP | Variable Importance in Projection |
| E-nose | Electronic nose |
References
- Spangenberg, J.E.; Baumann, J.; Zufferey, V. Classification of the Geographical Origin of Pinot Noir Wines from Southwestern Switzerland Using Isotopes, Volatile Organic Compounds, and Chemometrics. Food Chem. 2025, 491, 145147. [Google Scholar] [CrossRef] [PubMed]
- Lola, D.; Kalloniati, C.; Tzamourani, A.; Paramithiotis, S.; Dimopoulou, M.; Flemetakis, E.; Kotseridis, Y. The Use of Autochthonous Saccharomyces Cerevisiae Strains as a Strategy to Enhance Aroma Variability and Typicity of Savatiano Wines; RNAseq-Based Transcriptome Comparison of Indigenous Strains under Winemaking Conditions. Int. J. Food Microbiol. 2025, 440, 111249. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zheng, F.; Li, H.; Prejanò, M.; Marino, T.; Russo, N.; Tao, Y.; Li, Y. Effect of Hydroxycinnamic Acids on Ester-Improving and Aroma-Enhancing of Cabernet Sauvignon Red Wine and Its Mechanism. Food Res. Int. 2025, 217, 116763. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Capone, D.L.; Wilkinson, K.L.; Jeffery, D.W. Chemical and Sensory Profiles of Rosé Wines from Australia. Food Chem. 2016, 196, 682–693. [Google Scholar] [CrossRef]
- Goulioti, E.; Jeffery, D.W.; Gialouris, P.-L.; Nastou, E.; Dasenaki, M.; Kontoudakis, N.; Thomaidis, N.; Kotseridis, Y. Effect of Terroir on Aroma and Sensory Characteristics of Xinomavro (Vitis vinifera L. Cv.) Red Wines from Different Greek Protected Designations of Origin. Food Chem. 2025, 486, 144507. [Google Scholar] [CrossRef]
- Chen, H.; Bai, S.; Yang, B.; Ren, R.; Tang, Z.; Zhang, Z.; Zeng, Q. Inter- and Intra-Varietal Clonal Differences Influence the Aroma Compound Profiles of Wines Analyzed by GC–MS and GC-IMS. Food Chem. X 2025, 25, 102136. [Google Scholar] [CrossRef]
- Bianchi, A.; Pacifico, S.; Santini, G.; Pettinelli, S.; Alfieri, G.; Modesti, M.; Bellincontro, A.; Sanmartin, C.; Pittari, E.; Piccolella, S.; et al. Carbonic or Nitrogen Maceration of Wine Grape: Biochemical Differences of Grape and Wine Using Destructive and Non-Destructive Approach. Food Chem. 2025, 487, 144782. [Google Scholar] [CrossRef]
- Sanislav, T.; Mois, G.D.; Zeadally, S.; Folea, S.; Radoni, T.C.; Al-Suhaimi, E.A. A Comprehensive Review on Sensor-Based Electronic Nose for Food Quality and Safety. Sensors 2025, 25, 4437. [Google Scholar] [CrossRef]
- Li, C.; Pu, X.; Ye, P.; Fu, Q.; Zhou, Y.; Zhou, X.; Shi, X.; Wang, B. Flavor Characterization of Six White Wines from Xinjiang: Physicochemical Property, Antioxidant Activity, and Volatile Compounds Analysis. J. Agric. Food Res. 2025, 20, 101725. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, K.; Yang, X.; Li, J.; Li, X. Comparative Study of the Key Aromatic Compounds of Cabernet Sauvignon Wine from the Xinjiang Region of China. J. Food Sci. Technol. 2021, 58, 2109–2120. [Google Scholar] [CrossRef]
- Sun, Q.; Cui, R.; Zhao, Y. Regional Aroma Characteristics of Spontaneously Fermented Cabernet Sauvignon Wines Produced from Seven Sub-Regions in Shangri-La of China. Sci. Rep. 2024, 14, 24566. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Liu, N.; Sun, J.; Yao, R.; Shi, F.; Li, J.; Jiang, W.; Li, H.; Zhang, Q.; et al. Chemical and Sensory Properties of Young Cabernet Sauvignon and Marselan Wines from Subregions on the Eastern Foothills of Helan Mountains in Ningxia, China: Terroir Effect. Food Chem. X 2025, 25, 102191. [Google Scholar] [CrossRef]
