Centesimal Composition, Bioactive Compounds, Antioxidant and α-Glucosidase Inhibitory Activities of Commercial Edible Oyster Mushrooms at Different Maturity Stages in Northern Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Mushroom Strains
2.2. Inoculum and Substrate Preparation
2.3. Cultivation for Fruiting Body Production
2.4. Fruiting Body Harvesting, State Selection, and Sample Preparation
2.5. Determination of Centesimal Composition and Polysaccharide Content
2.6. Determination of Ergothioneine Content
2.7. Determination of Total Phenolic Content and Phenolic Compound Profiles
2.8. Determination of Antioxidant Activity
2.9. Determination of α-Glucosidase Inhibitory Activity
2.10. Statistical Analysis
3. Results and Discussion
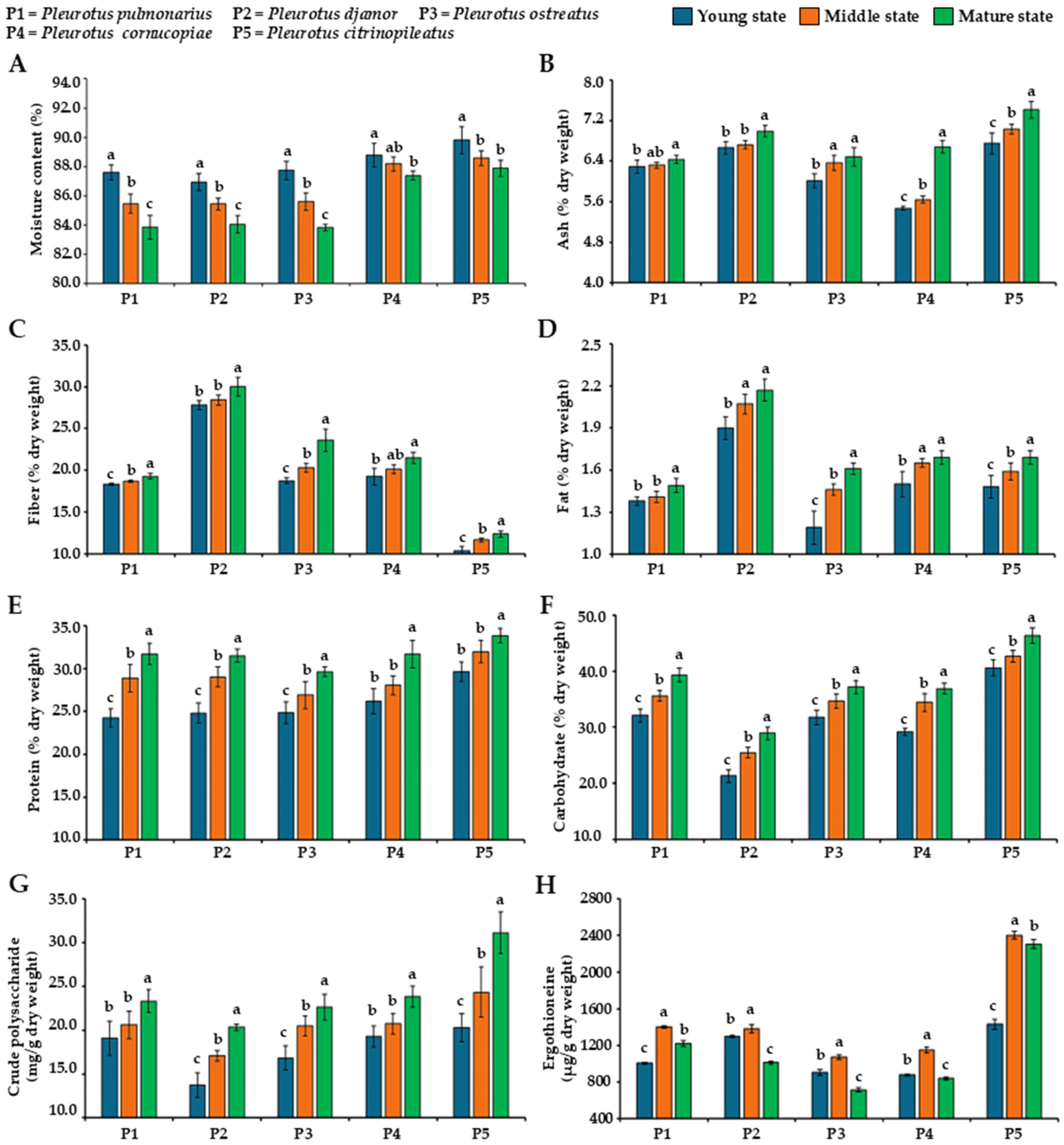
3.1. Moisture Content in Fruiting Bodies
3.2. Determination of Centesimal Composition
3.3. Determination of Polysaccharide Content
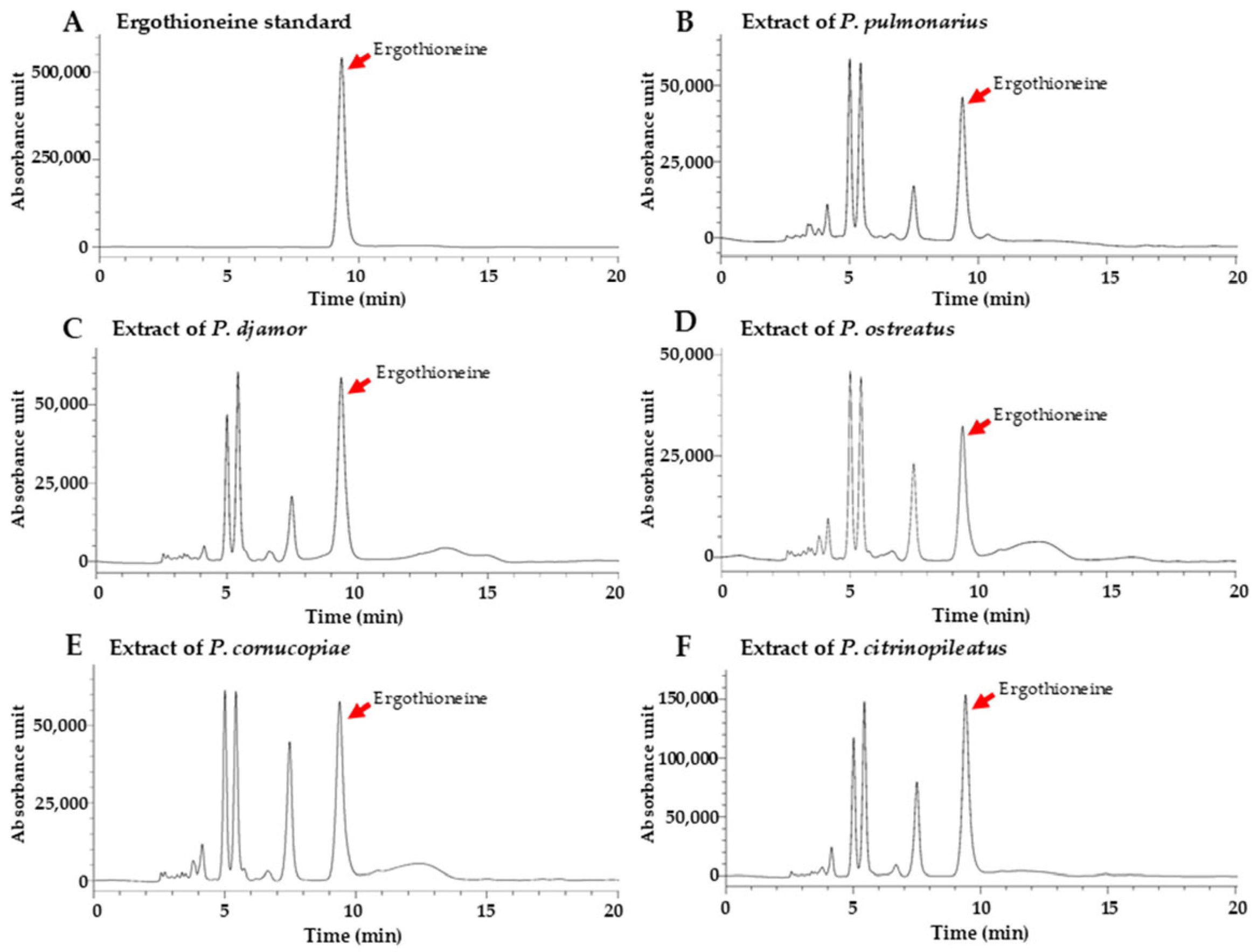
3.4. Determination of Ergothioneine Content
3.5. Determination of Total Phenolic Content and Phenolic Compound Profiles
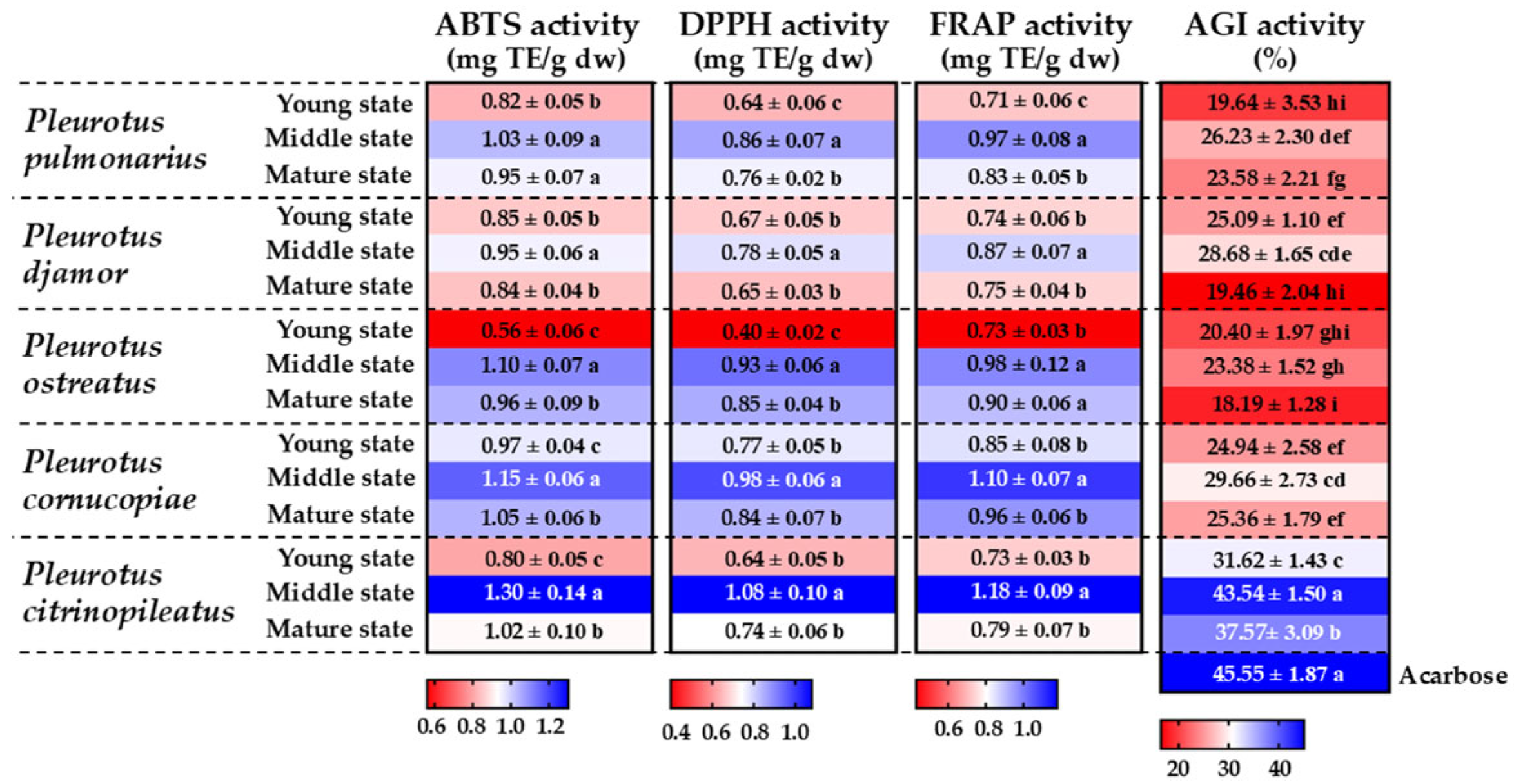
3.6. Determination of Antioxidant Activity
3.7. Determination of α-Glucosidase Inhibitory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, L.H.; Zulkifli, N.A.; Tan, T.C. Edible mushroom: Nutritional properties, potential nutraceutical values, and its utilisation in food product development. In An Introduction to Mushroom; IntechOpen: London, UK, 2020; pp. 1–19. [Google Scholar]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. Pharma. Nutr. 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernándea-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbial. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Cheung, P.C. Nutritional value and health benefits of mushrooms. Mushrooms Funct. Foods 2008, 2, 71–109. [Google Scholar]
