A Facile Electrode Modification Approach Based on Metal-Free Carbonaceous Carbon Black/Carbon Nanofibers for Electrochemical Sensing of Bisphenol A in Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments and Apparatus
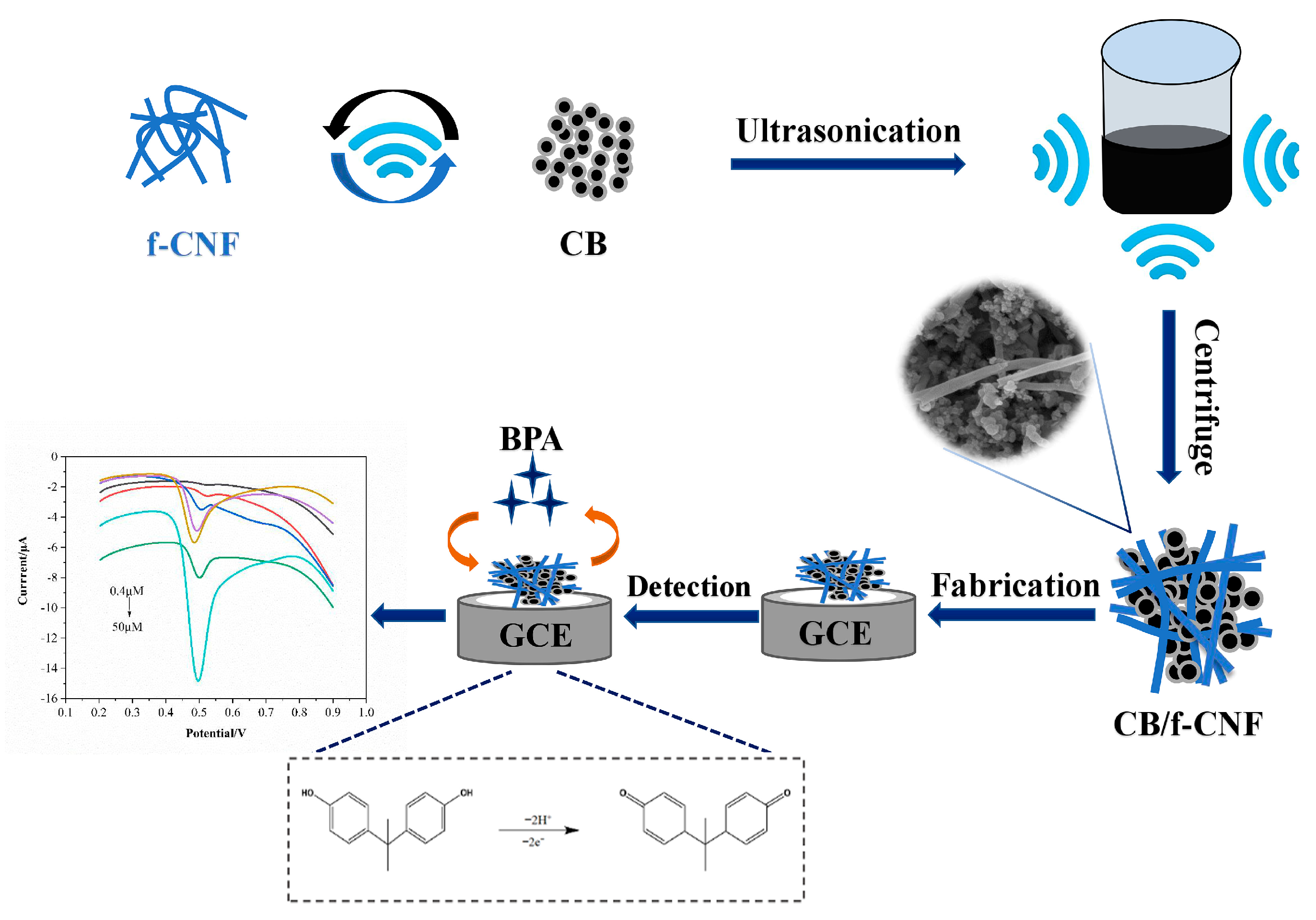
2.3. Construction of CB/f-CNF/GCE Sensor
2.4. Electrochemical Sensing of BPA
2.5. Preparation of Real Samples
2.6. Method Validation and Addition Recovery
3. Results and Discussion
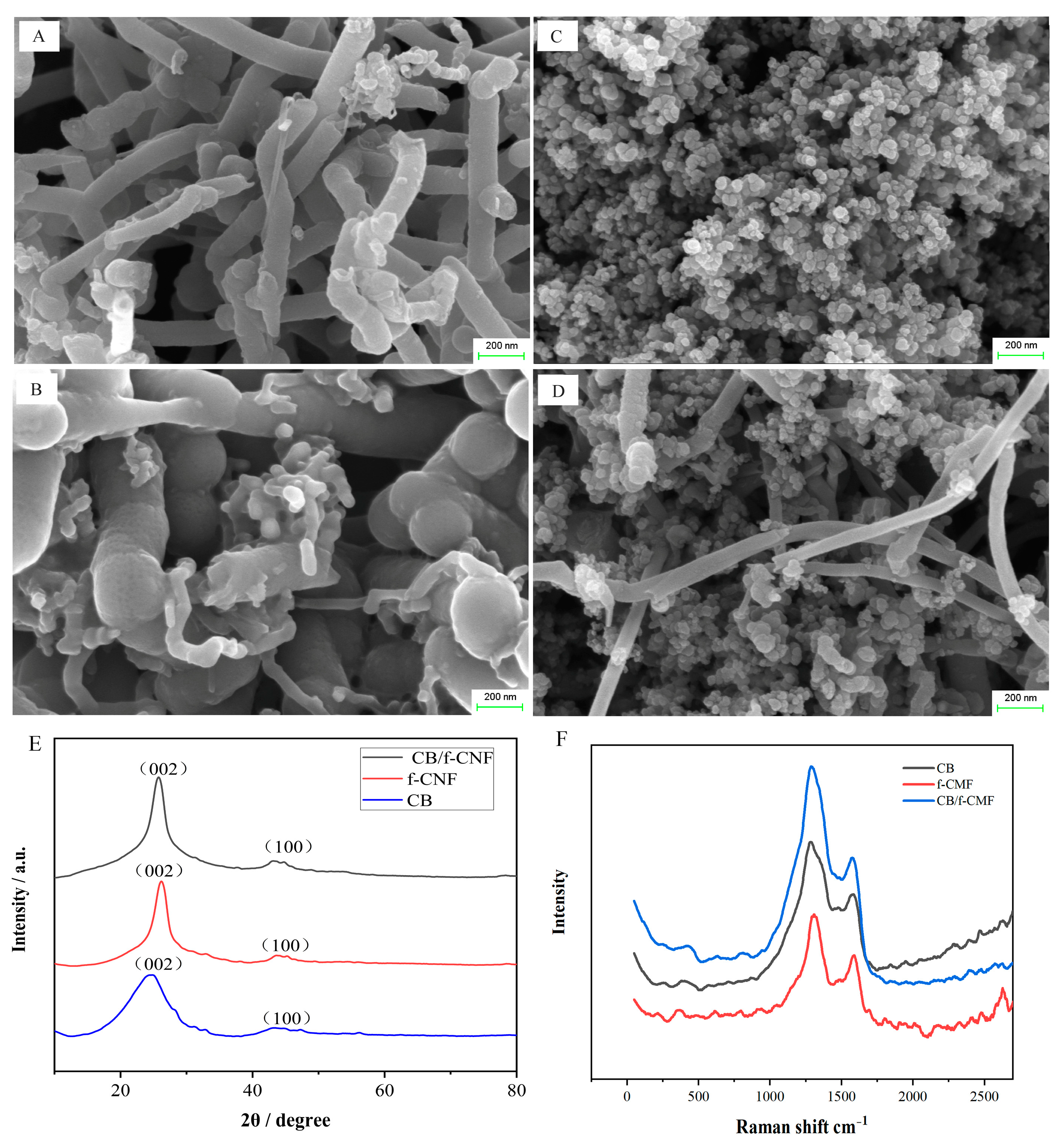
3.1. Morphology and Structure Characterization
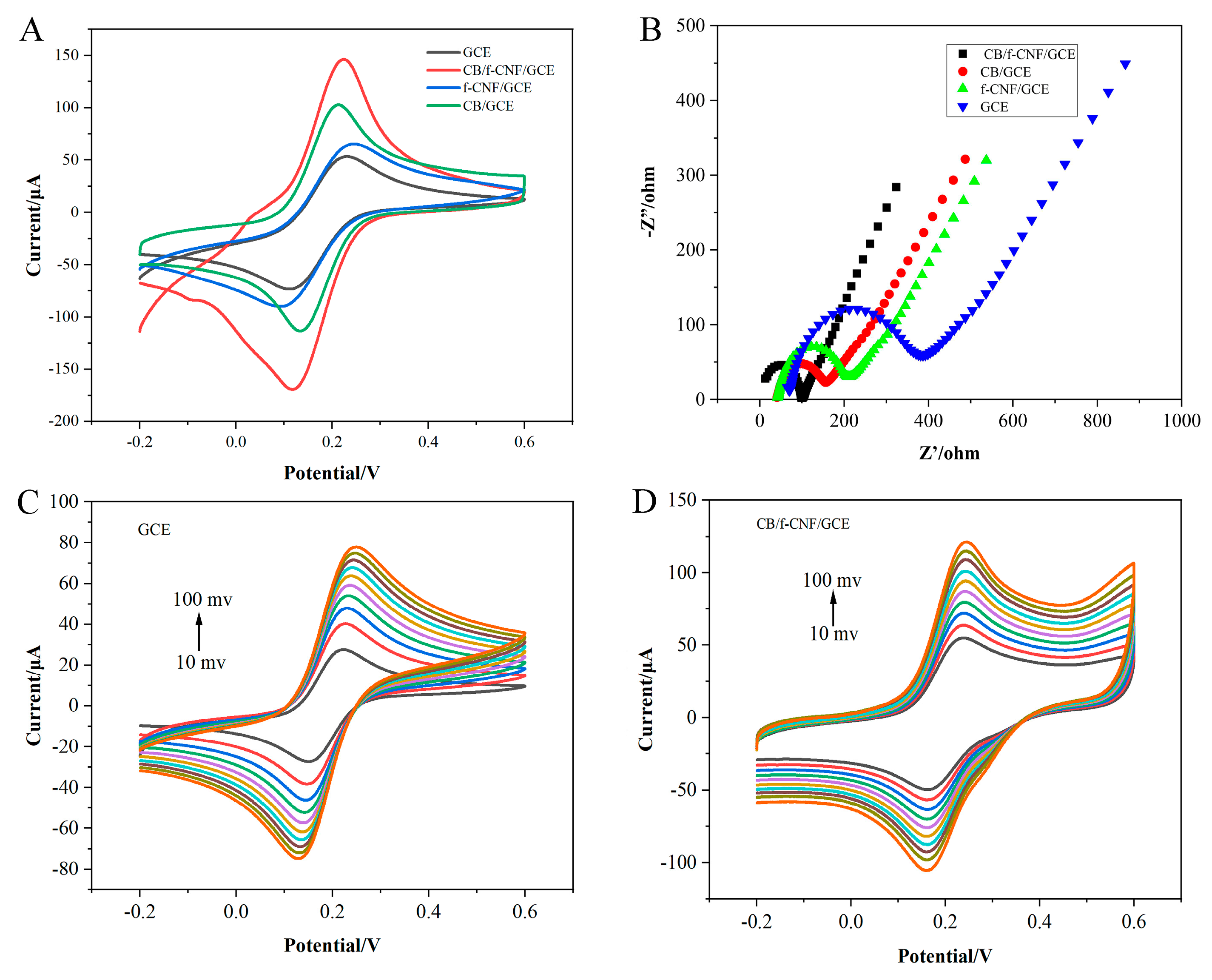
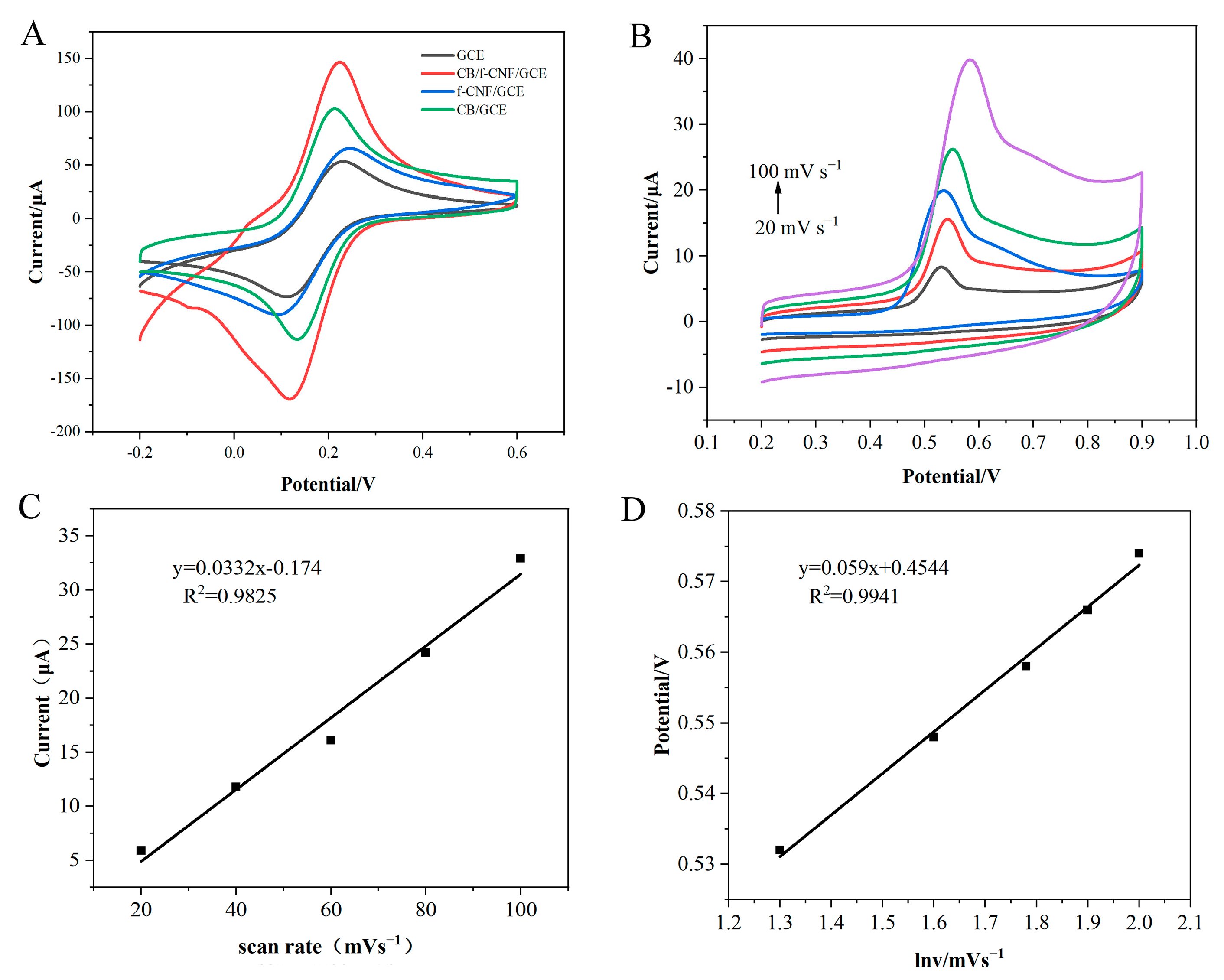
3.2. Electrochemical Characterization of CB/f-CNF/GCE
3.3. Electrochemical Response of CB/f-CNF/GCE Sensors Toward BPA
3.4. Optimization of Test Conditions
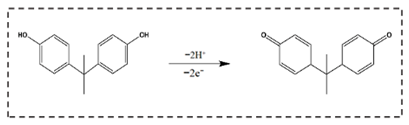
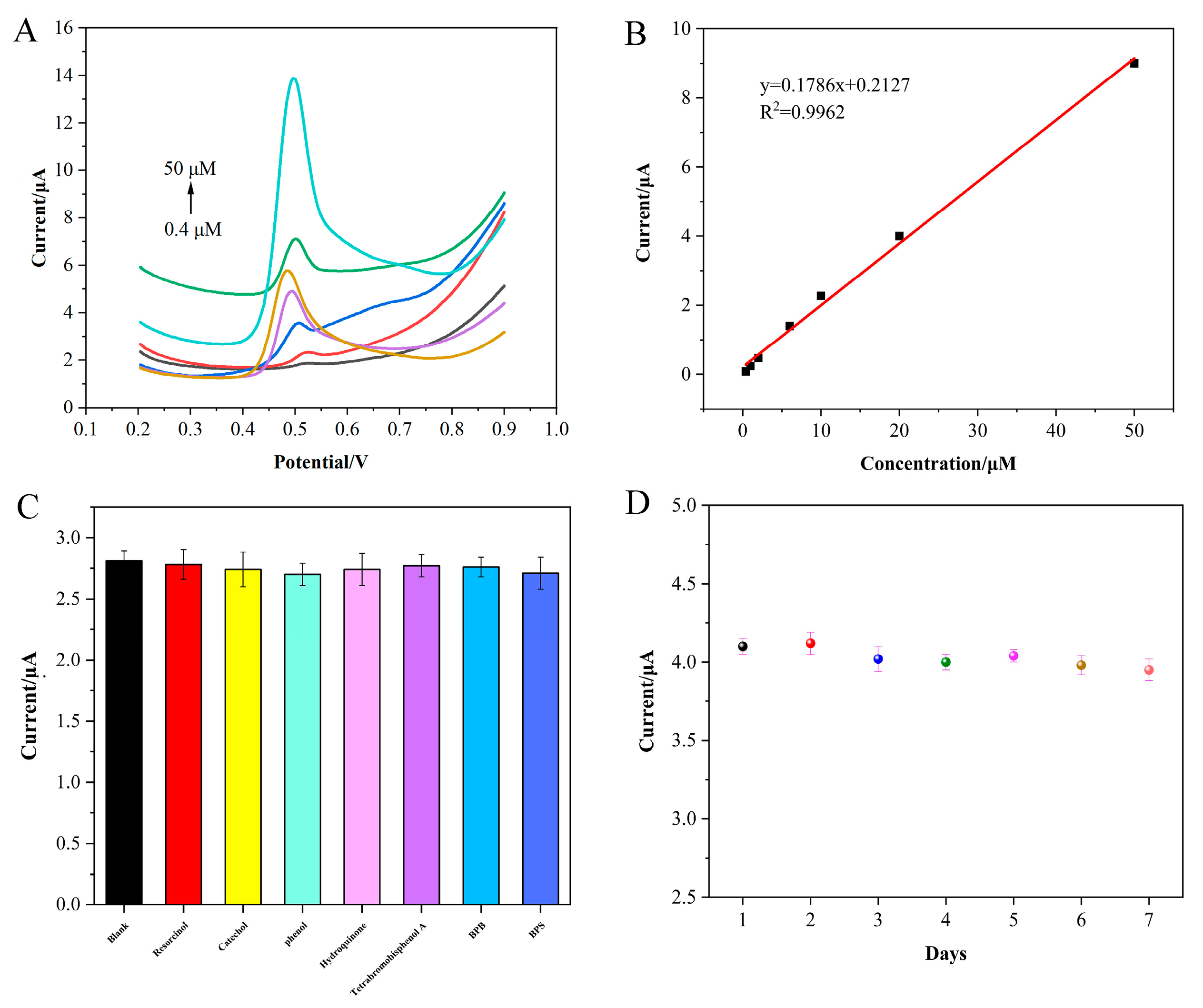
3.5. Electrochemical Sensing of BPA on the CB/f-CNF/GCE Sensor
3.6. The Repeatability, Reproducibility, Stability, and Anti-Interference of the CB/f-CNF/GCE Sensor
3.7. Accuracy and Applicability of the CB/f-CNF Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zhao, F.; Yang, L.; Zhang, J.; Park, H.-D.; Li, Z.; Chen, H.; Zhang, X.; Gao, M. Catalytic degradation of bisphenol a (BPA) in water by immobilizing silver-loaded graphene oxide (GO-Ag) in ultrafiltration membrane with finger-like structure. Chem. Eng. J. 2023, 474, 145577–145590. [Google Scholar] [CrossRef]
- Shaaban, H.; Mostafa, A.; Alqarni, A.M.; Almohamed, Y.; Abualrahi, D.; Hussein, D.; Alghamdi, M. Simultaneous determination of bisphenol A and its analogues in foodstuff using UPLC-MS/MS and assessment of their health risk in adult population. J. Food Compos. Anal. 2022, 110, 104549–104561. [Google Scholar] [CrossRef]
- Metwally, M.G.; Shehab, O.R.; Ibrahim, H.; El Nashar, R.M. Electrochemical detection of Bisphenol A in plastic bottled drinking waters and soft drinks based on molecularly imprinted polymer. J. Environ. Chem. Eng. 2022, 10, 107699–107711. [Google Scholar] [CrossRef]
