A Systematic Review of Evidence-Based Health Benefits of Oroxylum indicum and Its Functional Food Potential
Abstract
1. Introduction
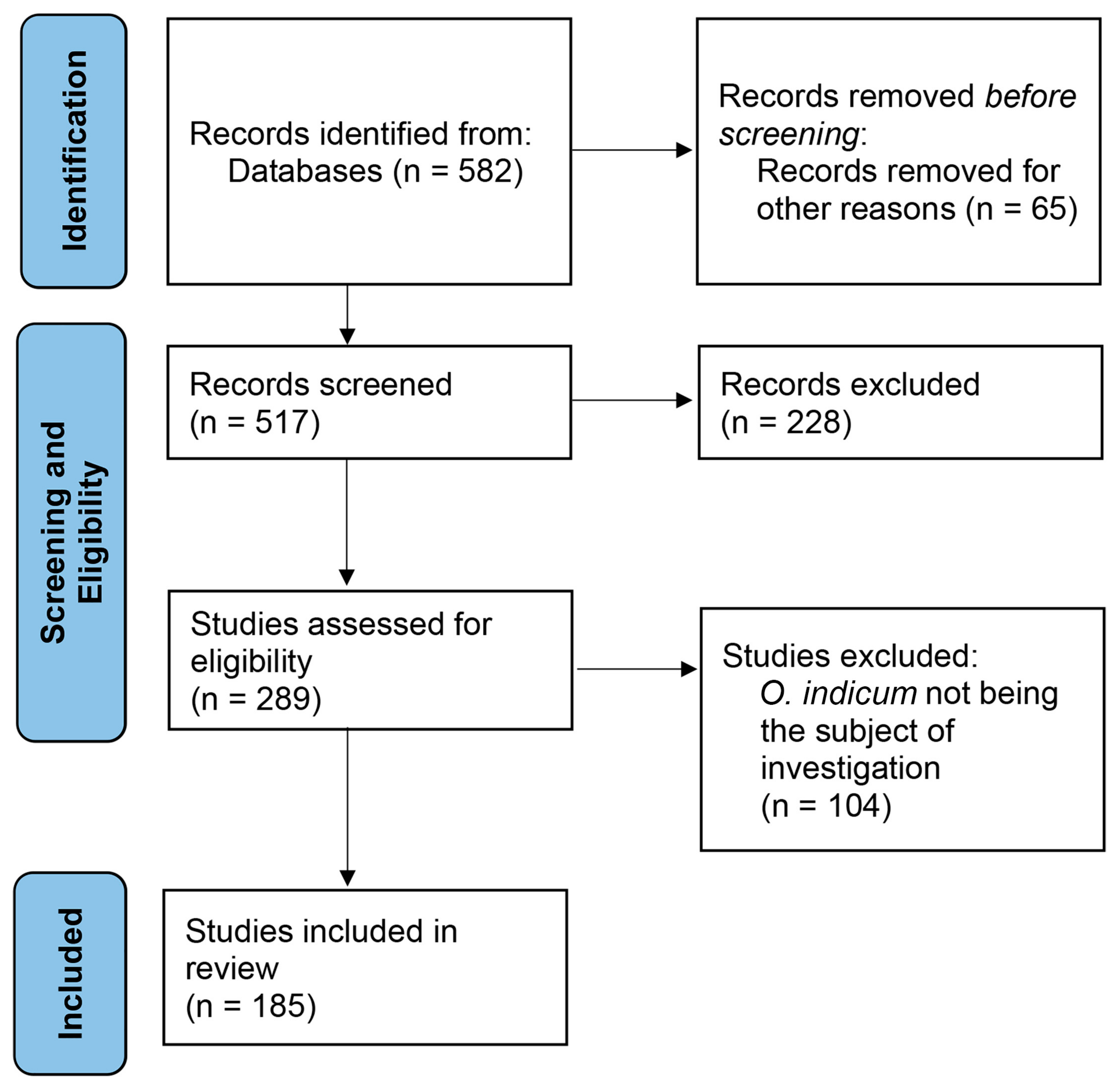
2. Methodology
2.1. Identification
2.2. Eligibility and Screening
2.3. Inclusion
2.4. Limitations
3. Results
3.1. Bibliometric Landscape
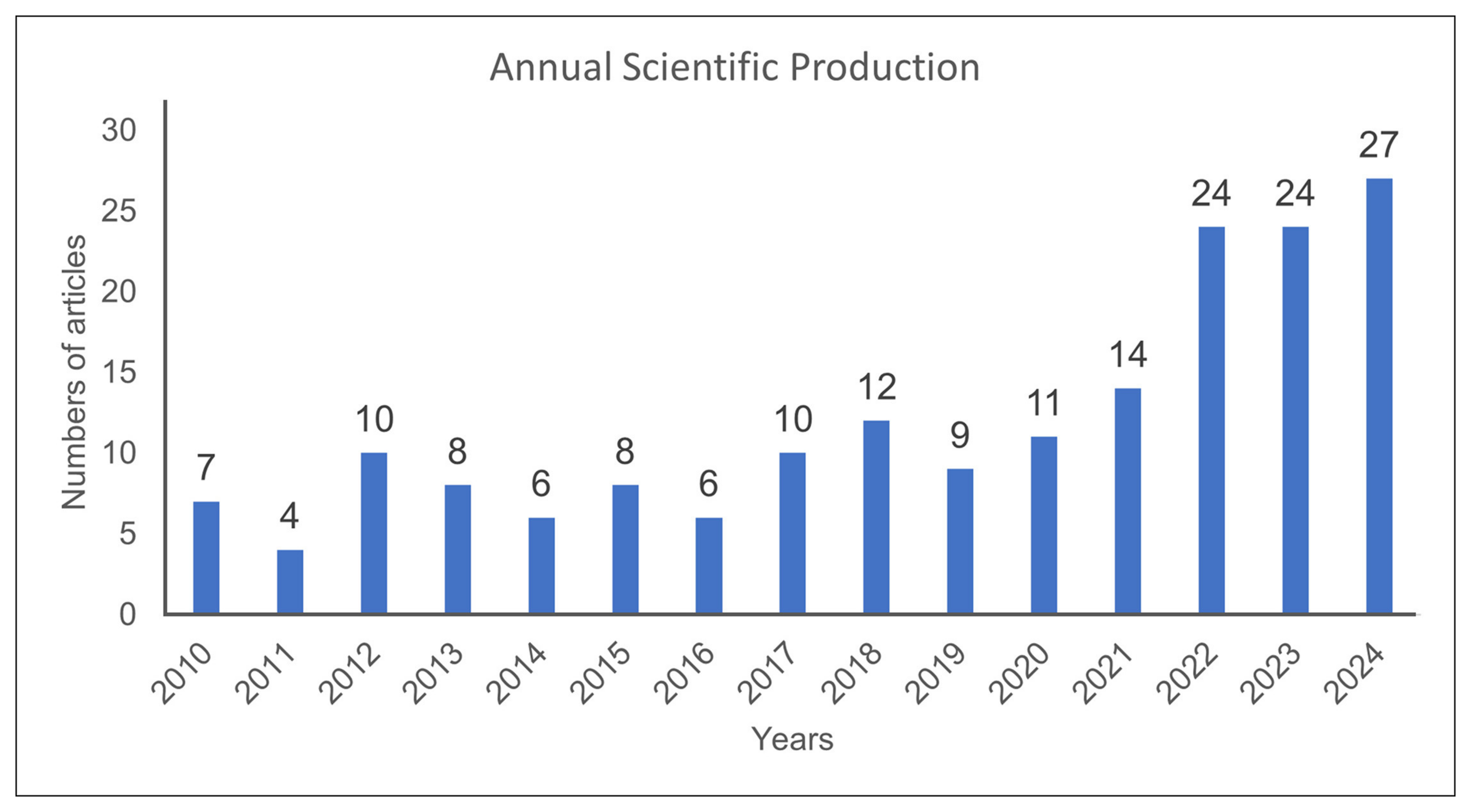
3.1.1. Publication Trends
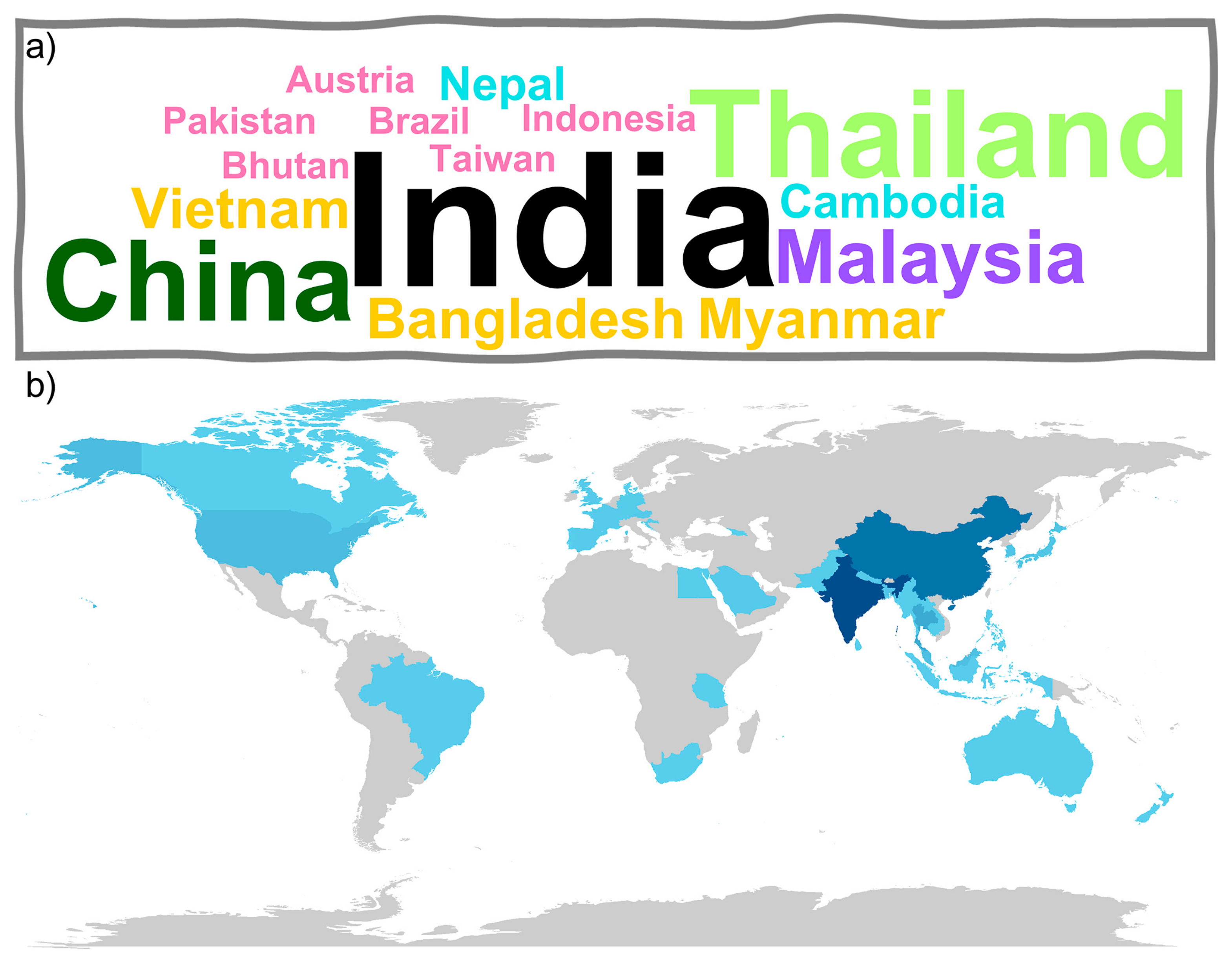
3.1.2. Geographical Distribution
3.1.3. Top Journals
3.1.4. Top-Cited Documents
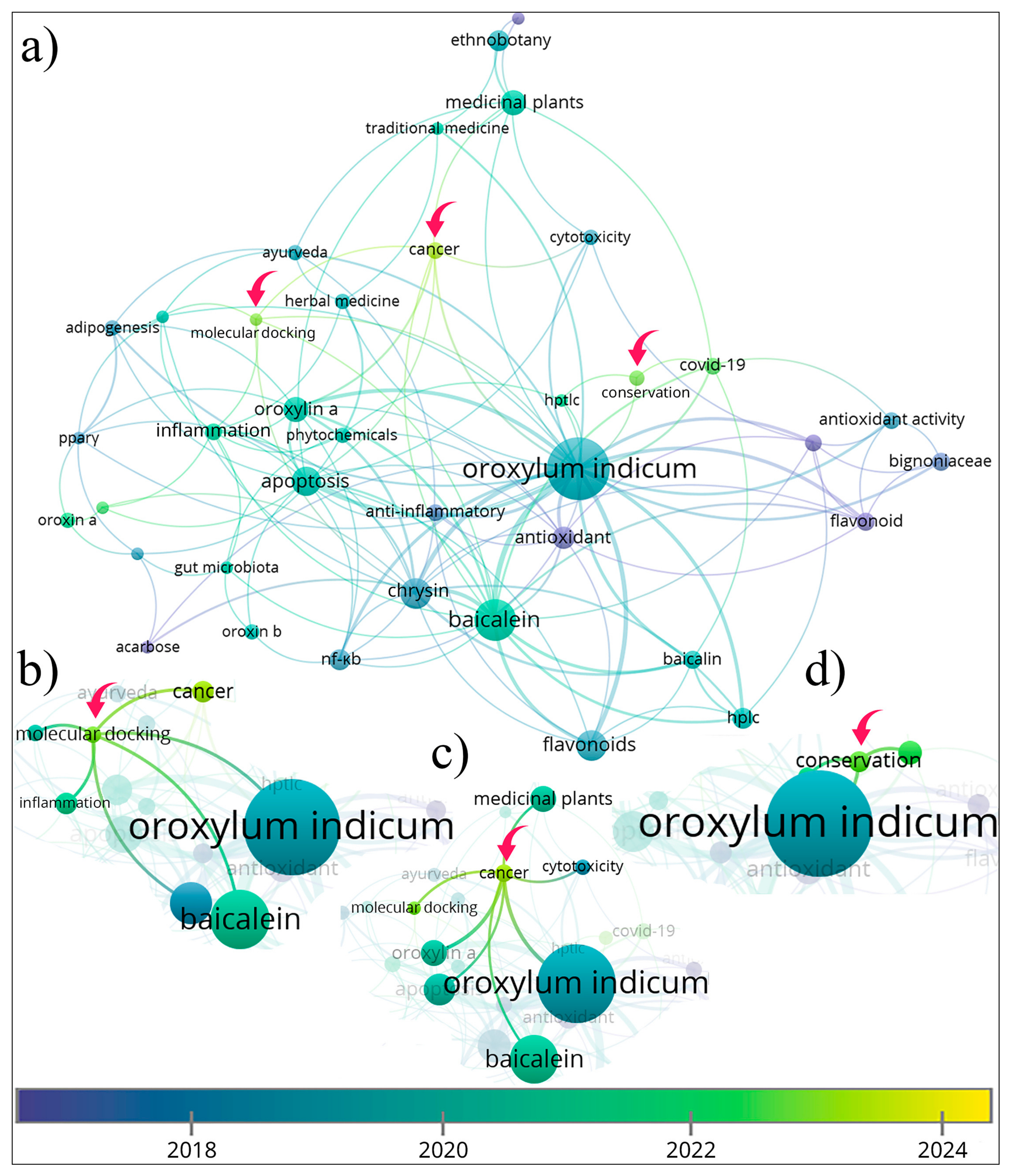
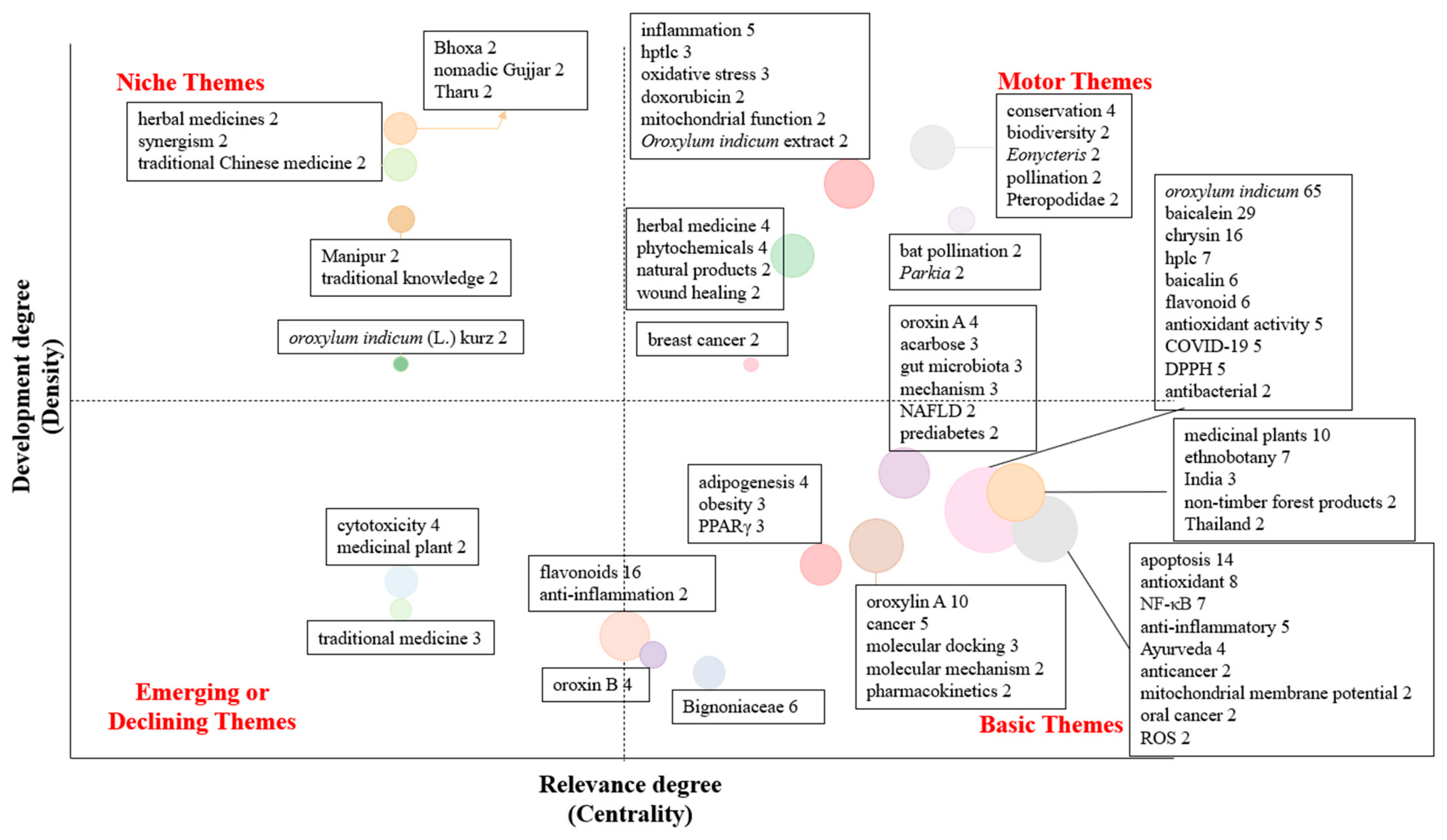
3.1.5. Author Keywords and Thematic Development
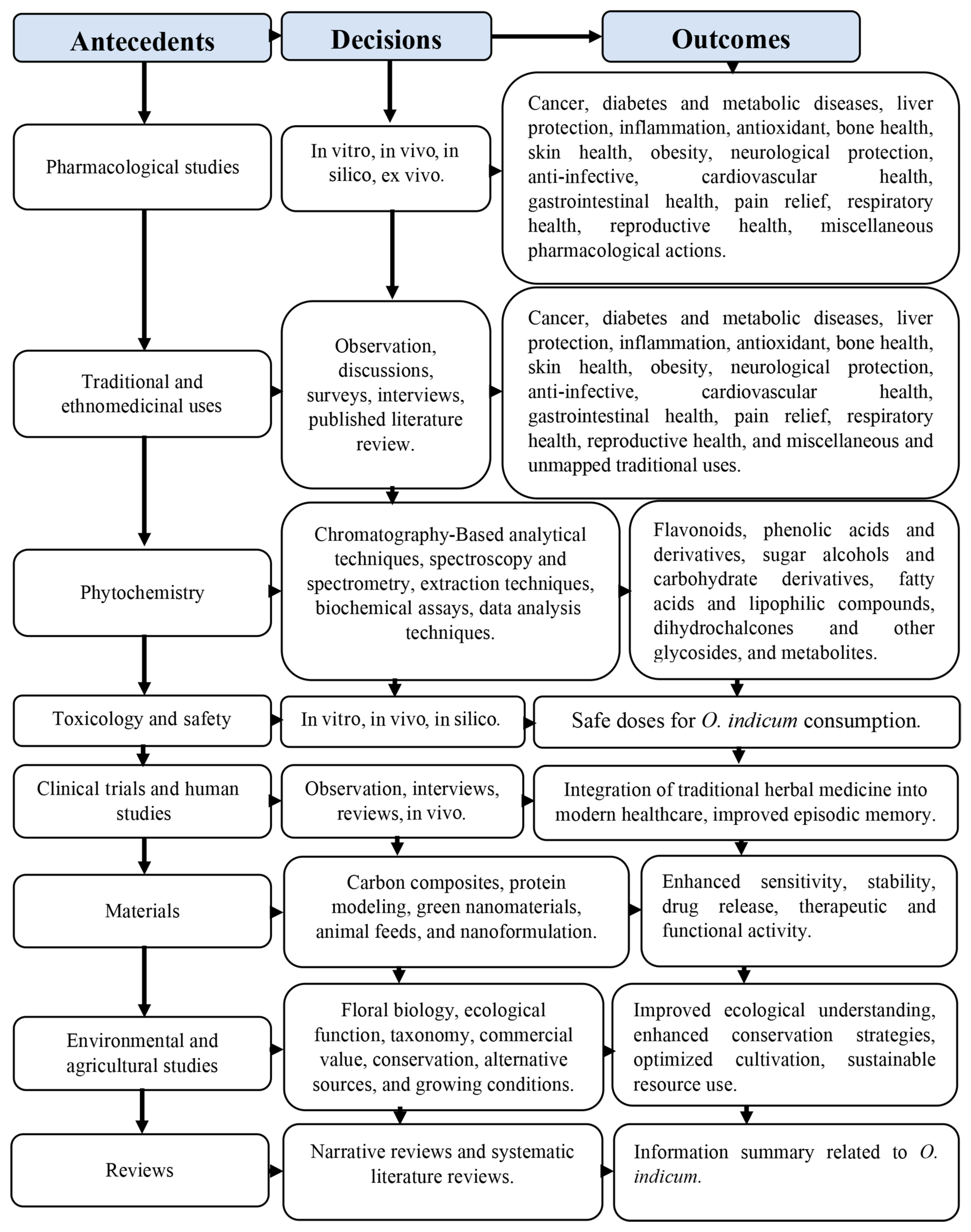
3.2. Research Classification Via the ADO Framework
3.3. Pharmacological Studies, Ethnomedicinal Uses, and Mapping
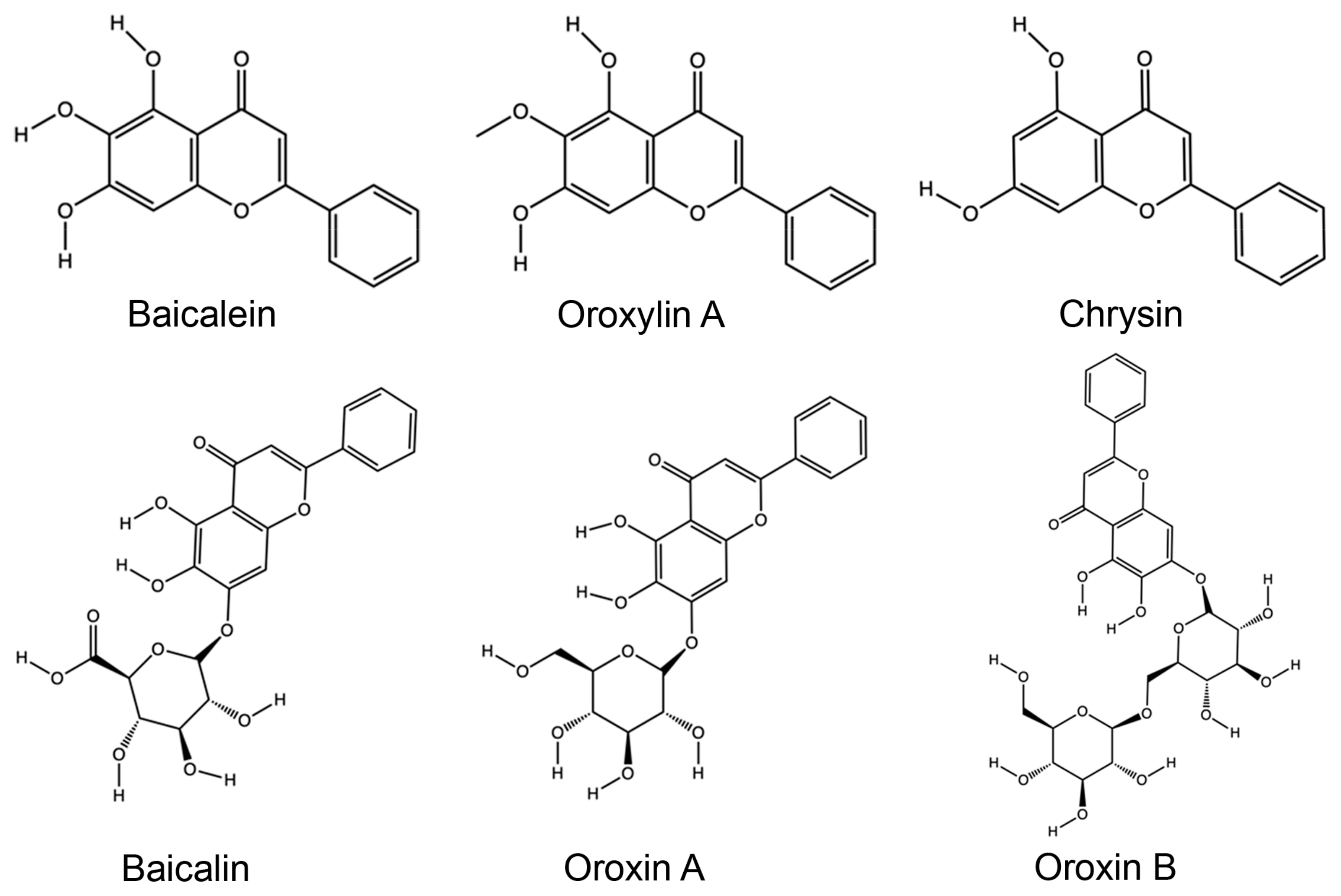
3.4. Phytochemistry
3.5. Toxicology, Safety, Clinical Trials, and Human Studies
3.6. Food Applications
3.6.1. Traditional and Current Food Uses
3.6.2. Bitterness as a Critical Challenge
3.6.3. Approaches to Detect and Manage Bitterness
3.6.4. Consumer Perception and Acceptability
3.6.5. Future Directions for Food Applications
3.7. Material, Environmental, and Agricultural Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADO | Antecedents–Decisions–Outcomes |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| NAFLD | Nonalcoholic fatty liver disease |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| HPTLC | High-Performance Thin-Layer Chromatography |
| ROS | Reactive Oxygen Species |
References
- Rojsanga, P.; Schwaiger, S.; Stuppner, H.; Sithisarn, P. Determination of phytochemical contents in extracts from different growth stages of Oroxylum indicum fruits using HPLC-DAD and QAMS methods. Molecules 2023, 28, 6837. [Google Scholar] [CrossRef]
- Tran, T.V.A.; Malainer, C.; Schwaiger, S.; Hung, T.; Atanasov, A.G.; Heiss, E.H.; Dirsch, V.M.; Stuppner, H. Screening of Vietnamese medicinal plants for NF-κB signaling inhibitors: Assessing the activity of flavonoids from the stem bark of Oroxylum indicum. J. Ethnopharmacol. 2015, 159, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Jha, K.; Smith-Hall, C. Three models to illustrate plant-people relationships in the medicinal plant hotspots of North East India. Ethnobot. Res. Appl. 2023, 26, 1–48. [Google Scholar] [CrossRef]
- Bhattarai, K.; Kunwar, R.; Baral, B. Phytochemical analysis and ethnomedicinal uses of Oroxylum indicum in Nepal. Ethnobot. Res. Appl. 2022, 24, 1–12. [Google Scholar] [CrossRef]
