Characteristics of Soft Wheat and Tiger Nut (Cyperus esculentus) Composite Flour Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Bread Preparation Method
2.3. Dough Rising Characteristics
2.4. Specific Volume
2.5. Imaging and Cross-Section Scanning
2.6. Texture Profile Analysis of Bread
2.7. Water Activity
2.8. Colour
2.9. Total Polyphenol Content
2.10. DPPH Radical Quenching
2.11. Reducing Sugar Content
2.12. Statistical Analyses
3. Results and Discussion
3.1. Dough Characteristics
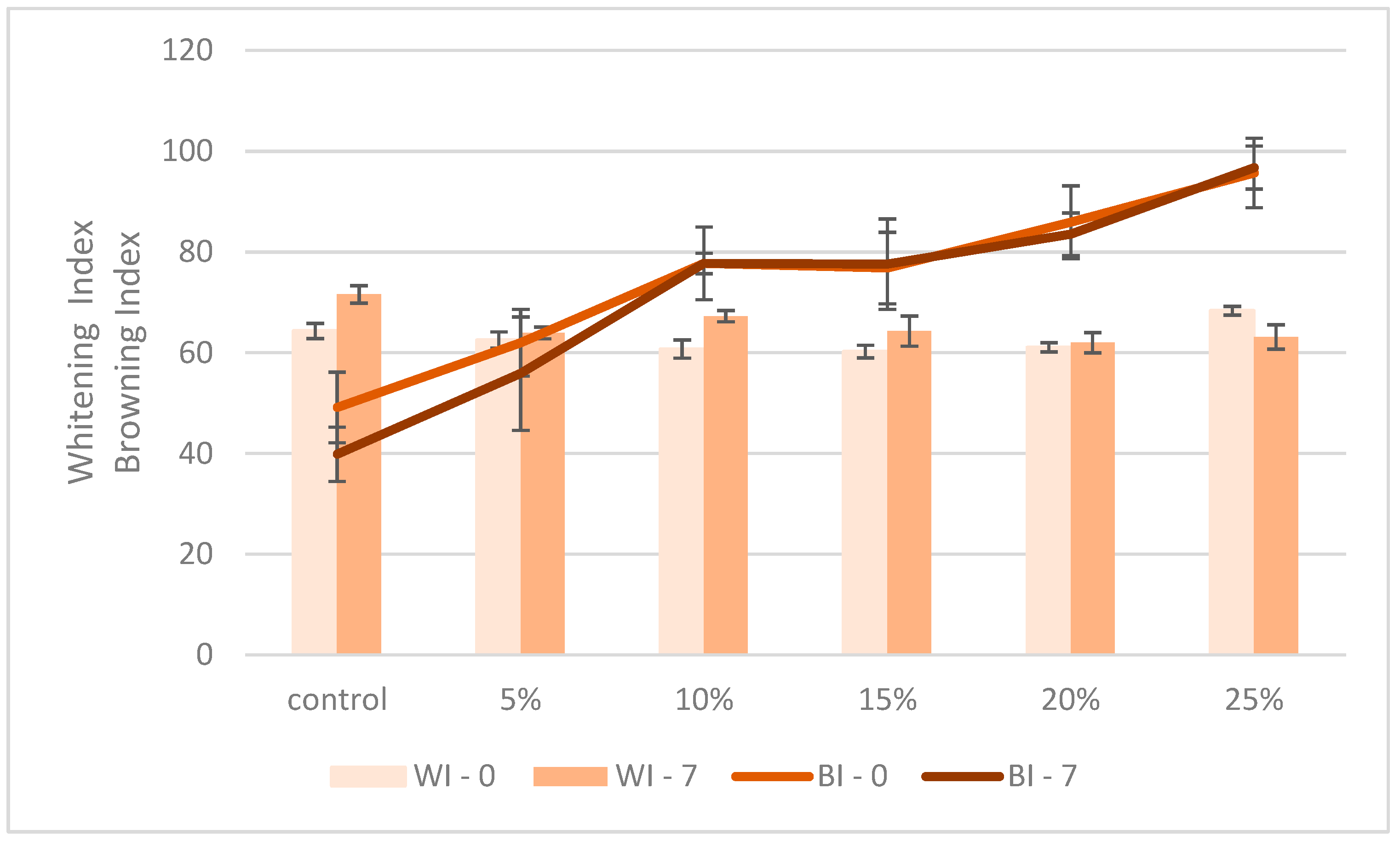
3.2. Quality Characteristics of the Composite Bread
3.3. Compositional Characteristics of the Composite Bread
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bender, D.; Schönlechner, R. Innovative Approaches towards Improved Gluten-Free Bread Properties. J. Cereal Sci. 2020, 91, 102904. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Mitelut, A.C.; Popa, E.E.; Popescu, P.A.; Popa, M.E. Trends of Innovation in Bread and Bakery Production. In Trends in Wheat and Bread Making; Academic Press: Cambridge, MA, USA, 2021; pp. 199–226. [Google Scholar] [CrossRef]
- Adejuyitan, J.A.; Otunola, E.T.; Akande, E.A.; Bolarinwa, I.F.; Oladokun, F.M. Effect of fermentation on the proximate composition of tigernut flour and its potentials in food formulation. Afr. J. Food Sci. Res. 2018, 6, 368–372. [Google Scholar]
- Nedviha, S.; Harasym, J. Functional and Antioxidative Characteristics of Soft Wheat and Tiger Nut (Cyperus esculentus) Flours Binary Blends. Foods 2024, 13, 596. [Google Scholar] [CrossRef]
- Özcan, M.M. Quality Evaluation of Bread Prepared from Wheat–Chufa Tuber Composite Flour. Foods 2023, 12, 444. [Google Scholar] [CrossRef]
- Guan, C.; Liu, T.; Li, Q.; Wang, D.; Zhang, Y. Analyzing the Effect of Baking on the Flavor of Defatted Tiger Nut Flour by E-Tongue, E-Nose and HS-SPME-GC-MS. Foods 2022, 11, 446. [Google Scholar] [CrossRef]
- Aguilar, N.; Albanell, E.; Miñarro, B.; Guamis, B.; Capellas, M. Effect of tiger nut-derived products in gluten-free batter and bread. Food Sci. Technol. Int. 2014, 21, 323–331. [Google Scholar] [CrossRef]
- Gasparre, N.; Pan, J.; da Silva Alves, P.L.; Rosell, C.M.; De J. Berrios, J. Tiger Nut (Cyperus esculentus) as a Functional Ingredient in Gluten-Free Extruded Snacks. Foods 2020, 9, 1770. [Google Scholar] [CrossRef]