- Beitollahi, H.; Shahsavari, M.; Sheikhshoaie, I.; Tajik, S.; Jahani, P.M.; Mohammadi, S.Z.; Afshar, A.A. Amplified electrochemical sensor employing screen-printed electrode modified with Ni-ZIF-67 nanocomposite for high sensitive analysis of Sudan I in present bisphenol A. Food Chem. Toxicol. 2022, 161, 112824–112832. [Google Scholar] [CrossRef]
- Wang, K.-P.; Hu, J.-M.; Zhang, X. Sensitive electrochemical detection of endocrine disruptor bisphenol A (BPA) in milk based on iodine-doped graphene. Microchem. J. 2022, 173, 107047–107054. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Z.-L.; Deng, X.-Y.; Wei, H.-D.; Wang, W.-L.; Xu, Z.; Feng, Y.; Shi, X. Metal-organic framework mixed-matrix membrane-based extraction combined HPLC for determination of bisphenol A in milk and milk packaging. Food Chem. 2022, 386, 132753–132761. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Abbaspour, M.; Mogaddam, M.R.A.; Ghorbanpour, H. Determination of some synthetic phenolic antioxidants and bisphenol A in honey using dispersive liquid–liquid microextraction followed by gas chromatography-flame ionization detection. Food Anal. Methods 2015, 8, 2035–2043. [Google Scholar] [CrossRef]
- Bodur, S.; Erarpat, S.; Bozyiğit, G.D.; Chormey, D.S.; Öz, E.; Özdoğan, N.; Bakırdere, S. A sensitive determination method for trace bisphenol A in bottled water and wastewater samples: Binary solvent liquid phase microextraction-quadrupole isotope dilution-gas chromatography-mass spectrometry. Microchem. J. 2020, 159, 105532–105539. [Google Scholar] [CrossRef]
- Pang, Y.; Cao, Y.; Han, J.; Xia, Y.; He, Z.; Sun, L.; Liang, J. A novel fluorescence sensor based on Zn porphyrin MOFs for the detection of bisphenol A with highly selectivity and sensitivity. Food Control 2022, 132, 108551–108560. [Google Scholar] [CrossRef]