- Ralte, L.; Sailo, H.; Singh, Y.T. Ethnobotanical study of medicinal plants used by the indigenous community of the western region of Mizoram, India. J. Ethnobiol. Ethnomed. 2024, 20, 2. [Google Scholar] [CrossRef]
- Hein, P.P.; Arunachalam, K.; Fu, Y.; Zaw, M.; Yang, Y.; Yang, X. Diversity of medicinal plants and their therapeutic usages of Kachin people (Jinghpaw) in the central part of Kachin State, Myanmar. J. Ethnopharmacol. 2023, 302, 115921. [Google Scholar] [CrossRef]
- Nagasaka, M.; Hashimoto, R.; Inoue, Y.; Ishiuchi, K.i.; Matsuno, M.; Itoh, Y.; Tokugawa, M.; Ohoka, N.; Morishita, D.; Mizukami, H. Anti-tumorigenic activity of chrysin from Oroxylum indicum via non-genotoxic p53 activation through the ATM-Chk2 pathway. Molecules 2018, 23, 1394. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, W.; Yu, N.; Sun, J.; Yu, X.; Li, X.; Xing, Y.; Yan, D.; Ding, Q.; Xiu, Z. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J. Funct. Foods 2018, 46, 256–267. [Google Scholar] [CrossRef]
- Choonong, R.; Waewaram, V.; Buraphaka, H.; Krittanai, S.; Boonsnongcheep, P.; Putalun, W. Anti-inflammatory potential of Oroxylum indicum flavonoids: Effects of traditional grilling on aglycone flavonoid content and activity against urban dust-induced inflammation. Food Biosci. 2024, 62, 105523. [Google Scholar] [CrossRef]
- Cardoso Reis, A.C.; Valente, G.M.; Silva, B.d.M.; de Brito Magalhães, C.L.; Kohlhoff, M.; Brandão, G.C. Anti-arboviral activity and chemical characterization of hispidulin and ethanolic extracts from Millingtonia hortensis Lf and Oroxylum indicum (L.) Kurz (Bignoniaceae). Nat. Prod. Res. 2023, 37, 613–617. [Google Scholar] [CrossRef]
- Panomai, P.; Thapphasaraphong, S.; Nualkaew, N. A Comparative Study of Two Oroxylum indicum (L.) Kurz. Phenotypes Based on Phytochemicals and Antioxidant Effects, and the Anti-Inflammatory Activity of Leaf and Pod Extracts. Plants 2024, 13, 2110. [Google Scholar] [CrossRef] [PubMed]
- Prapaipittayakhun, J.; Boonyuen, S.; Zheng, A.L.T.; Apinyauppatham, K.; Arpornmaeklong, P. Biologic effects of biosynthesized Oroxylum indicum/silver nanoparticles on human periodontal ligament stem cells. OpenNano 2023, 9, 100117. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Rahman, M.O.; Alqahtani, A.S.; Sultana, N.; Almarfadi, O.M.; Ali, M.A.; Lee, J. Anticancer potential of phytochemicals from Oroxylum indicum targeting Lactate Dehydrogenase A through bioinformatic approach. Toxicol. Rep. 2023, 10, 56–75. [Google Scholar] [CrossRef] [PubMed]
