Isolation and Evaluation of Rhizopus arrhizus Strains from Traditional Rice Wine Starters (Jiuqu): Enzyme Activities, Antioxidant Capacity, and Flavour Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
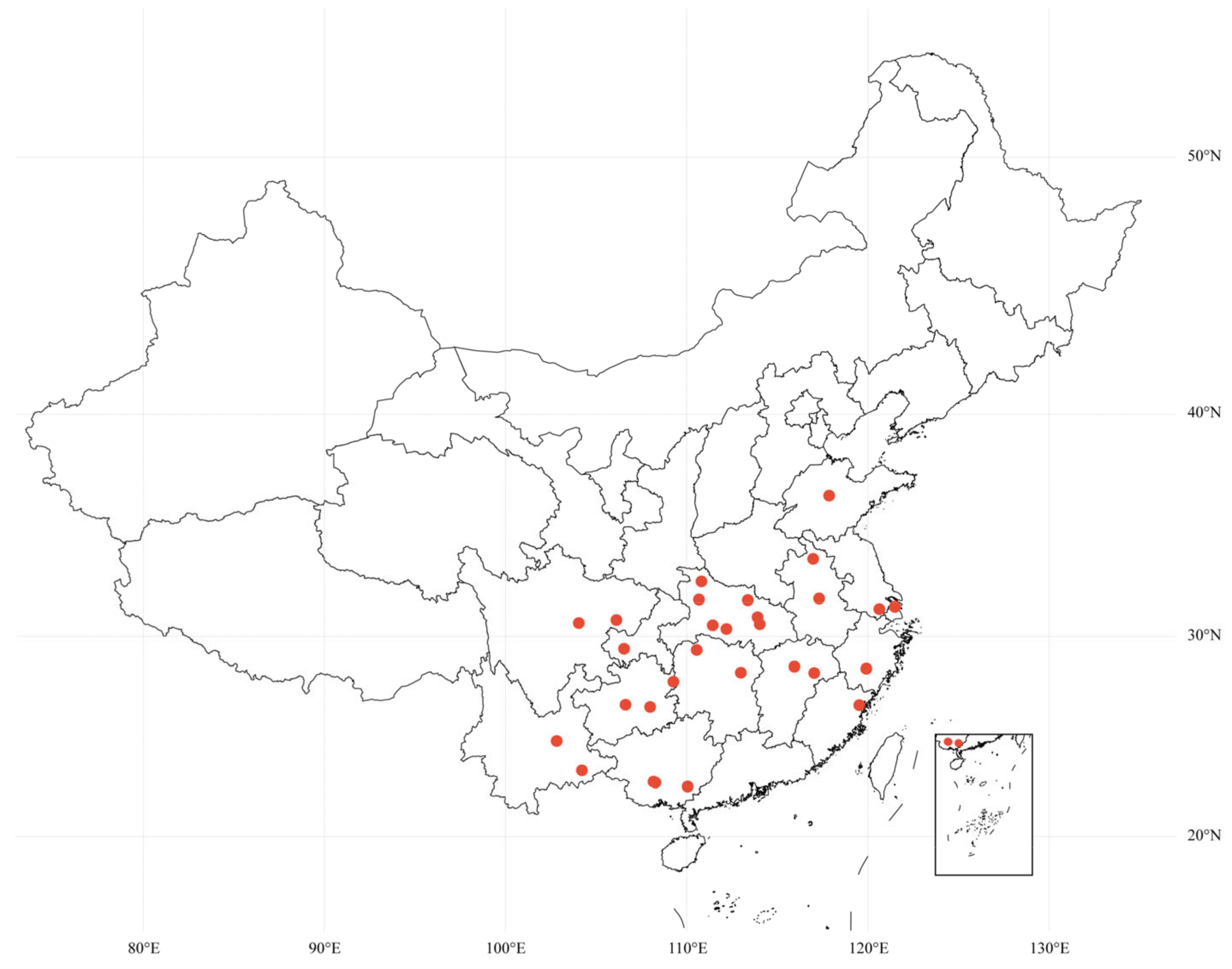
2.2. Sample Collection
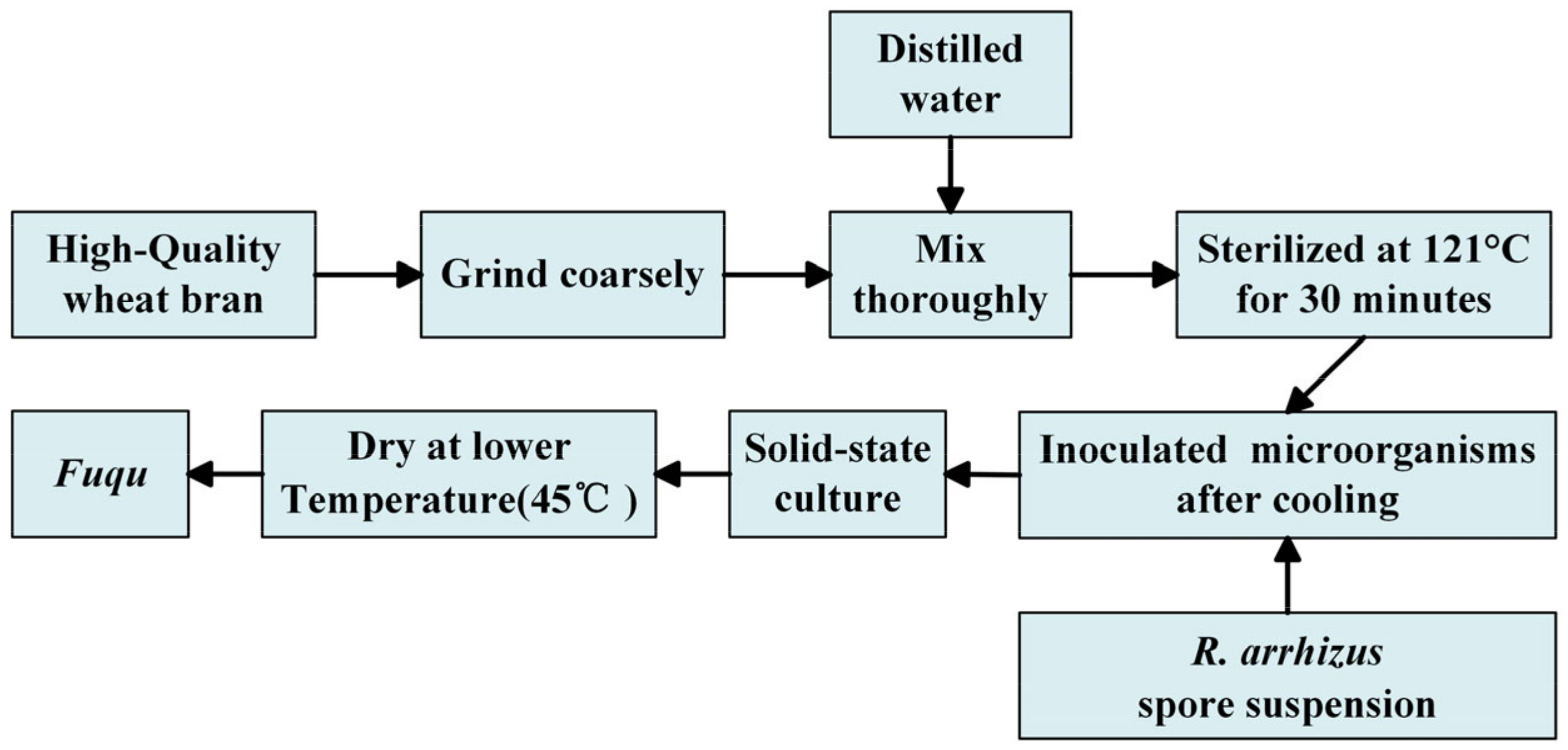
2.3. Isolation of Rhizopus
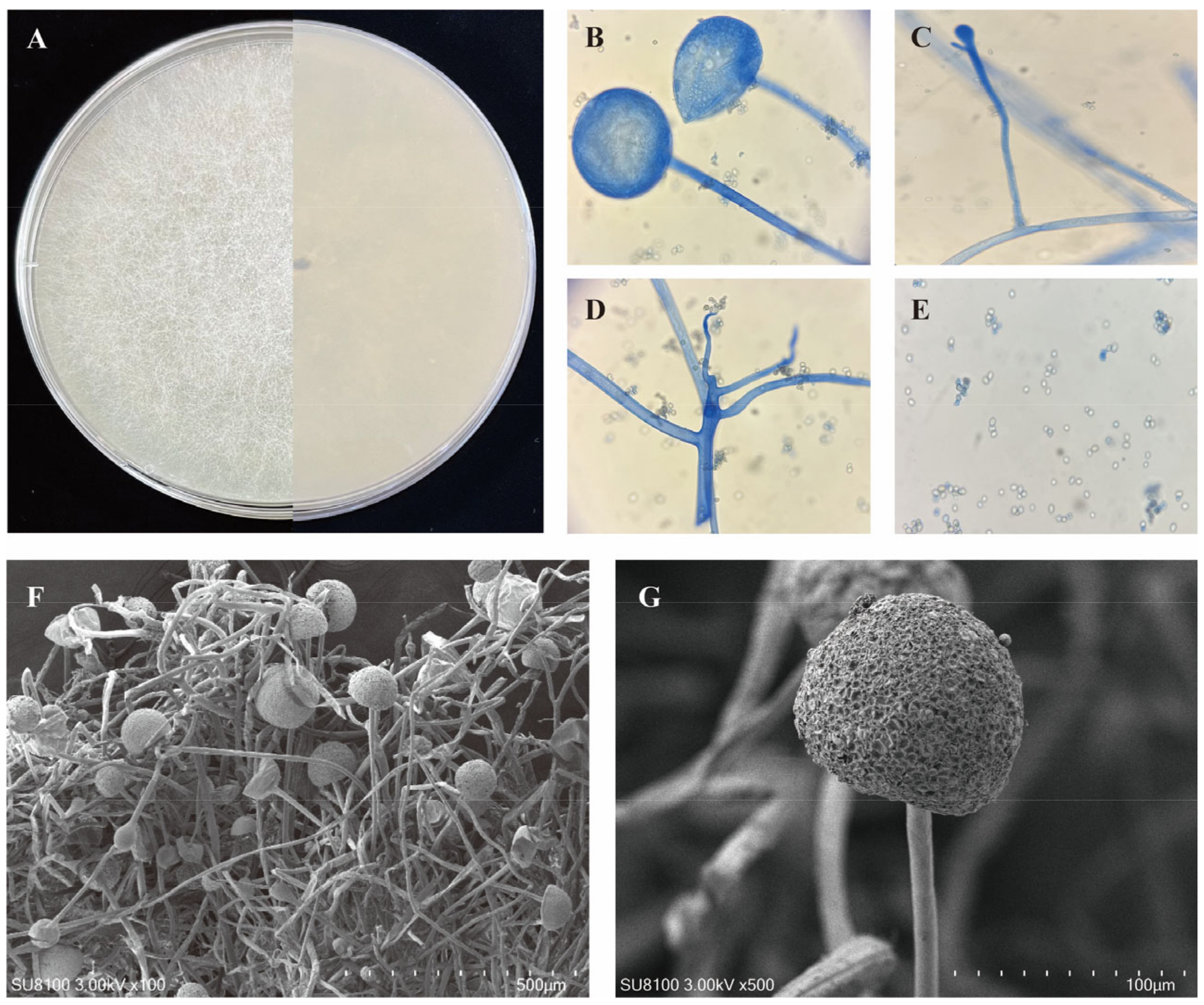
2.4. Morphological, Sporulation Ability, and Molecular Identification of Rhizopus
2.5. Evaluation of Enzyme Production Ability of R. arrhizus
2.6. Laboratory-Scale Brewing of Sweet Rice Wine
2.7. Determination of Physicochemical Properties, Bioactive Compounds Content, and Antioxidant Capacity
2.7.1. Physicochemical Properties
2.7.2. Bioactive Compounds Content
2.7.3. Antioxidant Capacity
2.8. Determination of Flavour Compounds
2.9. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Rhizopus
3.2. Sporulation Ability and Phylogenetic analyses
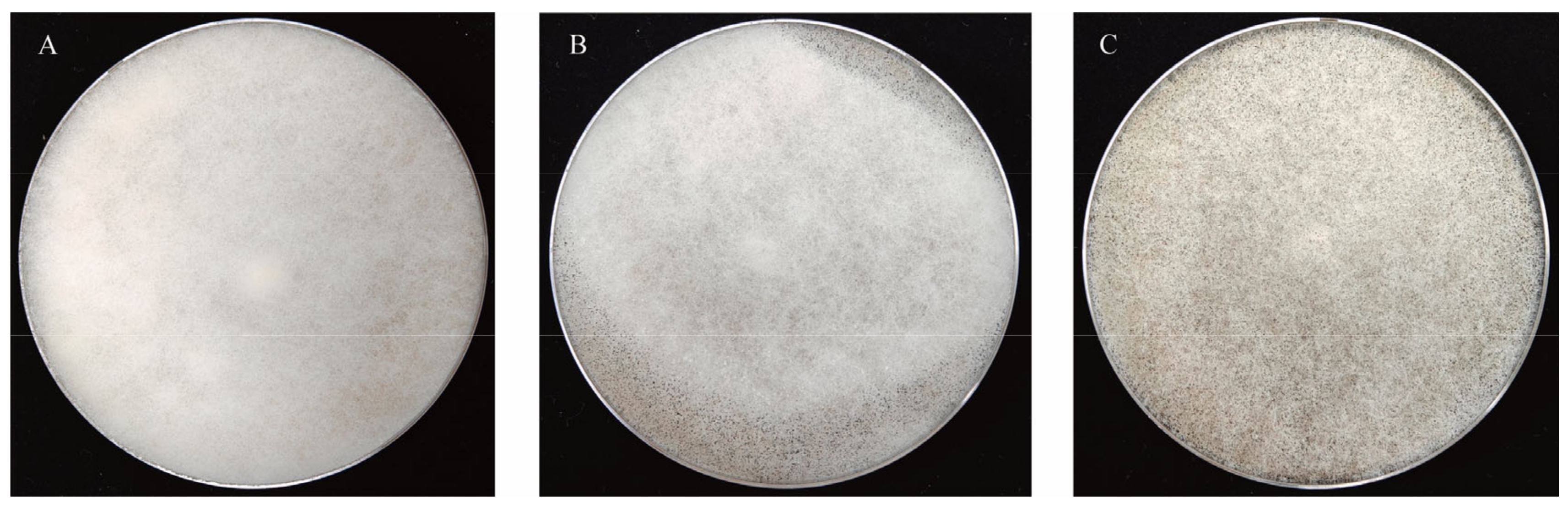
3.2.1. Sporulation Ability
3.2.2. Phylogenetic Tree
3.3. Enzyme Activity of Different R. arrhizus
3.4. Physicochemical Properties Analysis
3.4.1. Carbohydrate Metabolism
3.4.2. pH, Total Acid, Amino Nitrogen, and Total Peptide
3.4.3. Ethanol and Glycerol Content
3.5. Bioactive Compounds Content and Antioxidant Capacity
3.5.1. Total Flavonoid Content (TFC) and Total Phenolic Content (TPC)
3.5.2. ABTS Scavenging Rate and Total Antioxidant Capacity
3.5.3. Histamine
3.6. Characterisation of Flavour Compounds
3.6.1. Organic Acid
3.6.2. Amino Acid
3.6.3. Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Statement
References
- Lv, R.L.; Chantapakul, T.; Zou, M.M.; Li, M.; Zhou, J.W.; Ding, T.; Ye, X.Q.; Liu, D.H. Thermal inactivation kinetics of Bacillus cereus in Chinese rice wine and in simulated media based on wine components. Food Control 2018, 89, 308–313. [Google Scholar] [CrossRef]
- Yu, H.; Guo, W.; Ai, L.; Chen, C.; Tian, H. Unraveling the difference in aroma characteristics of Huangjiu from Shaoxing region fermented with different brewing water, using descriptive sensory analysis, comprehensive two-dimensional gas chromatography-quadrupole mass spectrometry and multivariate data analysis. Food Chem. 2022, 372, 131227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Li, T.; Zou, G.; Wei, Y.J.; Qu, L.B. Advancements and future directions in yellow rice wine production research. Fermentation 2024, 10, 40. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, C.; Gao, X.; Kang, Y.; Huang, M.; Wu, J.; Liu, Y.; Zhang, J.; Li, H.; Zhang, Y. Characterization of key aroma compounds in Huangjiu from northern China by sensory-directed flavor analysis. Food Res. Int. 2020, 134, 109238. [Google Scholar] [CrossRef]
- Huang, Z.R.; Guo, W.L.; Zhou, W.B.; Li, L.; Xu, J.X.; Hong, J.L.; Liu, H.P.; Zeng, F.; Bai, W.D.; Liu, B.; et al. Microbial communities and volatile metabolites in different traditional fermentation starters used for Hong Qu glutinous rice wine. Food Res. Int. 2019, 121, 593–603. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, X.; He, Z.; Su, H.; Li, W.; Ren, X. Amino acid and microbial community dynamics during the fermentation of Hong Qu glutinous rice wine. Food Microbiol. 2020, 90, 103467. [Google Scholar] [CrossRef]
- Pan, X.D.; Tang, J.; Chen, Q.; Wu, P.G.; Han, J.L. Evaluation of direct sampling method for trace elements analysis in Chinese rice wine by ICP-OES. Eur. Food Res. Technol. 2013, 236, 531–535. [Google Scholar] [CrossRef]
