Identification and In Vitro Evaluation of Milkfish (Chanos chanos) Frame Proteins and Hydrolysates with DPP-IV Inhibitory and Antioxidant Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Proximate Analysis
2.4. Preparation of Milkfish Frame Protein Isolate
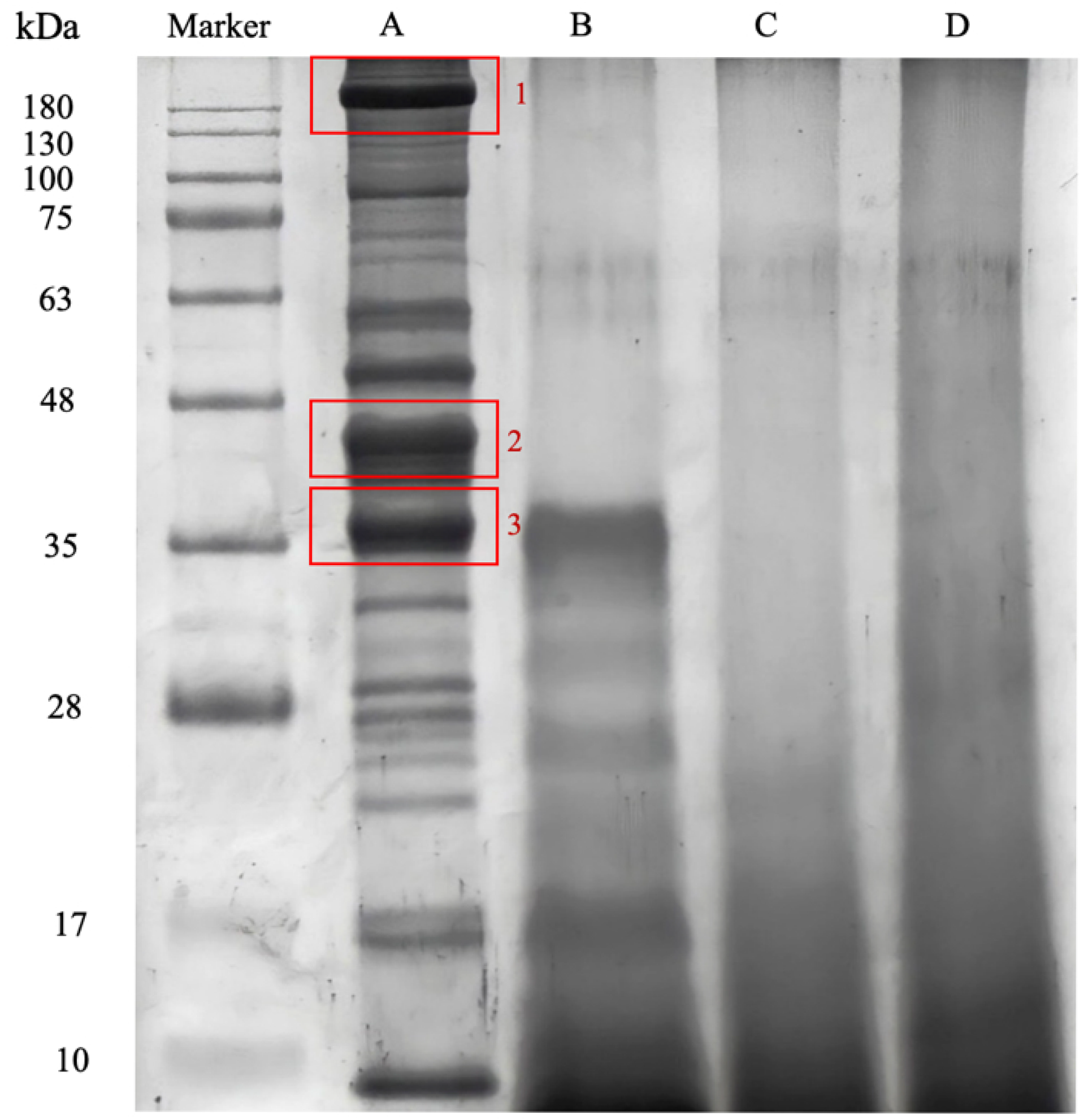
2.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
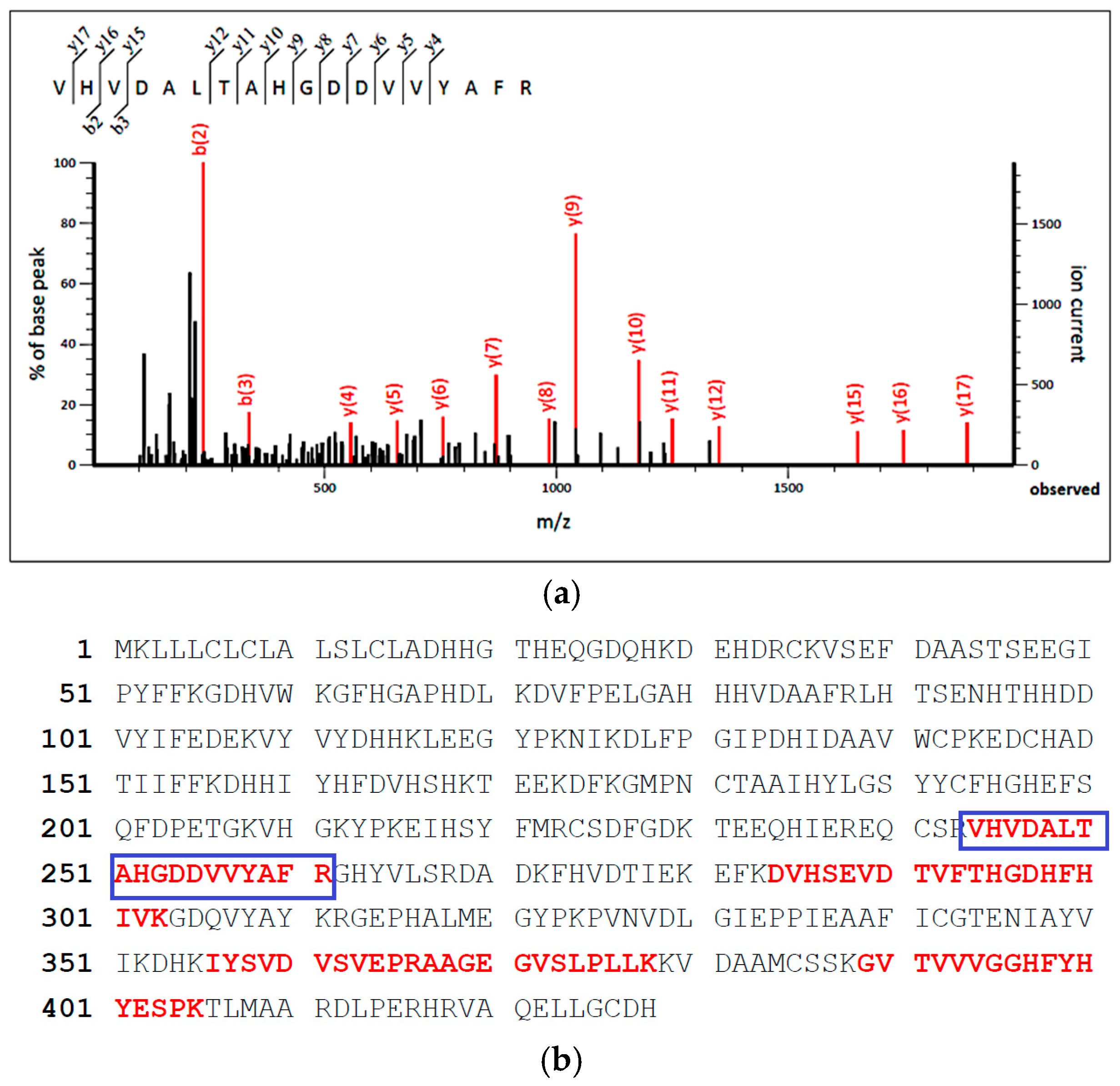
2.6. Proteomic Approach and Mascot Database Comparison
2.6.1. In-Gel Digestion
2.6.2. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.6.3. Tandem MS Database Comparison and Peptide Identification
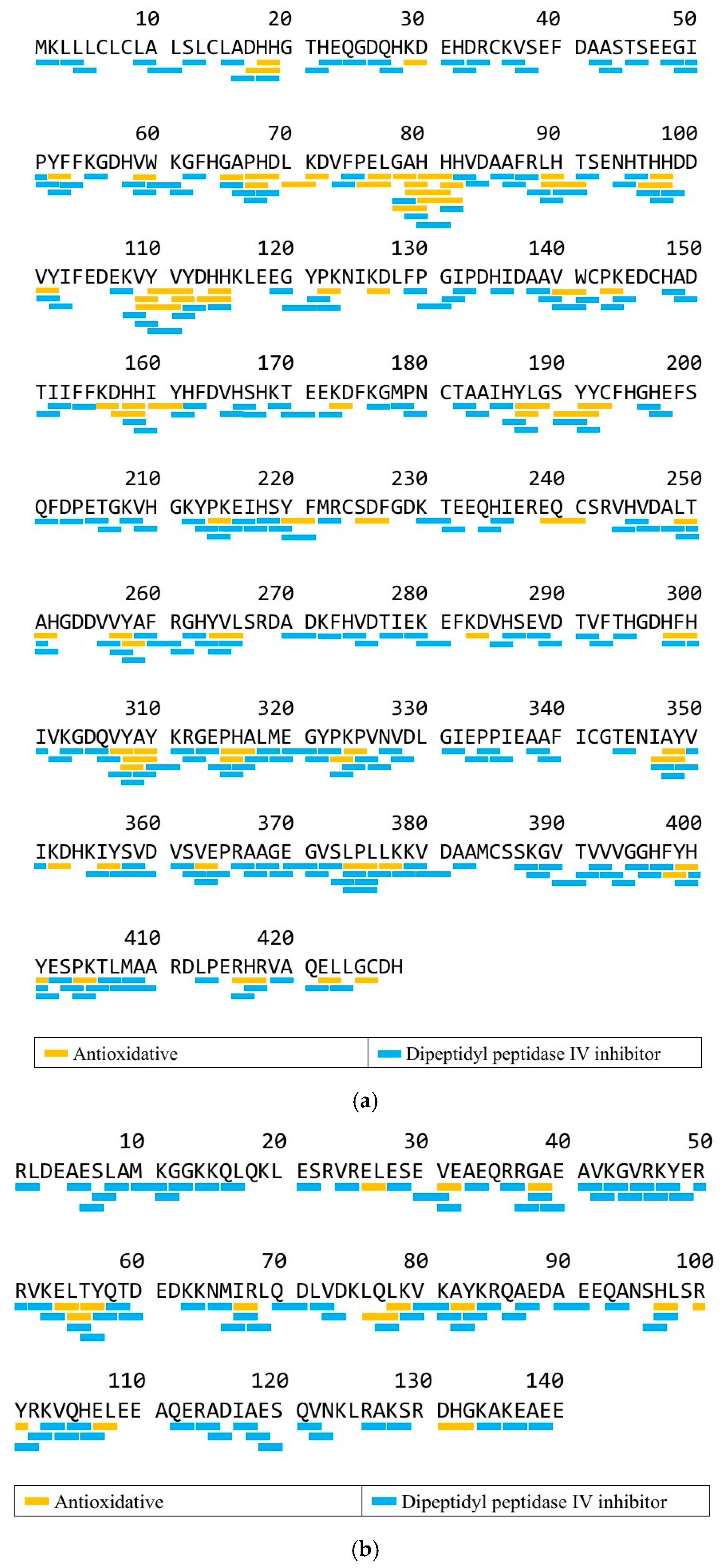
2.6.4. In Silico Analysis of Bioactive Peptides by BIOPEP-UWM Database Tools
2.7. In Vitro Analysis
2.7.1. Enzymatic Hydrolysis of Milkfish Frame Protein Isolate
2.7.2. Degree of Hydrolysis
2.7.3. Peptide Concentration Determination
2.7.4. Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Activity Assay
2.7.5. Antioxidant Activity Assay
2.7.6. Fractionation
2.7.7. Simulated Gastrointestinal Digestion
2.7.8. Cell Assay
FL83B Cell Culture
CCK8 Assay
Lactate Dehydrogenase (LDH) Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Composition of Milkfish Frame
3.2. Milkfish Frame Protein Extraction
3.3. Protein Identification
3.4. In Silico Analysis by BIOPEP-UWM
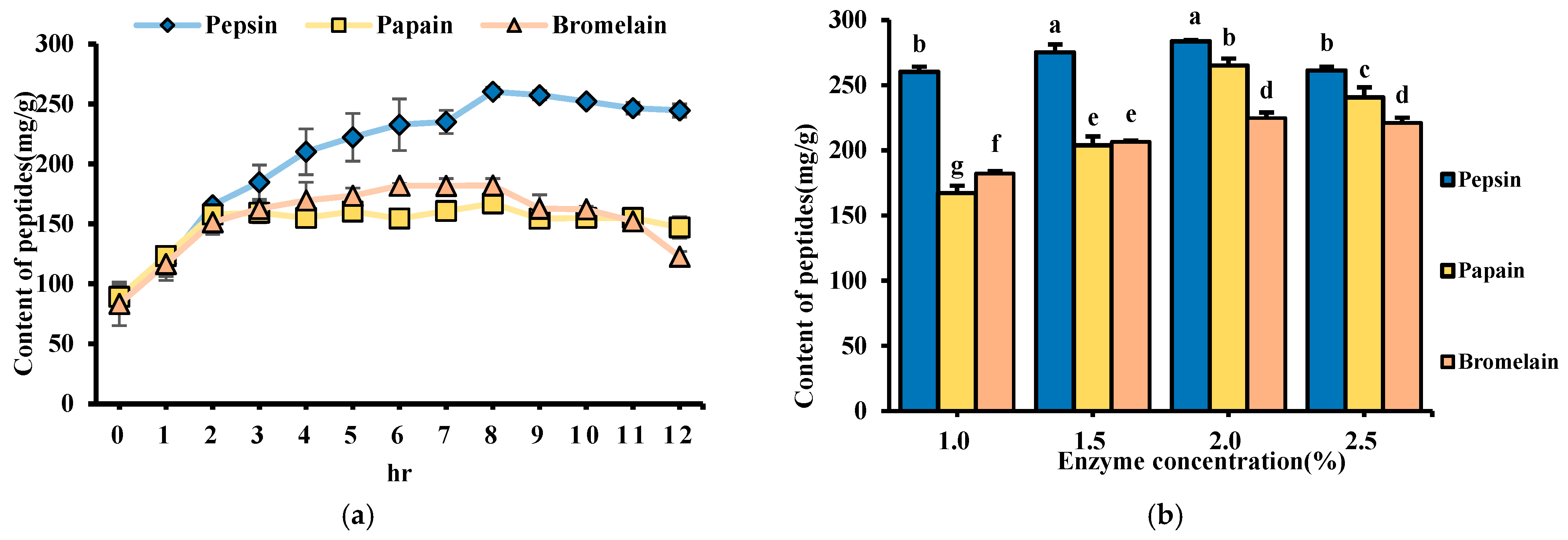
3.5. Peptide Content During Enzymatic Hydrolysis
3.6. Hydrolysis Rate of Hydrolysate
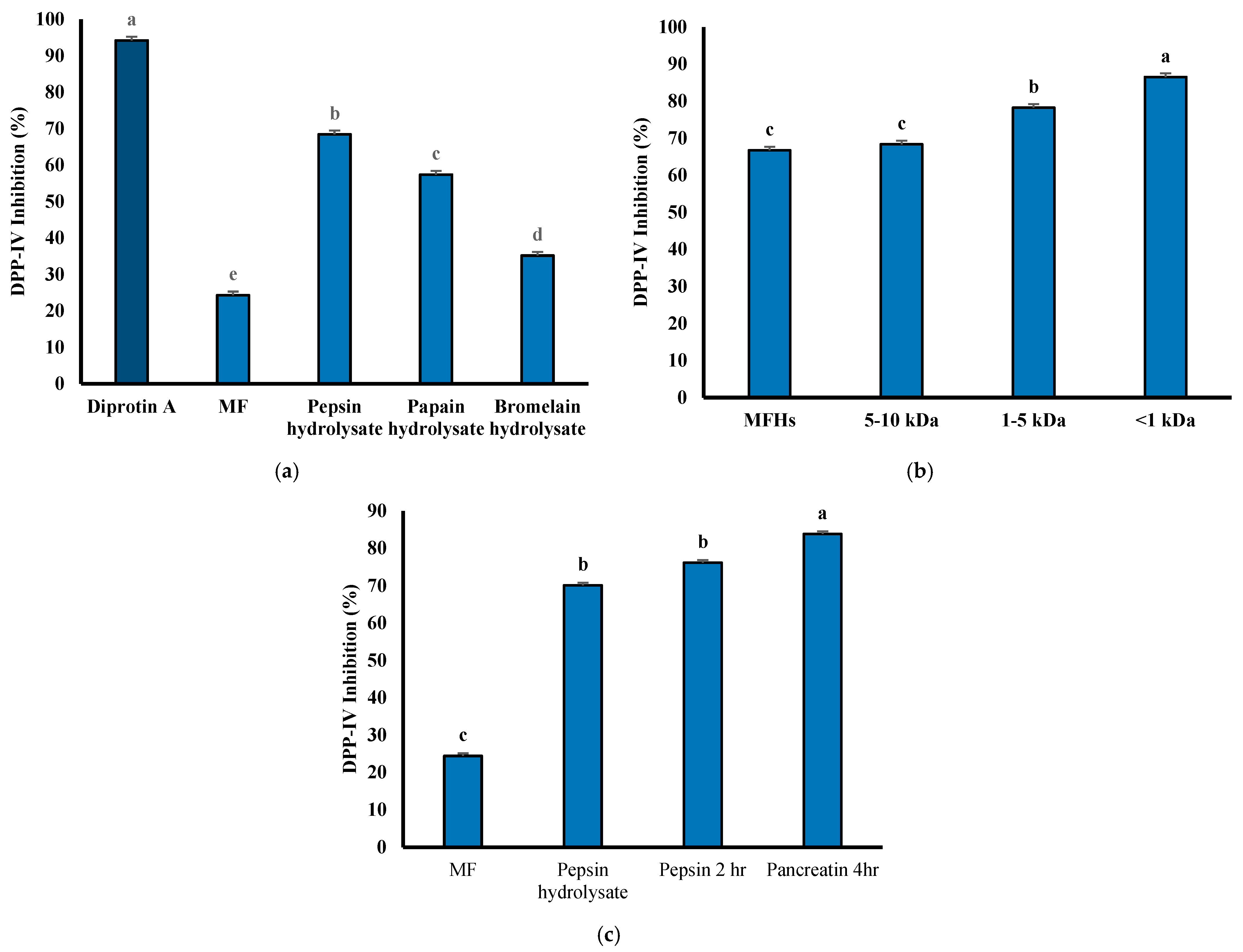
3.7. DPP-IV Inhibitory Activity
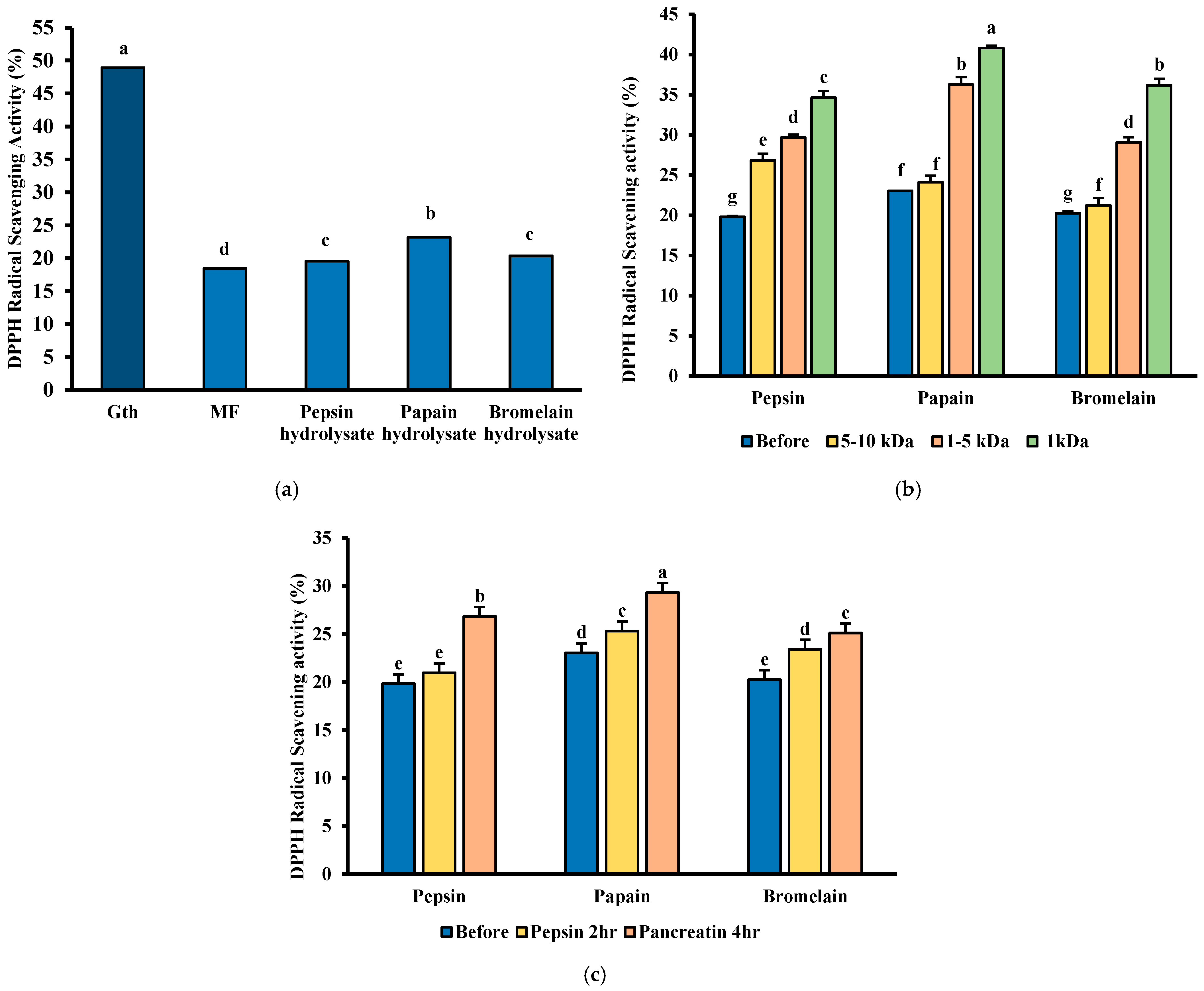
3.8. DPPH Free Radical Scavenging Ability
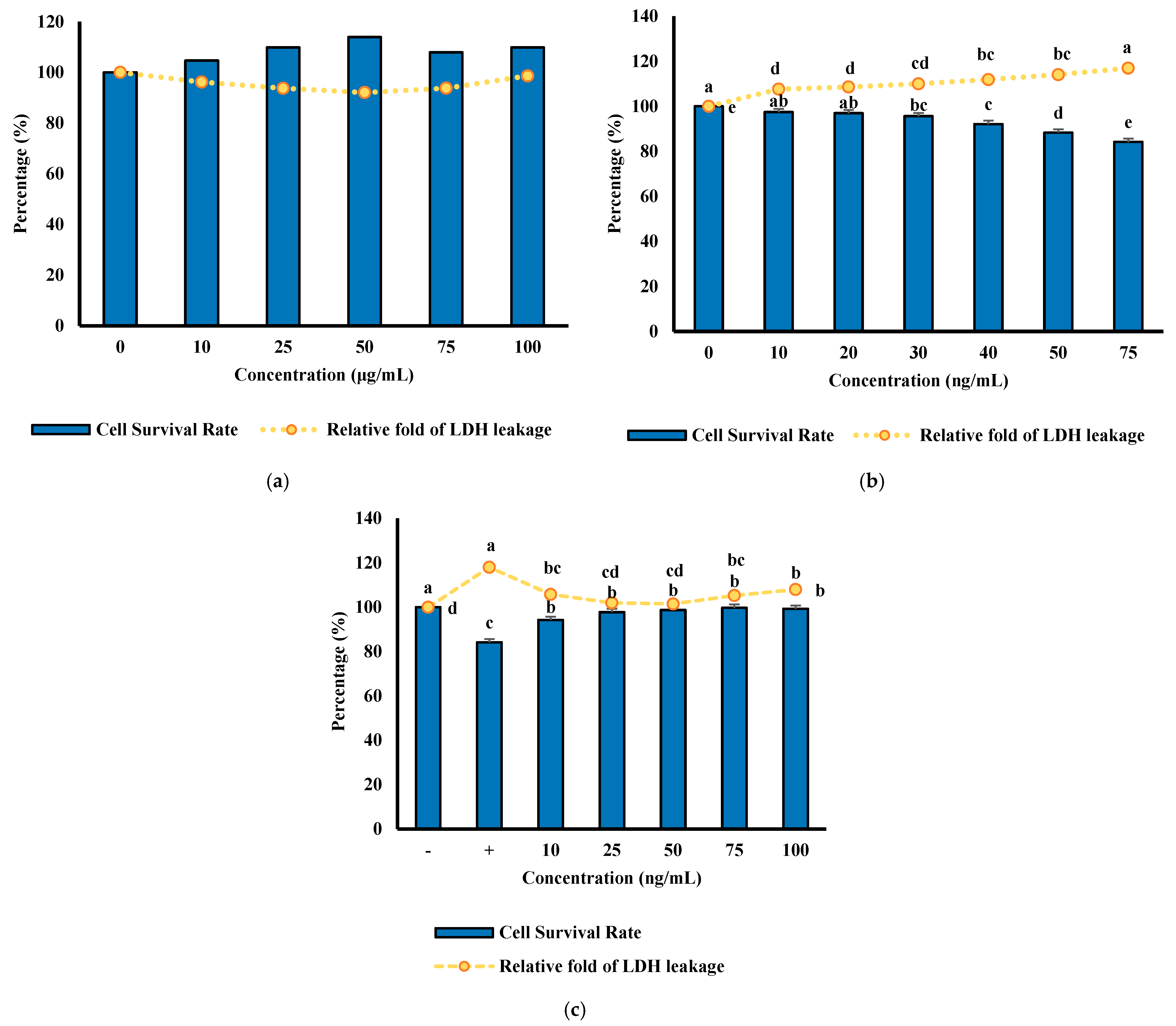
3.9. Cell Viability and Protective Effects of MFHs Against TNF-α-Induced Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation (IDF): Brussels, Belgium, 2021; ISBN 13. [Google Scholar]