- Edo, G.I.; Onoharigho, F.O.; Jikah, A.N.; Oloni, G.O.; Samuel, P.O.; Rapheal, O.A.; Ikpekoro, O.; Akpoghelie, P.O.; Agbo, J.J.; Ekokotu, H.A.; et al. Cyperus esculentus (tiger nut): An insight into its bioactive compounds, biological activities, nutritional and health benefits. Food Chem. Adv. 2023, 3, 100511. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, X.; Zhang, T.; Zhao, C.; Guan, S.; Pu, Y.; Gao, F. Tiger Nut (Cyperus esculentus L.): Nutrition, Processing, Function and Applications. Foods 2022, 11, 601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sabah, M.S.; Ahmed; Shaker, M.A.; Abbas, M.S.; Moursy, F.I. Nutritional value of tiger nut (Cyperus esculentus L.)tubers and its products. J. Biol. Chem. Environ. Sci. 2019, 14, 301–318. [Google Scholar]
- Nina, G.C.; Ogori, A.F.; Ukeyima, M.; Hleba, L.; Císarová, M.; Okuskhanova, E.; Vlasov, S.; Batishcheva, N.; Goncharov, A.; Shariati, M.A. Proximate, mineral and functional properties of tiger nut flour extracted from different tiger nuts cultivars. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 653–656. [Google Scholar] [CrossRef]
- Ani, C.P. Production, sensory and proximate evaluation of biscuit from blends of wheat, sweet potatoes and tiger nut flour. Int. J. Appl. Chem. Biol. Sci. 2021, 2, 111–117. [Google Scholar]
- Adelakun, O.E.; Oyinkansola, A.P. Oil Content and Fatty Acids Composition of Cookies Produced from Blends of Tigernut and Wheat Flour. Asian J. Agric. Food Sci. 2020, 8, 36–43. [Google Scholar] [CrossRef]
- Olędzki, R.; Harasym, J. Boiling vs. Microwave Heating—The Impact on Physicochemical Characteristics of Bell Pepper (Capsicum annuum L.) at Different Ripening Stages. Appl. Sci. 2023, 13, 8175. [Google Scholar] [CrossRef]
- Harasym, J.; Satta, E.; Kaim, U. Ultrasound Treatment of Buckwheat Grains Impacts Important Functional Properties of Resulting Flour. Molecules 2020, 25, 3012. [Google Scholar] [CrossRef]
- Djordjević, M.; Šoronja-Simović, D.; Nikolić, I.; Djordjević, M.; Šereš, Z.; Milašinović-Šeremešić, M. Sugar beet and apple fibres coupled with hydroxypropylmethylcellulose as functional ingredients in gluten-free formulations: Rheological, technological and sensory aspects. Food Chem. 2019, 295, 189–197. [Google Scholar] [CrossRef]
- Carpentieri, S.; Orkusz, A.; Ferrari, G.; Harasym, J. Effect of replacing durum wheat semolina with tenebrio molitor larvae powder on the techno-functional properties of the binary blends. Curr. Res. Food Sci. 2024, 8, 100672. [Google Scholar] [CrossRef]