- Sanko, V.; Şenocak, A.; Tümay, S.O.; Orooji, Y.; Demirbas, E.; Khataee, A. An electrochemical sensor for detection of trace-level endocrine disruptor bisphenol A using Mo2Ti2AlC3 MAX phase/MWCNT composite modified electrode. Environ. Res. 2022, 212, 113071. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Zhang, L.; Song, G.; Wang, N.; Xu, W.; Huang, W. A molecularly imprinted electrochemical BPA sensor based on multi-walled carbon nanotubes modified by CdTe quantum dots for the detection of bisphenol A. Microchem. J. 2021, 170, 106737–106747. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Jabbar, K.A.; Mahmoud, K.A. Platinum nanoparticles/Ti3C2Tx (MXene) composite for the effectual electrochemical sensing of Bisphenol A in aqueous media. J. Electroanal. Chem. 2021, 880, 114934–114943. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.-C.; Balram, D.; Al-Mubaddel, F.S.; Noman, M.T. Functionalization of CNFs surface with β-cyclodextrin and decoration of hematite nanoparticles for detection and degradation of toxic fungicide carbendazim. Appl. Surf. Sci. 2022, 586, 152666–152678. [Google Scholar] [CrossRef]
- Duan, D.; Ding, Y.; Li, L.; Ma, G. Rapid quantitative detection of melatonin by electrochemical sensor based on carbon nanofibers embedded with FeCo alloy nanoparticles. J. Electroanal. Chem. 2020, 873, 114422–114430. [Google Scholar] [CrossRef]
- Yang, S.; Yang, M.; Yao, X.; Fa, H.; Wang, Y.; Hou, C. A zeolitic imidazolate framework/carbon nanofiber nanocomposite based electrochemical sensor for simultaneous detection of co-existing dihydroxybenzene isomers. Sens. Actuators B Chem. 2020, 320, 128294–128303. [Google Scholar] [CrossRef]
- Priscillal, J.D.; Wang, S.F.; Kameoka, S. Investigation on the influence of reaction temperature on the structural and magnetic properties of morphologically diverse carbon nanotubes synthesized using LaNi5Pt0.5 alloy catalyst. J. Alloys Compd. 2023, 958, 170355. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; You, T. Carbon nanofiber based electrochemical biosensors: A review. Anal. Methods 2010, 2, 202–211. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, M.; Qiu, J.; Qiu, T.; Chen, Y.; Qi, S.; Wei, X.; Tian, X.; Xu, D. An electrochemical sensor based on CNF@ AuNPs for metronidazole hypersensitivity detection. Biosens. Bioelectron. X 2022, 10, 100102–100107. [Google Scholar] [CrossRef]