- Peng, L.; Ai-Lati, A.; Ji, Z.; Chen, S.; Mao, J. Polyphenols extracted from huangjiu have anti-inflammatory activity in lipopolysaccharide stimulated RAW264.7 cells. RSC Adv 2019, 9, 5295–5301. [Google Scholar] [CrossRef]
- Jeong, J.W.; Nam, P.W.; Lee, S.J.; Lee, K.G. Antioxidant activities of Korean rice wine concentrates. J. Agric. Food Chem. 2011, 59, 7039–7044. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, J.; Ni, T.; Lin, N.; Meng, L.; Gao, F.; Luo, H.; Liu, X.; Chi, J.; Guo, H. Yellow wine polyphenolic compounds prevent doxorubicin-induced cardiotoxicity through activation of the Nrf2 signalling pathway. J. Cell. Mol. Med. 2019, 23, 6034–6047. [Google Scholar] [CrossRef]
- Zhao, F.; Ji, Z.; Chi, J.; Tang, W.; Zhai, X.; Meng, L.; Guo, H. Effects of Chinese yellow wine on nitric oxide synthase and intercellular adhesion molecule-1 expressions in rat vascular endothelial cells. Acta Cardiol. 2016, 71, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next. Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z.; et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Caliceti, C.; Rizzo, P.; Giuliano, M. Role of natural compounds in oxidative stress and inflammation linked to cardiometabolic disorders: From biochemical aspects to clinical evidences. Oxidative Med. Cell. Longev. 2018, 2018, 1479309. [Google Scholar] [CrossRef]
- Rousta, N.; Aslan, M.; Yesilcimen Akbas, M.; Ozcan, F.; Sar, T.; Taherzadeh, M.J. Effects of fungal based bioactive compounds on human health: Review paper. Crit. Rev. Food Sci. Nutr. 2024, 64, 7004–7027. [Google Scholar] [CrossRef]
- Zhao, C.; Su, W.; Mu, Y.; Mu, Y.; Jiang, L. Integrative metagenomics-metabolomics for analyzing the relationship between microorganisms and non-volatile profiles of traditional Xiaoqu. Front. Microbiol. 2020, 11, 617030. [Google Scholar] [CrossRef]
- Went, F.A.F.C.; Prinsen Geerligs, H.C. Beobachtungen über die hefearten und zuckerbildenden pilze der arackfabrikation. Verh. K. Akad. Wet. 1895, 4, 1–31. [Google Scholar]
- Peng, Q.; Zheng, H.J.; Meng, K.; Yu, H.F.; Xie, G.F.; Zhang, Y.H.; Yang, X.Y.; Chen, J.L.; Xu, Z.Q.; Lin, Z.C.; et al. Quantitative study on core bacteria producing flavor substances in Huangjiu (Chinese yellow rice wine). LWT-Food Sci. Technol. 2022, 168, 113509. [Google Scholar] [CrossRef]
- Hong, X.; Chen, J.; Liu, L.; Wu, H.; Tan, H.; Xie, G.; Xu, Q.; Zou, H.; Yu, W.; Wang, L.; et al. Metagenomic sequencing reveals the relationship between microbiota composition and quality of Chinese rice wine. Sci. Rep. 2016, 6, 26621. [Google Scholar] [CrossRef]
- Londono-Hernandez, L.; Ramirez-Toro, C.; Ruiz, H.A.; Ascacio-Valdes, J.A.; Aguilar-Gonzalez, M.A.; Rodriguez-Herrera, R.; Aguilar, C.N. Rhizopus oryzae-Ancient microbial resource with importance in modern food industry. Int. J. Food Microbiol. 2017, 257, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zeng, Y.; Ma, J.; Cai, K.; Guo, T.; Tan, G.; Yu, X.; Hu, Y.; Peng, N.; Zhao, S. Characteristics and functions of dominant yeasts together with their applications during strong-flavor baijiu brewing. Foods 2024, 13, 2409. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Qu, G.; Wang, D.; Chen, J.; Du, G.; Fang, F. Synergistic fermentation with functional microorganisms improves safety and quality of traditional Chinese fermented foods. Foods 2023, 12, 2892. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.J.; Peng, P.; Yu, L.; Wan, B.; Liang, X.T.; Liu, P.L.; Liao, W.F.; Miao, L.H. Metagenomic analysis reveals the correlations between microbial communities and flavor compounds during the brewing of traditional Fangxian huangjiu. Food Biosci. 2024, 58, 103816. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Xiao, L.; Qu, L.; Zhang, X.; Wei, Y. Advancing insights into probiotics during vegetable fermentation. Foods 2023, 12, 3789. [Google Scholar] [CrossRef]
