1. Introduction
The food sector is constantly growing to meet the increasing demands of the global population, and the fruit and vegetable processing industry also contributes to this growth. The proportion of waste and by-products produced by fruits and vegetables processed in these businesses is reported to be 25–30%, varying from product to product. The circular bioeconomy, which focuses on the sustainable use of biological resources, positions these products as valuable raw materials for the recovery of bioactive compounds [
1,
2,
3,
4,
5]. This approach aligns with the green extraction framework and promotes polyphenol recovery using low-hazard solvents [
2,
6].
In this study, peel and core fractions were prepared as laboratory-simulated residues rather than industrial by-products, in order to minimize the confounding effects of enzymatic browning, microbial activity, and oxidative changes that are typical of industrial waste streams. This approach, commonly used in fruit by-product valorization research, ensures reproducibility and allows solvent-related effects to be isolated more clearly [
7,
8]. Quince (
Cydonia oblonga Mill.), introduced in the following section, was selected as the model fruit for this framework.
Quince belongs to the Maloideae subfamily of the Rosaceae family. Türkiye is the world’s leading producer of quince (192,237 tons in 2023), while global production exceeds 798 thousand tons [
9,
10]. Traditionally used in the production of products such as jam, marmalade, and fruit juice, quince is a firm-textured fruit that is also consumed fresh. Industrial quince processing generates peels and cores/seeds as by-products (~10% by mass), which is lower than in many fruits yet still substantial at production scale [
11,
12,
13]. Classic chemical profiling studies have shown that quince peel, which stands out among these by-products, has a significantly higher phenolic content compared to pulp [
14,
15]. Additionally, extracts obtained from quince by-products, particularly quince peel, have been demonstrated to have antioxidant, antimicrobial, and anti-inflammatory effects. This makes quince attractive as a functional food ingredient [
11,
16,
17].
Previous studies show that quince peel extracts are phenolic-rich and exhibit measurable in vitro antioxidant activity, supporting their use as functional ingredients [
18,
19]. At the same time, in industrial quince processing, peel and core fractions are frequently diverted to low-value uses (e.g., feed, compost) or discarded, indicating limited valorization in many settings [
20], whereas recent assessments support the potential of quince by-products as functional ingredients owing to their composition and antioxidant capacity [
21].
Organic solvents have traditionally been used for polyphenol recovery. However, environmental and safety limitations persist for these solvents [
22]. Therefore, natural deep eutectic solvents (NADES), composed of bio-compatible components (e.g., choline chloride + organic acid/sugar), are emerging as biodegradable, adaptable, and highly effective extraction alternatives [
2,
6,
23]. The type of hydrogen donor component (HBD) and water content of NADES have been shown to significantly affect phenolic solubility by adjusting the viscosity and polarity of the solvent. It is also reported to offer favorable antimicrobial/antioxidant profiles [
23,
24,
25]. Consistent with this rationale, choline chloride-based NADES, with viscosity adjusted by water, have increased phenolic extraction and supported extract stability in orange peel [
26,
27], and improved polyphenol recovery and extraction kinetics in cocoa bean shells relative to conventional media [
25].
Low-temperature conditions during phenolic extraction are critical for preventing their degradation. Ultrasound-assisted extraction is often used to accelerate the extraction process and obtain high yields. When combined with NADES, the process achieves high yields at lower temperatures and in shorter times, reflecting a more favorable environmental profile [
28,
29]. Consistent with this, choline chloride-based NADES optimized via viscosity modulation by water markedly increase total phenolic content (TPC) and total antioxidant capacity (TAC) in polyphenol extraction from plant peels [
25,
30]. In a study conducted on walnut shells from walnut by-products, solvent–matrix interaction was examined from a bioaccessibility perspective, and it was reported that phenolics were efficiently extracted with ultrasound-assisted extraction (UAE) + NADES, and the resulting fractions were examined within the in vitro bioaccessibility window [
31].
While studies conducted explicitly on quince indicate that it is rich in the phenolic profile and bioactivity of traditional organic solvent extracts [
12,
14,
15], data on the post-digestion bioaccessibility of extracts obtained from quince peel and core using NADES have not been investigated. However, the decisive factor for consumer health is the release of extract components during digestion and their transition to the soluble phase, i.e., their bioaccessibility; a high extract content alone is not sufficient [
31]. Furthermore, NADES formulation (hydrogen-bond acceptor (HBA)/HBD selection and water percentage) and UAE application may have different effects on the persistence of individual phenolics and their interactions in the matrix [
23,
25]. The present study addresses this limitation by (i) comparing polyphenol recovery from quince peel (QP) and quince core (QC, seed included inner fraction) by UAE with conventional ethanol and NADES, (ii) determination of individual phenolic content by LC-MS/MS system, (iii) evaluation of total and individual bioaccessibility of phenolic compounds after static in vitro digestion model. Thus, it aims to provide a feasible framework for translating the green solvent–process combination into the development of functional components from quince by-products.