- FAOSTAT. Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/#home (accessed on 20 May 2025).
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Cruz-Martins, N.; Dhanjal, D.S.; Singh, R.; Chopra, C.; Verma, R.; Abd-Elsalam, K.A.; et al. Potential usage of edible mushrooms and their residues to retrieve valuable supplies for industrial applications. J. Fungi. 2021, 7, 427. [Google Scholar] [CrossRef] [PubMed]
- Jasinska, A. Sustainability of mushroom cultivation systems. Horticulturae 2023, 9, 1191. [Google Scholar] [CrossRef]
- Grimm, D.; Wösten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [PubMed]
- Stabnikova, O.; Stabnikov, V.; Paredes-López, O. Wild and cultivated mushrooms as food, pharmaceutical and industrial products. Ukrainian Food J. 2024, 13, 20–59. [Google Scholar] [CrossRef]
- Chang, S.T.; Miles, P.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press LLC.: New York, NY, USA, 2004; pp. 1–431. [Google Scholar]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and future prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef]
- Kumla, J.; Thangrongthong, S.; Kaewnunta, A.; Suwannarach, N. Research advances in fungal polysaccharides: Production, extraction, characterization, properties, and their multifaceted applications. Front. Cell. Infect. Microbiol. 2025, 15, 1604184. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, Y.; Zhao, Q.; Patrick, M.H.; Su, L.; Liu, D.; Huang, X.; Long, D.; Tang, Z.; Zhang, Y. The benefits of edible mushroom polysaccharides for health and their influence on gut microbiota: A review. Front. Nutr. 2023, 10, 1213010. [Google Scholar] [CrossRef] [PubMed]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Zhu, M.; Ma, H.; Hu, X.; Ren, L. Recent progress in mushroom-derived ergothioneine: Techniques and applications. Food Biosci. 2024, 62, 105533. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Wang, Z.; Lei, Z.; Jia, Y.; Chen, W.; Shi, R.; Wang, C. A review of novel antioxidant ergothioneine: Biosynthesis pathways, production, function and food applications. Foods 2025, 14, 1588. [Google Scholar] [CrossRef]
- Thomas, T.A.; Francis, R.O.; Zimring, J.C.; Kao, J.P.; Nemkov, T.; Spitalnik, S.L. The role of ergothioneine in red blood cell biology: A review and perspective. Antioxidants 2024, 13, 717. [Google Scholar] [CrossRef]
- Aditya; Neeraj; Jarial, R.S.; Jarial, K.; Bhatia, J.N. Comprehensive review on oyster mushroom species (Agaricomycetes): Morphology, nutrition, cultivation and future aspects. Heliyon 2024, 10, 26539. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and nutritional value of prominent Pleurotus spp.: An overview. Mycobiology 2020, 49, 1–14. [Google Scholar] [CrossRef]
- Devi, P.V.; Islam, J.; Narzary, P.; Sharma, D.; Sultana, F. Bioactive compounds, nutraceutical values and its application in food product development of oyster mushroom. J. Future Foods 2024, 4, 335–342. [Google Scholar]
- The Collection of Production and Marketing Data for Economic Mushrooms and Edible Wild Mushrooms in Thailand. Available online: https://waa.inter.nstda.or.th/stks/pub/2021/20210817-economic-mushroom-market.pdf (accessed on 27 September 2025).