- Chhabria, S.; Mathur, S.; Vadakan, S.; Sahoo, D.K.; Mishra, P.; Paital, B. A Review on Phytochemical and Pharmacological Facets of Tropical Ethnomedicinal Plants as Reformed DPP-IV Inhibitors to Regulate Incretin Activity. Front. Endocrinol. 2022, 13, 1027237. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Cozzolino, D.; Bellastella, G.; Maiorino, M.I.; Chiodini, P.; Ceriello, A.; Giugliano, D. Dipeptidyl Peptidase-4 Inhibitors and HbA1c Target of <7% in Type 2 Diabetes: Meta-Analysis of Randomized Controlled Trials. Diabetes Obes. Metab. 2011, 13, 594–603. [Google Scholar] [CrossRef]
- Pospisilik, J.A.; Martin, J.; Doty, T.; Ehses, J.A.; Pamir, N.; Lynn, F.C.; Piteau, S.; Demuth, H.-U.; McIntosh, C.H.S.; Pederson, R.A. Dipeptidyl Peptidase IV Inhibitor Treatment Stimulates β-Cell Survival and Islet Neogenesis in Streptozotocin-Induced Diabetic Rats. Diabetes 2003, 52, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Van Genugten, R.E.; Van Raalte, D.H.; Diamant, M. Dipeptidyl Peptidase-4 Inhibitors and Preservation of Pancreatic Islet-cell Function: A Critical Appraisal of the Evidence. Diabetes Obes. Metab. 2012, 14, 101–111. [Google Scholar] [CrossRef]
- Kaji, K.; Yoshiji, H.; Ikenaka, Y.; Noguchi, R.; Aihara, Y.; Douhara, A.; Moriya, K.; Kawaratani, H.; Shirai, Y.; Yoshii, J.; et al. Dipeptidyl Peptidase-4 Inhibitor Attenuates Hepatic Fibrosis via Suppression of Activated Hepatic Stellate Cell in Rats. J. Gastroenterol. 2014, 49, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Kasina, S.V.S.K.; Baradhi, K.M. Dipeptidyl Peptidase IV (DPP IV) Inhibitors; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gujarathi, D.; Trivedi, D. DPP-4 Inhibitors in Type 2 Diabetes Mellitus—A Panoramic Review. Ann. Clin. Med. Case Rep. 2023, 11, 1–7. [Google Scholar]
- Kung, H.-F.; Lin, C.-S.; Liu, S.-S.; Huang, C.-Y.; Chiu, K.; Lee, Y.-C.; Tsai, Y.-H. High Pressure Processing Extend the Shelf Life of Milkfish Flesh during Refrigerated Storage. Food Control 2022, 134, 108768. [Google Scholar] [CrossRef]
- Lu, Y.-H.; Huang, Y.-W.; Lee, J.-J.; Huang, S.-J. Evaluation of the Technical Efficiency of Taiwan’s Milkfish Polyculture in Consideration of Differences in Culturing Models and Environments. Fishes 2022, 7, 224. [Google Scholar] [CrossRef]