2. Materials and Methods
2.1. Materials and Sample Preparation
Quince fruits were supplied from the local market. Peel (QP) and core (QC) were prepared as laboratory-simulated by-products. The washed quinces were peeled, the fruit was cut open, and the core was removed. Peels were removed manually with a stainless-steel knife to a target thickness of approximately 1–1.5 mm. Both the QP and the QC were dried in a freeze-dryer (INOFD-12PUP, Innova Bio-Meditech Co., Ltd., Qingdao, China) at −86 °C for 40 h, under a vacuum range of 10–20 Pa, making them ready for grinding. The dried samples were pulverized using an analytical grinder (A11 basic, IKA
®-Werke GmbH & Co. KG, Staufen, Germany). Powders were passed through a 250 µm stainless-steel sieve (200 × 50 mm, 60 mesh, ASTM E11; Retsch GmbH, Haan, Germany) to standardize particle size (<250 µm) and then stored at −20 °C until analysis. All experimental parameters are reported in SI units, and centrifugal forces are expressed as relative g-force (×
g) rather than revolutions per minute (rpm) to ensure reproducibility across instruments. Standardizing peel thickness and particle size in this manner minimizes variability in extraction efficiency associated with diffusion path length and surface area, as also emphasized in previous studies on fruit by-products [
7,
32].
2.2. Preparation of Choline Chloride-Based NADES
In preparing the NADES, choline chloride (ChCl, ≥98%; Sigma-Aldrich, Steinheim, Germany) was used as the hydrogen-bond acceptor. The hydrogen-bond donors were lactic acid (LA, >85%; Sigma-Aldrich, Steinheim, Germany), malic acid (MA, >99.0%; TCI, Tokyo, Japan), citric acid (CA, >98.0%; TCI, Tokyo, Japan), glucose (Glu, >98.0%; Merck, Darmstadt, Germany), glycerol (Gly, >99.0%; Merck, Darmstadt, Germany), and xylitol (Xyl > 98.5%; Kimyalab, Istanbul, Turkey). NADES mixtures were prepared at the molar ratios reported in previous studies and mixed at 80 °C in a shaking water bath (Nuve ST 30, Ankara, Turkey) until homogeneous, colorless liquids formed. Distilled water (30%,
v/
v) was then added to lower viscosity; the mixtures were vortexed for 60 s and treated in an ultrasonic bath (Isolab, Wertheim, Germany) for 5 min. The 30% water addition was intended to enhance mass transfer and improve interactions with plant matrices by reducing inherent viscosity. This level was chosen based on reports that 30% water can notably improve extraction performance without disrupting the eutectic structure or hydrogen-bonding capacity of NADES [
33,
34]. Maintaining this balance is critical: too much water weakens the unique solvent properties of NADES, whereas too little leaves them highly viscous and less practical to use [
34,
35]. A 70% ethanol solution was used for comparison (
Table 1). Ethanol (70%) is commonly used as a benchmark for extracting plant phenolics because hydroethanolic mixtures in the ~30–70%
v/
v range balance polarity and mass transfer; accordingly, many studies and reviews adopt ~70% ethanol as a standard condition [
36,
37,
38].
2.3. Characterization of NADES
2.3.1. Density
One-milliliter aliquots of each NADES were weighed at room temperature with a semi-micro balance (AUW220D, Shimadzu, Tokyo, Japan).
The density was then calculated using the formula: ρ = mNADES/VNADES.
2.3.2. Fourier Transform İnfrared (FTIR) Spectroscopy
FTIR spectra of NADES were measured at room temperature (Shimadzu IRTracer-100, Tokyo, Japan) in the wavelength range of 4000 to 500 cm−1, with a resolution of 4 cm−1 and 32 scans per spectrum.