- Pasakawee, K.; Banjongsinsiri, P.; Donrung, N.; Satiankomsorakrai, J. Nutritional and antioxidant properties of selected-commercial mushroom in Thailand. J. Food Sci. Agric. Technol. 2018, 4, 36–40. [Google Scholar]
- Kwon, H.; Thatithatgoon, S. Mushroom Growing for a Living Worldwide: Mushroom Growing in Northern Thailand. In Mushroom Growers’ Handbook1: Oyster Mushroom Cultivation; Gush, R., Ed.; Mush World-Heineart Inc.: Seoul, Republic of Korea, 2004. [Google Scholar]
- Kumla, J.; Suwannarach, N.; Jaiyasen, A.; Bussaban, B.; Lumyong, S. Development of an edible wild strain of Thai oyster mushroom for economic mushroom production. Chiang Mai J. Sci. 2013, 40, 161–172. [Google Scholar]
- Jinanukul, P.; Kumla, J.; Aiduang, W.; Thamjaree, W.; Oranratmanee, R.; Shummadtayar, U.; Tongtuam, Y.; Lumyong, S.; Suwannarach, N.; Waroonkun, T. Comparative evaluation of mechanical and physical properties of mycelium composite boards made from Lentinus sajor-caju with various ratios of corn husk and sawdust. J. Fungi 2024, 10, 634. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Liu, Y.S.; Tanruean, K.; Lumyong, S. Survey of edible Amanita in northern thailand and their nutritional value, total phenolic content, antioxidant and α-glucosidase inhibitory activities. J. Fungi 2023, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Sarker, N.; Kakon, A.; Syed, S.; Elahi, M.T.; Biswas, S.; Tang, S.; Rahman, A.; Paul, D. Comparison of major nutritional constituents and genetic diversity analysis of five strains of oyster mushrooms. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 405–414. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1996. [Google Scholar]
- Saetang, N.; Rattanapot, T.; Manmai, N.; Amornlerdpison, D.; Ramaraj, R.; Unpaprom, Y. Effect of hot water extraction process on schizophyllan from split gill mushroom. Biomass Convers. Biorefin. 2024, 14, 1017–1026. [Google Scholar]
- Zhu, M.; Han, Y.; Hu, X.; Gong, C.; Ren, L. Ergothioneine production by submerged fermentation of a medicinal mushroom Panus conchatus. Fermentation 2022, 8, 431. [Google Scholar] [CrossRef]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Lumyong, S. Phenolic profile of various wild edible mushroom extracts from Thailand and their antioxidant properties, anti-tyrosinase and hyperglycaemic inhibitory activities. J. Funct. Foods 2016, 27, 352–364. [Google Scholar] [CrossRef]
- Thitilertdecha, N.; Teerawutgulrag, A.; Rakariyatham, N. Antioxidant and antimicrobial activities of Nephelium lappacium L. extracts. LWT Food Sci. Technol. 2008, 41, 2029–2035. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Oki, T.; Matsui, T.; Osajima, Y. Inhibitory effect of α-glucosidase inhibitors varies according to its origin. J. Agric. Food Chem. 1999, 47, 550–553. [Google Scholar] [CrossRef]
- Tanruean, K.; Kaewnarin, K.; Suwannarach, N.; Lumyong, S. Comparative evaluation of phytochemicals, and antidiabetic and antioxidant activities of Cuscuta reflexa grown on different hosts in northern Thailand. Nat. Prod. Commun. 2017, 12, 51–54. [Google Scholar] [CrossRef]
- Toler, K.D.; Abera, S. Nutritional quality of oyster mushroom (Pleurotus ostreatus) as affected by osmotic pretreatments and drying methods. Food Sci. Nutr. 2017, 5, 989–996. [Google Scholar] [CrossRef]
- Piskov, S.; Timchenko, L.; Grimm, W.D.; Rzhepakovsky, I.; Avanesyan, S.; Sizonenko, M.; Kurchenko, V. Effects of various drying methods on some physico-chemical properties and the antioxidant profile and ACE inhibition activity of oyster mushrooms (Pleurotus ostreatus). Foods 2020, 9, 160. [Google Scholar] [CrossRef]
- Effiong, M.E.; Umeokwochi, C.P.; Afolabi, I.S.; Chinedu, S.N. Assessing the nutritional quality of Pleurotus ostreatus (oyster mushroom). Front. Nutri. 2024, 10, 1279208. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Srivastav, P.P.; Mishra, H.N. Optimization of microwave-convective drying of oyster mushrooms (Pleurotus ostreatus) using response-surface methodology. Int. Food Res. J. 2014, 21, 1575–1581. [Google Scholar]
- Radzi, M.P.M.F.; Azizah, M.; Maininah, T.; Sumaiyah, A. Growth, yield and antioxidant activity of grey oyster mushroom (Pleurotus pulmonarius) grown in sawdust substrate with the supplementation of alkaline materials. J. Anim. Plant Sci. 2021, 31, 1699–1711. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Qi, Z.; Deng, Z.; Han, A.; Long, H.; Wang, J.; Yao, W.; et al. Advances in postharvest storage and preservation strategies for Pleurotus eryngii. Foods 2023, 12, 1046. [Google Scholar] [CrossRef]