- Gao, F.; Guan, L.; Zeng, G.; Hao, X.; Li, H.; Wang, H. Preliminary Characterization of Chemical and Sensory Attributes for Grapes and Wines of Different Cultivars from the Weibei Plateau Region in China. Food Chem. X 2024, 21, 101091. [Google Scholar] [CrossRef]
- Shi, N.; Zhao, Y.-F.; Lu, H.-C.; Tian, M.-B.; Li, M.-Y.; Deng, J.-Y.; Wei, J.-Y.; Chen, A.-S.; Cheng, C.-F.; Li, S.-D.; et al. Flavoromics Analysis and Sensory Evaluation of Wines Produced from Nine Red Grape Cultivars in Manas Region, Xinjiang, China: Implications for Terroir Adaptation. Food Chem. 2025, 490, 145096. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, W.; Zhang, B.; Qin, H.; Wang, Y.; Yang, Y.; Xu, P.; Wang, Y.; Fan, S.; Li, C.; et al. Effects of Different Shaping Methods and Loading on Fruit Quality and Volatile Compounds in ‘Beibinghong’ Grapes. Foods 2025, 14, 772. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Y.; Pei, X.-X.; Shi, N.; Yang, Y.-M.; Fan, S.-T.; Sun, Y.-F.; Kong, Q.-S.; Duan, C.-Q.; Yu, K.; Wang, J. Volatomic Differences among Vitis Amurensis Cultivars and Its Hybrids with V. Vinifera Revealed the Effects of Genotype, Region, and Vintage on Grape Aroma. Food Res. Int. 2024, 191, 114726. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, B.-Q.; Wang, J.-J.; Ma, Y.; Li, Y.-L. CO2 Response Model Fitting and Evaluation of Vitis amurensis. J. Agric. Sci. Technol. 2024, 26, 58–66. [Google Scholar] [CrossRef]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Wang, Y.; Lu, W. Widely Targeted Metabolomics Was Used to Reveal the Differences between Non-Volatile Compounds in Different Wines and Their Associations with Sensory Properties. Foods 2023, 12, 290. [Google Scholar] [CrossRef]
- GB/T 15038-2006; Analytical Methods of Wine and Fruit Wine. Standards Press of China: Beijing, China, 2016.
- Sun, Y.; Yuan, P.; Liu, G.; Yang, Y.; Shu, N.; Lu, W. Analysis of the Effects of Post-Fermentation Freezing Treatment on the Flavor Characteristics of Beibinghong Ice Wine by HPLC and HS-GC-IMS. Foods 2025, 14, 1631. [Google Scholar] [CrossRef]
- Coelho, E.M.; da Silva Padilha, C.V.; Miskinis, G.A.; de Sá, A.G.B.; Pereira, G.E.; de Azevêdo, L.C.; dos Santos Lima, M. Simultaneous Analysis of Sugars and Organic Acids in Wine and Grape Juices by HPLC: Method Validation and Characterization of Products from Northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef]
- He, Y.; Qin, H.; Wen, J.; Cao, W.; Yan, Y.; Sun, Y.; Yuan, P.; Sun, B.; Fan, S.; Lu, W.; et al. Characterization of Key Compounds of Organic Acids and Aroma Volatiles in Fruits of Different Actinidia Argute Resources Based on High-Performance Liquid Chromatography (HPLC) and Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS). Foods 2023, 12, 3615. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lei, W.; Guo, S.; Gao, W.; Ma, K.; Li, J.; Li, H.; Ma, L. Spontaneous Fermentation under Adversity Shapes the Aromatic Profile of Vidal Blanc Icewine: A Main Contribution to the Evolution of Higher Alcohols and Esters. Food Biosci. 2025, 69, 106919. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, Y.; Cui, M.; Chen, Y.; Li, Y.; Hu, Y.; Niu, J.; Cheng, W.; Gao, F. Exploring the Effect of Inoculation Strategies with Saccharomyces Cerevisiae and Lactobacillus Paracasei SMN-LBK on Chemical, Flavor and Metabolic Profiles of Prune Wine. Food Res. Int. 2025, 217, 116867. [Google Scholar] [CrossRef] [PubMed]