2.4. Ultrasound-Assisted Extraction (UAE) of Phenolic Compounds
Extraction of phenolic compounds was performed as previously described in the literature [
39]. As indicated in
Table 1, 10 mL aliquots of NADES solutions or ethanol (70%) were added to each of the prepared QP and QC powders (0.5 g). Extractions were conducted in 50 mL conical tubes (10 mL working volume), capped, and held upright in the bath; the water level was adjusted to match the liquid level in the tubes. The mixture was placed in an ultrasonic bath (Isolab, Eschau, Germany) at 60 °C, 40 kHz, and 180 W for 30 min. The mixtures were then centrifuged at 11,180×
g (Hitachi CF15RN, Tokyo, Japan) for 10 min, and supernatants were transferred to clean tubes. All extracts obtained were stored at 4 °C until analysis.
2.5. Total Phenolic Content
The TPC of the samples was determined as previously described [
40]. Results were obtained using a gallic acid standard curve (prepared in distilled water immediately before use, concentration range: 10–400 mg L
−1; R
2 > 0.999) and reported as milligrams of gallic acid equivalent (mg GAE) per gram of dry weight (mg GAE g
−1 dw). For each measurement, 100 μL of extract was added into a cuvette, followed by 750 μL of Folin–Ciocalteu reagent. After 5 min of incubation at room temperature, 750 μL of 6% (
w/
v) Na
2CO
3 solution was added, and the mixture was incubated for 90 min in the dark. To reduce matrix effects caused by NADES, given their reducing character, all mixtures were centrifuged at 11,180×
g for 5 min before absorbance measurement. After this step, which helps to remove particulate matter and increase the accuracy of spectrophotometric readings, the mixtures were transferred to cuvettes and absorbance was measured at 725 nm in a spectrophotometer (Cary 60, Agilent Technologies, Santa Clara, CA, USA). Baseline absorbance was corrected against matched digestion blanks (gastric and intestinal) processed in parallel for each solvent system (70% ethanol or the corresponding NADES), i.e., each extract was read against its own digestion blank.
2.6. Total Antioxidant Capacity Assays (DPPH and CUPRAC)
The TAC of the samples was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and cupric ion-reducing antioxidant capacity (CUPRAC) assays [
41,
42].
DPPH assay was performed by mixing 100 µL of extract with 2.0 mL of DPPH working solution, incubating in the dark for 30 min at room temperature, and reading at 517 nm [
41]. Trolox standards (10–200 mg L
−1, prepared immediately before use) yielded R
2 ≥ 0.999. Results were expressed as mg Trolox equivalents per g dry weight (mg TE g
−1 dw).
CUPRAC assay was carried out by combining 100 µL of extract sequentially with 1.0 mL of Cu (II) chloride solution, 1.0 mL of neocuproine solution, 1.0 mL of ammonium acetate solution, and 1.0 mL of deionized water, which were incubated in the dark for 30 min at room temperature, and a reading was performed at 450 nm [
42]. Trolox standards (100–600 mg L
−1) provided R
2 ≥ 0.996. DPPH and CUPRAC readings were blank-subtracted against solvent- and phase-matched digestion blanks (70% ethanol or the relevant NADES processed through the same gastric/intestinal step without sample), so that observed differences between solvents reflect extract composition rather than matrix effects.
2.7. Identification and Quantification of Phenolic Compounds Using UPLC-ESI-MS/MS
Phenolic compounds were identified and quantified using an ultra-performance liquid chromatography–electrospray ionization tandem mass spectrometry (UPLC-ESI-MS/MS) system (Shimadzu LC-MS/MS 8060, Kyoto, Japan). A C18 column (100 × 3 mm, 3 µm; GL Sciences, Tokyo, Japan) was used for analysis, and the MS was operated in both negative and positive ESI modes. Ultrapure water (Purelab Classic, ELGA LabWater, High Wycombe, UK) and acetonitrile (LC-MS Grade; Merck, Darmstadt, Germany) were used as mobile phases in a gradient elution (
Table S1). Analyses were performed at a flow rate of 0.40 mL min
−1, with the column temperature at 40 °C and the autosampler maintained at 4 °C; the injection volume was 10 µL [
43]. The MRM transitions and MS parameters are summarized in
Table 2, and the calibration/validation data (slope, intercept, R
2, LOD, LOQ) are provided in
Table 3.
Sample spectra were recorded in multiple reaction monitoring (MRM) mode, and data acquisition was performed using Shimadzu LabSolution software (version 5.91, Shimadzu, Kyoto, Japan). Compounds were quantified using the following external standards: 3,4-dimethoxycinnamic acid (3,4-DMCA), epicatechin ((-)-epicatechin), rutin, quercetin, kaempferol, and chlorogenic acid (3-caffeoylquinic acid) from TRC (Toronto Research Chemicals, Vaughan, ON, Canada); isoquercetin (quercetin-3-glucoside) and p-coumaric acid (4-hydroxycinnamic acid) were from Sigma-Aldrich. Standards were stored at −20 °C until preparation, in dry form, and their purity was ≥95–99%. The purity of methanol (Merck) used for stock solutions was LC-MS grade. Results are reported as µg g−1 (dw).