- Zheng, R.Y.; Chen, G.Q.; Huang, H.; Liu, X.Y. A monograph of Rhizopus. Sydowia 2007, 59, 273–372. [Google Scholar]
- Zhao, H.; Nie, Y.; Zong, T.K.; Wang, K.; Lv, M.L.; Cui, Y.J.; Tohtirjap, A.; Chen, J.J.; Zhao, C.L.; Wu, F.; et al. Species diversity, updated classification and divergence times of the phylum Mucoromycota. Fungal Divers. 2023, 123, 49–157. [Google Scholar] [CrossRef]
- Liu, X.Y.; Huang, H.; Zheng, R.Y. Delimitation of Rhizopus varieties based on IGS rDNA sequences. Sydowia 2008, 60, 93–112. [Google Scholar]
- Dolatabadi, S.; de Hoog, G.S.; Meis, J.F.; Walther, G. Species boundaries and nomenclature of Rhizopus arrhizus (syn. R. oryzae). Mycoses 2014, 57, 108–127. [Google Scholar] [CrossRef]
- Saito, K.; Saito, A.; Ohnishi, M.; Oda, Y. Genetic diversity in Rhizopus oryzae strains as revealed by the sequence of lactate dehydrogenase genes. Arch. Microbiol. 2004, 182, 30–36. [Google Scholar] [CrossRef]
- Yao, L.D.; Ju, X.; James, T.Y.; Qiu, J.Z.; Liu, X.Y. Relationship between saccharifying capacity and isolation sources for strains of the Rhizopus arrhizus complex. Mycoscience 2018, 59, 409–414. [Google Scholar] [CrossRef]
- Ju, X.; Zhang, M.Z.; Zhao, H.; Liu, Z.; Jia, B.S.; Timothy, Y.J.; Qiao, M.; Liu, X.R. Genomic SNPs reveal population structure of Rhizopus arrhizus. Mycosystema 2020, 39, 2285–2303. [Google Scholar] [CrossRef]
- Yao, L.D.; Ju, X.; Timothy, Y.J.; Liu, X.Y.; Qiu, J.Z. Diversity of growth kinetic models for Rhizopus arrhizus. Microbiol. China 2019, 46, 42–53. [Google Scholar] [CrossRef]
- Liu, X.L.; Ju, X.; Jia, B.S.; Timothy, Y.J.; Qiao, M.; Liu, X.Y. The correlations of fermentation metabolites with sporulation capability and varieties of Rhizopus arrhizus. Acta Microbiol. Sin. 2022, 62, 1131–1149. [Google Scholar] [CrossRef]
- Wu, H.; Liu, H.N.; Ma, A.M.; Zhou, J.Z.; Xia, X.D. Synergetic effects of Lactobacillus plantarum and Rhizopus oryzae on physicochemical, nutritional and antioxidant properties of whole-grain oats (Avena sativa L.) during solid-state fermentation. LWT-Food Sci. Technol. 2022, 154, 112687. [Google Scholar] [CrossRef]
- Cantabrana, I.; Perise, R.; Hernández, I. Uses of Rhizopus oryzae in the kitchen. Int. J. Gastron. Food Sci. 2015, 2, 103–111. [Google Scholar] [CrossRef]
- Araujo, L.P.; Vilela, H.; Solinho, J.; Pinheiro, R.; Belo, I.; Lopes, M. Enrichment of fruit peels’ nutritional value by solid-state fermentation with Aspergillus ibericus and Rhizopus oryzae. Molecules 2024, 29, 3563. [Google Scholar] [CrossRef]
- Drabo, M.S.; Savadogo, A.; Raes, K. Effects of tempeh fermentation using Rhizopus oryzae on the nutritional and flour technological properties of Zamnè (Senegalia macrostachya seeds): Exploration of processing alternatives for a hard-to-cook but promising wild legume. Food Biosci. 2023, 54, 102823. [Google Scholar] [CrossRef]
- Balogun, O.I.; Epriliati, I.; Otunola, E.T.; Agboola, H.A. Rhizopus oryzae FNCC 6010, Rhizopus oligosporus FNCC 6011, and their hybrid lowered antioxidant capacity in velvet beans compared to germination. Int. Food Res. J. 2017, 24, 1606–1613. [Google Scholar]
- Zhao, H.; Ju, X.; Nie, Y.; James, T.Y.; Liu, X.Y. High-throughput screening carbon and nitrogen sources to promote growth and sporulation in Rhizopus arrhizus. AMB Express 2024, 14, 76. [Google Scholar] [CrossRef]
