Natural Food Colorant Obtained from Wild Berberis vulgaris L. by Ultrasound-Assisted Extraction: Optimization and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Determination of the Optimal Extraction Conditions Through RSM
2.2.1. Experimental Design
2.2.2. Ultrasound-Assisted Extraction Process
2.2.3. Response Criteria Used to Evaluate the Extraction Process
2.2.4. Mathematical Model: Response Surface Methodology (RSM)
2.3. Analytical Determinations
2.3.1. Physical–Chemical Analysis
2.3.2. Color Characterization: CIELAB Parameters
2.3.3. Anthocyanin Characterization: Total Monomeric Anthocyanin and Individual Anthocyanin Profile
Determination of Total Monomeric Anthocyanin Content
Individual Anthocyanin Profile
2.4. Determination of Total Polyphenols and Phenolic Families by QUENCHER Methodology
2.4.1. Determination of Total Polyphenols by QUENCHER Methodology
2.4.2. Determination of Hydroxybenzoic Acids by QUENCHER Methodology
2.4.3. Determination of Hydroxycinnamic Acids by QUENCER Methodology
2.4.4. Determination of Flavonols by QUENCHER Methodology
2.5. Bioactive Properties of Berberis vulgaris and Its Optimized Extract
2.5.1. Antioxidant Activity
Folin–Ciocalteu Assay
DPPH Assay
Antihemolytic Activity: OxHLIA Assay
2.5.2. Antibacterial Activity
2.5.3. Antifungal Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Berberis vulgaris Fresh Fruit Sample (BVF)
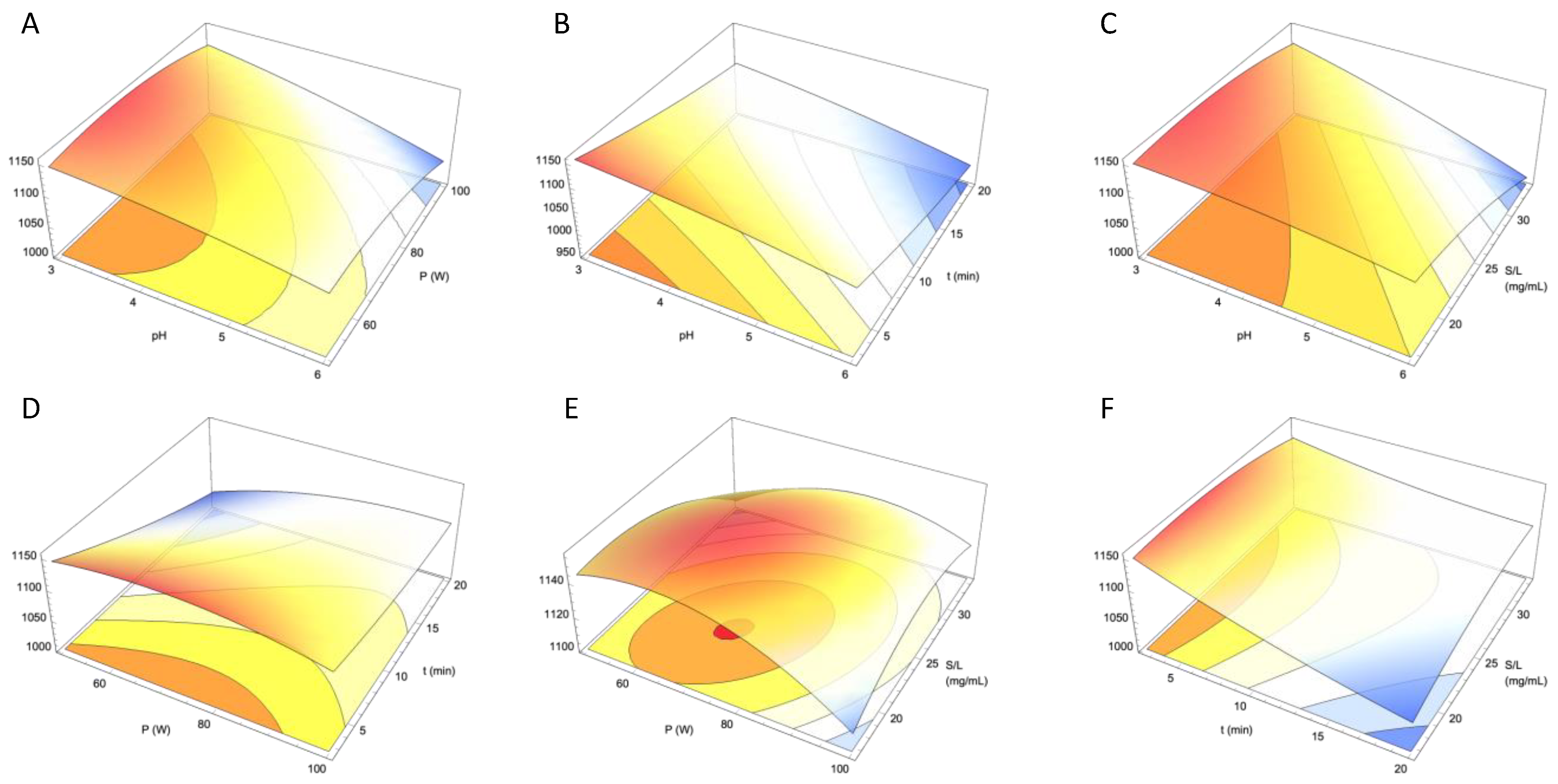
3.2. Determination of the Optimal Extraction Conditions Through RSM
3.3. Anthocyanin Characterization in Berberis vulgaris Freeze-Dried Fruit Fraction (BVFF) and Optimized Colorant Extract (BVE)
3.4. Total Polyphenols and Phenolic Families in Berberis vulgaris Freeze-Dried Fruit Fraction (BVFF) and Optimized Colorant Extract (BVE)
3.5. Bioactive Properties of B. vulgaris Freeze-Dried Fraction Fruit Sample (BVFF) and Its Optimized Colorant Extract (BVE)
3.5.1. Antioxidant Activity
3.5.2. Antibacterial Activity
3.5.3. Antifungal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spence, C. On the Manipulation, and Meaning(s), of Color in Food: A Historical Perspective. J. Food Sci. 2022, 88, A5–A20. [Google Scholar] [CrossRef] [PubMed]
- EFSA Food Colours. Available online: https://www.efsa.europa.eu/en/topics/topic/food-colours (accessed on 29 January 2024).
- Vega, E.N.; Ciudad-Mulero, M.; Fernández-Ruiz, V.; Barros, L.; Morales, P. Natural Sources of Food Colorants as Potential Substitutes for Artificial Additives. Foods 2023, 12, 4102. [Google Scholar] [CrossRef] [PubMed]