- Lisovska, T.; Tyupova, A.; Olędzki, R.; Harasym, J. Microwave-Supported Modulation of Functional Characteristics of Gluten-Free Breads. Appl. Sci. 2023, 13, 12716. [Google Scholar] [CrossRef]
- Bhajan, C.; Soulange, J.G.; Sanmukhiya, V.M.R.; Olędzki, R.; Harasym, J. Phytochemical Composition and Antioxidant Properties of Tambourissa ficus, a Mauritian Endemic Fruit. Appl. Sci. 2023, 13, 10908. [Google Scholar] [CrossRef]
- Cotovanu, I.; Mironeasa, C.; Mironeasa, S. Incorporation of Buckwheat Flour at Different Particle Sizes and Distinctive Doses in Wheat Flour to Manufacture an Improved Wheat Bread. Foods 2023, 12, 1730. [Google Scholar] [CrossRef] [PubMed]
- Dahdah, P.; Cabizza, R.; Farbo, M.G.; Fadda, C.; Mara, A.; Hassoun, G.; Piga, A. Improving the Rheological Properties of Dough Obtained by Partial Substitution of Wheat Flour with Freeze-Dried Olive Pomace. Foods 2024, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Bojňanská, T.; Vollmannová, A.; Musilová, J. Milk thistle flour effect on dough rheological properties. Potravin. Slovak J. Food Sci. 2020, 14, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu-Iuga, M.; Atudorei, D.; Codină, G.G.; Mironeasa, S. Rheological Approaches of Wheat Flour Dough Enriched with Germinated Soybean and Lentil. Appl. Sci. 2021, 11, 11706. [Google Scholar] [CrossRef]
- Xu, Y.; Chall, C.; Manthey, F. Effect of Flaxseed Flour on Rheological Properties of Wheat Flour Dough and on Bread Characteristics. J. Food Res. 2014, 3. [Google Scholar] [CrossRef][Green Version]
- Bresciani, A.; Sergiacomo, A.; De Stefani, A.; Marti, A. Impact of Sprouted Chickpea Grits and Flour on Dough Rheology and Bread Features. Foods 2024, 13, 2698. [Google Scholar] [CrossRef]
- Aguilar, N.; Albanell, E.; Miñarro, B.; Capellas, M. Chickpea and Tiger Nut Flours As Alternatives To Emulsifier And Shortening In Gluten-Free Bread. LWT-Food Sci. Technol. 2015, 62, 225–232. [Google Scholar] [CrossRef]
- Kizzie-Hayford, N.; Abano, E.E.; Akanson, J.; Dankwa, E.; Rohm, H.; Ampofo-Asiama, J. Effects of sprouting duration on the nutrient, functional, and phytochemical properties of tiger nut flour, and the sensory properties of bread made thereof. J. Food Sci. 2023, 88, 3681–3693. [Google Scholar] [CrossRef]
- Naqvi, S.N.; Liaquat, M.; Kazmi, A.; Sammi, S.; Ali, A.; Luna-Arias, J.P.; Sherzad, I.U. Sesame-enriched delights: A comparative exploration of physicochemical and sensory attributes in fine and whole wheat flour cookies. Food Sci. Nutr. 2024, 12, 7751–7765. [Google Scholar] [CrossRef]