- Ibarruri, J.; Cebrián, M.; Hernández, I. Solid state fermentation of brewer’s spent grain using Rhizopus sp. to enhance nutritional value. Waste Biomass Valorization 2019, 10, 3687–3700. [Google Scholar] [CrossRef]
- Mu, Y.; Huang, Y.; Li, D.; Zhu, Z.; Yu, S.; Xie, F. Revealing the comprehensive effect of mechanization on sauce-flavor Daqu through high-throughput sequencing and multi-dimensional metabolite profiling. Food Res. Int. 2024, 191, 114645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Su, W.; Mu, Y.; Luo, L.; Zhao, M.; Qiu, S.; Su, G.; Jiang, L. Effects of Jiuqu inoculating Rhizopus oryzae Q303 and Saccharomyces cerevisiae on chemical components and microbiota during black glutinous rice wine fermentation. Int. J. Food Microbiol. 2023, 385, 110012. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Li, Z.Q.; Zheng, D.W.; Chen, C.; Ge, C.; Tian, H.X. Exploring microbial dynamics and metabolic pathways shaping flavor profiles in Huangjiu through metagenomic analysis. Food Res. Int. 2024, 196, 115036. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Liu, B.; Feng, S.B.; Ma, X.; Wang, S.X.; Zhang, Y.T. Correlation between microbe, physicochemical properties of Jiuqu in different plateau areas and volatile flavor compounds of highland barley alcoholic drink. Food Biosci. 2023, 51, 102276. [Google Scholar] [CrossRef]
- Yang, Y.R.; Zhong, H.Y.; Yang, N.; Xu, S.Z.; Yang, T. Quality improvement of sweet rice wine fermented with Rhizopus delemar on key aroma compounds content, phenolic composition, and antioxidant capacity compared to Rhizopus oryzae. J. Food Sci. Technol.-Mysore 2022, 59, 2339–2350. [Google Scholar] [CrossRef]
- Kim, M.; Seo, J.A. Fermentation profiling of rice wine produced by Aspergillus oryzae KSS2 and Rhizopus oryzae KJJ39 newly isolated from Korean fermentation starter. Appl. Biol. Chem. 2021, 64, 25. [Google Scholar] [CrossRef]
- Yu, H.; Liu, S.; Qin, H.; Zhou, Z.; Zhao, H.; Zhang, S.; Mao, J. Artificial intelligence-based approaches for traditional fermented alcoholic beverages’ development: Review and prospect. Crit. Rev. Food Sci. Nutr. 2024, 64, 2879–2889. [Google Scholar] [CrossRef]
- Ma, D.; Liu, S.; Liu, H.; Zhang, S.; Xu, Y.; Mao, J. Environmental factors drive microbial community succession in biofortified wheat Qu and its improvement on the quality of Chinese huangjiu. J. Biosci. Bioeng. 2024, 137, 124–133. [Google Scholar] [CrossRef]
- Dong, W.W.; Yang, Q.; Liao, Y.X.; Liu, Y.C.; Hu, Y.L.; Peng, N.; Liang, Y.X.; Zhao, S.M. Characterisation and comparison of the microflora of traditional and pure culture xiaoqu during the baijiu liquor brewing process. J. Inst. Brew. 2020, 126, 213–220. [Google Scholar] [CrossRef]
- Chen, X.; Song, C.; Zhao, J.; Xiong, Z.; Peng, L.; Zou, L.; Liu, B.; Li, Q. Effect of a new fermentation strain combination on the fermentation process and quality of highland barley yellow wine. Foods 2024, 13, 2193. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, S.; Walther, G.; Gerrits van den Ende, A.H.G.; Hoog, G.S. Diversity and delimitation of Rhizopus microsporus. Fungal Divers. 2013, 64, 145–163. [Google Scholar] [CrossRef]