- Muniyasamy, R.; Manjubala, I. Elucidating anti-sclerostin mechanism of baicalein using LRP6-Sclersotin complex of canonical Wnt/β-catenin signaling pathway. J. Biomol. Struct. Dyn. 2025, 43, 5009–5019. [Google Scholar] [CrossRef]
- Gokhale, M.; Faraz, R.; Deshpande, I.; Garg, A. Isolation of bio-molecule Baicalein (5, 6, 7-Trihydroxy flavone) from root of Oroxylum indicum L. Vent and its prospective interaction with COVID-19 Viral S-Protein Receptor Binding Domain. Res. J. Pharm. Technol. 2022, 15, 5050–5056. [Google Scholar] [CrossRef]
- Sala, E.; Guasch, L.; Iwaszkiewicz, J.; Mulero, M.; Salvadó, M.-J.; Bladé, C.; Ceballos, M.; Valls, C.; Zoete, V.; Grosdidier, A. Identification of human IKK-2 inhibitors of natural origin (Part II): In Silico prediction of IKK-2 inhibitors in natural extracts with known anti-inflammatory activity. Eur. J. Med. Chem. 2011, 46, 6098–6103. [Google Scholar] [CrossRef]
- Doshi, K.; Ilanchezhian, R.; Acharya, R.; Patel, B.; Ravishankar, B. Anti-inflammatory activity of root bark and stem bark of Shyonaka. J. Ayurveda Integr. Med. 2012, 3, 194. [Google Scholar] [CrossRef]
- Pondugula, S.R.; Harshan, A.; Ramesh, S.; Govindarajulu, M.; Almaghrabi, M.; Majrashi, M.; Abbott, K.L.; Nadar, R.; Alturki, M.; Salamat, J.M. Cardioprotective effects of Oroxylum indicum extract against doxorubicin and cyclophosphamide-induced cardiotoxicity. Cardiovasc. Toxicol. 2022, 22, 67–77. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, C.; Wang, M.; Xiong, T.; Song, X.; Sun, W.; Li, J. Oroxin B improves metabolic-associated fatty liver disease by alleviating gut microbiota dysbiosis in a high-fat diet-induced rat model. Eur. J. Pharmacol. 2023, 951, 175788. [Google Scholar] [CrossRef]
- Dinda, B.; SilSarma, I.; Dinda, M.; Rudrapaul, P. Oroxylum indicum (L.) Kurz, an important Asian traditional medicine: From traditional uses to scientific data for its commercial exploitation. J. Ethnopharmacol. 2015, 161, 255–278. [Google Scholar] [CrossRef]
- Deka, D.; Kumar, V.; Prasad, C.; Kumar, K.; Gogoi, B.; Singh, L.; Srivastava, R. Oroxylum indicum–a medicinal plant of North East India: An overview of its nutritional, remedial, and prophylactic properties. J. Appl. Pharm. Sci. 2013, 3, S104–S112. Available online: https://japsonline.com/admin/php/uploads/951_pdf.pdf (accessed on 6 October 2025).