- Nelis, J.L.; Migliorelli, D.; Mühlebach, L.; Generelli, S.; Stewart, L.; Elliott, C.T.; Campbell, K. Highly sensitive electrochemical detection of the marine toxins okadaic acid and domoic acid with carbon black modified screen printed electrodes. Talanta 2021, 228, 122215–122224. [Google Scholar] [CrossRef]
- Jebril, S.; Cubillana-Aguilera, L.; Palacios-Santander, J.M.; Dridi, C. A novel electrochemical sensor modified with green gold sononanoparticles and carbon black nanocomposite for bisphenol A detection. Mater. Sci. Eng. B 2021, 264, 114951–114961. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Electrochemical immunosensor based on chitosan/conductive carbon black composite modified disposable ITO electrode: An analytical platform for p53 detection. Biosens. Bioelectron. 2018, 121, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Guo, S.; Ding, Y.; Lou, B.; Shi, N.; Wen, F.; Yang, X.; Li, G.; Wu, B.; Zhu, W. Preparation of mesocarbon microbeads as anode material for lithium-ion battery by co-carbonization of FCC decant oil and conductive carbon black. Fuel Process. Technol. 2022, 227, 107110–107123. [Google Scholar] [CrossRef]
- Choi, W.Y.; Seo, D.J.; Choi, H.; Lee, M.H.; Choi, S.W.; Yoon, Y.G.; Kim, T.Y.; Kim, H.; Jung, C.-Y. Ionomer immobilized onto nitrogen-doped carbon black as efficient and durable electrode binder and electrolyte for polymer electrolyte fuel cells. Electrochim. Acta 2022, 421, 140427–140437. [Google Scholar] [CrossRef]
- Ma, C.; Wu, L.; Dirican, M.; Cheng, H.; Li, J.; Song, Y.; Shi, J.; Zhang, X. Carbon black-based porous sub-micron carbon fibers for flexible supercapacitors. Appl. Surf. Sci. 2021, 537, 147914–147925. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hsu, C.