- Hota, S.; Achary, K.G.; Singh, S. Identification and molecular characterization of Rhizopus delemar from Eastern Ghats of state of India and its biotechnological applications. Geomicrobiol. J. 2023, 41, 429–439. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. Corrigendum to: IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 2461. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Du, J.; Cao, C.; Cai, G.; Sun, J.; Wu, D.; Lu, J. Development of a novel multi-strain wheat Qu with high enzyme activities for Huangjiu fermentation. J. Sci. Food Agric. 2021, 101, 4808–4817. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 2002, 31, 426–428. [Google Scholar] [CrossRef]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: A biotechnological perspective. Process Biochem. 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Si, G.R.; Rao, Z.M.; Li, J.L.; Zhang, X.; Mei, J.; Wang, J.S.; Ye, M.; Zhou, P. High yield of tetramethylpyrazine in functional Fuqu using bacillus amyloliquefaciens. Food Biosci. 2019, 31, 100435. [Google Scholar] [CrossRef]
- Gilham, D.; Lehner, R. Techniques to measure lipase and esterase activity in vitro. Methods 2005, 36, 139–147. [Google Scholar] [CrossRef]
- Yuan, H.W.; Zhang, C.; Chen, S.Y.; Zhao, Y.; Tie, Y.; Yin, L.G.; Chen, J.; Wu, Q.D.; Wang, Y.T.; Xu, Z.; et al. Effect of different moulds on oenological properties and flavor characteristics in rice wine. LWT Food Sci. Technol. 2023, 173, 114201. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Qian, H.; Yang, Y.; Zhu, X.; Wu, Q.; Mu, Y.; Huang, Z. Analysis of saccharification products of high-concentration glutinous rice fermentation by Rhizopus nigricans Q3 and alcoholic fermentation of Saccharomyces cerevisiae GY-1. ACS Omega 2021, 6, 8038–8044. [Google Scholar] [CrossRef] [PubMed]
- GB/T 13662-2018; Huangjiu. Standards Press of China: Beijing, China, 2018.
- Liu, P.; Miao, L. Multiple batches of fermentation promote the formation of functional microbiota in Chinese miscellaneous-flavor baijiu fermentation. Front. Microbiol. 2020, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Q.; Zou, H.; Yu, Y.; Zhou, Z.; Mao, J.; Zhang, S. A metagenomic analysis of the relationship between microorganisms and flavor development in Shaoxing mechanized huangjiu fermentation mashes. Int. J. Food Microbiol. 2019, 303, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Zhou, Z.L.; Yu, Y.J.; Liu, S.P.; Zhu, S.H.; Jian, D.Z.; Cui, P.J.; Zhong, F.; Mao, J. Investigation of the 5-hydroxymethylfurfural and furfural content of Chinese traditional fermented vinegars from different regions and its correlation with the saccharide and amino acid content. LWT Food Sci. Technol. 2020, 124, 109175. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Wu, Q.; Jiang, X.; Liu, H.; Wang, C.; Huang, M.; Wu, J.; Zhang, J.; Yu, Y. Sensomics-assisted flavor decoding of coarse cereal Huangjiu. Food Chem. 2022, 381, 132296. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.C.; Lassabliere, B.; Yu, B.; Liu, S.Q. Green tea fermentation with Saccharomyces boulardii CNCM I-745 and Saccharomyces boulardii 299V. LWT Food Sci. Technol. 2022, 157, 113081. [Google Scholar] [CrossRef]
- Lucking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansa, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding. IMA Fungus 2020, 11, 14. [Google Scholar] [CrossRef]
- Yang, Q.; Yao, H.; Liu, S.; Mao, J. Interaction and application of molds and yeasts in Chinese fermented foods. Front. Microbiol. 2021, 12, 664850. [Google Scholar] [CrossRef]
- Sjamsuridzal, W.; Khasanah, M.; Febriani, R.; Vebliza, Y.; Oetari, A.; Santoso, I.; Gandjar, I. The effect of the use of commercial tempeh starter on the diversity of Rhizopus tempeh in Indonesia. Sci. Rep. 2021, 11, 23932. [Google Scholar] [CrossRef]