- Feingold, B.F. Hyperkinesis and Learning Disabilities Linked to the Lngestion of Artificial Food Colors and Flavors. J. Learn. Disabil. 1976, 9, 551–557. [Google Scholar] [CrossRef]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food Additives and Hyperactive Behaviour in 3-Year-Old and 8/9-Year-Old Children in the Community: A Randomised, Double-Blinded, Placebo-Controlled Trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Amin, K.A.; Fawzia, S.A.-S. Toxicological and Safety Assessment of Tartrazine as a Synthetic Food Additive on Health Biomarkers: A Review. Afr. J. Biotechnol. 2018, 17, 139–149. [Google Scholar] [CrossRef]
- Rahimi-Madiseh, M.; Lorigoini, Z.; Zamani-gharaghoshi, H.; Rafieian-kopaei, M. Berberis vulgaris: Specifications and Traditional Uses. Iran. J. Basic. Med. Sci. 2017, 20, 569–587. [Google Scholar]
- Yang, L.; Zhang, Z.; Hu, X.; You, L.; Khan, R.A.A.; Yu, Y. Phenolic Contents, Organic Acids, and the Antioxidant and Bio Activity of Wild Medicinal Berberis Plants- as Sustainable Sources of Functional Food. Molecules 2022, 27, 2497. [Google Scholar] [CrossRef]
- Akbulut, M.; Çališir, S.; Marakoglu, T.; Çoklar, H. Some Physicomechanical and Nutritional Properties of Barberry (Berberis vulgaris L.) Fruits. J. Food Process Eng. 2009, 32, 497–511. [Google Scholar] [CrossRef]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and Therapeutic Effects of Berberis vulgaris and Its Active Constituent, Berberine. Phytother. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Protocol for the Scientific Opinion on the Evaluation of the Safety in Use of Plant Preparations Containing Berberine; (EFSA) Supporting Publications; Wiley: Hoboken, NJ, USA, 2023; Volume 20. [Google Scholar] [CrossRef]
- Khoshandam, A.; Imenshahidi, M.; Hosseinzadeh, H. Pharmacokinetic of Berberine, the Main Constituent of Berberis vulgaris L.: A Comprehensive Review. Phytother. Res. 2022, 36, 4063–4079. [Google Scholar] [CrossRef]
- Hanachi, P. Using HPLC to Determination the Composition and Antioxidant Activity of Berberis vulgaris. Eur. J. Sci. Res. 2009, 29, 47–54. [Google Scholar]
- Bonfigli, M.; Godoy, E.; Reinheimer, M.A.; Scenna, N.J. Comparison between Conventional and Ultrasound-Assisted Techniques for Extraction of Anthocyanins from Grape Pomace. Experimental Results and Mathematical Modeling. J. Food Eng. 2017, 207, 56–72. [Google Scholar] [CrossRef]