- Koo, C.D.; Cho, S.Y. Relationship between water content and osmotic potential of Lentinula edodes. Mycobiology 2008, 36, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Sun, H.; Ni, Y.; Tang, J.; Yu, J.; Wu, J.; Yan, X.; Deng, X. Dynamics of water status during fruiting body development of Agaricus bisporus and its relationship to mushroom quality. Chem. Biol. Technol. Agric. 2025, 12, 14. [Google Scholar] [CrossRef]
- Herman, K.C.; Bleichrodt, R. Go with the flow: Mechanisms driving water transport during vegetative growth and fruiting. Fungal Biol. Rev. 2022, 41, 10–23. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hossain, A.; Rahman, M.S.; Bashir, N.M.B.; Mia, R. Study on maturity level of Pleurotus cystidiosus-2 maple oyster mushroom emphasized on organoleptic taste and nutrient content. J. Nutr. Sci. Res. 2021, 6, 153. [Google Scholar]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Bak, W.C.; Park, J.H.; Park, Y.A.; Ka, K.H. Determination of glucan contents in the fruiting bodies and mycelia of Lentinula edodes cultivars. Mycobiology 2014, 42, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Dikeman, C.L.; Bauer, L.L.; Flickinger, E.A.; Fahey, G.C. Effects of stage of maturity and cooking on the chemical composition of select mushroom varieties. J. Agric. Food Chem. 2005, 53, 1130–1138. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional composition and biological properties of sixteen edible mushroom species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Timm, T.G.; Arantes, M.S.T.; de Oliveira, E.H.S.; Tavares, L.B.B.; Mathias, Á.L.; da Silva, V.R.; Helm, C.V. Substrate effects on the growth, yield, and nutritional composition of edible mushrooms. Adv. Appl. Microbiol. 2025, 130, 159–190. [Google Scholar]
- Fernando, L.D.; Widanage, M.C.D.; Penfield, J.; Lipton, A.S.; Washton, N.; Latgé, J.-P.; Wang, P.; Zhang, L.; Wang, T. Structural polymorphism of chitin and chitosan in fungal cell walls from solid-state NMR and principal component analysis. Front. Mol. Biosci. 2021, 8, 727053. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Zhang, Y.; Zhao, X. Structural characterization and antioxidant activity of a polysaccharide from the fruiting bodies of Pleurotus ostreatus at different maturity stages. Int. J. Biol. Macromol. 2017, 103, 570–576. [Google Scholar]
- Petraglia, T.; Latronico, T.; Fanigliulo, A.; Crescenzi, A.; Liuzzi, G.M.; Rossano, R. Antioxidant activity of polysaccharides from the edible mushroom Pleurotus eryngii. Molecules 2023, 28, 2176. [Google Scholar] [CrossRef]
- Toh, C.J.Y.S.; Bi, X.; Lee, H.W.; Yeo, M.T.Y.; Henry, C.J. Is mushroom polysaccharide extract a better fat replacer than dried mushroom powder for food applications? Front. Nutr. 2023, 10, 1111955. [Google Scholar] [CrossRef]
- Baeva, E.; Bleha, R.; Lavrova, E.; Sushytskyi, L.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Polysaccharides from basidiocarps of cultivating mushroom Pleurotus ostreatus: Isolation and structural characterization. Molecules 2019, 24, 2740. [Google Scholar] [CrossRef]
- Leong, Y.K.; Yang, F.C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef]
- Abreu, H.; Zavadinack, M.; Smiderle, F.R.; Cipriani, T.R.; Cordeiro, L.M.C.; Iacomini, M. Polysaccharides from Pleurotus eryngii: Selective extraction methodologies and their modulatory effects on THP-1 macrophages. Carbohyd. Polym. 2021, 15, 117177. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Smiderle, F.R.; Morales, D.; Iacomini, M.; Soler-Rivas, C. Strengths and weaknesses of the aniline-blue method used to test mushroom (1→3)-β-d-glucans obtained by microwave-assisted extractions. Carbohydr. Polym. 2019, 217, 135–143. [Google Scholar] [CrossRef]
- Rodríguez-Seoane, P.; Torres Perez, M.D.; Fernández de Ana, C.; Sinde-Stompel, E.; Domínguez, H. Antiradical and functional properties of subcritical water extracts from edible mushrooms and from commercial counterparts. Int. J. Food Sci. Technol. 2022, 57, 1420–1428. [Google Scholar] [CrossRef]