- Chira, K.; Lorrain, B.; Ky, I.; Teissedre, P.-L. Tannin Composition of Cabernet-Sauvignon and Merlot Grapes from the Bordeaux Area for Different Vintages (2006 to 2009) and Comparison to Tannin Profile of Five 2009 Vintage Mediterranean Grapes Varieties. Molecules 2011, 16, 1519–1532. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Herrera-Bustamante, B.; López-Solís, R. Ripening-Associated Flattening out of Inter-Varietal Differences in Some Groups of Phenolic Compounds in the Skins of Six Emblematic Grape Wine Varieties. J. Food Compos. Anal. 2021, 99, 103858. [Google Scholar] [CrossRef]
- Lian, Y.; Meng, Y.; Wang, M.; Song, J.; Suo, H.; Zhang, Y. Pre- and Post-Transformation Changes in Two Mulberry Varieties for Semi-Sweet Wine Production. Food Chem. X 2025, 29, 102728. [Google Scholar] [CrossRef]
- Ferrero, L.; Beria D’Argentina, S.; Paissoni, M.A.; Río Segade, S.; Rolle, L.; Giacosa, S. Phenolic Budget in Red Winemaking: Influence of Maceration Temperature and Time. Food Chem. 2025, 482, 144159. [Google Scholar] [CrossRef]
- Niu, S.; Liu, C.; Liu, Q.; Fan, X.; Zhang, Y.; Sun, L.; Zhang, X.; Jiang, J. Composition and Contents of Organic Acids in Different Grape Germplasms. Food Sci. 2022, 43, 228–234. [Google Scholar]
- Rodríguez-Rey, J.; Balmaseda, A.; Bordons, A.; Rozès, N.; Reguant, C. Influence of Nitrogen Compounds from Different Yeast Lees on Malolactic Fermentation. LWT 2025, 229, 118169. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Zhang, Z.; Yang, Z.; Zhou, W.; He, J.; Song, A.; Wu, Y.; Qiu, S.; Han, L. Targeting Indigenous Schizosaccharomyces Japonicus for Genotype Exploration and Organic Acid Degradation Analysis. Front. Microbiol. 2025, 16, 1569585. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research Progress of Wine Aroma Components: A Critical Review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef]
- Marrufo-Curtido, A.; Ferreira, V.; Escudero, A. Can Aldehyde Accumulation Rates of Red Wines Undergoing Oxidation Be Predicted in Accelerated Conditions? The Controverted Role of Aldehyde–Polyphenol Reactivity. J. Sci. Food Agric. 2022, 102, 3869–3878. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, X.; Li, Y.; Huang, Y. Aromatic Composition and Sensory Characteristics of Passion Fruit Wine Fermented by Different Indigenous Non-Saccharomyces Yeast Strains. Food Biosci. 2025, 68, 106576. [Google Scholar] [CrossRef]
- Jiang, J.; Yin, R.; Xie, Y.; Ma, X.; Cui, M.; Chen, Y.; Li, Y.; Hu, Y.; Niu, J.; Cheng, W.; et al. Effects of Cofermentation of Saccharomyces cerevisiae and Different Lactic Acid Bacteria on the Organic Acid Content, Soluble Sugar Content, Biogenic Amines, Phenol Content, Antioxidant Activity and Aroma of Prune Wine. Food Chem. X 2024, 22, 101502. [Google Scholar] [CrossRef]