- Wang, M.; van Vliet, T.; Hamer, R.J. How gluten properties are affected by pentosans. J. Cereal Sci. 2004, 39, 395–402. [Google Scholar] [CrossRef]
- Mancebo, C.M.; San Miguel, M.Á.; Martínez, M.M.; Gómez, M. Optimisation of rheological properties of gluten-free doughs with HPMC, psyllium and different levels of water. J. Cereal Sci. 2021, 93, 102981. [Google Scholar] [CrossRef]
- Witczak, M.; Ziobro, R.; Juszczak, L.; Korus, J. Starch and starch derivatives in gluten-free systems—A review. J. Cereal Sci. 2022, 69, 371–386. [Google Scholar] [CrossRef]
- Martín-Esparza, M.E.; Raigón, M.D.; Raga, A.; Albors, A. Functional, Thermal and Rheological Properties of High Fibre Fresh Pasta: Effect of Tiger Nut Flour and Xanthan Gum Addition. Food Bioprocess Technol. 2018, 11, 2131–2141. [Google Scholar] [CrossRef]
- García-Ramón, F.; Sotelo-Méndez, A.; Alvarez-Chancasanampa, H.; Norabuena, E.; Sumarriva, L.; Yachi, K.; Gonzales Huamán, T.; Naupay Vega, M.; Cornelio-Santiago, H.P. Influence of Peruvian Andean grain flours on the nutritional, rheological, physical, and sensory properties of sliced bread. Front. Sustain. Food Syst. 2023, 7, 1202322. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, T.; Su, C.; Li, Q.; Yu, X. Fortification of Chinese steamed bread with flaxseed flour and evaluation of its physicochemical and sensory properties. Food Chem. X 2022, 13, 100267. [Google Scholar] [CrossRef]
- Wang, K.; Lu, F.; Li, Z.; Zhao, L.; Han, C. Recent developments in gluten-free bread baking approaches: A review. Food Chem. 2023, 404, 133396. [Google Scholar] [CrossRef]
- Rao, S.; Ashwath Kumar, K.; Indrani, D. Low carbohydrate high fat flour: Its rheology, bread making, physico-sensory and staling characteristics. J. Food Sci. Technol. 2022, 59, 2220–2230. [Google Scholar] [CrossRef]
| Phase | Parameters | Control | 5% | 10% | 15% | 20% | 25% |
|---|---|---|---|---|---|---|---|
| Dough development | Hm [mm] | 29.8 ± 0.8 e | 27.3 ± 1.3 d | 21.5 ± 0.2 c | 19.7 ± 0.4 ab | 19.2 ± 0.2 a | 21.2 ± 0.1 bc |
| h [mm] | 28.4 ± 0.5 e | 23.5 ± 0.3 d | 21.5 ± 0.2 c | 19.2 ± 0.4 ab | 18.5 ± 0.1 a | 19.7 ± 0.1 b | |
| (Hm-h)/Hm [%] | 4.9 ± 1.0 bc | 13.7 ± 3.2 d | 0.0 ± 0.0 a | 2.3 ± 0.4 ab | 4.9 ± 2.5 bc | 7.1 ± 0.6 c | |
| T1 [min] | 158.3 ± 9.5 cd | 132.5 ± 7.8 b | 180.0 ± 0.0 e | 160.0 ± 0.7 d | 145.8 ± 2.5 c | 103.0 ± 3.5 a | |