- Byun, H.-G.; Kim, S.-K. Purification and Characterization of Angiotensin I Converting Enzyme (ACE) Inhibitory Peptides from Alaska Pollack (Theragra chalcogramma) Skin. Process Biochem. 2001, 36, 1155–1162. [Google Scholar] [CrossRef]
- Miao, Z.; Jiao, Z.; Liu, J.; Chen, F.; Chen, W.; Zhou, S.; Chen, Z.; Zhong, W.; Yang, W.; Chen, Y. Fisheries Agency, Council of Agriculture, Executive Yuan Fisheries Statistical Annual Report 2023; Council of Agriculture Fisheries Agency: Taiwan, China, 2023. [Google Scholar]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; De Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bao, H.N.D.; Dang, H.T.T.; Tómasson, T.; Arason, S.; Gudjónsdóttir, M. Protein Characteristics and Bioactivity of Fish Protein Hydrolysates from Tra Catfish (Pangasius hypophthalmus) Side Stream Isolates. Foods 2022, 11, 4102. [Google Scholar] [CrossRef]
- Ng, W.-J.; Wong, F.-C.; Abd Manan, F.; Chow, Y.-L.; Ooi, A.-L.; Ong, M.-K.; Zhang, X.; Chai, T.-T. Antioxidant Peptides and Protein Hydrolysates from Tilapia: Cellular and In Vivo Evidences for Human Health Benefits. Foods 2024, 13, 2945. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhao, L.; Xie, Y.; Tan, Y. Purification and Identification of Novel Dipeptidyl Peptidase IV Inhibitory Peptides Derived from Bighead Carp (Hypophthalmichthys nobilis). Foods 2024, 13, 2644. [Google Scholar] [CrossRef]
- Amini Sarteshnizi, R.; Sahari, M.A.; Ahmadi Gavlighi, H.; Regenstein, J.M.; Nikoo, M.; Udenigwe, C.C. Influence of Fish Protein Hydrolysate-Pistachio Green Hull Extract Interactions on Antioxidant Activity and Inhibition of α-Glucosidase, α-Amylase, and DPP-IV Enzymes. LWT 2021, 142, 111019. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Liang, C.-H.; Wu, H.-T.; Pang, H.-Y.; Chen, C.; Wang, G.-H.; Chan, L.-P. Antioxidant and Anti-Inflammatory Capacities of Collagen Peptides from Milkfish (Chanos chanos) Scales. J. Food Sci. Technol. 2018, 55, 2310–2317. [Google Scholar] [CrossRef]