- Li, S.; Rao, C.; Zang, X.; Yang, Y.; Yang, W.; Huang, X.; Li, J.; Sun, J.; Liu, Y.; Ye, D. Characterization of Aroma Active Compounds and Microbial Communities in Spontaneously Fermented Vitis quinquangularis Wines. Food Res. Int. 2025, 214, 116676. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the Volatile Compounds in Fresh and Thermally Treated Watermelon Juice via Headspace-Gas Chromatography-Ion Mobility Spectrometry and Comprehensive Two-Dimensional Gas Chromatography-Olfactory-Mass Spectrometry Analysis. LWT 2021, 137, 110478. [Google Scholar] [CrossRef]
- Pueyo, E.; Martínez-Rodríguez, A.; Polo, M.C.; Santa-María, G.; Bartolomé, B. Release of Lipids during Yeast Autolysis in a Model Wine System. J. Agric. Food Chem. 2000, 48, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, C.; Liu, Y.; Tang, K.; Chen, M.; Qiu, J.; Yuan, L.; Shen, H.; Wu, Z. Flavor Characteristics of Different Varieties of Flue-Cured Tobacco Based on Headspace Gas Chromatography–Ion Mobility Spectrometry and Headspace Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry. ACS Omega 2025, 10, 20382–20401. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, H.; Sun, J.; Cheng, X.; Lei, J.; Lian, W.; Han, C.; Huang, W.; Zhang, M.; Chen, Y. Succession of Microbiota and Its Influence on the Dynamics of Volatile Compounds in the Semi-Artificial Inoculation Fermentation of Mulberry Wine. Food Chem. X 2024, 21, 101223. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Zhang, C.; Li, S.; Wang, H.; Yu, P.; Shao, H.; Wang, S.; Jin, F. Unveiling the Key Aroma-Active Volatiles Influencing Consumer Preferences in Typical Oriental Melon Varieties by Molecular Sensory Science Methods. J. Food Compos. Anal. 2025, 143, 107527. [Google Scholar] [CrossRef]
- Yang, H.; Qu, T.; Liu, J.; Zhang, C.; Chen, M. Aroma-Producing Saccharomyces cerevisiae Improved the Quality of Dangshan Pear (Pyrus Spp.) Wine through the Biosynthesis of Key Esters. Food Biosci. 2025, 69, 106799. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, P.; Zhao, Y.; Qu, F.; Cheng, Y. Metabolome Analyses of Two Varieties of Anhua Dark Tea. Int. J. Food Prop. 2025, 28, 2519844. [Google Scholar] [CrossRef]
- Liu, P.-T.; Zhang, B.-Q.; Duan, C.-Q.; Yan, G.-L. Pre-Fermentative Supplementation of Unsaturated Fatty Acids Alters the Effect of Overexpressing ATF1 and EEB1 on Esters Biosynthesis in Red Wine. LWT 2020, 120, 108925. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Zhao, M.; Han, Y.; Guan, R.; Wang, B.; Zhang, B.; Ma, T.; Li, J.; Feng, L.; et al. Benzothiadiazole Application Promotes Aroma Accumulation in ‘Chardonnay’ Grapes by Controlling Lipoxygenase Metabolism. Eur. Food Res. Technol. 2025, 251, 3349–3363. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, X.s.; Mao, Y.l.; Zhao, D.d.; Li, Y.c. Influence of Saccharomyces Cerevisiae Autochthonous MQ3 Strain on Terpenes during the Alcoholic Fermentation of Chardonnay Dry White Wine. Aust. J. Grape Wine Res. 2022, 28, 41–49. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on Varietal Aromas during Wine Making: A Review of the Impact of Varietal Aromas on the Flavor of Wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef]