- Rodríguez, L.; Plaza, A.; Méndez, D.; Carrasco, B.; Tellería, F.; Palomo, I.; Fuentes, E. Antioxidant Capacity and Antiplatelet Activity of Aqueous Extracts of Common Bean (Phaseolus vulgaris L.) Obtained with Microwave and Ultrasound Assisted Extraction. Plants 2022, 11, 1179. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- Loughrey, K. Overview of Color Analysis. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2002; Volume 3, pp. F51.1–F51.13. [Google Scholar]
- Gonnet, J.-F. Colour Effects of Co-Pigmentation of Anthocyanins Revisited—1. A Colorimetric Definition Using the CIELAB Scale. Food Chem. 1998, 63, 409–415. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Del Pino-García, R.; García-Lomillo, J.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. Adaptation and Validation of QUick, Easy, New, CHEap, and Reproducible (QUENCHER) Antioxidant Capacity Assays in Model Products Obtained from Residual Wine Pomace. J. Agric. Food Chem. 2015, 63, 6922–6931. [Google Scholar] [CrossRef]
- Fogliano, V.; Maria Monti, S.; Musella, T.; Randazzo, G.; Ritieni, A. Formation of Coloured Maillard Reaction Products in a Gluten-Glucose Model System. Food Chem. 1999, 66, 293–299. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Soares, A.A.; Correa, R.C.G.; Barros, L.; Haminiuk, C.W.I.; Peralta, R.M.; Ferreira, I.C.F.R.; Bracht, A. Merlot Grape Pomace Hydroalcoholic Extract Improves the Oxidative and Inflammatory States of Rats with Adjuvant-Induced Arthritis. J. Funct. Foods 2017, 33, 408–418. [Google Scholar] [CrossRef]
- Vega, E.N.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Morales, P. A Rapid and Simple UHPLC- DAD Method for Individual Anthocyanin Analysis: Optimization and Validation in Wild Mediterranean Berries. Food Anal. Methods 2024, 1–16. [Google Scholar] [CrossRef]
- Medina, M.B. Determination of the Total Phenolics in Juices and Superfruits by a Novel Chemical Method. J. Funct. Foods 2011, 3, 79–87. [Google Scholar] [CrossRef]
- Palombini, S.V.; Claus, T.; Maruyama, S.A.; Carbonera, F.; Montanher, P.F.; Visentainer, J.V.; Gomes, S.T.M.; Matsushita, M. Optimization of a New Methodology for Determination of Total Phenolic Content in Rice Employing Fast Blue BB and QUENCHER Procedure. J. Braz. Chem. Soc. 2016, 27, 1188–1194. [Google Scholar] [CrossRef]
- Vega, E.N.; García-Herrera, P.; Ciudad-Mulero, M.; Dias, M.I.; Matallana-González, M.C.; Cámara, M.; Tardío, J.; Molina, M.; Pinela, J.; Pires, T.C.; et al. Wild Sweet Cherry, Strawberry and Bilberry as Underestimated Sources of Natural Colorants and Bioactive Compounds with Functional Properties. Food Chem. 2023, 414, 135669. [Google Scholar] [CrossRef]