- Kusumaningtyas, E.; Nurilmala, M.; Sibarani, D. Antioxidant and Antifungal Activities of Collagen Hydrolysates from Skin of Milkfish (Chanos chanos) Hydrolyzed Using Various Bacillus Proteases. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012040. [Google Scholar] [CrossRef]
- Nugraha, R.; Kurniawan, F.; Abdullah, A.; Lopata, A.L.; Ruethers, T. Antihypertensive and Antidiabetic Drug Candidates from Milkfish (Chanos chanos)—Identification and Characterization through an Integrated Bioinformatic Approach. Foods 2024, 13, 2594. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. Association of Official Analytical Chemists International. In Official Methods of Analysis of AOAC International; Current Through Revision 1; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Huang, C.-Y.; Kuo, J.-M.; Wu, S.-J.; Tsai, H.-T. Isolation and Characterization of Fish Scale Collagen from Tilapia (Oreochromis sp.) by a Novel Extrusion–Hydro-Extraction Process. Food Chem. 2016, 190, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Redmile-Gordon, M.A.; Armenise, E.; White, R.P.; Hirsch, P.R.; Goulding, K.W.T. A Comparison of Two Colorimetric Assays, Based upon Lowry and Bradford Techniques, to Estimate Total Protein in Soil Extracts. Soil Biol. Biochem. 2013, 67, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-B.; Lin, H.-C.; Chang, Y.-W. Analysis of Proteins and Potential Bioactive Peptides from Tilapia (Oreochromis spp.) Processing Co-Products Using Proteomic Techniques Coupled with BIOPEP Database. J. Funct. Foods 2015, 19, 629–640. [Google Scholar] [CrossRef]
- Lin, C.; Tejano, L.A.; Panjaitan, F.C.A.; Permata, V.N.S.; Sevi, T.; Chang, Y. Protein Identification and Potential Bioactive Peptides from Pumpkin (Cucurbita maxima) Seeds. Food Nutr. 2024, 12, 5388–5402. [Google Scholar] [CrossRef]