- Rosyida, V.T.; Hayati, S.N.; Wiyono, T.; Darsih, C.; Ratih, D. Effect of aqueous extraction method on total water-soluble polysaccharides content and phytochemical properties of white oyster mushroom (Pleurotus ostreatus). IOP Conf. Ser. Earth Environ. Sci. 2024, 1377, 012064. [Google Scholar] [CrossRef]
- Nachimuthu, S.; Kandasamy, R.; Ponnusamy, R.; Deruiter, J.; Dhanasekaran, M.; Thilagar, S. L-Ergothioneine: A Potential Bioactive Compound from Edible Mushrooms. In Medicinal Mushrooms; Agrawal, D., Dhanasekaran, M., Eds.; Springer: Singapore, 2019. [Google Scholar]
- Chen, S.Y.; Ho, K.J.; Hsieh, Y.J.; Wang, L.T.; Mau, J.L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT-Food Sci. Technol. 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Giri, A.; Ohshima, T. A rapid HPLC post-column reaction analysis for the quantification of ergothioneine in edible mushrooms and in animals fed a diet supplemented with extracts from the processing waste of cultivated mushrooms. Food Chem. 2012, 133, 585–591. [Google Scholar] [CrossRef]
- Chilanti, G.; da Rosa, L.O.; Poleto, L.; Branco, C.S.; Camassola, M.; Fontana, R.C.; Dillon, A.J. Effect of different substrates on Pleurotus spp. cultivation in Brazil-Ergothioneine and lovastatin. J. Food Compos. Anal. 2022, 107, 104367. [Google Scholar] [CrossRef]
- Tsiantas, K.; Tsiaka, T.; Koutrotsios, G.; Siapi, E.; Zervakis, G.I.; Kalogeropoulos, N.; Zoumpoulakis, P. On the identification and quantification of ergothioneine and lovastatin in various mushroom species: Assets and challenges of different analytical approaches. Molecules 2021, 26, 1832. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kato, M.; Tsuchida, H.; Harada, E.; Niwa, T.; Osawa, T. Ergothioneine as an anti-oxidative/anti-inflammatory component in several edible mushrooms. Food Sci. Technol. Res. 2011, 17, 103–110. [Google Scholar] [CrossRef]
- Kataoka, R.; Nigaki, A.; Barua, B.S.; Yamashita, K. Ergothioneine circulation in mushroom cultivation using food waste recycling. Recycling 2025, 10, 91. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Dašić, M. Mushrooms as possible antioxidant and antimicrobial agents. Iran. J. Pharm. Res. 2012, 11, 1095. [Google Scholar]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, antiradical potential and phenolic compounds of thirty-one polish mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef]
- Chilanti, G.; Todescatto, K.; Andrade, L.B.; Branco, C.S.; Salvador, M.; Camassola, M.; Fontana, R.C.; Dillon, A.J.P. Polyphenolic content and antioxidant activity of mycelia and basidiomes of oyster mushrooms Pleurotus spp. (Agaricomycetes) from Brazil. Int. J. Med. Mushrooms 2021, 23, 13–23. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Y.; Ren, J.; Qin, N. Yield, nutritional content, and antioxidant activity of Pleurotus ostreatus on corncobs supplemented with herb residues. Mycobiology 2018, 46, 24–32. [Google Scholar] [CrossRef]
- González-Palma, I.; Escalona-Buendía, H.B.; Ponce-Alquicira, E.; Téllez-Téllez, M.; Gupta, V.K.; Díaz-Godínez, G.; Soriano-Santos, J. Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Front. Microbiol. 2016, 7, 1099. [Google Scholar] [CrossRef]
- El-Razek, A.; Amal, M.; Ibrahim, A.; Elattar, A.; Asker, D. Utilization of agro-wastes to produce oyster mushroom (Pleurotus ostreatus) with high antioxidant and antimicrobial activities. Alex. J. Food Sci. Technol. 2020, 17, 1–15. [Google Scholar] [CrossRef]
- Yim, H.S., Jr.; Chye, F.Y.; Tan, C.T.; Ng, Y.C.; Ho, C.W. Antioxidant activities and total phenolic content of aqueous extract of Pleurotus ostreatus (cultivated oyster mushroom). Malays. J. Nutr. 2010, 16, 281–291. [Google Scholar]
- Yin, C.; Fan, X.; Liu, C.; Fan, Z.; Shi, D.; Yao, F.; Cheng, W.; Gao, H. The antioxidant properties, tyrosinase and α-glucosidase inhibitory activities of phenolic compounds in different extracts from the golden oyster mushroom, Pleurotus citrinopileatus (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 865–874. [Google Scholar] [CrossRef]
- Tan, Y.S.; Baskaran, A.; Nallathamby, N.; Chua, K.H.; Kuppusamy, U.R.; Sabaratnam, V. Influence of customized cooking methods on the phenolic contents and antioxidant activities of selected species of oyster mushrooms (Pleurotus spp.). J. Food Sci. Tech. 2015, 52, 3058–3064. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 2016, 242, 723–732. [Google Scholar] [CrossRef]