| Gas production | H′m [mm] | 32.0 ± 2.5 b | 33.6 ± 1.3 b | 30.0 ± 2.1 ab | 27.3 ± 0.7 a | 32.3 ± 0.3 b | 39.3 ± 0.5 c |
| T′1 [min] | 177.0 ± 0.0 c | 177.5 ± 3.5 c | 170.5 ± 6.4 bc | 179.0 ± 1.4 c | 161.0 ± 2.8 b | 120.5 ± 12.0 a | |
| Tx [min] | 146.0 ± 2.8 c | 126.0 ± 2.8 b | 147.5 ± 9.2 c | 146.0 ± 7.1 c | 126.0 ± 4.2 b | 80.0 ± 1.4 a | |
| Total volume [mL] | 652.5 ± 65.8 ab | 817.5 ± 40.3 d | 694.0 ± 42.4 bc | 603.5 ± 3.5 a | 777.0 ± 5.7 cd | 966.5 ± 4.9 e | |
| Volume of CO2 lost [mL] | 6.0 ± 1.4 a | 19.5 ± 0.7 b | 17.5 ± 2.1 b | 8.5 ± 0.7 a | 23.5 ± 3.5 b | 48.5 ± 6.4 c | |
| Volume of retention [mL] | 646.5 ± 64.3 a | 798.0 ± 39.6 c | 676.5 ± 44.5 ab | 595.5 ± 2.1 a | 756.0 ± 7.1 bc | 917.5 ± 10.6 d | |
| Retention coefficient [%] | 99.1 ± 0.0 c | 97.6 ± 0.0 b | 98.3 ± 0.6 bc | 98.6 ± 0.1 c | 97.4 ± 0.1 b | 95.0 ± 0.6 a |
| Sample | Days | Volume [mL] | Weight [g] | Specific Volume [mL/g] | Porosity [%] | Water Activity |
|---|---|---|---|---|---|---|
| Control | 0 | 236.3 ± 0.5 de | 104.9 ± 0.4 ab | 2.3 ± 0.1 c | 62.3 ± 5.0 bc | 0.972 ± 0.001 b |
| 7 | 233.2 ± 2.5 CD | 101.8 ± 0.4 BC | 2.3 ± 0.0 C | 62.2 ± 10.3 B | 0.976 ± 0.001 B | |
| 5% | 0 | 207.0 ± 2.5 c | 104.7 ± 0.5 ab | 2.0 ± 0.0 b | 64.3 ± 9.3 cd | 0.974 ± 0.001 b |
| 7 | 204.1 ± 4.4 B | 99.0 ± 0.5 A | 2.1 ± 0.1 B | 59.1 ± 3.8 B | 0.974 ± 0.001 BC | |
| 10% | 0 | 243.6 ± 3.2 e | 104.0 ± 0.3 a | 2.4 ± 0.1 c | 67.0 ± 5.0 d | 0.978 ± 0.001 c |
| 7 | 239.6 ± 4.8 DE | 99.6 ± 1.8 AB | 2.4 ± 0.0 C | 53.3 ± 1.9 A | 0.970 ± 0.001 AB | |
| 15% | 0 | 232.6 ± 4.2 d | 103.7 ± 0.6 a | 2.3 ± 0.1 c | 67.6 ± 8.2 d | 0.978 ± 0.001 c |
| 7 | 230.7 ± 3.9 C | 100.3 ± 0.5 ABC | 2.3 ± 0.0 C | 54.4 ± 2.9 A | 0.970 ± 0.004 AB | |
| 20% | 0 | 195.8 ± 2.3 b | 105.8 ± 0.8 b | 1.9 ± 0.1 a | 59.7 ± 8.4 b | 0.969 ± 0.001 a |
| 7 | 195.4 ± 1.7 A | 100.7 ± 1.5 ABC | 2.0 ± 0.1 AB | 54.4 ± 2.6 A | 0.968 ± 0.003 A | |
| 25% | 0 | 186.8 ± 4.2 a | 104.9 ± 0.1 ab | 1.8 ± 0.0 a | 48.0 ± 2.2 a | 0.9675 ± 0.001 a |
| 7 | 188.5 ± 2.9 A | 102.2 ± 0.3 C | 1.9 ± 0.1 A | 55.0 ± 1.5 A | 0.968 ± 0.004 A | |
| Sample | *** | * | *** | *** | *** | |
| Days | ns | *** | * | *** | * | |
| Sample × days | ns | ns | ns | *** | ** | |
| Sample | Day | Hardness [N] | Cohesiveness | Springiness | Chewiness [N] | Resilience |
|---|---|---|---|---|---|---|
| Control | 0 | 8.14 ± 1.20 bc | 0.781 ± 0.045 c | 0.845 ± 0.065 c | 5.345 ± 0.723 c | 0.50 ± 0.04 e |