- Perkins, D.N.; Pappin, D.J.C.; Creasy, D.M.; Cottrell, J.S. Probability-Based Protein Identification by Searching Sequence Databases Using Mass Spectrometry Data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Esua, O.J.; Sun, D.-W.; Cheng, J.-H.; Wang, H.; Chen, C. Hybridising Plasma Functionalized Water and Ultrasound Pretreatment for Enzymatic Protein Hydrolysis of Larimichthys polyactis: Parametric Screening and Optimization. Food Chem. 2022, 385, 132677. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Hao, L.; Li, X.; Zhao, B.; Song, X.; Zhang, Y.; Liang, Q. Enzymatic Hydrolysis Optimization of Yak Whey Protein Concentrates and Bioactivity Evaluation of the Ultrafiltered Peptide Fractions. Molecules 2024, 29, 1403. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Dipeptidyl Peptidase IV Inhibitory and Antioxidative Properties of Milk Protein-Derived Dipeptides and Hydrolysates. Peptides 2013, 39, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.; Gao, L.; Rao, L.; Li, P.; Zhang, Y.; Han, J.; Li, J. Bioactive Peptides Identified from Enzymatic Hydrolysates of Sturgeon Skin. J. Sci. Food Agric. 2022, 102, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Teng, X.; Shang, J.; Wang, D.; Liu, N. Identification of Novel DPP–IV Inhibitory Peptides from Atlantic Salmon (Salmo salar) Skin. Food Res. Int. 2020, 133, 109161. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Production and Identification of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides from Discarded Sardine Pilchardus Protein. Food Chem. 2020, 328, 127096. [Google Scholar] [CrossRef]
- American Type Culture Collection Animal Cell Culture Guide: Tips and Techniques for Continuous Cell Lines; ATCC: Manassas, VA, USA, 2012.
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef]
- Weyermann, J.; Lochmann, D.; Zimmer, A. A Practical Note on the Use of Cytotoxicity Assays. Int. J. Pharm. 2005, 288, 369–376. [Google Scholar] [CrossRef]
- Amiza, M.A.; Oon, X.X.; Norizah, M.S. Optimization of Enzymatic Hydrolysis Conditions on the Degree of Hydrolysis of Edible Bird’s Nest Using Alcalase® and Nutritional Composition of the Hydrolysate. Food Res. 2019, 3, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish Viscera Protein Hydrolysates: Production, Potential Applications and Functional and Bioactive Properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-Derived Peptidic Antioxidants: A Review of Their Production, Assessment, and Potential Applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Xu, Q.; Zheng, L.; Huang, M.; Zhao, M. Exploring Structural Features of Potent Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides Derived from Tilapia (Oreochromis niloticus) Skin Gelatin by an Integrated Approach of Multivariate Analysis and Gly-Pro-Based Peptide Library. Food Chem. 2022, 397, 133821. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, G.; Zhou, F.; Lin, L.; Liu, X.; Zhao, M. Alcalase-Hydrolyzed Oyster (Crassostrea rivularis) Meat Enhances Antioxidant and Aphrodisiac Activities in Normal Male Mice. Food Res. Int. 2019, 120, 178–187. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Dipeptidyl Peptidase-IV Inhibitory Activity of Dairy Protein Hydrolysates. Int. Dairy J. 2012, 25, 97–102. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Yang, W.; Li, X.; Qi, D.; Chen, H.; Liu, H.; Yu, S.; Pan, Y.; Liu, Y.; et al. Identification, Screening, and Comprehensive Evaluation of Novel DPP-IV Inhibitory Peptides from the Tilapia Skin Gelatin Hydrolysate Produced Using Ginger Protease. Biomolecules 2022, 12, 1866. [Google Scholar] [CrossRef]
- Sila, A.; Martinez-Alvarez, O.; Haddar, A.; Gómez-Guillén, M.C.; Nasri, M.; Montero, M.P.; Bougatef, A. Recovery, Viscoelastic and Functional Properties of Barbel Skin Gelatine: Investigation of Anti-DPP-IV and Anti-Prolyl Endopeptidase Activities of Generated Gelatine Polypeptides. Food Chem. 2015, 168, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, J.; Zhu, R.; Zhang, H.; Li, D.; Li, H.; Tang, H.; Chen, L.; Peng, X.; Xu, X.; et al. Mechanistic Study of Novel Dipeptidyl Peptidase IV Inhibitory Peptides from Goat’s Milk Based on Peptidomics and In Silico Analysis. Foods 2024, 13, 1194. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Hsieh, C.-H.; Hung, C.-C.; Jao, C.-L.; Chen, M.-C.; Hsu, K.-C. Fish Skin Gelatin Hydrolysates as Dipeptidyl Peptidase IV Inhibitors and Glucagon-like Peptide-1 Stimulators Improve Glycaemic Control in Diabetic Rats: A Comparison between Warm- and Cold-Water Fish. J. Funct. Foods 2015, 19, 330–340. [Google Scholar] [CrossRef]