- Gryganskyi, A.P.; Golan, J.; Muszewska, A.; Idnurm, A.; Dolatabadi, S.; Mondo, S.J.; Kutovenko, V.B.; Kutovenko, V.O.; Gajdeczka, M.T.; Anishchenko, I.M.; et al. Sequencing the genomes of the first terrestrial fungal lineages: What have we learned. Microorganisms 2023, 11, 1830. [Google Scholar] [CrossRef]
- Marsit, S.; Leducq, J.B.; Durand, E.; Marchant, A.; Filteau, M.; Landry, C.R. Evolutionary biology through the lens of budding yeast comparative genomics. Nat. Rev. Genet. 2017, 18, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wu, Z.; Liu, H.; He, X.; Liao, Z.; Luo, W.; Li, L.; Yin, L.; Wu, F.; Zhang, L.; et al. Screening, identification, and characterization of molds for brewing rice wine: Scale-up production in a bioreactor. PLoS ONE 2024, 19, e0300213. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Y.; Li, J.L.; Liu, S.; He, S.D.; Sun, H.J.; Yao, S.F.; Xu, S.Y. Effect of enzymes addition on the fermentation of Chinese rice wine using defined fungal starter. LWT-Food Sci. Technol. 2021, 143, 111101. [Google Scholar] [CrossRef]
- Chen, L.H.; Wang, S.X.; Ren, L.X.; Li, D.N.; Ma, X.; Rong, Y.Z. Flavour characteristics of rice wine fermented with mixed starter by moulds and yeast strains. Int. J. Food Sci. Tech. 2021, 56, 5791–5798. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, W.; Liang, X.; Liu, J.; Zhu, H.; Cai, T.; Zhang, Q.; Tang, J. Fungal biomarkers in traditional starter determine the chemical characteristics of turbid rice wine from the rim of the Sichuan Basin, China. Foods 2023, 12, 585. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.P.; Ma, D.L.; Xu, Y.Z.; Yang, C.; Mao, J. Stabilization of jiuyao quality for huangjiu brewing by fortifying functional strains based on core microbial community analysis. Food Biosci. 2023, 52, 102370. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, J.; Ou, W.; Chen, Y.; Wang, R.; Li, K.; Sun, X.M.; Li, Y.; Xu, Q.; Huang, H. Transcriptome analysis of Rhizopus oryzae seed pellet formation using triethanolamine. Biotechnol. Biofuels Bioprod. 2021, 14, 230. [Google Scholar] [CrossRef]
- Qian, M.; Ruan, F.; Zhao, W.; Dong, H.; Bai, W.; Li, X.; Huang, X.; Li, Y. The dynamics of physicochemical properties, microbial community, and flavor metabolites during the fermentation of semi-dry Hakka rice wine and traditional sweet rice wine. Food Chem. 2023, 416, 135844. [Google Scholar] [CrossRef]
- Jin, Z.; Cai, G.; Wu, C.; Hu, Z.; Xu, X.; Xie, G.; Wu, D.; Lu, J. Profiling the key metabolites produced during the modern brewing process of Chinese rice wine. Food Res. Int. 2021, 139, 109955. [Google Scholar] [CrossRef]
- Jiang, L.; Su, W.; Mu, Y.; Mu, Y. Major metabolites and microbial community of fermented black glutinous rice wine with different starters. Front. Microbiol. 2020, 11, 593. [Google Scholar] [CrossRef]
- Zhou, M.; Bu, T.; Zheng, J.; Liu, L.; Yu, S.; Li, S.; Wu, J. Peptides in brewed wines: Formation, structure, and function. J. Agric. Food Chem. 2021, 69, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Yao, L.D.; Bai, Y.C.; Qiu, J.Z.; Liu, X.Y. Exploration of alcohol production and alcohol tolerance of Rhizopus arrhizus. In Proceedings of the 2018 Annual Meeting of Mycological Society of China, Taian, China, 10–12 August 2018. [Google Scholar]
- Liu, H.; Xiao, Q.; Yue, Y.; Huang, X.L.; Zhang, Y.K.; Deng, L.; Wang, F. Influence analysis of glycerol in fumaric acid co-fermentation process by Rhizopus arrhizus. J. Environ. Chem. Eng. 2021, 9, 104750. [Google Scholar] [CrossRef]