- Nik Salleh, N.N.H.; Othman, F.A.; Kamarudin, N.A.; Tan, S.C. The biological activities and therapeutic potentials of baicalein extracted from Oroxylum indicum: A systematic review. Molecules 2020, 25, 5677. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Dsouza, S.; Joshi, M.; Antoun, R.; Phan, D.H.T. Environmental, social and governance investing: Systematic literature review using ADO model. J. Account. Lit. 2025, in press. [CrossRef]
- Singh, V.; Chaudhary, A. A review on the taxonomy, ethnobotany, chemistry and pharmacology of Oroxylum indicum Vent. Indian J. Pharm. Sci. 2011, 73, 483. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Van Eck, N.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Kumar, D.; Rawat, S.; Joshi, R. Predicting the current and future suitable habitat distribution of the medicinal tree Oroxylum indicum (L.) Kurz in India. J. Appl. Res. Med. Aromat. Plants 2021, 23, 100309. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Sumbul, S.; Ahmad, M.A.; Mohd, A.; Mohd, A. Role of phenolic compounds in peptic ulcer: An overview. J. Pharm. Bioallied Sci. 2011, 3, 361–367. [Google Scholar] [CrossRef]
- Siriwatanametanon, N.; Fiebich, B.L.; Efferth, T.; Prieto, J.M.; Heinrich, M. Traditionally used Thai medicinal plants: In vitro anti-inflammatory, anticancer and antioxidant activities. J. Ethnopharmacol. 2010, 130, 196–207. [Google Scholar] [CrossRef]
- Panda, S.K.; Mohanta, Y.K.; Padhi, L.; Park, Y.-H.; Mohanta, T.K.; Bae, H. Large scale screening of ethnomedicinal plants for identification of potential antibacterial compounds. Molecules 2016, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Gairola, S.; Gaur, R.; Painuli, R. The treatment of jaundice with medicinal plants in indigenous communities of the Sub-Himalayan region of Uttarakhand, India. J. Ethnopharmacol. 2012, 143, 262–291. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Gairola, S.; Gaur, R.; Painuli, R.; Siddiqi, T. Ethnomedicinal plants used for treating epilepsy by indigenous communities of sub-Himalayan region of Uttarakhand, India. J. Ethnopharmacol. 2013, 150, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, J.; Xiang, Z.; Cai, L.; Lu, W. A hierarchical Co@ mesoporous C/macroporous C sheet composite derived from bimetallic MOF and Oroxylum indicum for enhanced microwave absorption. Carbon 2022, 187, 477–487. [Google Scholar] [CrossRef]
- Saha, D.; Sundriyal, R. Utilization of non-timber forest products in humid tropics: Implications for management and livelihood. For. Policy Econ. 2012, 14, 28–40. [Google Scholar] [CrossRef]
- Kumari, P.; Chandra joshi, G.; Mohan TEWARI, L. Biodiversity status, distribution and use pattern of some ethno-medicinal plants. Int. J. Conserv. Sci. 2012, 3, 309. Available online: https://ijcs.ro/pub/IJCS-12-31-Kumary.pdf (accessed on 6 October 2025).
- Lalou, C.; Basak, A.; Mishra, P.; Mohanta, B.; Banik, R.; Dinda, B.; Khatib, A. Inhibition of tumor cells proliferation and migration by the flavonoid furin inhibitor isolated from Oroxylum indicum. Curr. Med. Chem. 2013, 20, 583–591. [Google Scholar] [CrossRef]
- Zhang, B.-W.; Sang, Y.-B.; Sun, W.-L.; Yu, H.-S.; Ma, B.-P.; Xiu, Z.-L.; Dong, Y.-S. Combination of flavonoids from Oroxylum indicum seed extracts and acarbose improves the inhibition of postprandial blood glucose: In vivo and in vitro study. Biomed. Pharmacother. 2017, 91, 890–898. [Google Scholar] [CrossRef]
- Cheng, X.-J.; Kong, D.-Z.; Li, Y.-H.; Bian, G.-L.; Li, D.-Q. Screening of dual targeted inhibitors of 5-lipoxygenase and cyclooxygenase-2 from Oroxylum indicum by off-line two-dimensional liquid chromatography coupled with mass spectrometry. Ind. Crops Prod. 2022, 186, 115243. [Google Scholar] [CrossRef]
- Thrigulla, S.R.; Singh, G.; Soni, H.; Tandon, S.; Koulgi, S.; Uppuladinne, M.V.; Jani, V.; Sonavane, U.; Joshi, R.; Gandhi, Y. In-silico evaluation of Oroxylum indicum vent compounds in the plausible treatment and prevention of nasopharyngeal cancer. J. Ayurveda Integr. Med. 2024, 15, 100986. [Google Scholar] [CrossRef]
- Pathak, P.; Novak, J.; Naumovich, V.; Grishina, M.; Balkrishna, A.; Sharma, N.; Sharma, V.; Potemkin, V.; Verma, A. Polyphenolic rich extract of Oroxylum indicum alleviate β-glucuronidase activity via down-regulate oxidative stress: Experimental and computational studies. Biocatal. Agric. Biotechnol. 2020, 29, 101804. [Google Scholar] [CrossRef]
- Hengpratom, T.; Lowe, G.M.; Eumkeb, G. An insight into anti-adipogenic properties of an Oroxylum indicum (L.) Kurz extract. Med. Ther. 2020, 20, 319. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, B.; Sumathi, T. 5, 6, 7 trihydroxy flavone armoured neurodegeneration caused by quinolinic acid induced huntington’s like disease in rat striatum-reinstating the level of brain neurotrophins with special reference to cognitive-socio behaviour, biochemical and histopathological aspects. Neurosci. Res. 2022, 174, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.N.; Nik Salleh, N.N.H.; Chung, W.J.; Lee, C.Y.; Tan, S.C. Baicalein-enriched fraction extracted from Oroxylum indicum (L.) Benth. ex kurz leaves exerts antioxidant and inhibitory effects against glioblastoma multiforme. Processes 2019, 7, 963. [Google Scholar] [CrossRef]
- Somsakeesit, L.-o.; Senawong, T.; Senawong, G.; Kumboonma, P.; Samankul, A.; Namwan, N.; Yenjai, C.; Phaosiri, C. Evaluation and molecular docking study of two flavonoids from Oroxylum indicum (L.) Kurz and their semi-synthetic derivatives as histone deacetylase inhibitors. J. Nat. Med. 2024, 78, 236–245. [Google Scholar] [CrossRef]
- Yang, P.; Fu, S.; Cao, Z.; Liao, H.; Huo, Z.; Pan, Y.; Zhang, G.; Gao, A.; Zhou, Q. Oroxin B selectively induces tumor-suppressive ER stress and concurrently inhibits tumor-adaptive ER stress in B-lymphoma cells for effective anti-lymphoma therapy. Toxicol. Appl. Pharmacol. 2015, 288, 269–279. [Google Scholar] [CrossRef]
- Menon, S.; Albaqami, J.J.; Hamdi, H.; Lawrence, L.; Divya, M.K.; Antony, L.; Padikkala, J.; Mathew, S.E.; Narayanankutty, A. Root bark extract of Oroxylum indicum Vent. inhibits solid and ascites tumors and prevents the development of DMBA-induced skin papilloma formation. Molecules 2022, 27, 8459. [Google Scholar] [CrossRef]
- Menon, S.; Albaqami, J.J.; Hamdi, H.; Lawrence, L.; Padikkala, J.; Mathew, S.E.; Narayanankutty, A. Oroxylum indicum vent root bark extract inhibits the proliferation of cancer cells and induce apoptotic cell death. Processes 2023, 11, 188. [Google Scholar] [CrossRef]
- Shah, R.K.; Upadhyay, B.; Buragohain, J.; Rai, M. Phytochemical Analysis, Antioxidant, Antimicrobial and Anticancer Activity of Nigella sativa and Oroxylum indicum. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2024, 94, 1059–1065. [Google Scholar] [CrossRef]
- Rai, D.; Ram, H.A.; Patel, K.N.; Babu, U.; Kumar, L.S.; Kannan, R. In vitro immuno-stimulatory and anticancer activities of Oroxylum indicum (L.) Kurz.: An evidence for substitution of aerial parts for conservation. J. Ayurveda Integr. Med. 2022, 13, 100523. [Google Scholar] [CrossRef]
- Chassagne, F.; Haddad, M.; Amiel, A.; Phakeovilay, C.; Manithip, C.; Bourdy, G.; Deharo, E.; Marti, G. A metabolomic approach to identify anti-hepatocarcinogenic compounds from plants used traditionally in the treatment of liver diseases. Fitoterapia 2018, 127, 226–236. [Google Scholar] [CrossRef]
- Pal-Bhadra, M.; Ramaiah, M.J.; Reddy, T.L.; Krishnan, A.; Pushpavalli, S.; Babu, K.S.; Tiwari, A.K.; Rao, J.M.; Yadav, J.S.; Bhadra, U. Plant HDAC inhibitor chrysin arrest cell growth and induce p21 WAF1 by altering chromatin of STAT response element in A375 cells. BMC Cancer 2012, 12, 180. [Google Scholar] [CrossRef]
- Sisin, N.N.T.; Kong, A.R.; Edinur, H.A.; Jamil, N.I.N.; Che Mat, N.F. Silencing E6/E7 Oncoproteins in SiHa Cells Treated with siRNAs and Oroxylum indicum Extracts Induced Apoptosis by Upregulating p53/pRb Pathways. Appl. Biochem. Biotechnol. 2024, 196, 4234–4255. [Google Scholar] [CrossRef]
- Zhong, W.; Hou, H.; Liu, T.; Su, S.; Xi, X.; Liao, Y.; Xie, R.; Jin, G.; Liu, X.; Zhu, L. Cartilage oligomeric matrix protein promotes epithelial-mesenchymal transition by interacting with transgelin in colorectal cancer. Theranostics 2020, 10, 8790. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, K.; Yang, L.; Zhang, G. Bladder cancer cell viability inhibition and apoptosis induction by baicalein through targeting the expression of anti-apoptotic genes. Saudi J. Biol. Sci. 2018, 25, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Singh, S.; Rai, N.; Verma, A.; Tiwari, H.; Kamble, S.C.; Gautam, H.K.; Gautam, V. Unveiling the cytotoxic and anti-proliferative potential of green-synthesized silver nanoparticles mediated by Colletotrichum gloeosporioides. RSC Adv. 2024, 14, 4074–4088. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.; Keshri, P.K.; Gupta, P.; Verma, A.; Kamble, S.C.; Singh, S.K.; Gautam, V. Bioprospecting of fungal endophytes from Oroxylum indicum (L.) Kurz with antioxidant and cytotoxic activity. PLoS ONE 2022, 17, e0264673. [Google Scholar] [CrossRef]
- Singh, A.R.; Singh, S.A.; Singh, T.D.; Singh, N.T.; Machathoibi, T.C.; Singh, O.M.; Singh, L.S. Bioassay-Guided Isolation of 2-[p-(2-Carboxyhydrazino) phenoxy]-6-(hydroxymethyl) tetrahydro-2H-pyran-3, 4, 5-triol from Oroxylum indicum and the Investigation of Its Molecular Mechanism Action of Apoptosis Induction. Pharmaceuticals 2022, 15, 559. [Google Scholar] [CrossRef]
- Kameyanda Poonacha, S.; Harishkumar, M.; Radha, M.; Varadarajan, R.; Nalilu, S.K.; Shetty, S.S.; Shetty, P.K.; Chandrashekharappa, R.B.; Sreenivas, M.G.; Bhandary Bavabeedu, S.K. Insight into oroxylina-7-o-β-d-glucuronide-enriched Oroxylum indicum bark extract in oral cancer hsc-3 cell apoptotic mechanism: Role of mitochondrial microenvironment. Molecules 2021, 26, 7430. [Google Scholar] [CrossRef]