Target compounds were quantified by UPLC–ESI–MS/MS in MRM mode using compound-specific transitions and collision energies (
Table 2). Quantification used external calibration with authentic standards; linearity across the working ranges and LOD/LOQ are reported in
Table 3. Analyte identity was accepted when the retention time fell within the predefined window and the quantifier/qualifier ion ratio was within tolerance. Quantification relied on MRM selectivity; full baseline separation was not required when these criteria were met. Representative chromatograms are provided in
Supplementary Figures S2 and S3.
2.8. In Vitro Digestion Simulation
The in vitro digestion model applied by Minekus et al. [
46] was used in the study. Gastric and intestinal electrolyte solutions were freshly prepared in accordance with the INFOGEST protocol, including the relevant buffer salts and enzymes for each phase (e.g., NaCl, KCl, NaHCO
3, and CaCl
2; pepsin for the gastric step; pancreatin and bile salts for the intestinal step). Stock acid and base solutions (1 M HCl and 1 M NaOH) were prepared for pH adjustments as recommended in the protocol, with minor variations depending on the solvent systems.
Following gastric and intestinal digestions, 5 mL aliquots were taken from each sample. At each stage, aliquots were immediately adjusted with 0.1% of formic acid, centrifuged at 11,180× g for 10 min, and the clear supernatants were analyzed as the bioaccessible fractions (and stored at −20 °C until measurement). The remaining mixture proceeded to the next digestion step.
In parallel with the digestion experiments, blank digestions were performed for each solvent system (all NADES formulations and 70% ethanol) without the addition of enzymes and bile. In these blanks, the respective solvent was subjected to the same gastric and intestinal electrolyte solutions and incubation conditions as the test samples. Aliquots were collected from these blanks and processed under identical analytical procedures, and the resulting values were used as matched blanks in spectrophotometric assays. This approach allowed us to control for the intrinsic reducing properties of NADES and ethanol, as well as the potential contributions of the digestion medium itself, thereby ensuring that the reported TPC and TAC values reflect only the effects of the extracts [
46].
Bioaccessibility (%) was calculated as the ratio of the compound concentration detected in the intestinal supernatant (post-digestion) to its initial concentration in the undigested extract, expressed as a percentage according to the following equation:
where C
intestinal supernatant is the concentration of the analyte quantified in the aqueous fraction of the intestinal digesta, and C
undigested extract represents the concentration of the same compound in the original undigested sample.
Finally, after the intestinal step, digesta were centrifuged under the same ×
g conditions, and the clear supernatants (intestinal micellar phase) were analyzed directly without further extraction, as recommended in static digestion protocols [
46,
47]. To ensure comparability with undigested extracts, matrix-matched blanks (electrolyte solutions without sample) and enzyme-free controls (electrolytes + bile, no enzymes) were included. These controls confirmed that the observed post-digest changes reflected sample responses rather than the background effects of the digestion media.
2.9. Statistical Analysis
Analyses were performed in triplicate, and results are expressed as the mean ± standard deviation. Data were statistically analyzed using SPSS software (version 25; Chicago, IL, USA). Differences between groups were evaluated using one-way analysis of variance followed by Tukey’s post hoc test, with p < 0.05 considered statistically significant, and were applied to TPC, TAC, and compound-level data reported in the tables; % bioaccessibility values were presented descriptively without post hoc comparisons. Prior to ANOVA, the assumptions of normality and homogeneity of variances were verified. Shapiro–Wilk’s test indicated that the data did not deviate from normal distribution (p > 0.05), and Levene’s test confirmed the homogeneity of variances (p > 0.05). These results supported the validity of applying parametric analysis (one-way ANOVA followed by Tukey’s post hoc test).