- Chen, J.X.; Chen, Y.l.; Hu, J.J.; He, C.; Peng, X.Z.; Li, Z.J.; Wang, Y.L.; Zhu, M.Z.; Xiao, Y. Solid-state fermentation with Rhizopus oryzae HC-1 improves the metabolites profiling, antioxidant activity and gut microbiota modulation effect of soybeans. LWT-Food Sci. Technol. 2023, 187, 115253. [Google Scholar] [CrossRef]
- Lim, J.; Nguyen, T.T.H.; Pal, K.; Gil Kang, C.; Park, C.; Kim, S.W.; Kim, D. Phytochemical properties and functional characteristics of wild turmeric (Curcuma aromatica) fermented with Rhizopus oligosporus. Food Chem. X 2022, 13, 100198. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Chen, T.; Ding, Y.; Yu, D.; Zhang, J.; Zhang, R.; Zhang, Y.; Wang, X.; Xu, T.; Li, J. Effects of Rhizopus arrhizus 31-assisted pretreatment on the extraction and bioactivity of total flavonoids from Hibiscus manihot L. Molecules 2024, 29, 1046. [Google Scholar] [CrossRef]
- Silva, L.C.; Kupski, L.; Beserra da Silva de Souza, S.; Bolanho Barros, B.C. Influence of fermentation conditions by Rhizopus oryzae on the release of phenolic compounds, composition, and properties of brewer’s spent grain. Food Biosci. 2024, 61, 104591. [Google Scholar] [CrossRef]
- Selo, G.; Planinic, M.; Tisma, M.; Martinovic, J.; Perkovic, G.; Bucic-Kojic, A. Bioconversion of grape pomace with Rhizopus oryzae under solid-state conditions: Changes in the chemical composition and profile of phenolic compounds. Microorganisms 2023, 11, 956. [Google Scholar] [CrossRef]
- He, Z.; Zhou, Y.; Li, S.; Li, W.; Zhang, Y.; Guo, C.; Guo, Z.; Wei, B.; Bi, Y. Bioactive Peptides and Evaluation of Cardiac Cytoprotective Effects of Red Millet Yellow Wine as Functional Food. Foods 2024, 13, 4111. [Google Scholar] [CrossRef]
- Tsuji, A.; Koyanagi, T. Significant contribution of amino acids to antioxidant capacity of Japanese rice wine (sake). J. Sci. Food Agric. 2025, 105, 227–234. [Google Scholar] [CrossRef]
- Ren, N.; Gong, W.; Zhao, Y.; Zhao, D.G.; Xu, Y. Innovation in sweet rice wine with high antioxidant activity: Eucommia ulmoides leaf sweet rice wine. Front. Nutr. 2022, 9, 1108843. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Hu, L.; Wang, N.; Cui, F.; Ying, S.; Hu, F. Research on the brewing technology of Dangshen Huangjiu with low biogenic amines and high functional factors. J. Sci. Food Agric. 2024, 104, 6330–6341. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Liu, S.; Qiu, J.; Lin, H.; Li, D.; Jiang, J. Identification and quality evaluation of Chinese rice wine using UPLC-PDA-QTOF/MS with dual-column separation. Phytomedicine 2023, 108, 154498. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.Y.; Wu, Z.Y.; Zhang, W.X. Evaluation of six commercial koji on the formation of biogenic amines and higher alcohols in rice wine. J. Inst. Brew. 2022, 128, 124–132. [Google Scholar] [CrossRef]
- Yan, Y.; Liang, Z.; Huo, Y.; Wu, Q.; Ni, L.; Lv, X. A comparative study of microbial communities, biogenic amines, and volatile profiles in the brewing process of rice wines with Hongqu and Xiaoqu as fermentation starters. Foods 2024, 13, 2452. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, W.; Yuan, Y.; Liang, Z.; Yan, Y.; Chen, Y.; Ni, L.; Lv, X. Metagenomic insights into the regulatory effects of microbial community on the formation of biogenic amines and volatile flavor components during the brewing of Hongqu rice wine. Foods 2023, 12, 3075. [Google Scholar] [CrossRef]
- Zhao, C.; Su, W.; Mu, Y.; Jiang, L.; Mu, Y. Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Res. Int. 2020, 138, 109800. [Google Scholar] [CrossRef]