- Pandey, K.; Ghosh, S.K.; Sanyal, T.; Bera, T.; Pal, S. Mycochemistry, antioxidant content, and antioxidant potentiality of the ethanolic extract of Pleurotus florida and its anti-cancerous effect on HeLa cancer cell line, and antitumor effect on HeLa-implanted mice. Int. J. Health Sci. 2023, 17, 18–35. [Google Scholar]
- Matkovits, A.; Fodor, M.; Jókai, Z. Analysis of polyphenol patterns of Pleurotus ostreatus cultivars by UHPLC-ESI-MS/MS; application of ft-nir and chemometric methods, classification options. Chemosensors 2024, 12, 19. [Google Scholar] [CrossRef]
- Piska, K.; Sułkowska-Ziaja, K.; Muszyńska, B. Edible mushroom Pleurotus ostreatus (oyster mushroom)–its dietary significance and biological activity. Acta Sci. Pol. Hortorum. Cultus. 2017, 16, 151–161. [Google Scholar]
- Villalobos-Pezos, M.; Muñoz-Fariña, O.; Ah-Hen, K.S.; Garrido-Figueroa, M.F.; García-Figueroa, O.; González-Esparza, A.; de Medina, L.G.-P.; Bastías-Montes, J.M. Optimization of phenolic content extraction and effects of drying treatments on physicochemical characteristics and antioxidant properties of edible mushroom Pleurotus ostreatus (Jacq.) P. Kumm (oyster mushroom). Antioxidants 2024, 13, 1581. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Mourão, F.; Umeo, S.H.; Takemura, O.S.; Linde, G.A.; Colauto, N.B. Antioxidant activity of Agaricus brasiliensis basidiocarps on different maturation phases. Braz. J. Microbiol. 2011, 42, 197–202. [Google Scholar] [CrossRef]
- Raman, J.; Lakshmanan, H.; Jang, K.Y.; Oh, M.; Oh, Y.L.; Im, J.H. Nutritional composition and antioxidant activity of pink oyster mushrooms (Pleurotus djamor var. roseus) grown on a paddy straw substrate. J. Mushrooms 2020, 18, 189–200. [Google Scholar]
- Bristy, A.T.; Islam, T.; Ahmed, R.; Hossain, J.; Reza, H.M.; Jain, P. Evaluation of total phenolic content, HPLC analysis, and antioxidant potential of three local varieties of mushroom: A comparative study. Int. J. Food Sci. 2022, 2022, 3834936. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H.; Seo, S.H.; Kang, E.-Y.; Kim, S.-L.; Park, Y.-J. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Dubost, N.J.; Ou, B.; Beelman, R.B. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert Opi. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef]
- Agrawal, N.; Sharma, M.; Singh, S.; Goyal, A. Recent advances of α-glucosidase inhibitors: A comprehensive review. Curr. Top. Med. Chem. 2022, 22, 2069–2086. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpushkar, A.; Agrawal, D.; Chourasia, A.; Shrivastava, A.; Dubey, N. In vitro α-amylase and α-glucosidase inhibitory potential of Pleurotus ostreatus cv. florida extract. Indian J. Biochem. Biophys. 2022, 59, 1016–1019. [Google Scholar]
- Wahab, N.A.; Abdullah, N.; Aminudin, N. Characterisation of potential antidiabetic-related proteins from Pleurotus pulmonarius (Fr.) Quél. (grey oyster mushroom) by MALDI-TOF/TOF mass spectrometry. BioMed Res. Int. 2014, 2024, 131607. [Google Scholar]
- Im, K.H.; Nguyen, T.K.; Choi, J.; Lee, T.S. In vitro antioxidant, anti-diabetes, anti-dementia, and inflammation inhibitory effect of Trametes pubescens fruiting body extracts. Molecules 2016, 21, 639. [Google Scholar] [CrossRef]
- Sknepnek, A.; Miletić, D.; Stupar, A.; Salević-Jelić, A.; Nedović, V.; Cvetanovic Kljakic, A. Natural solutions for diabetes: The therapeutic potential of plants and mushrooms. Front. Nutr. 2025, 12, 1511049. [Google Scholar] [CrossRef]
- Nagarani, G.; Abirami, A.; Siddhuraju, P. A comparative study on antioxidant potentials, inhibitory activities against key enzymes related to metabolic syndrome, and anti-inflammatory activity of leaf extract from different Momordica species. Food Sci. Hum. Wellness 2014, 3, 36–46. [Google Scholar] [CrossRef]
- Shodehinde, S.A.; Oboh, G. In vitro antioxidant activities and inhibitory effects of aqueous extracts of unripe plantain pulp (Musa paradisiaca) on enzymes linked with type 2 diabetes and hypertension. J. Toxicol. Environ. Health Sci. 2012, 4, 65–75. [Google Scholar]


| Pleurotus Species | Morphological Characteristics | ||
|---|---|---|---|
| Young Stage | Middle Stage | Mature Stage | |
| P. citrinopileatus | Cap: 5–20 mm in diameter, bright yellow, convex with curled edges. Stipe: 10–20 mm long. | Cap: 21–40 mm in diameter, yellow, expanded and beginning to flatten. Stipe: elongated, 21–40 mm long. | Cap: ≥41 mm in diameter, yellow to pale yellow, flat with slightly downturned edges. Stipe: ≥41 mm long, cylindrical, and slender. |