-C. Synergetic effect of carbon black as co-catalyst for enhanced visible-light photocatalytic activity and stability on ZnO nanoparticles. Solid State Sci. 2020, 107, 106366–106373. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Mazzaracchio, V.; Scognamiglio, V.; Amine, A.; Moscone, D. Carbon black as an outstanding and affordable nanomaterial for electrochemical (bio) sensor design. Biosens. Bioelectron. 2020, 156, 112033–112049. [Google Scholar] [CrossRef]
- dos Santos, W.T.; Amin, H.M.; Compton, R.G. A nano-carbon electrode optimized for adsorptive stripping voltammetry: Application to detection of the stimulant selegiline in authentic saliva. Sens. Actuators B Chem. 2019, 279, 433–439. [Google Scholar] [CrossRef]
- Koterwa, A.; Kaczmarzyk, I.; Mania, S.; Cieslik, M.; Tylingo, R.; Ossowski, T.; Bogdanowicz, R.; Niedziałkowski, P.; Ryl, J. The role of electrolysis and enzymatic hydrolysis treatment in the enhancement of the electrochemical properties of 3D-printed carbon black/poly (lactic acid) structures. Appl. Surf. Sci. 2022, 574, 151587–151600. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.; Xu, Z.; Xu, L. A novel fluorescent sensor based on a magnetic covalent organic framework-supported, carbon dot-embedded molecularly imprinted composite for the specific optosensing of bisphenol A in foods. Sens. Actuators B Chem. 2022, 361, 131729–131738. [Google Scholar] [CrossRef]
- Haghgoo, M.; Ansari, R.; Hassanzadeh-Aghdam, M.K. The effect of nanoparticle conglomeration on the overall conductivity of nanocomposites. Int. J. Eng. Sci. 2020, 157, 103392–103416. [Google Scholar] [CrossRef]
- Hassanzadeh-Aghdam, M.K.; Mahmoodi, M.J.; Ansari, R. Creep performance of CNT polymer nanocomposites-An emphasis on viscoelastic interphase and CNT agglomeration. Compos. Part B Eng. 2019, 168, 274–281. [Google Scholar] [CrossRef]
- Haidyrah, A.S.; Sundaresan, P.; Venkatesh, K.; Ramaraj, S.K.; Thirumalraj, B. Fabrication of functionalized carbon nanofibers/carbon black composite for electrochemical investigation of antibacterial drug nitrofurantoin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127112–127121. [Google Scholar] [CrossRef]