| 7 | 23.99 ± 5.27 B | 0.598 ± 0.198 B | 0.727 ± 0.057 D | 10.137 ± 3.417 C | 0.37 ± 0.10 AB | |
| 5% | 0 | 10.14 ± 2.25 cd | 0.734 ± 0.098 c | 0.777 ± 0.101 bc | 5.773 ± 1.499 c | 0.42 ± 0.05 a |
| 7 | 27.56 ± 5.73 B | 0.439 ± 0.030 A | 0.687 ± 0.047 CD | 8.285 ± 1.730 BC | 0.28 ± 0.03 A | |
| 10% | 0 | 5.48 ± 1.40 a | 0.668 ± 0.030 b | 0.721 ± 0.031 ab | 2.620 ± 0.564 a | 0.40 ± 0.03 ab |
| 7 | 14.56 ± 4.63 A | 0.494 ± 0.175 AB | 0.685 ± 0.081 CD | 4.684 ± 1.252 A | 0.43 ± 0.26 B | |
| 15% | 0 | 6.38 ± 1.93 ab | 0.646 ± 0.037 b | 0.719 ± 0.116 ab | 2.904 ± 0.851 a | 0.37 ± 0.04 bc |
| 7 | 17.41 ± 3.92 A | 0.428 ± 0.029 A | 0.640 ± 0.047 BC | 4.839 ± 1.480 A | 0.28 ± 0.03 A | |
| 20% | 0 | 10.08 ± 2.04 cd | 0.585 ± 0.013 a | 0.695 ± 0.054 ab | 4.094 ± 0.897 b | 0.32 ± 0.01 cd |
| 7 | 25.44 ± 1.58 B | 0.416 ± 0.025 A | 0.546 ± 0.114 A | 5.744 ± 1.094 A | 0.28 ± 0.03 A | |
| 25% | 0 | 11.00 ± 1.38 d | 0.572 ± 0.025 a | 0.671 ± 0.077 a | 4.208 ± 0.600 b | 0.34 ± 0.03 d |
| 7 | 26.19 ± 2.03 B | 0.399 ± 0.028 A | 0.599 ± 0.029 AB | 6.288 ± 0.936 AB | 0.28 ± 0.03 A | |
| Sample | *** | *** | *** | *** | *** | |
| Day | *** | *** | *** | *** | *** | |
| Sample × day | * | ns | ns | ns | ns | |
| Sample | Days | Crumb | Crust | ||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | ||
| Control | 0 | 73.18 ± 1.17 d | 0.3 ± 0.1 a | 16.8 ± 0.3 a | 77.14 ± 2.59 e | 5.8 ± 1.0 a | 27.9 ± 2.3 a |
| 7 | 75.09 ± 1.77 C | 0.3 ± 0.1 A | 13.6 ± 0.5 A | 77.44 ± 1.33 E | 5.2 ± 0.8 A | 23.4 ± 2.3 A | |
| 5% | 0 | 68.74 ± 1.65 c | 1.5 ± 0.3 b | 17.1 ± 0.4 ab | 69.48 ± 2.21 d | 9.3 ± 1.0 b | 28.9 ± 1.5 a |
| 7 | 70.69 ± 1.16 B | 1.0 ± 0.2 B | 14.5 ± 0.9 B | 69.05 ± 2.97 D | 8.5 ± 1.8 B | 26.2 ± 3.0 B | |
| 10% | 0 | 66.83 ± 2.03 b | 1.7 ± 0.3 b | 17.3 ± 0.8 ab | 64.14 ± 0.79 c | 12.0 ± 0.2 d | 31.2 ± 0.4 b |
| 7 | 67.46 ± 1.24 A | 1.4 ± 0.3 C | 15.5 ± 0.5 C | 63.15 ± 2.16 C | 12.0 ± 0.9 C | 30.6 ± 1.1 C | |
| 15% | 0 | 64.89 ± 1.75 a | 2.2 ± 0.5 c | 17.4 ± 0.6 ab | 63.70 ± 2.16 c | 11.9 ± 1.1 c | 30.6 ± 1.0 b |
| 7 | 67.74 ± 2.99 A | 1.7 ± 0.6 D | 15.1 ± 0.8 BC | 62.83 ± 3.21 C | 11.9 ± 1.3 C | 30.3 ± 1.0 C | |
| 20% | 0 | 64.51 ± 1.39 a | 2.5 ± 0.4 cd | 17.7 ± 0.5 b | 59.46 ± 2.52 b | 13.3 ± 0.9 e | 30.7 ± 0.9 b |
| 7 | 66.04 ± 2.13 A | 2.3 ± 0.3 E | 16.8 ± 0.4 D | 59.78 ± 1.90 B | 12.8 ± 0.6 C | 30.3 ± 1.1 C | |
| 25% | 0 | 65.15 ± 1.10 a | 2.5 ± 0.4 d | 17.1 ± 1.2 ab | 54.95 ± 3.15 a | 14.3 ± 1.0 f | 30.4 ± 0.6 b |