| P. cornucopiae | Cap: 10–25 mm in diameter, gray, convex with inrolled margins. Stipe: 20–30 mm long | Cap: 26–50 mm in diameter, gray to light brownish, flattened with margins starting to expand. Stipe: elongated, 31–40 mm long. | Cap: ≥51 mm in diameter, pale brown, fully expanded or slightly depressed in the center. Stipe: ≥41 mm long, often curved or tapering. |
| P. djamor | Cap: 10–25 mm in diameter, bright pink with edges curled inward. Stipe: 5–10 mm long. | Cap: 26–50 mm in diameter, pink to light pink, flat with wavy edges. Stipe: 11–25 mm long. | Cap: ≥51 mm in diameter, pale pink to salmon pink, wide and flat with wavy edges that may curl upward. Stipe: ≥26 mm long. |
| P. ostreatus | Cap: 10–25 mm in diameter, gray to brown with tightly curved edges. Stipe: 5–15 mm long. | Cap: 26–50 mm in diameter, light gray with tightly curved edges. Stipe: 16–30 mm long. | Cap: ≥51 mm in diameter, pale gray to white, flat to funnel shaped. Stipe: ≥31 mm long, cylindrical, and slender. |
| P. pulmonarius | Cap: 10–30 mm in diameter, light gray, convex with curled edges. Stipe: 5–20 mm long. | Cap: 31–60 mm in diameter, light gray to pale gray, flattened. Stipe: 21–40 mm long. | Cap: ≥61 mm in diameter, pale gray to light brown, flat to funnel-shaped. Stipe: ≥41 mm long, cylindrical, and slender. |
| Parameter | Pearson Correlation Coefficients with Increasing Maturity (r/p-Value) | ||||
|---|---|---|---|---|---|
| P. pulmonarius | P. djamor | P. ostreatus | P. cornucopiae | P. citrinopileatus | |
| Moisture | −0.922 */<0.01 | −0.928 */<0.01 | −0.960 */<0.01 | −0.737 */0.02 | −0.784 */<0.01 |
| Ash | 0.549 */0.03 | 0.787 */<0.01 | 0.783 */<0.01 | 0.941 */<0.01 | 0.872 */<0.01 |
| Fiber | 0.864 */<0.01 | 0.763 */<0.01 | 0.914 */<0.01 | 0.831 */<0.01 | 0.896 */<0.01 |
| Fat | 0.741 */0.02 | 0.833 */<0.01 | 0.921 */<0.01 | 0.793 */<0.01 | 0.833 */<0.01 |
| Protein | 0.924 */<0.01 | 0.934 */<0.01 | 0.871 */<0.01 | 0.862 */<0.01 | 0.909 */<0.01 |
| Carbohydrate | 0.948 */<0.01 | 0.955 */<0.01 | 0.893 */<0.01 | 0.932 */<0.01 | 0.889 */<0.01 |
| Polysaccharide | 0.761 */<0.01 | 0.858 */<0.01 | 0.887 */<0.01 | 0.853 */<0.01 | 0.896 */<0.01 |
| Ergothioneine | 0.542/0.06 | 0.733 */<0.01 | 0.522/0.08 | 0.113/0.72 | 0.83 */<0.01 |
| TPC | 0.455/0.14 | 0.587/0.55 | 0.347/0.27 | 0.573/0.86 | 0.466/0.12 |
| ABTS Activity | 0.499/0.98 | 0.121/0.71 | 0.398/0.72 | 0.370/0.23 | 0.389/0.21 |
| DPPH Activity | 0.496/0.10 | 0.08/0.78 | 0.780 */<0.01 | 0.283/0.37 | 0.185/0.56 |
| FRAP Activity | 0.397/0.20 | 0.267/0.93 | 0.467/0.40 | 0.368/0.24 | 0.114/0.723 |
| AGI Activity | 0.446/0.14 | 0.560/0.58 | 0.353/0.26 | 0.584/0.85 | 0.466/0.13 |
| Parameter | Pearson Correlation Coefficients (r/p-Value) | |||
|---|---|---|---|---|
| Antioxidant Activity | α-Glucosidase Inhibitory Activity | |||
| ABTS Activity | DPPH Activity | FRAP Activity | ||
| Total phenolic content | 0.594 */0.02 | 0.493/0.06 | 0.589 */0.04 | 0.995 */<0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumla, J.; Lumyong, S.; Suwannarach, N. Centesimal Composition, Bioactive Compounds, Antioxidant and α-Glucosidase Inhibitory Activities of Commercial Edible Oyster Mushrooms at Different Maturity Stages in Northern Thailand. Foods 2025, 14, 3511. https://doi.org/10.3390/foods14203511
Kumla J, Lumyong S, Suwannarach N. Centesimal Composition, Bioactive Compounds, Antioxidant and α-Glucosidase Inhibitory Activities of Commercial Edible Oyster Mushrooms at Different Maturity Stages in Northern Thailand. Foods. 2025; 14(20):3511. https://doi.org/10.3390/foods14203511
Chicago/Turabian StyleKumla, Jaturong, Saisamorn Lumyong, and Nakarin Suwannarach. 2025. "Centesimal Composition, Bioactive Compounds, Antioxidant and α-Glucosidase Inhibitory Activities of Commercial Edible Oyster Mushrooms at Different Maturity Stages in Northern Thailand" Foods 14, no. 20: 3511. https://doi.org/10.3390/foods14203511
APA StyleKumla, J., Lumyong, S., & Suwannarach, N. (2025). Centesimal Composition, Bioactive Compounds, Antioxidant and α-Glucosidase Inhibitory Activities of Commercial Edible Oyster Mushrooms at Different Maturity Stages in Northern Thailand. Foods, 14(20), 3511. https://doi.org/10.3390/foods14203511