- Yu, H.; Li, Q.; Guo, W.; Chen, C.; Ai, L.; Tian, H. Dynamic analysis of volatile metabolites and microbial community and their correlations during the fermentation process of traditional Huangjiu (Chinese rice wine) produced around winter solstice. Food Chem. X 2023, 18, 100620. [Google Scholar] [CrossRef]
- El-Zawawy, N.A.; Ali, S.S.; Nouh, H.S. Exploring the potential of Rhizopus oryzae AUMC14899 as a novel endophytic fungus for the production of L-tyrosine and its biomedical applications. Microb. Cell Fact. 2023, 22, 31. [Google Scholar] [CrossRef]
- Liang, Z.C.; Lin, X.Z.; He, Z.G.; Su, H.; Li, W.X.; Guo, Q.Q. Comparison of microbial communities and amino acid metabolites in different traditional fermentation starters used during the fermentation of Hong Qu glutinous rice wine. Food Res. Int. 2020, 136, 109329. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Zhao, Z.; Li, X.; Sun, B. Flavor mystery of Chinese traditional fermented baijiu: The great contribution of ester compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, T.; Chen, H.; Dai, Y.; Li, Z.; He, J.; Ju, R.; Hou, A. Study on the Fermented Grain Characteristics and Volatile Flavor Substances during the Tuqu Fermentation of Hunan Light-Flavor Baijiu. Foods 2024, 13, 899. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiao, H.; Zhang, R.; Zhang, W.; Wen, P. Microbial Diversity and Volatile Flavor Compounds in Tibetan Flavor Daqu. Foods 2023, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Tan, X.; Wang, L.; Li, Z.; Hou, A.; Zhu, Y.; Lai, L.; Wang, Y. GC-MS Coupled with Rate-All-That-Apply (RATA) to Analyse the Volatile Flavor Substances of Yellow Wine during Fermentation. Foods 2023, 12, 1992. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; He, F.; Pan, Z.; Du, Y.; Chen, Z.; He, Y.; Sun, Y.; Li, M. Effect of microbial communities on flavor profile of Hakka rice wine throughout production. Food Chem. X 2024, 21, 101121. [Google Scholar] [CrossRef]
- Xiang, W.; Xu, Q.; Zhang, N.; Rao, Y.; Zhu, L.; Zhang, Q. Mucor indicus and Rhizopus oryzae co-culture to improve the flavor of Chinese turbid rice wine. J. Sci. Food Agric. 2019, 99, 5577–5585. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic diagramming platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2024, 52, D1234–D1245. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, B.; Tian, T.; Xiong, Y.; Wang, S.; Luo, X.; Liao, W.; Liu, P.; Miao, L.; Gao, R. Isolation and Evaluation of Rhizopus arrhizus Strains from Traditional Rice Wine Starters (Jiuqu): Enzyme Activities, Antioxidant Capacity, and Flavour Compounds. Foods 2025, 14, 312. https://doi.org/10.3390/foods14020312
Wan B, Tian T, Xiong Y, Wang S, Luo X, Liao W, Liu P, Miao L, Gao R. Isolation and Evaluation of Rhizopus arrhizus Strains from Traditional Rice Wine Starters (Jiuqu): Enzyme Activities, Antioxidant Capacity, and Flavour Compounds. Foods. 2025; 14(2):312. https://doi.org/10.3390/foods14020312
Chicago/Turabian StyleWan, Bo, Tian Tian, Ying Xiong, Siqi Wang, Xinyu Luo, Weifang Liao, Pulin Liu, Lihong Miao, and Ruijie Gao. 2025. "Isolation and Evaluation of Rhizopus arrhizus Strains from Traditional Rice Wine Starters (Jiuqu): Enzyme Activities, Antioxidant Capacity, and Flavour Compounds" Foods 14, no. 2: 312. https://doi.org/10.3390/foods14020312
APA StyleWan, B., Tian, T., Xiong, Y., Wang, S., Luo, X., Liao, W., Liu, P., Miao, L., & Gao, R. (2025). Isolation and Evaluation of Rhizopus arrhizus Strains from Traditional Rice Wine Starters (Jiuqu): Enzyme Activities, Antioxidant Capacity, and Flavour Compounds. Foods, 14(2), 312. https://doi.org/10.3390/foods14020312