- Ketnawa, S.; Suwal, S.; Huang, J.; Liceaga, A.M. Selective Separation and Characterisation of Dual ACE and DPP—IV Inhibitory Peptides from Rainbow Trout (Oncorhynchus mykiss) Protein Hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 1062–1073. [Google Scholar] [CrossRef]
- Je, J.-Y.; Qian, Z.-J.; Byun, H.-G.; Kim, S.-K. Purification and Characterization of an Antioxidant Peptide Obtained from Tuna Backbone Protein by Enzymatic Hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Je, J.; Park, P.; Byun, H.; Jung, W.; Kim, S. Angiotensin I Converting Enzyme (ACE) Inhibitory Peptide Derived from the Sauce of Fermented Blue Mussel. Bioresour. Technol. 2005, 96, 1624–1629. [Google Scholar] [CrossRef]
- Ketnawa, S.; Benjakul, S.; Martínez-Alvarez, O.; Rawdkuen, S. Fish Skin Gelatin Hydrolysates Produced by Visceral Peptidase and Bovine Trypsin: Bioactivity and Stability. Food Chem. 2017, 215, 383–390. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Martínez De Marañón, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. JCM 2019, 8, 1385. [Google Scholar] [CrossRef]

| Composition | Original (%) | After Degreasing (%) |
|---|---|---|
| Moisture | 1.59 ± 0.08 | 1.76 ± 0.07 |
| Crude protein | 53.61 ± 0.27 | 69.15 ± 0.54 |
| Crude fat | 22.15 ± 0.18 | 1.08 ± 0.18 |
| Ash | 11.76 ± 0.31 | 13.38 ± 0.81 |
| Carbohydrate | 10.90 ± 0.80 | 14.63 ± 0.94 |
| Protein Content (%) | Peptide Content (mg/g) | Yield (%) 1 | |
|---|---|---|---|
| Milkfish frame protein isolate | 71.25 ± 0.71 | 87.81 ± 3.56 | 30.05 ± 0.82 |
| Protease | Myosin Heavy Chain | 65 kDA Warm Temperature Acclimation Protein 1 (WAP65-1) | ||
|---|---|---|---|---|
| DPP-IV Inhibitory | Antioxidative | DPP-IV Inhibitory | Antioxidative | |
| Ficin | 10 | 5 | 30 | 11 |