4. Conclusions
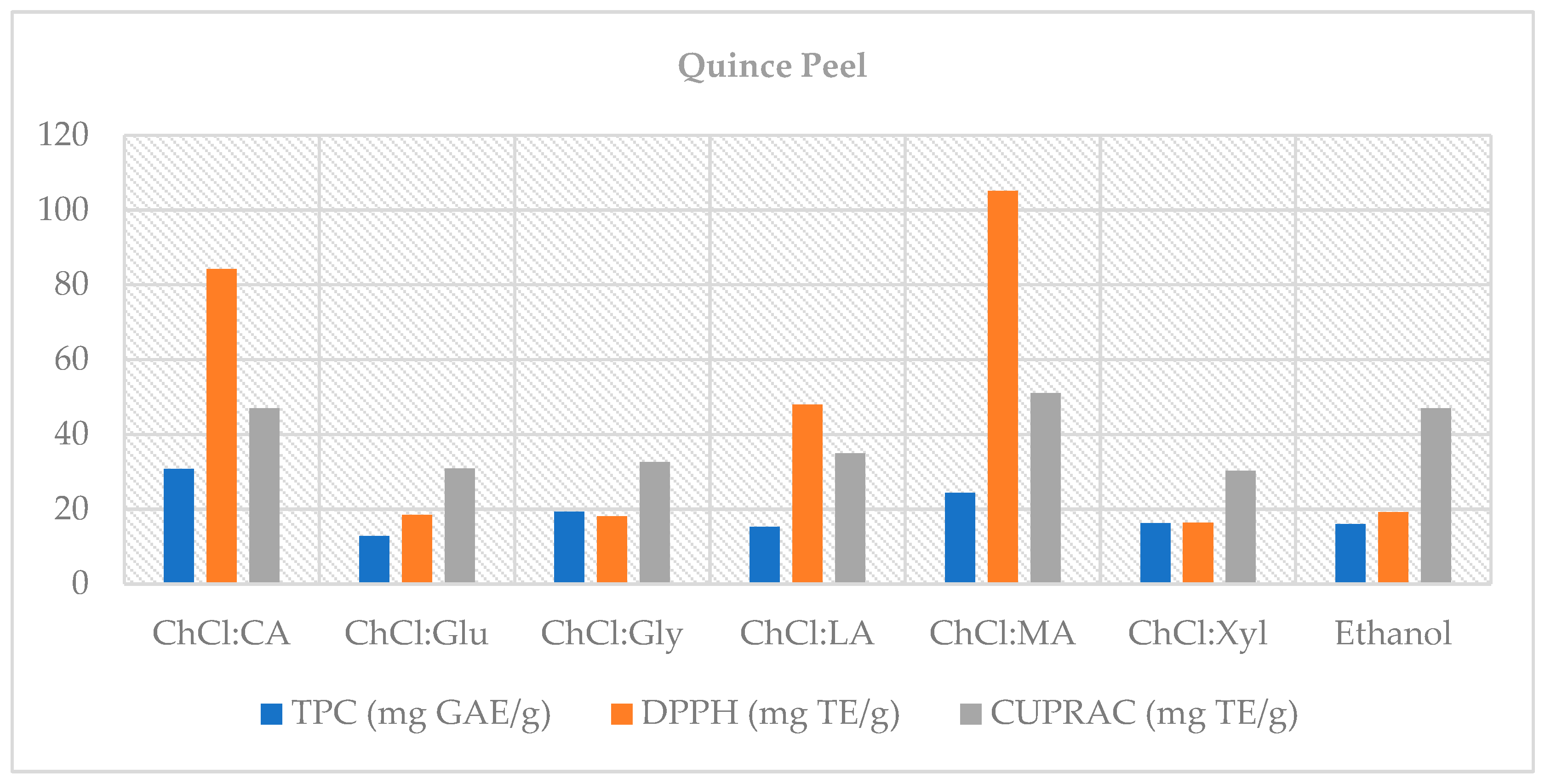
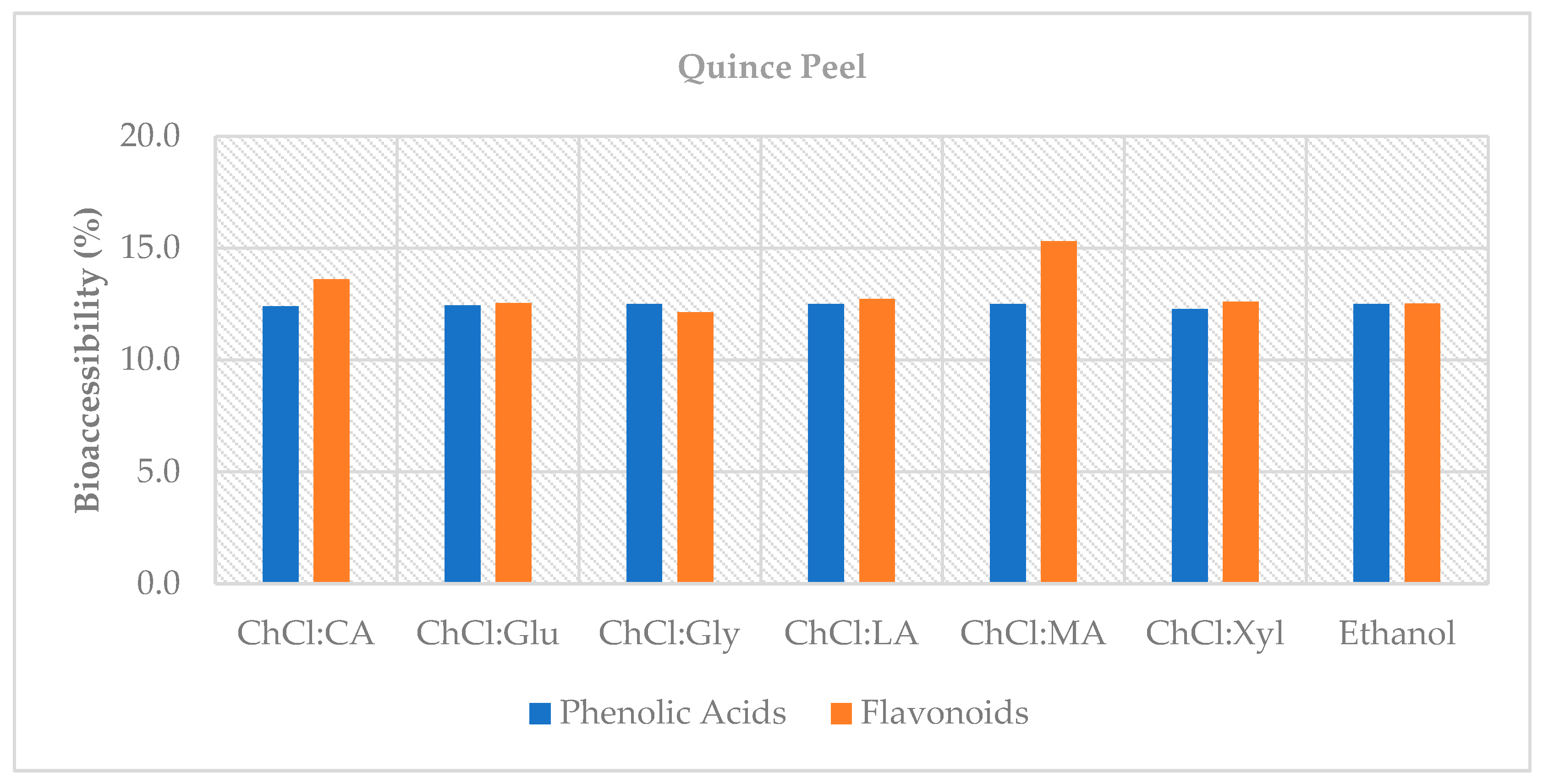
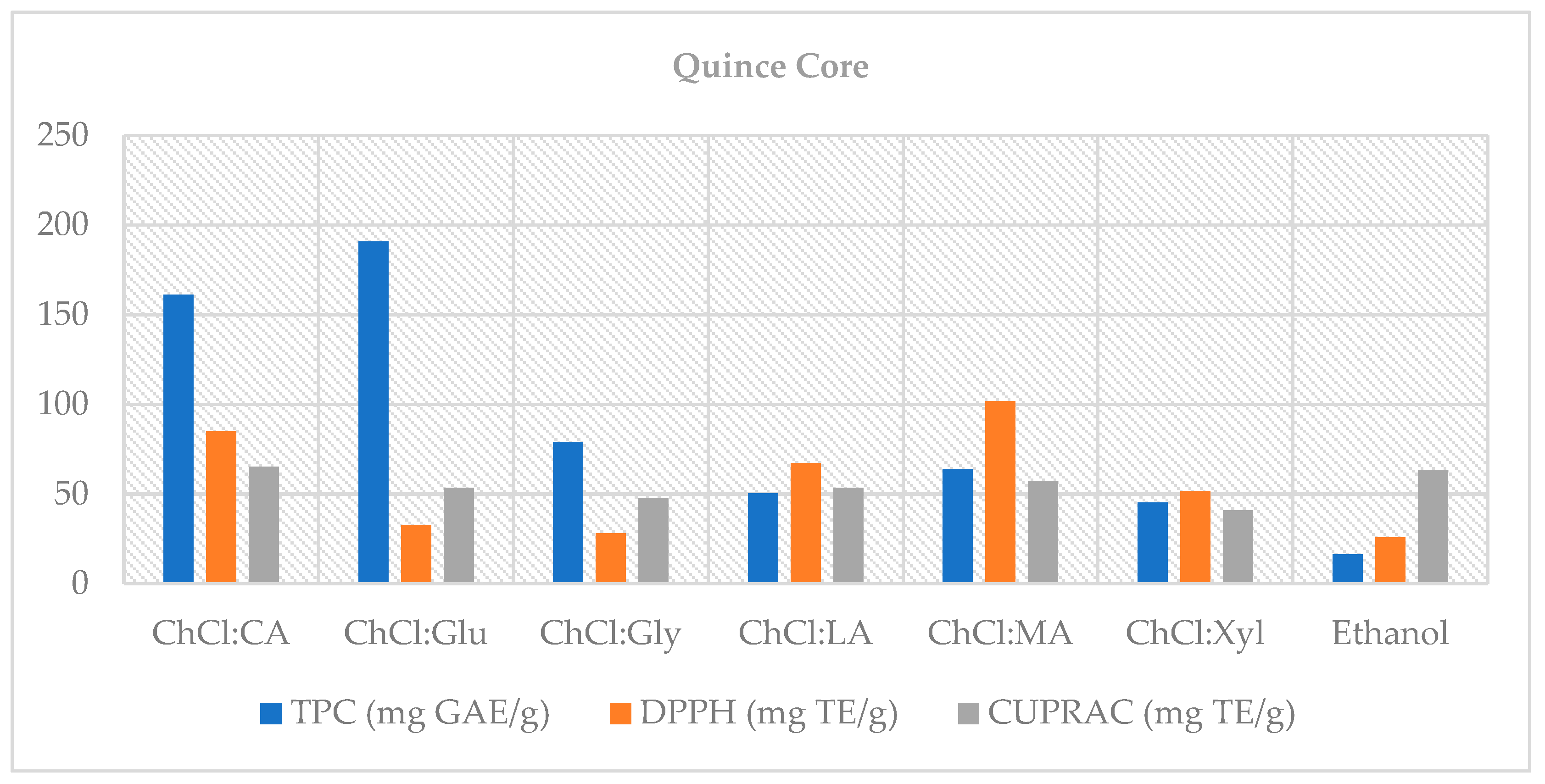
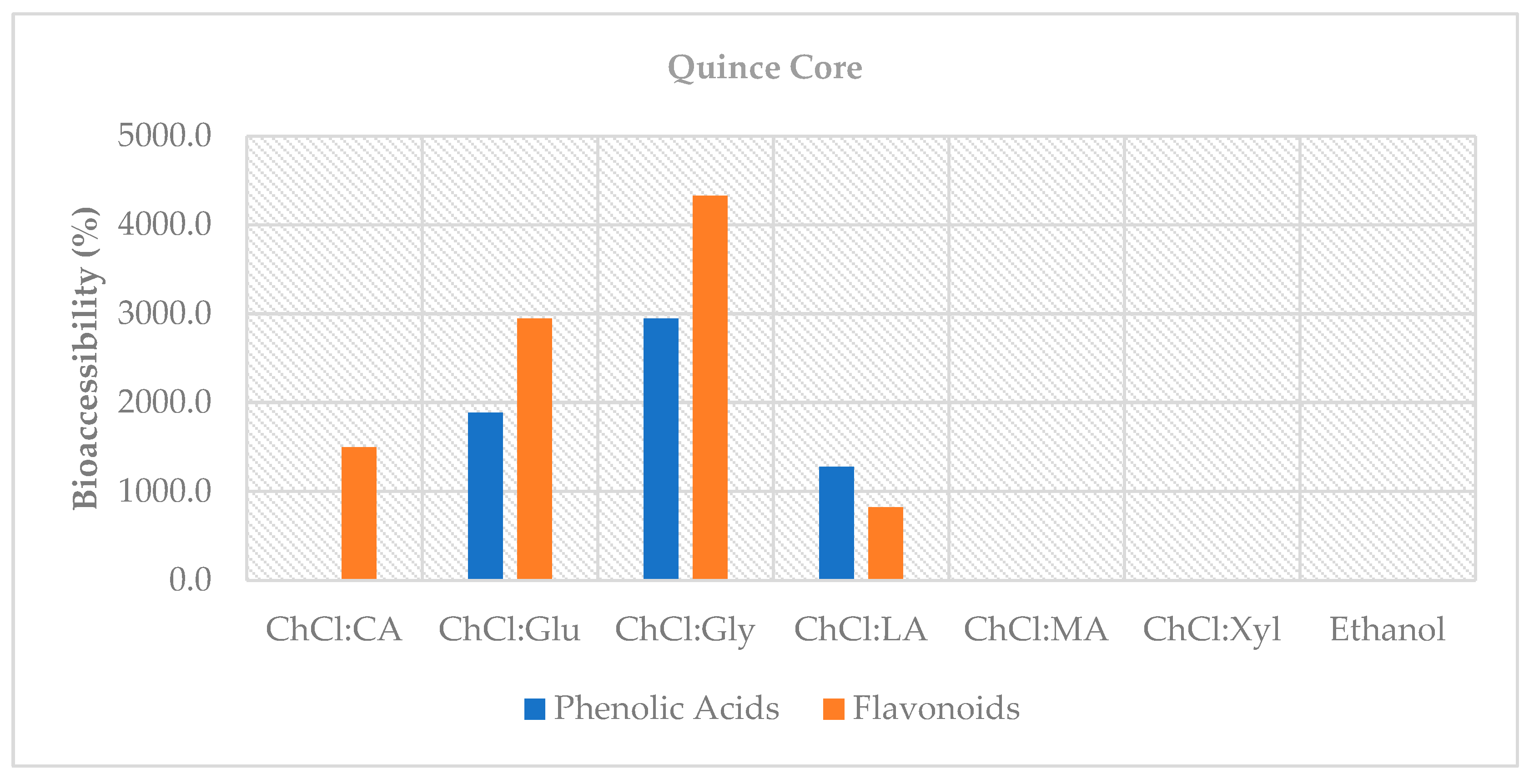
This study demonstrates that choline chloride-based natural deep eutectic solvents (NADES)—particularly systems with organic-acid donors such as malic and lactic acid—offer efficient, lower-hazard alternatives to the conventional solvent (70% ethanol) for recovering phenolic compounds from quince by-products. In the peel, organic acid-based NADES (notably ChCl:MA 2:1 and ChCl:LA 1:1) achieved up to ~45% higher total phenolic content than ethanol and maintained superior antioxidant capacity after digestion; in the core fraction, glucose- and glycerol-based NADES exceeded 100% bioaccessibility, indicating the release of previously bound phenolics under digestive conditions and highlighting its potential as a functional