- Parvin, M.; Rahaman, A.; Sarkar, A.; Debnath, S.; De, U.C.; Mandal, D.P.; Bhattacharjee, S. Oroxylum indicum stem bark extract reduces tumor progression by inhibiting the EGFR-PI3K-AKT pathway in an in vivo 4NQO-induced oral cancer model. J. Am. Nutr. Assoc. 2023, 42, 573–587. [Google Scholar] [CrossRef]
- Buranrat, B.; Noiwetch, S.; Suksar, T.; Ta-Ut, A. Inhibition of cell proliferation and migration by Oroxylum indicum extracts on breast cancer cells via Rac1 modulation. J. Pharm. Anal. 2020, 10, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Lalrinzuali, K.; Vabeiryureilai, M.; Jagetia, G.C. Sonapatha (Oroxylum indicum) mediates cytotoxicity in cultured HeLa cells by inducing apoptosis and suppressing NF-κB, COX-2, RASSF7 and NRF2. Bioorg. Chem. 2021, 114, 105126. [Google Scholar] [CrossRef]
- Rai, N.; Gupta, P.; Verma, A.; Singh, S.K.; Gautam, V. Isolation and characterization of N-(2-Hydroxyethyl) hexadecanamide from Colletotrichum gloeosporioides with apoptosis-inducing potential in breast cancer cells. Biofactors 2023, 49, 663–683. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; Sarkar, A.; Debnath, S.; Debnath, B.; Ghosh, R.; Zaki, M.E.; Al-Hussain, S.A. α-Glucosidase inhibitory potential of Oroxylum indicum using molecular docking, molecular dynamics, and in vitro evaluation. Saudi Pharm. J. 2024, 32, 102095. [Google Scholar] [CrossRef]
- Kumar, G.S.; Tiwari, A.K.; Rao, V.R.S.; Prasad, K.R.; Ali, A.Z.; Babu, K.S. Synthesis and biological evaluation of novel benzyl-substituted flavones as free radical (DPPH) scavengers and α-glucosidase inhibitors. J. Asian Nat. Prod. Res. 2010, 12, 978–984. [Google Scholar] [CrossRef]
- Sun, W.; Sang, Y.; Zhang, B.; Yu, X.; Xu, Q.; Xiu, Z.; Dong, Y. Synergistic effects of acarbose and an Oroxylum indicum seed extract in streptozotocin and high-fat-diet induced prediabetic mice. Biomed. Pharmacother. 2017, 87, 160–170. [Google Scholar] [CrossRef]
- Sun, W.; Liu, P.; Yang, B.; Wang, M.; Wang, T.; Sun, W.; Wang, X.; Zheng, W.; Song, X.; Li, J. A network pharmacology approach: Inhibition of the NF-κB signaling pathway contributes to the NASH preventative effect of an Oroxylum indicum seed extract in oleic acid-stimulated HepG2 cells and high-fat diet-fed rats. Phytomedicine 2021, 88, 153498. [Google Scholar] [CrossRef]
- Sun, W.; Sun, J.; Zhang, B.; Xing, Y.; Yu, X.; Li, X.; Xiu, Z.; Dong, Y. Baicalein improves insulin resistance via regulating SOCS3 and enhances the effect of acarbose on diabetes prevention. J. Funct. Foods 2017, 37, 339–353. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, H.; Zhang, D.; Jiang, H.; Xiu, Z.; Dong, Y. Baicalein, a dietary flavonoid, enhances the insulin-sensitizing effect of metformin to prevent type 2 diabetes via the regulation of lipid metabolism and gut microenvironment. Food Front. 2024, 5, 668–690. [Google Scholar] [CrossRef]
- Singh, J.; Kakkar, P. Modulation of liver function, antioxidant responses, insulin resistance and glucose transport by Oroxylum indicum stem bark in STZ induced diabetic rats. Food Chem. Toxicol. 2013, 62, 722–731. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, B.; Yu, X.; Zhuang, C.; Li, X.; Sun, J.; Xing, Y.; Xiu, Z.; Dong, Y. Oroxin A from Oroxylum indicum prevents the progression from prediabetes to diabetes in streptozotocin and high-fat diet induced mice. Phytomedicine 2018, 38, 24–34. [Google Scholar] [CrossRef]
- Cai, T.; Xu, X.; Dong, L.; Liang, S.; Xin, M.; Wang, T.; Li, T.; Wang, X.; Zheng, W.; Wang, C. Oroxin A from Oroxylum indicum improves disordered lipid metabolism by inhibiting SREBPs in oleic acid-induced HepG2 cells and high-fat diet-fed non-insulin-resistant rats. Heliyon 2024, 10, e29168. [Google Scholar] [CrossRef]
- Lalrinzuali, K.; Vabeiryureilai, M.; Jagetia, G.C. Topical application of stem bark ethanol extract of Sonapatha, Oroxylum indicum (L.) Kurz accelerates healing of deep dermal excision wound in Swiss albino mice. J. Ethnopharmacol. 2018, 227, 290–299. [Google Scholar] [CrossRef]
- Abdulhafiz, F.; Reduan, M.F.H.; Hisam, A.H.; Mohammad, I.; Abdul Wahab, I.R.; Abdul Hamid, F.F.; Mohammed, A.; Nordin, M.L.; Shaari, R.; Bakar, L.A. LC–TOF-MS/MS and GC-MS based phytochemical profiling and evaluation of wound healing activity of Oroxylum indicum (L.) Kurz (Beka). Front. Pharmacol. 2022, 13, 1050453. [Google Scholar] [CrossRef]
- Zhao, P.; Alam, M.B.; An, H.; Choi, H.-J.; Cha, Y.H.; Yoo, C.-Y.; Kim, H.-H.; Lee, S.-H. Antimelanogenic effect of an Oroxylum indicum seed extract by suppression of MITF expression through activation of MAPK signaling protein. Int. J. Mol. Sci. 2018, 19, 760. [Google Scholar] [CrossRef]
- Kumar, R.A.; Rajkumar, V.; Guha, G.; Mathew, L. Therapeutic potentials of Oroxylum indicum bark extracts. Chin. J. Nat. Med. 2010, 8, 121–126. [Google Scholar] [CrossRef]
- Mrazek, N.; Watla-iad, K.; Deachathai, S.; Suteerapataranon, S. Rapid antioxidant capacity screening in herbal extracts using a simple flow injection-spectrophotometric system. Food Chem. 2012, 132, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Cao, Y.; Yang, B. HPLC-DPPH screening method for evaluation of antioxidant compounds extracted from Semen Oroxyli. Molecules 2014, 19, 4409–4417. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, J.; Li, S. High-performance liquid chromatography coupled with post-column dual-bioactivity assay for simultaneous screening of xanthine oxidase inhibitors and free radical scavengers from complex mixture. J. Chromatogr. A 2014, 1345, 50–56. [Google Scholar] [CrossRef]
- Mim, J.; Sultana, M.S.; Dhar, P.K.; Hasan, M.K.; Dutta, S.K. Green mediated synthesis of cerium oxide nanoparticles by using Oroxylum indicum for evaluation of catalytic and biomedical activity. RSC Adv. 2024, 14, 25409–25424. [Google Scholar] [CrossRef]
- Ranabhat, K.; Regmi, K.P.; Parajuli, S.; Thapa, R.; Timilsina, A.P.; Katuwal, S.; Fleming, S.; Mishra, A.D.; Sharma, K.R.; Regmi, B.P. Evaluation of Antioxidant, Antimicrobial, and Cytotoxic Activities and Correlation with Phytoconstituents in Some Medicinal Plants of Nepal. J. Chem. 2022, 2022, 4725801. [Google Scholar] [CrossRef]
- Chhouk, K.; Kanda, H.; Goto, M. Efficacy of supercritical carbon dioxide integrated hydrothermal extraction of Khmer medicinal plants with potential pharmaceutical activity. J. Environ. Chem. Eng. 2018, 6, 2944–2956. [Google Scholar] [CrossRef]
- Masood, N.; Yadav, A.K.; Kumar, N.; Gupta, M.M.; Luqman, S. Density functional theory-based quantum rationalization of flavones from Oroxylum indicum, their correlation with redox effect, molecular interaction studies and osmotic hemolysis. Curr. Sci. 2018, 115, 2085–2094. Available online: https://www.jstor.org/stable/26978555 (accessed on 6 October 2025). [CrossRef]
- Srimawong, C.; Putalun, W. Consolidated extraction and conversion of baicalin to baicalein from Oroxylum indicum using a novel NADES-UAE system and bioactivity evaluation in RAW 264.7 cells. Food Biosci. 2025, 65, 106031. [Google Scholar] [CrossRef]
- Singh, J.; Chaudhari, B.P.; Kakkar, P. Baicalin and chrysin mixture imparts cyto-protection against methylglyoxal induced cytotoxicity and diabetic tubular injury by modulating RAGE, oxidative stress and inflammation. Environ. Toxicol. Pharmacol. 2017, 50, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.-Y.; Cao, Y.-Y.; Chen, C.-Y.; Dai, H.-Q.; Yu, S.-X.; Wei, J.-L.; Li, H.; Yang, B. Antioxidant flavonoids from the seed of Oroxylum indicum. Fitoterapia 2011, 82, 841–848. [Google Scholar] [CrossRef]
- Tang, Q.; Jia, H.; Qin, X.; Lu, Z.; Huang, W.; Wang, Y.; Cao, Z. Scutellarein ameliorates dextran sulfate sodium-induced ulcerative colitis by inhibiting colonic epithelial cell proinflammation and barrier disruption. Front. Pharmacol. 2024, 15, 1479441. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, C.; Xie, J.; Zhai, K.; Wei, S.; Cheng, X.; Zhang, R. Oroxin A ameliorates the oleic acid-induced A549 cell injury through the suppression of pyroptosis and degradation of alveolar surfactant. An. Acad. Bras. Cienc. 2022, 94, e20211400. [Google Scholar] [CrossRef]
- Jelić, D.; Lower-Nedza, A.D.; Brantner, A.H.; Blažeković, B.; Bian, B.; Yang, J.; Brajša, K.; Vladimir-Knežević, S. Baicalin and Baicalein Inhibit Src Tyrosine Kinase and Production of IL-6. J. Chem. 2016, 2016, 2510621. [Google Scholar] [CrossRef]
- Chen, D.-H.; Zheng, G.; Zhong, X.-Y.; Lin, Z.-H.; Yang, S.-W.; Liu, H.-X.; Shang, P. Oroxylin A attenuates osteoarthritis progression by dual inhibition of cell inflammation and hypertrophy. Food Funct. 2021, 12, 328–339. [Google Scholar] [CrossRef]
- Huang, J.-M.; Wang, C.-Z.; Lu, S.-Y.; Wang, Z.; Yan, Z.-Q. Oroxin B attenuates ovariectomy-induced bone loss by suppressing osteoclast formation and activity. Drug Des. Devel. Ther. 2021, 15, 4811–4825. [Google Scholar] [CrossRef]
- Muniyasamy, R.; Manjubala, I. Identification of potential sclerostin inhibiting flavonoids from Oroxylum indicum: An insilico approach. J. Biomol. Struct. Dyn. 2024, 42, 6588–6599. [Google Scholar] [CrossRef] [PubMed]
- Mangal, P.; Khare, P.; Jagtap, S.; Bishnoi, M.; Kondepudi, K.K.; Bhutani, K.K. Screening of six Ayurvedic medicinal plants for anti-obesity potential: An investigation on bioactive constituents from Oroxylum indicum (L.) Kurz bark. J. Ethnopharmacol. 2017, 197, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Yang, R.; Yang, Q.; Wan, W.; Wei, X. Proteomic screening identifies the direct targets of chrysin anti-lipid depot in adipocytes. J. Ethnopharmacol. 2021, 267, 113361. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kakkar, P. Oroxylin A, a constituent of Oroxylum indicum inhibits adipogenesis and induces apoptosis in 3T3-L1 cells. Phytomedicine 2014, 21, 1733–1741. [Google Scholar] [CrossRef]