| Papain | 10 | 1 | 22 | 1 |
| Pepsin (pH > 2) | 15 | 1 | 47 | 16 |
| Chymotrypsin C | 10 | 1 | 16 | 5 |
| Proteinase K | 5 | 1 | 23 | 4 |
| Thermolysin | 6 | 4 | 20 | 1 |
| Cathepsin G | 3 | 12 | 14 | 5 |
| Bromelain | 16 | 3 | 25 | 4 |
| Enzyme | Concentration (%) | Soluble Protein (%) | Yield 1 (%) |
|---|---|---|---|
| Pepsin | 1.0 | 61.80 ± 0.92 Ac | 62.69 |
| 1.5 | 73.79 ± 0.70 Ab | 70.44 | |
| 2.0 | 86.46 ± 0.49 Aa | 82.54 | |
| 2.5 | 74.00 ± 0.41 Ab | 78.42 | |
| Papain | 1.0 | 39.43 ± 0.52 Cc | 32.74 |
| 1.5 | 56.25 ± 0.47 Bb | 40.65 | |
| 2.0 | 59.44 ± 0.39 Ca | 63.42 | |
| 2.5 | 27.54 ± 0.58 Cd | 61.03 | |
| Bromelain | 1.0 | 46.85 ± 0.40 Bd | 41.43 |
| 1.5 | 53.23 ± 0.49 Cc | 52.56 | |
| 2.0 | 61.28 ± 0.75 Ba | 68.25 | |
| 2.5 | 58.66 ± 0.85 Bb | 62.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cagabhion, A.T., III; Ko, W.-L.; Chuang, T.-J.; Aluko, R.E.; Chang, Y.-W. Identification and In Vitro Evaluation of Milkfish (Chanos chanos) Frame Proteins and Hydrolysates with DPP-IV Inhibitory and Antioxidant Activities. Foods 2025, 14, 3456. https://doi.org/10.3390/foods14203456
Cagabhion AT III, Ko W-L, Chuang T-J, Aluko RE, Chang Y-W. Identification and In Vitro Evaluation of Milkfish (Chanos chanos) Frame Proteins and Hydrolysates with DPP-IV Inhibitory and Antioxidant Activities. Foods. 2025; 14(20):3456. https://doi.org/10.3390/foods14203456
Chicago/Turabian StyleCagabhion, Anastacio T., III, Wen-Ling Ko, Ting-Jui Chuang, Rotimi E. Aluko, and Yu-Wei Chang. 2025. "Identification and In Vitro Evaluation of Milkfish (Chanos chanos) Frame Proteins and Hydrolysates with DPP-IV Inhibitory and Antioxidant Activities" Foods 14, no. 20: 3456. https://doi.org/10.3390/foods14203456
APA StyleCagabhion, A. T., III, Ko, W.-L., Chuang, T.-J., Aluko, R. E., & Chang, Y.-W. (2025). Identification and In Vitro Evaluation of Milkfish (Chanos chanos) Frame Proteins and Hydrolysates with DPP-IV Inhibitory and Antioxidant Activities. Foods, 14(20), 3456. https://doi.org/10.3390/foods14203456