- Lu, H.-C.; Han, X.; Tian, M.-B.; Shi, N.; Li, M.-Y.; Duan, C.-Q.; He, F.; Wang, J. Integrated Metabolome and Transcriptome Analyses Reveal Insights into How Macro-Terroir Affects Polyphenols of Cabernet Sauvignon Grapes. Sci. Hortic. 2025, 341, 113996. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Li, J.; Wang, B.; Li, M.; Ma, T.; Jiang, Y.; Zhang, B. Volatile Organic Compound Dynamics in Ugni Blanc and Vidal Wines during Fermentation in the Hexi Corridor (China): Insights from E-Nose, GC-MS, GC-IMS, and Multivariate Statistical Models. LWT 2025, 217, 117440. [Google Scholar] [CrossRef]





| Sample Number | Variety Name |
|---|---|
| BBH | Beibinghong |
| XLH | Xuelanhong |
| SH | Shuanghong |
| ZYH | Zuoyouhong |
| Parameter | Condition | Parameter | Condition |
|---|---|---|---|
| Vial | 20 mL | Trap Initial Temp. | 50 °C |
| Sample Amount | 1 g/mL | Trap Split Flow | 10 min |
| Incubation Temp. | 50 °C | Trap Hold Time | 34 s |
| Incubation Time | 30 min | Trap Final Temp. | 240 °C |
| Shaker Speed | 500 r/min | Oven Initial Temp. | 50 °C |
| Injection Volume | 3000 μL | Temperature Program | 2 °C/s to 250 °C |
| Injection Speed | 125 μL/s | Acquisition Time | 110 s |
| Inlet Temp. | 200 °C | Detector Temp. | 260 °C |
| Injection Duration | 29 s | FID Gain | 12 |
| Compound | Flavor Profile | Odor Threshold (µg/kg) | OAV > 1 | |||
|---|---|---|---|---|---|---|
| BBH | SH | XLH | ZYH | |||
| Ethyl isobutyrate | Sweet, Fruit, Cheese | 0.089 | 13,693.83 ± 209.89 b | 14,060.92 ± 194.22 a | 5985.70 ± 193.39 c | 4393.84 ± 7.05 d |
| Ethyl acetate | Aromatic, Brandy, Grape | 5 | 3325.01 ± 2.99 a | 3063.88 ± 2.92 b | 2568.70 ± 13.24 c | 1870.44 ± 3.10 d |
| Ethyl butyrate | Apple, Butter, Cheese, Pineapple, Strawberry | 0.9 | 1281.64 ± 91.78 b | 1268.66 ± 73.52 b | 1848.73 ± 14.15 a | 1369.19 ± 1.45 b |
| Isoamyl acetate | Apple, Banana, Pear | 19 | 193.68 ± 3.45 a | 111.26 ± 2.15 c | 128.72 ± 2.80 b | 101.61 ± 0.36 d |
| Ethyl propionate | Apple, Pineapple, Rum, Strawberry | 10 | 138.14 ± 0.98 b | 195.89 ± 1.82 a | 123.63 ± 2.14 c | 27.18 ± 0.53 d |
| Ethyl acrylate | Pungent, Fragrant, | 6.7 | 71.00 ± 2.18 a | 52.74 ± 0.62 b | 22.17 ± 0.67 c | 7.96 ± 0.19 d |
| Isobutyl acetate | Apple, Banana, Floral, Herb | 25 | 65.96 ± 1.16 a | 21.63 ± 0.25 b | 20.71 ± 0.48 b | 7.36 ± 0.17 c |
| Propyl butanoate | Apricot, Fruit, Pineapple, Solvent | 18 | 40.24 ± 0.53 c | 38.43 ± 0.75 d | 42.76 ± 0.38 a | 41.84 ± 0.02 b |
| Pentyl acetate | Apple, Banana, Pear | 43 | 35.73 ± 1.05 a | 32.44 ± 0.65 c | 33.43 ± 0.53 bc | 34.28 ± 0.44 b |
| Butyl butyrate | Floral | 400 | 1.21 ± 0.01 a | 1.16 ± 0.00 b | 1.21 ± 0.01 a | 1.21 ± 0.00 a |
| Ethyl 3-hydroxybutyrate | Marshmallow, Roasted Nut | 2500 | 1.89 ± 0.16 a | 1.13 ± 0.20 b | 0.81 ± 0.08 c | 0.82 ± 0.04 c |
| Methyl butyrate | Apple, Banana, Cheese, Ester, Floral | 59 | 0.88 ± 0.05 d | 9.47 ± 0.12 a | 3.01 ± 0.10 c | 5.91 ± 0.10 b |
| Isoamyl alcohol | Burnt, Cocoa, Floral, Malt | 4 | 108.92 ± 3.20 a | 109.59 ± 1.85 a | 106.15 ± 2.10 a | 106.12 ± 1.86 a |
| Heptanol | Fat, Pungent | 5.4 | 97.58 ± 3.14 a | 81.00 ± 2.49 b | 50.70 ± 1.35 c | 19.06 ± 0.70 d |
| 1-Butanol | Fruit | 459.2 | 11.62 ± 0.03 c | 13.34 ± 0.10 a | 12.68 ± 0.03 b | 11.59 ± 0.03 c |
| trans-3-Hexen-1-ol | Green | 110 | 0.29 ± 0.04 c | 0.45 ± 0.05 b | 0.40 ± 0.04 bc | 2.70 ± 0.14 a |
| cis-4-Heptenal | Green, Fruity | 0.06 | 1015.82 ± 5.31 d | 2611.15 ± 126.00 a | 2218.63 ± 53.97 b | 1386.48 ± 22.36 c |