- Menon, S.; Lawrence, L.; Sivaram, V.P.; Padikkala, J. Oroxylum indicum root bark extract prevents doxorubicin-induced cardiac damage by restoring redox balance. J. Ayurveda Integr. Med. 2019, 10, 159–165. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Sasikumar, S.; Puhari, S.S.M.; Ramprasath, T.; Baskaran, N.; Vasudevan, V.; Selvam, G.S. Chrysin reduces hypercholesterolemia-mediated atherosclerosis through modulating oxidative stress, microflora, and apoptosis in experimental rats. J. Food Biochem. 2022, 46, e14349. [Google Scholar] [CrossRef]
- Babu, T.H.; Manjulatha, K.; Kumar, G.S.; Hymavathi, A.; Tiwari, A.K.; Purohit, M.; Rao, J.M.; Babu, K.S. Gastroprotective flavonoid constituents from Oroxylum indicum Vent. Bioorg. Med. Chem. Lett. 2010, 20, 117–120. [Google Scholar] [CrossRef]
- Chalermwongkul, C.; Khamphukdee, C.; Maneenet, J.; Daodee, S.; Monthakantirat, O.; Boonyarat, C.; Chotritthirong, Y.; Awale, S.; Kijjoa, A.; Chulikhit, Y. Antidepressant-like effect of Oroxylum indicum seed extract in mice model of unpredictable chronic mild stress. Nutrients 2023, 15, 4742. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Majeed, M.; Drummond, P.D. Effects of an Oroxylum indicum Extract (Sabroxy®) on cognitive function in adults with self-reported mild cognitive impairment: A randomized, double-blind, placebo-controlled study. Front. Aging Neurosci. 2021, 13, 728360. [Google Scholar] [CrossRef]
- Mairuae, N.; Connor, J.R.; Buranrat, B.; Lee, S.Y. Oroxylum indicum (L.) extract protects human neuroblastoma SH-SY5Y cells against β-amyloid-induced cell injury. Mol. Med. Rep. 2019, 20, 1933–1942. [Google Scholar] [CrossRef]
- Pondugula, S.R.; Majrashi, M.; Almaghrabi, M.; Ramesh, S.; Abbott, K.L.; Govindarajulu, M.; Gill, K.; Fahoury, E.; Narayanan, N.; Desai, D. Oroxylum Indicum ameliorates chemotherapy induced cognitive impairment. PLoS ONE 2021, 16, e0252522. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.G.; Arai, M.A.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M. Phenolic compounds from the bark of Oroxylum indicum activate the Ngn2 promoter. J. Nat. Med. 2015, 69, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Thi Vien, L.; Thi Hong Hanh, T.; Quang, T.H.; Cuong, N.T.; Cuong, N.X.; Oh, H.; Sinh, N.V.; Nam, N.H.; Van Minh, C. Phenolic glycosides from Oroxylum indicum. Nat. Prod. Res. 2022, 36, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- Summat, R.; Waiwut, P.; Daodee, S.; Nualkaew, N.; Phemphunananchai, K.; Arsito, P.N.; Chulikhit, Y.; Montakantirat, O.; Khamphukdee, C.; Boonyarat, C. Phytomedicine Potential of Oroxylum indicum Root and Its Constituents: Targeting Alzheimer’s Disease. Plants 2025, 14, 223. [Google Scholar] [CrossRef]
- Sohn, S.-H.; Yoon, M.; Kim, J.; Choi, H.-L.; Shin, M.; Hong, M.; Bae, H. Screening herbal medicines for the recovery of alpha-synuclein-induced Parkinson’s disease model of yeast. Mol. Cell. Toxicol. 2012, 8, 343–348. [Google Scholar] [CrossRef]
- Kang, I.N.; Lee, C.Y.; Tan, S.C. Selection of best reference genes for qRT-PCR analysis of human neural stem cells preconditioned with hypoxia or baicalein-enriched fraction extracted from Oroxylum indicum medicinal plant. Heliyon 2019, 5, e02156. [Google Scholar] [CrossRef]
- Sreedharan, S.; Pande, A.; Pande, A.; Majeed, M.; Cisneros-Zevallos, L. The neuroprotective effects of Oroxylum indicum extract in SHSY-5Y neuronal cells by upregulating BDNF gene expression under LPS induced inflammation. Nutrients 2024, 16, 1887. [Google Scholar] [CrossRef]
- Muniyasamy, R.; Manjubala, I. Synergistic combination of baicalein and rifampicin against Staphylococcus aureus biofilms. Front. Microbiol. 2024, 15, 1458267. [Google Scholar] [CrossRef]
- Feng, W.-Y.; Cheang, U.-I.; Wong, K.-I.; Cheong, H.-I.; Meng, L.-R.; Fong, P. Synergistic activity of Coptis Chinensis and clotrimazole against Candida Albicans. Pharmacol. Res. Mod. Chin. Med. 2023, 8, 100287. [Google Scholar] [CrossRef]
- Sithisarn, P.; Nantateerapong, P.; Rojsanga, P.; Sithisarn, P. Screening for antibacterial and antioxidant activities and phytochemical analysis of Oroxylum indicum fruit extracts. Molecules 2016, 21, 446. [Google Scholar] [CrossRef]
- Ratanakomol, T.; Roytrakul, S.; Wikan, N.; Smith, D.R. Oroxylin A shows limited antiviral activity towards dengue virus. BMC Res. Notes 2022, 15, 154. [Google Scholar] [CrossRef]
- Fan, Q.-F.; Hu, Z.-Y.; Na, Z.; Tang, H.-S.; Zuo, G.-Y.; Song, Q.-S. One new flavonoid from Oroxylum indicum. Nat. Prod. Res. 2015, 29, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Gan, R.-Y.; Zhang, D.; Farha, A.K.; Habimana, O.; Mavumengwana, V.; Li, H.-B.; Wang, X.-H.; Corke, H. Large-scale screening of 239 traditional Chinese medicinal plant extracts for their antibacterial activities against multidrug-resistant Staphylococcus aureus and cytotoxic activities. Pathogens 2020, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Palachum, W.; Chisti, Y.; Choorit, W. In-vitro assessment of probiotic potential of Lactobacillus plantarum WU-P19 isolated from a traditional fermented herb. Ann. Microbiol. 2018, 68, 79–91. [Google Scholar] [CrossRef]
- Sithisarn, P.; Rojsanga, P.; Sithisarn, P. Inhibitory effects on clinical isolated bacteria and simultaneous HPLC quantitative analysis of flavone contents in extracts from Oroxylum indicum. Molecules 2019, 24, 1937. [Google Scholar] [CrossRef]
- Sithisarn, P.; Rojsanga, P.; Sithisarn, P. Flavone-rich fractions and extracts from Oroxylum indicum and their antibacterial activities against clinically isolated zoonotic bacteria and free radical scavenging effects. Molecules 2021, 26, 1773. [Google Scholar] [CrossRef]
- Deori, K.; Yadav, A.K. Anthelmintic effects of Oroxylum indicum stem bark extract on juvenile and adult stages of Hymenolepis diminuta (Cestoda), an in vitro and in vivo study. Parasitol. Res. 2016, 115, 1275–1285. [Google Scholar] [CrossRef]
- Singh, K.; Yadav, A.; Khan, S.; Shukla, A.; Alam, M.; Verma, A.K.; Tiwari, N.; Khan, F.; Yadav, P.N.; Dev, K. Baicalein isolated from Oroxylum indicum acts as a potent µ-and κ-opioid receptor antagonist agent via the reversal of agonist-mediated cAMP inhibition. Nat. Prod. Res. 2024, in press. 1–9. [Google Scholar] [CrossRef]
- Kiratipaiboon, C.; Wasana, P.W.D.; Sukrong, S.; Ruangrungsri, N.; Towiwat, P. Herbal root extracts in Ben-Cha-Moon-Yai remedy attenuated pain-like behaviors and inflammation through the opioid and prostaglandin systems. J. Ethnopharmacol. 2022, 290, 115088. [Google Scholar] [CrossRef]
- Lee, A.-Y.; Kang, S.; Park, S.-J.; Huang, J.; Im, D.-S. Anti-Allergic Effect of Oroxylin A from Oroxylum indicum Using in vivo and in vitro Experiments. Biomol. Ther. 2016, 24, 283. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Mahapatra, M.; Gurung, B.; Dey, A.; Nongalleima, K.; Das, S.; Talukdar, A.D.; Chowdhury, A.; Choudhury, M.D.; Deb, L. Antifertility activity of Oroxylum indicum Vent. stem bark on female Wistar rats. J. Tradit. Knowl. 2024, 23, 189–199. [Google Scholar] [CrossRef]
- Liu, R.P.; Wang, X.Q.; Wang, J.; Dan, L.; Li, Y.H.; Jiang, H.; Xu, Y.N.; Kim, N.H. Oroxin A reduces oxidative stress, apoptosis, and autophagy and improves the developmental competence of porcine embryos in vitro. Reprod. Domest. Anim. 2022, 57, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-Q.; Zhao, J.; Li, S.-P.; Zhang, Q.-W. Discovery of xanthine oxidase inhibitors from a complex mixture using an online, restricted-access material coupled with column-switching liquid chromatography with a diode-array detection system. Anal. Bioanal. Chem. 2014, 406, 1975–1984. [Google Scholar] [CrossRef]
- Lone, B.A.; Sharma, N.; Kour, D.; Bhushan, A.; Rani, D.; Kumar, A.; Gupta, P.K.; Gupta, P. In-vitro anti-sickling potential of baicalin and naringenin isolated from Oroxylum indicum and Citrus aurantium on human sickle red blood cells. Nat. Prod. Res. 2023, 37, 3902–3908. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wang, N.; Liu, J.; Zhang, L.-T.; Zhang, Z.-Q.; Li, D.-Q. The mysteries of pharmacokinetics and in vivo metabolism of Oroxylum indicum (L.) Kurz: A new perspective from MSOP method. Heliyon 2024, 10, e33234. [Google Scholar] [CrossRef]
- Chakraborty, R.; Roy, S.; Mandal, V. Assessment of traditional knowledge of the antidiabetic plants of Darjeeling and Sikkim Himalayas in the context of recent phytochemical and pharmacological advances. J. Integr. Med. 2016, 14, 336–358. [Google Scholar] [CrossRef]
- Yaipharembi, N.; Huidrom, E.; Nongalleima, K.; Singh, H.B. An Ethnobotanical study on the dietary use of wild trees as traditional vegetables by three ethnic communities in Manipur, North East India. Econ. Bot. 2023, 77, 324–339. [Google Scholar] [CrossRef]
- Panmei, R.; Gajurel, P.; Singh, B. Ethnobotany of medicinal plants used by the Zeliangrong ethnic group of Manipur, northeast India. J. Ethnopharmacol. 2019, 235, 164–182. [Google Scholar] [CrossRef]
- Tangjang, S.; Namsa, N.D.; Aran, C.; Litin, A. An ethnobotanical survey of medicinal plants in the Eastern Himalayan zone of Arunachal Pradesh, India. J. Ethnopharmacol. 2011, 134, 18–25. [Google Scholar] [CrossRef]
- Deb, L.; Laishram, S.; Khumukcham, N.; Ningthoukhongjam, D.; Nameirakpam, S.S.; Dey, A.; Moirangthem, D.S.; Talukdar, N.C.; Ningthoukhongjam, T.R. Past, present and perspectives of Manipur traditional medicine: A major health care system available for rural population in the North-East India. J. Ethnopharmacol. 2015, 169, 387–400. [Google Scholar] [CrossRef]