| 7 | 66.69 ± 2.19 A | 2.1 ± 0.3 E | 15.6 ± 1.1 C | 54.23 ± 2.20 A | 14.3 ± 0.8 D | 30.2 ± 0.8 C | |
| Sample | *** | *** | *** | *** | *** | *** | |
| Day | *** | *** | *** | ns | ns | *** | |
| Sample × day | ns | ns | *** | ns | ns | *** | |
| Sample | Crust mg Glu/g DM | Crumb mg Glu/g DM |
|---|---|---|
| Control | 7.13 ± 0.04 b | 7.07 ± 0.15 a |
| 5% | 3.21 ± 0.13 a | 7.93 ± 0.11 ab |
| 10% | 9.43 ± 0.23 c | 8.55 ± 0.10 b |
| 15% | 9.64 ± 0.02 c | 13.29 ± 0.52 d |
| 20% | 10.89 ± 0.37 d | 10.59 ± 0.66 c |
| 25% | 13.36 ± 0.45 e | 9.67 ± 0.61 c |
| Sample | Crumb | Crust |
|---|---|---|
| mg GA/100 g DM | ||
| Control | 5.16 ± 0.03 ab | 2.86 ± 2.02 a |
| 5% | 4.29 ± 0.78 a | 2.75 ± 1.41 a |
| 10% | 6.16 ± 1.74 ab | 3.57 ± 0.01 a |
| 15% | 7.88 ± 0.26 b | 4.10 ± 0.30 a |
| 20% | 7.07 ± 2.26 ab | 4.32 ± 0.01 a |
| 25% | 5.85 ± 0.11 ab | 4.24 ± 0.08 a |
| Sample | Solvent | TE mg/g DM | |
|---|---|---|---|
| Crumb | Crust | ||
| Control | E | 15.31 ± 4.33 a | 31.41 ± 3.30 b |
| W | 15.68 ± 1.85 a | 72.48 ± 1.92 d | |
| 5% | E | 11.90 ± 2.64 a | 13.80 ± 1.62 a |
| W | 23.81 ± 1.12 b | 6.49 ± 1.02 a | |
| 10% | E | 37.96 ± 14.96 b | 14.51 ± 0.82 a |
| W | 25.64 ± 1.05 bc | 7.72 ± 0.52 a | |
| 15% | E | 43.47 ± 0.86 b | 34.16 ± 4.83 b |
| W | 28.01 ± 1.68 c | 20.31 ± 2.72 b | |
| 20% | E | 51.08 ± 5.42 b | 60.93 ± 9.76 c |
| W | 75.86 ± 1.42 d | 49.66 ± 2.60 c | |
| 25% | E | 111.31 ± 2.53 c | 111.59 ± 3.22 d |
| W | 97.66 ± 0.95 e | 114.24 ± 9.12 d | |
| Part | ns | ||
| Sample | *** | ||
| Solvent | ns | ||
| Sample × part | *** | ||
| Part × solvent | ns | ||
| Sample × solvent | *** | ||
| Sample × solvent × part | *** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedviha, S.; Harasym, J. Characteristics of Soft Wheat and Tiger Nut (Cyperus esculentus) Composite Flour Bread. Foods 2025, 14, 229. https://doi.org/10.3390/foods14020229
Nedviha S, Harasym J. Characteristics of Soft Wheat and Tiger Nut (Cyperus esculentus) Composite Flour Bread. Foods. 2025; 14(2):229. https://doi.org/10.3390/foods14020229
Chicago/Turabian StyleNedviha, Svitlana, and Joanna Harasym. 2025. "Characteristics of Soft Wheat and Tiger Nut (Cyperus esculentus) Composite Flour Bread" Foods 14, no. 2: 229. https://doi.org/10.3390/foods14020229
APA StyleNedviha, S., & Harasym, J. (2025). Characteristics of Soft Wheat and Tiger Nut (Cyperus esculentus) Composite Flour Bread. Foods, 14(2), 229. https://doi.org/10.3390/foods14020229