| 2-Nonenal | Paper | 0.19 | 692.60 ± 11.73 c | 963.96 ± 39.89 b | 692.07 ± 14.07 c | 1420.00 ± 20.49 a |
| Heptanal | Citrus, Fat, Green, Nut | 2.8 | 466.62 ± 4.65 ab | 447.31 ± 11.70 c | 475.64 ± 5.25 a | 454.02 ± 3.99 bc |
| 2-Octenal | Dandelion, Fat, Fruit, Grass, Green, Spice | 3 | 223.70 ± 6.62 b | 451.24 ± 1.91 a | 205.52 ± 1.86 c | 137.64 ± 2.49 d |
| Octylaldehyde | Citrus, Fat, Green, Oil, Pungent | 0.587 | 146.70 ± 7.71 d | 1432.67 ± 114.32 a | 565.29 ± 28.05 b | 258.30 ± 3.95 c |
| 2-Hexenal | Cheese | 88.5 | 97.67 ± 1.59 d | 104.57 ± 0.22 c | 114.05 ± 0.90 a | 111.18 ± 0.27 b |
| 2,6-Dimethyl-5-heptenal | Fruit, Green, Melon | 16 | 38.41 ± 0.46 a | 9.62 ± 0.97 d | 15.00 ± 0.52 c | 19.01 ± 1.49 b |
| Hexanal | Apple, Fat, Fresh, Green, Oil | 5 | 31.78 ± 1.46 a | 24.29 ± 0.99 c | 27.24 ± 0.93 b | 16.38 ± 1.46 d |
| Valeraldehyde | Almond, Bitter, Malt, Oil, Pungent | 12 | 6.65 ± 0.22 c | 10.13 ± 0.32 a | 4.64 ± 0.03 d | 8.45 ± 0.19 b |
| Butanal | Banana, Green, Pungent | 17 | 4.33 ± 0.52 c | 7.27 ± 0.25 b | 7.64 ± 0.11 b | 25.80 ± 0.29 a |
| 2-Acetylthiazole | Nut, Popcorn, Roast, Sulfur | 3 | 234.79 ± 5.10 a | 150.37 ± 1.70 b | 87.57 ± 2.67 c | 45.56 ± 0.95 d |
| 2-Nonanone | Fragrant, Fruit, Green, Hot Milk | 41 | 16.62 ± 0.72 b | 25.58 ± 0.44 a | 15.30 ± 0.08 c | 9.45 ± 0.28 d |
| 3-Pentanone | Green | 40 | 10.02 ± 0.32 b | 49.33 ± 0.35 a | 7.96 ± 0.28 c | 9.67 ± 0.23 b |
| 3-Hydroxy-2-butanone | Butter, Creamy, Green Pepper | 14 | 8.94 ± 0.49 bc | 14.86 ± 0.69 a | 9.51 ± 0.11 b | 8.43 ± 0.34 c |
| 2-Octanone | Fat, Fragrant, Mold | 50.2 | 0.94 ± 0.04 c | 1.33 ± 0.18 b | 1.20 ± 0.01 bc | 2.75 ± 0.40 a |
| Acetone | Pungent | 832 | 0.72 ± 0.04 d | 2.17 ± 0.03 a | 1.72 ± 0.02 c | 1.78 ± 0.01 b |
| Limonene | Citrus, Fresh, Pine | 200 | 0.17 ± 0.02 c | 0.30 ± 0.01 b | 0.18 ± 0.01 c | 1.25 ± 0.01 a |
| Terpinolene | Pine | 200 | 0.53 ± 0.12 d | 1.12 ± 0.10 b | 0.83 ± 0.06 c | 2.31 ± 0.20 a |
| 1-Octene | Oil | 0.5 | 449.79 ± 12.23 c | 865.59 ± 6.27 a | 741.50 ± 14.34 b | 751.05 ± 4.98 b |
| 2-Ethyl-5-methylpyrazine | Fruit, Green, Citrus, | 100 | 2.63 ± 0.24 b | 10.01 ± 0.44 a | 2.17 ± 0.06 c | 1.06 ± 0.04 d |
| 2-Pentylfuran | Butter, Floral, Fruit, Green Bean | 5.8 | 177.85 ± 4.61 d | 203.56 ± 3.01 c | 211.26 ± 4.44 b | 238.86 ± 1.19 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, M.; Cao, W.; Ma, M.; Xu, P.; Li, C.; Pan, Y.; Lu, W. Analysis of Volatile Organic Compounds in Wines from Vitis amurensis Varieties in Xinjiang, China. Foods 2025, 14, 3521. https://doi.org/10.3390/foods14203521
Sun Y, Wang M, Cao W, Ma M, Xu P, Li C, Pan Y, Lu W. Analysis of Volatile Organic Compounds in Wines from Vitis amurensis Varieties in Xinjiang, China. Foods. 2025; 14(20):3521. https://doi.org/10.3390/foods14203521
Chicago/Turabian StyleSun, Yining, Mengqi Wang, Weiyu Cao, Mingjie Ma, Peilei Xu, Changyu Li, Yue Pan, and Wenpeng Lu. 2025. "Analysis of Volatile Organic Compounds in Wines from Vitis amurensis Varieties in Xinjiang, China" Foods 14, no. 20: 3521. https://doi.org/10.3390/foods14203521
APA StyleSun, Y., Wang, M., Cao, W., Ma, M., Xu, P., Li, C., Pan, Y., & Lu, W. (2025). Analysis of Volatile Organic Compounds in Wines from Vitis amurensis Varieties in Xinjiang, China. Foods, 14(20), 3521. https://doi.org/10.3390/foods14203521