- Moyseowicz, A.; Gryglewicz, S.; Gryglewicz, G. Enhancing electrochemical capacitor performance through the application of nanostructured carbon materials as conducting additives. Chem. Eng. Process.-Process Intensif. 2021, 169, 108647–108657. [Google Scholar] [CrossRef]
- Fan, S.X.; Tang, W. Synthesis, characterization and mechanism of electrospun carbon nanofibers decorated with ZnO nanoparticles for flexible ammonia gas sensors at room temperature. Sens. Actuators B Chem. 2022, 362, 131789–131799. [Google Scholar] [CrossRef]
- Zhao, H.; Ran, Q.; Li, Y.; Li, B.; Liu, B.; Ma, H.; Zhang, M.; Komarneni, S. Highly sensitive detection of gallic acid based on 3D interconnected porous carbon nanotubes/carbon nanosheets modified glassy carbon electrode. J. Mater. Res. Technol. 2020, 9, 9422–9433. [Google Scholar] [CrossRef]
- Roushani, M.; Rahmati, Z. Development of electrochemical sensor based on molecularly imprinted copolymer for detection of nitrofurantoin. J. Iran. Chem. Soc. 2019, 16, 999–1006. [Google Scholar] [CrossRef]
- Krejčová, Z.; Barek, J.; Vyskočil, V. Voltammetric determination of nitrofurantoin at a mercury meniscus modified silver solid amalgam electrode. Electroanalysis 2015, 27, 185–192. [Google Scholar] [CrossRef]
- Li, Z.L.; Deng, L.B.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Sun, P.; Xu, K.; Guang, S.; Xu, H. Controlling assembly-induced single layer RGO to achieve highly sensitive electrochemical detection of Pb (II) via synergistic enhancement. Microchem. J. 2021, 162, 105883–105893. [Google Scholar] [CrossRef]
- Priscillal, J.; Wang, S.F. Hierarchically Ordered Tungsten Antimonate Nanoflowers Anchored on Carbon Nanofibers for Electrochemical Detection of a Food Additive. ACS Appl. Nano Mater. 2022, 5, 10331–10340. [Google Scholar] [CrossRef]
- Alam, A.U.; Deen, M.J. Bisphenol A electrochemical sensor using graphene oxide and β-cyclodextrin-functionalized multi-walled carbon nanotubes. Anal. Chem. 2020, 92, 5532–5539. [Google Scholar] [CrossRef] [PubMed]