source. Importantly, NADES enhanced extraction efficiency and helped retain antioxidant capacity after in vitro digestion, underscoring their value in valorizing quince by-products.
Nonetheless, the work is limited to a static in vitro digestion model, which cannot fully replicate the complexity of human gastrointestinal conditions. Moreover, the matrices were laboratory-simulated rather than factory-collected, and potential process-related differences were not evaluated. In addition, the study evaluated solely the soluble fraction, while insoluble bound phenolics were not considered, although their release during digestion could substantially contribute to the observed outcomes. Furthermore, only selected NADES formulations were assessed; the effects of solvent composition (including water content), solid-to-liquid ratio, temperature/time, and other process parameters warrant further optimization.
Future research should employ dynamic digestion systems, conduct in vivo validation, and advance pilot-scale trials to evaluate stability, safety, food-grade compliance, sensory and technological performance in real matrices, and solvent recyclability/reuse. In parallel, NADES should be assessed as mixtures, with measurement of residual solvent levels in the final product, basic cytotoxicity screening, and verification of compliance with applicable regulatory limits. From an application standpoint, the solvent components used here (choline chloride paired with organic acids or sugars) are commercially available in food-compatible grades. The feasibility of scale-up will depend primarily on viscosity control, the solid-to-liquid ratio, and operation at moderate temperatures. These parameters should be verified at pilot-scale together with solvent recovery and reuse. A focused techno-economic assessment is warranted to quantify costs and delineate the required unit operations.
Overall, the findings indicate that quince by-products are suitable raw materials and that ChCl-based NADES can be used to obtain phenolic ingredients for incorporation into food matrices. From a process point of view, choosing between ChCl-based NADES and 70% ethanol should be based on clear technical and economic factors: solvent cost and potential for reuse, viscosity and the energy needed to handle it, achievable extract concentration, and waste management. In this regard, large-scale adoption of NADES will likely depend on closed-loop solvent recovery/reuse and a demonstrable cost–benefit advantage over ethanol, as highlighted in techno-economic assessments of bio-based processes. To place the reported extraction gains in context, future work should therefore combine bioefficacy data with solvent-recycling metrics and life-cycle analysis.