- Singh, S.S.; Ralte, L.; Sailo, H.; Pinokiyo, A.; Devi, M.R.; Khomdram, S.D.; Singh, Y.T. Ethnobotanical study of medicinal plants used by Lois community of Kakching district, Manipur, India. Trees For. People 2025, 19, 100765. [Google Scholar] [CrossRef]
- Chotchoungchatchai, S.; Saralamp, P.; Jenjittikul, T.; Pornsiripongse, S.; Prathanturarug, S. Medicinal plants used with Thai Traditional Medicine in modern healthcare services: A case study in Kabchoeng Hospital, Surin Province, Thailand. J. Ethnopharmacol. 2012, 141, 193–205. [Google Scholar] [CrossRef]
- Kadir, M.F.; Sayeed, M.S.B.; Setu, N.I.; Mostafa, A.; Mia, M. Ethnopharmacological survey of medicinal plants used by traditional health practitioners in Thanchi, Bandarban Hill Tracts, Bangladesh. J. Ethnopharmacol. 2014, 155, 495–508. [Google Scholar] [CrossRef]
- Aliani, M.; Eskin, M.N. Bitterness: Perception, Chemistry and Food Processing; John Wiley & Sons: Chichester, UK, 2017; ISBN 9781118590232. [Google Scholar]
- Cavallo, C.; Cicia, G.; Del Giudice, T.; Sacchi, R.; Vecchio, R. Consumers’ perceptions and preferences for bitterness in vegetable foods: The case of extra-virgin olive oil and brassicaceae—A narrative review. Nutrients 2019, 11, 1164. [Google Scholar] [CrossRef]
- Behrens, M.; Gu, M.; Fan, S.; Huang, C.; Meyerhof, W. Bitter substances from plants used in traditional Chinese medicine exert biased activation of human bitter taste receptors. Chem. Biol. Drug Des. 2018, 91, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Z.; Akbar, A.; Kamran, K.; Achakzai, J.K.; Wong, L.S.; Sadiq, M.B. Unlocking the therapeutic and antimicrobial potential of Prunus armeniaca L. Seed kernel oil. Int. J. Food Sci. 2024, 2024, 5589506. [Google Scholar] [CrossRef]
- Lopes, A.P.; Galuch, M.B.; Petenuci, M.E.; Oliveira, J.H.; Canesin, E.A.; Schneider, V.V.A.; Visentainer, J.V. Quantification of phenolic compounds in ripe and unripe bitter melons (Momordica charantia) and evaluation of the distribution of phenolic compounds in different parts of the fruit by UPLC–MS/MS. Chem. Pap. 2020, 74, 2613–2625. [Google Scholar] [CrossRef]
- Dai, W.; Xiang, A.; Pan, D.; Xia, Q.; Sun, Y.; Wang, Y.; Wang, W.; Cao, J.; Zhou, C. Insights into the identification of bitter peptides from Jinhua ham and its taste mechanism by molecular docking and transcriptomics analysis. Food Res. Int. 2024, 189, 114534. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, M.; Hayes, J.E.; Duffy, V.B. Masking vegetable bitterness to improve palatability depends on vegetable type and taste phenotype. Chemosens. Percept. 2013, 6, 8–19. [Google Scholar] [CrossRef]
- Ke, J.; Wang, Y.; Luo, T.; Liang, Y.; Wang, X.; Ma, Y.; Zhao, L.; Zhang, Z. Study on the effect of different bitter masking inhibitors on the bitter masking of Zanthoxylum bungeanum Maxim. Int. J. Gastron. Food Sci. 2024, 35, 100894. [Google Scholar] [CrossRef]
- Kan, R.; Yu, Z.; Zhao, W. Identification and molecular action mechanism of novel TAS2R14 blocking peptides from egg white proteins. LWT 2023, 180, 114716. [Google Scholar] [CrossRef]
- Cox, D.N.; Melo, L.; Zabaras, D.; Delahunty, C.M. Acceptance of health-promoting Brassica vegetables: The influence of taste perception, information and attitudes. Public Health Nutr. 2012, 15, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Worakitjaroenphon, S.; Shanmugam, P.; Boonyuen, S.; Smith, S.M.; Chookamnerd, K. Green synthesis of silver and gold nanoparticles using Oroxylum indicum plant extract for catalytic and antimicrobial activity. Biomass Convers. Biorefin. 2023, in press. 1–12. [Google Scholar] [CrossRef]
- Greene, A.M.; Panyadee, P.; Inta, A.; Huffman, M.A. Asian elephant self-medication as a source of ethnoveterinary knowledge among Karen mahouts in northern Thailand. J. Ethnopharmacol. 2020, 259, 112823. [Google Scholar] [CrossRef]
- Sritongchuay, T.; Bumrung, S.; Meesawat, U.; Mazer, S.J. Stigma closure and re-opening in Oroxylum indicum (Bignoniaceae): Causes and consequences. Am. J. Bot. 2010, 97, 136–143. [Google Scholar] [CrossRef]
- Sonia, R.; Shaheen, S.; Waheed, M.; Imran, S.; Haq, S.M.; Muhammad, M.; Hashem, A.; Al Shehri, S.; Abd-Allah, E.F. Anatomical characterization of Semi-arid Bignoniaceae using light and scanning electron microscopy. BMC Plant Biol. 2025, 25, 125. [Google Scholar] [CrossRef]
- Yin, X.-s.; Zhong, Z.-f.; Bian, G.-l.; Cheng, X.-j.; Li, D.-q. Ultra-rapid, enhanced and eco-friendly extraction of four main flavonoids from the seeds of Oroxylum indicum by deep eutectic solvents combined with tissue-smashing extraction. Food Chem. 2020, 319, 126555. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, P.; Yu, Q.; Li, P.; Dong, N. Column-free matrix solid-phase dispersion extraction based on cucurbit[8]uril used for the determination of the flavonoid content in Oroxylum indicum (L.) Vent. Ind. Crops Prod. 2022, 175, 114277. [Google Scholar] [CrossRef]
- Rojsanga, P.; Bunsupa, S.; Sithisarn, P. Flavones contents in extracts from Oroxylum indicum seeds and plant tissue cultures. Molecules 2020, 25, 1545. [Google Scholar] [CrossRef]
- Xie, Z.; Li, G.; Fu, Y.; Sun, M.; Ye, B. Sensitive, simultaneous determination of chrysin and baicalein based on Ta2O5-chitosan composite modified carbon paste electrode. Talanta 2017, 165, 553–562. [Google Scholar] [CrossRef]
- Tamta, B.; Kumar, R.; Uniyal, S.; Uniyal, A. Exploration and selection of elite germplasm of Oroxylum indicum (L.) Vent.(Shyonak) in the forest divisions of Punjab, India. Curr. Sci. 2022, 122, 1401–1406. [Google Scholar] [CrossRef]
- Othman, F.A.; Mat Zin, A.A.; Zakaria, Y.; Nik Salleh, N.N.H.; Mohd Satar, A.; Tan, S.C. Pre-clinical acute oral toxicity and subacute neurotoxicity risk assessments on sprague dawley rats treated with single dose or repeated doses of flavonoid-enriched fraction extracted from Oroxylum indicum leaves. Drug Chem. Toxicol. 2025, 48, 1104–1120. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.S.; Aparna, A.S. High Performance Thin Layer Chromatographic determination of chrysin in Oroxylum indicum vent. from different geographical regions of India. J. Chem. 2012, 9, 313–317. [Google Scholar] [CrossRef]
- Xie, Z.; Wu, Y.; Kai, S.; Li, G.; Ye, B. A newly competitive electrochemical sensor for sensitive determination of chrysin based on electrochemically activated Ta2O5 particles modified carbon paste electrode. Electroanalysis 2017, 29, 835–842. [Google Scholar] [CrossRef]
- Treesuwan, W.; Ichikawa, S.; Wang, Z.; Neves, M.A.; Uemura, K.; Nakajima, M.; Kobayashi, I. Formulation and storage stability of baicalein-loaded oil-in-water emulsions. Eur. J. Lipid Sci. Technol. 2013, 115, 1115–1122. [Google Scholar] [CrossRef]
- Zhou, L.; Jing, T.; Zhang, P.; Zhang, L.; Cai, S.; Liu, T.; Fan, H.; Yang, G.; Lin, R.; Zhang, J. Kinetics and modeling for extraction of chrysin from Oroxylum indicum seeds. Food Sci. Biotechnol. 2015, 24, 2045–2050. [Google Scholar] [CrossRef]
- Sharma, H.; Narshimhaji, C.V.; Singh, R. Relative estimation of beta-sitosterol in alcoholic extracts of roots and small branches of Oroxylum indicum (L.) Kurz by HPTLC for plant part substitution, medicinal uses and conservation. J. Appl. Res. Med. Aromat. Plants 2023, 35, 100496. [Google Scholar] [CrossRef]
- Dong, N.; Yang, L.; Li, X.; Zhao, A. A novel green sample pretreatment method column-free matrix solid-phase dispersion extraction: Application in a high-performance liquid chromatography experiment for undergraduate chemistry. J. Chem. Educ. 2024, 101, 3369–3376. [Google Scholar] [CrossRef]
- Feng, R.; Zhang, X.; Yin, J.; Zhang, Y.; Ma, Y.; Zhang, X.; Zhang, L.; Li, D. A comprehensive study of the metabolism of flavonoid oroxin B in vivo and in vitro by UHPLC-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2021, 197, 113905. [Google Scholar] [CrossRef]
- Krüger, A.; Ganzera, M. Oroxylum indicum seeds–Analysis of flavonoids by HPLC–MS. J. Pharm. Biomed. Anal. 2012, 70, 553–556. [Google Scholar] [CrossRef]
- Liu, R.; Xu, L.; Li, A.; Sun, A. Preparative isolation of flavonoid compounds from Oroxylum indicum by high-speed counter-current chromatography by using ionic liquids as the modifier of two-phase solvent system. J. Sep. Sci. 2010, 33, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Manika, N.; Bagchi, G.D.; Gupta, M.M. Simultaneous determination of flavonoids in Oroxylum indicum by RP-HPLC. Med. Chem. Res. 2013, 22, 2222–2227. [Google Scholar] [CrossRef]
- Chandewar, A.V.; Kochar, N.I.; Shrirao, A.V.; Karpe, S.T. Phytochemical screening, chromatographic and quantitative study of phenols and flavonoids in leaves of Oroxylum indicum and Pongamia pinnata. Res. J. Pharm. Technol. 2023, 16, 2604–2608. [Google Scholar] [CrossRef]
- Bhusari, S.; Morey, S.; Nikam, K.; Wakte, P. Comparative evaluation of baicalein from Oroxylum indicum by using conventional and non-conventional extraction methodology. Res. J. Pharm. Technol. 2019, 12, 1817–1822. [Google Scholar] [CrossRef]
- Shi, S.; Wei, Y.; Feng, J.; Zhou, C.; Zuo, J.; Yao, L.; Ding, J.; Li, K.; He, Q. Facile and ultrasensitive electrochemical detection of baicalein on bismuth oxide-carboxylated multi-walled carbon nanotube/glassy carbon electrode. J. Food Compos. Anal. 2023, 123, 105557. [Google Scholar] [CrossRef]
- Deshmukh, A.B.; Datir, S.S.; Bhonde, Y.; Kelkar, N.; Samdani, P.; Tamhane, V.A. De novo root transcriptome of a medicinally important rare tree Oroxylum indicum for characterization of the flavonoid biosynthesis pathway. Phytochemistry 2018, 156, 201–213. [Google Scholar] [CrossRef]