- Priscillal, J.; Wang, S.F. Synchronously activated strontium aluminate nanoflakes anchored functionalized carbon nanofiber nanocomposite for sensitive amperometric detection of food additive: Propyl gallate. Food Chem. 2022, 389, 133119. [Google Scholar] [CrossRef] [PubMed]
- Mofokeng, M.; Nthunya, L.N.; Gutierrez, L.; Matabola, P.; Mishra, S.; Nxumalo, E.N. Perflurooctyltriethoxy silane and carbon nanotubes-modified PVDF superoleophilic nanofibre membrane for oil-in-water adsorption and recovery. J. Environ. Chem. Eng. 2020, 8, 104497–104508. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Y.; Zhang, S.; Wang, T.; Zhuang, X.; Tian, C.; Luan, F.; Ni, S.-Q.; Fu, X. Enhanced electrochemical sensor based on gold nanoparticles and MoS2 nanoflowers decorated ionic liquid-functionalized graphene for sensitive detection of bisphenol A in environmental water. Microchem. J. 2021, 161, 105769–105777. [Google Scholar] [CrossRef]
- Mita, D.; Attanasio, A.; Arduini, F.; Diano, N.; Grano, V.; Bencivenga, U.; Rossi, S.; Amine, A.; Moscone, D. Enzymatic determination of BPA by means of tyrosinase immobilized on different carbon carriers. Biosens. Bioelectron. 2007, 23, 60–65. [Google Scholar] [CrossRef]
- Cammarota, M.; Lepore, M.; Portaccio, M.; Di Tuoro, D.; Arduini, F.; Moscone, D.; Mita, D. Laccase biosensor based on screen-printed electrode modified withthionine-carbon black nanocomposite, for Bisphenol A detection. Electrochim. Acta 2013, 109, 340–347. [Google Scholar]
- Zhang, X.; Zhu, J.; Wu, Z.; Wen, W.; Zhang, X.; Wang, S. Electrochemical sensor based on confined synthesis of gold nanoparticles@ covalent organic frameworks for the detection of bisphenol A. Anal. Chim. Acta 2023, 1239, 340743–340751. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Yu, Y.; Luo, Z.; Li, G.; Lin, X. Nanoporous electrode with stable polydimethylsiloxane coating for direct electrochemical analysis of bisphenol A in complex wine media. Food Chem. 2023, 405, 134806–134815. [Google Scholar] [CrossRef]