- Gurupasad, K.; Mascarenhas, R.; Gopinath, P.; Satyamoorthy, K. Studies on Brahma rasayana in male Swiss albino mice: Chromosomal aberrations and sperm abnormalities. J. Ayurveda Integr. Med. 2010, 1, 40. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3149391/ (accessed on 6 October 2025). [CrossRef]
| Top 35 Journals and Number of Publications | ||
|---|---|---|
| Journal of Ethnopharmacology (18) | Cancers (2) | Journal of Chemistry (2) |
| Molecules (11) | Current Science (2) | Journal of Functional Foods (2) |
| Natural Product Research (6) | Economic Botany (2) | Journal of Natural Medicines (2) |
| Journal of Ayurveda and Integrative Medicine (5) | Ethnobotany Research and Applications (2) | Journal of Pharmaceutical and Biomedical Analysis (2) |
| Phytomedicine (4) | European Journal of Pharmacology (2) | Journal of Pharmacy and Bioallied Sciences (2) |
| European Journal of Medicinal Chemistry (3) | Fitoterapia (2) | Medicinal Chemistry Research (2) |
| Heliyon (3) | Food Bioscience (2) | Pharmacological Research-Modern Chinese Medicine (2) |
| Indian Journal of Traditional Knowledge (3) | Food Chemistry (2) | Plants (2) |
| Journal of Biomolecular Structure and Dynamics (3) | Frontiers in Pharmacology (2) | PLoS ONE (2) |
| Nutrients (3) | Industrial Crops and Products (2) | Processes (2) |
| Research Journal of Pharmacy and Technology (3) | Journal of Applied Pharmaceutical Science (2) | RSC Advances (2) |
| Biomedicine and Pharmacotherapy (2) | Journal of Applied Research on Medicinal and Aromatic Plants (2) | |
| Group | Related Activities | Extract/ Compound | Plant Part | Model | References |
|---|---|---|---|---|---|
| Cancer | anticancer, anti-tumor, cytotoxic, anti-metastatic, histone deacetylase (HDAC) inhibition, and anti-lymphoma. | Endophytic fungus extract, ethanol extract, ethyl acetate extract, methanol extract, aqueous extract, hydroalcoholic extract, fungal endophyte metabolites, baicalein, chrysin, oroxylin A, oroxylin A glycoside, oroxylin A-7-O-glucuronide, oroxyquinone, methoxy-chrysin, scutellarein-7-rutinoside, oroxin B, chrysin-7-O-glucuronide, oroxindin, oroxin A, and semi-synthetic derivatives. | Stem bark, bark, leaves, fruit, young stems, root, and root bark. | In vitro, in vivo, in silico. | [7,13,30,37,40,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63] |
| Diabetes | Anti-prediabetes, antidiabetic, and anti-NASH (non-alcoholic steatohepatitis). | Methanol extract, seed extract, baicalein, chrysin, baicalein-7-O-glucoside, baicalein-7-O-diglucoside, baicalin, oroxylin A, flavone derivatives, and glycosides. | Seed, stem bark, root, and bark. | In vitro, in vivo, in silico. | [8,38,64,65,66,67,68,69,70,71] |
| Liver Protection | Hepatoprotective and MAFLD (metabolic associated fatty liver disease). | Ethanol extract, apigenin, baicalein, chrysin, oroxylin A, scutellarin, tetuin, oroxin B, and oroxin A. | Stem bark and seed. | In vitro, in vivo, in silico. | [19,41,72] |
| Skin Health | Wound healing and skin-whitening. | Ethanol extract, aqueous extracts, and chrysin. | Leaf, seed, and stem bark. | In vitro, in vivo. | [73,74,75] |
| Antioxidant | Antioxidant. | Methanol extract, aqueous extract, ethanol extract, acetone extract, hexane extract, SCeCO2eH extract (supercritical carbon dioxide integrated hydrothermal extract), hydrothermal extract, Soxhlet methanol extract, cerium oxide nanoparticles, baicalin, chrysin, baicalein, oroxylin A, scutellarein, baicalein-7-O-gentiobioside, baicalein-7-O-glucoside, and hispidulin. | Bark, fruit, seed, leaf, branch, stem, twig, root, and immature pod. | In vitro, in vivo, in silico. | [11,76,77,78,79,80,81,82,83,84,85,86] |
| Anti-inflammatory | Anti-inflammatory. | Ethanol extract, dichloromethane extract, ethyl acetate extract, decoction, baicalein, scutellarein, oroxylin A, chrysin, hispidulin, oroxin A, and baicalin. | Bark, leaves, seeds, shoots, fruits, grilled fruits, stem bark, and root bark. | In vitro, in vivo, in silico. | [2,9,16,17,39,87,88,89] |
| Bone Health | Anti-osteoarthritic, anti-osteoporotic, osteogenic support, and skeletal protective activity. | Ethanol extract, oroxin B, oroxylin A, baicalein, and other major flavonoids. | Stem bark. | In vitro, in vivo, in silico. | [12,14,90,91,92] |
| Obesity | Anti-adipogenic and anti-obesity. | Ethyl acetate extract, ethanol extract, baicalein, oroxylin A, luteolin, apigenin, and chrysin. | Fruit and bark. | In vitro, in silico. | [42,93,94,95] |
| Cardiovascular Health | Anti-atherosclerotic and cardioprotective. | Sabroxy (O. indicum extract), methanol extract, and chrysin. | Stem bark and root bark. | In vivo. | [18,96,97] |
| Gastrointestinal Health | Anti-ulcer. | Dihydrooroxylin A-7-O-methyl glucuronide and chrysin. | Stem bark. | In vivo. | [98] |
| Neurological Protection | Neuroprotective, neuroregenerative, anti-Alzheimer, antidepressants, and anti-neuroinflammatory | Sabroxy, water extract, methanol extract, ethanol extract, baicalein, oroxylin A, chrysin, hispidulin, apigenin, baicalin, and isoverbascoside. | Leaf, bark, seed, root, root bark, and fruit pod. | In vitro, in vivo, in silico. | [43,99,100,101,102,103,104,105,106,107,108] |
| Anti-infective | Antibacterial, antimicrobial, antiviral, anthelmintic, and antifungal. | Aqueous extract, methanol extract, ethanol extract, Lactobacillus plantarum WU-P19, baicalein, baicalin, 5,6,7-trimethoxyflavone-8-O-β-D-glucopyranoside, oroxylin A-7-O-β-D-glucuronide butyl ester, chrysin, 6-methoxybaicalein, oroxylin A-7-O-glucoside, and oroxylin A. | Seeds, young fruits, flowers, fermented fruit, leaf, stem, bark, and root. | In vitro, in silico. | [10,15,31,109,110,111,112,113,114,115,116,117,118] |
| Pain Relief | Anti-nociceptive. | Ethanol extract, water extract, and baicalein. | Stem barks and roots. | In vitro, in vivo, in silico. | [119,120] |
| Respiratory Health | Anti-allergic and anti-asthmatic. | Oroxylin A. | Not specified. | In vitro, in vivo. | [121] |
| Reproductive Health | Antifertility and embryo support | Aqueous extract, methanol extract, and oroxin A. | Stem bark. | Ex vivo, in vivo, in vitro. | [122,123] |
| Miscellaneous Pharmacological Actions | Pharmacokinetics, anti-gout, and anti-sickling | Ethanol extract, methanol extract, baicalin, oroxylumoside A, oroxylumoside B, darendoside A, and leucosceptoside A. | Seeds and stem bark. | In vitro, in vivo, ex vivo | [124,125,126] |
| Group | Ethnomedicinal Uses | Comparison with Pharmacological Activities | References |
|---|---|---|---|
| Cancer | Cancer. | Strongly supported | [3] |
| Diabetes | Antidiabetic. | Strongly supported | [3,93,127,128] |
| Liver Protection | Jaundice, liver problems, hepatitis, and hepatoprotective. | Strongly supported | [3,4,32] |
| Skin Health | Leukoderma, urticaria, infantile erythema, cuts and wounds, burns, skin disorder, and skin diseases. | Moderately supported | [3,5,129] |
| Anti-inflammatory | Arthritis, inflammation, and rheumatism. | Strongly supported | [3,4] |
| Antioxidant | Detoxification and rejuvenation. | Strongly supported | [2,93] |
| Bone Health | Arthritis and rheumatism. | Strongly supported | [3,4] |
| Obesity | Obesity. | Moderately supported | [93] |
| Cardiovascular Health | Cardiac disorders, high blood pressure, hypertension, and heart problems. | Strongly supported | [3,129,130] |
| Neurological Protection | Headache, neuralgia, epilepsy, and paralysis. | Moderately supported | [3,33,131] |
| Gastrointestinal Health | Stomach problems, diarrhea, carminative, stomachache, dysentery, purgative, astringent, stomachic, dyspepsia, gastropathy, gastralgia, stomach cleaning, colic, indigestion, bloody stool, stomach ulcer, constipation, abdominal pain, gastric ulcer, ulcer, piles, colitis, hemorrhoids, and other digestive ailments. | Strongly supported | [3,4,5,6,131,132] |
| Anti-infective | Fever, malaria, tuberculosis, smallpox, cholera, measles, typhoid, gonorrhea, pneumonia, and anthelmintic. | Moderately supported | [3,5,129] |
| Pain Relief | Muscle pain, chest pain, body pain, sprains, and analgesic. | Strongly supported | [3,4,120] |
| Respiratory Health | Cough, bronchitis, pharyngodymia, asthma, sore throat, laryngitis, hoarseness, allergic disease, and tonsillitis. | Strongly supported | [3] |
| Reproductive Health | Placental problem, menstrual disorders, womb ailment, leucorrhea, and a blood tonic. | Moderately supported | [3,4,129,133] |
| Miscellaneous and Unmapped Traditional Uses | Hair tonic, tonic, dropsy, urinary problems, scrotal swelling, and dog bite, enlarged spleen, antipyretic agent, hemorrhage, and scorpion sting | Unclear or unsupported | [3,4,5,120,134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.L.; Sae-Eaw, A.; Tran, D.Q.; Prinyawiwatkul, W.; Chulikhit, Y. A Systematic Review of Evidence-Based Health Benefits of Oroxylum indicum and Its Functional Food Potential. Foods 2025, 14, 3465. https://doi.org/10.3390/foods14203465
Nguyen HL, Sae-Eaw A, Tran DQ, Prinyawiwatkul W, Chulikhit Y. A Systematic Review of Evidence-Based Health Benefits of Oroxylum indicum and Its Functional Food Potential. Foods. 2025; 14(20):3465. https://doi.org/10.3390/foods14203465
Chicago/Turabian StyleNguyen, Hai Linh, Amporn Sae-Eaw, Dinh Quyen Tran, Witoon Prinyawiwatkul, and Yaowared Chulikhit. 2025. "A Systematic Review of Evidence-Based Health Benefits of Oroxylum indicum and Its Functional Food Potential" Foods 14, no. 20: 3465. https://doi.org/10.3390/foods14203465
APA StyleNguyen, H. L., Sae-Eaw, A., Tran, D. Q., Prinyawiwatkul, W., & Chulikhit, Y. (2025). A Systematic Review of Evidence-Based Health Benefits of Oroxylum indicum and Its Functional Food Potential. Foods, 14(20), 3465. https://doi.org/10.3390/foods14203465