| Sensor | Linear Range (μM) | LOD (μM) | EC Analytical Methods | Ref. |
|---|---|---|---|---|
| Tyr-MWCNT/CPE | 1–16 | 1.0 | Amperometry | [45] |
| Laccase-thionine-CB/GCE | 0.5–50 | 0.2 | Amperometry | [46] |
| AuNPs@TpBD-COFs/GCE | 5–1000 | 1.0 | DPV | [47] |
| PDMS@SNCM/ITO | 1.0–20.0 | 0.23 | DPV | [48] |
| CB/f-CNF/GCE | 0.4–50 | 0.059 | DPV | This work |
| Samples | Added Level (μg kg−1) | Original Level (μg kg−1) | Found Level (μg kg−1) | Recovery (%) (mean ± SD, n = 3) |
|---|---|---|---|---|
| Canned peach in glass jars | 10 | 29.8 | 38.4 | 86.0 ± 2.3 |
| 30 | 29.8 | 60.6 | 102.7 ± 3.9 | |
| 50 | 29.8 | 79.3 | 99.0 ± 3.4 | |
| Milk in paper cartons | 10 | 22.4 | 32.6 | 102.0 ± 3.6 |
| 30 | 22.4 | 52.8 | 101.3 ± 2.7 | |
| 50 | 22.4 | 72.1 | 99.4 ± 4.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yang, Z.; Gu, S.; Pan, M.; Xu, L. A Facile Electrode Modification Approach Based on Metal-Free Carbonaceous Carbon Black/Carbon Nanofibers for Electrochemical Sensing of Bisphenol A in Food. Foods 2025, 14, 314. https://doi.org/10.3390/foods14020314
Wang J, Yang Z, Gu S, Pan M, Xu L. A Facile Electrode Modification Approach Based on Metal-Free Carbonaceous Carbon Black/Carbon Nanofibers for Electrochemical Sensing of Bisphenol A in Food. Foods. 2025; 14(2):314. https://doi.org/10.3390/foods14020314
Chicago/Turabian StyleWang, Jin, Zhen Yang, Shuanghuan Gu, Mingfei Pan, and Longhua Xu. 2025. "A Facile Electrode Modification Approach Based on Metal-Free Carbonaceous Carbon Black/Carbon Nanofibers for Electrochemical Sensing of Bisphenol A in Food" Foods 14, no. 2: 314. https://doi.org/10.3390/foods14020314
APA StyleWang, J., Yang, Z., Gu, S., Pan, M., & Xu, L. (2025). A Facile Electrode Modification Approach Based on Metal-Free Carbonaceous Carbon Black/Carbon Nanofibers for Electrochemical Sensing of Bisphenol A in Food. Foods, 14(2), 314. https://doi.org/10.3390/foods14020314