- Bonoli, M.; Verardo, V.; Marconi, E.; Caboni, M.F. Antioxidant Phenols in Barley (Hordeum vulgare L.) Flour: Comparative Spectrophotometric Study among Extraction Methods of Free and Bound Phenolic Compounds. J. Agric. Food Chem. 2004, 52, 5195–5200. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical Features and Bioactivities of Cornflower (Centaurea cyanus L.) Capitula: The Blue Flowers and the Unexplored Non-Edible Part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and Antimicrobial Properties of Dried Portuguese Apple Variety (Malus domestica Borkh. Cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, I.C.F.R.; Esteves, A.P.; Ćirić, A.; Glamočlija, J.; Martins, A.; Soković, M.; Queiroz, M.J.R.P. Antimicrobial and Demelanizing Activity of Ganoderma lucidum Extract, p-Hydroxybenzoic and Cinnamic Acids and Their Synthetic Acetylated Glucuronide Methyl Esters. Food Chem. Toxicol. 2013, 58, 95–100. [Google Scholar] [CrossRef]
- Eroğlu, A.Y.; Çakır, Ö.; Sağdıç, M.; Dertli, E. Bioactive Characteristics of Wild Berberis vulgaris and Berberis crataegina Fruits. J. Chem. 2020, 2020, 8908301. [Google Scholar] [CrossRef]
- Özgen, M.; Saraçoğlu, O.; Geçer, E.N. Antioxidant Capacity and Chemical Properties of Selected Barberry (Berberis vulgaris L.) Fruits. Hortic. Environ. Biotechnol. 2012, 53, 447–451. [Google Scholar] [CrossRef]
- Yildiz, H.; Ercisli, S.; Sengul, M.; Topdas, E.F.; Beyhan, O.; Cakir, O.; Narmanlioglu, H.K.; Orhan, E. Some Physicochemical Characteristics, Bioactive Content and Antioxidant Characteristics of Non-Sprayed Barberry (Berberis vulgaris L.) Fruits from Turkey. Erwerbs-Obstbau 2014, 56, 123–129. [Google Scholar] [CrossRef]
- Moghaddam, P.R.; Fallahi, J.; Shajari, M.A.; Mahallati, M.N. Effects of Harvest Date, Harvest Time, and Post-Harvest Management on Quantitative and Qualitative Traits in Seedless Barberry (Berberis vulgaris L.). Ind. Crops Prod. 2013, 42, 30–36. [Google Scholar] [CrossRef]
- Hoshyar, R.; Mahboob, Z.; Zarban, A. The Antioxidant and Chemical Properties of Berberis vulgaris and Its Cytotoxic Effect on Human Breast Carcinoma Cells. Cytotechnology 2016, 68, 1207–1213. [Google Scholar] [CrossRef]
- Barba, F.J.; Rajha, H.N.; Debs, E.; Abi-Khattar, A.-M.; Khabbaz, S.; Dar, B.N.; Simirgiotis, M.J.; Castagnini, J.M.; Maroun, R.G.; Louka, N. Optimization of Polyphenols’ Recovery from Purple Corn Cobs Assisted by Infrared Technology and Use of Extracted Anthocyanins as a Natural Colorant in Pickled Turnip. Molecules 2022, 27, 5222. [Google Scholar] [CrossRef]
- Avila, L.B.; Barreto, E.R.C.; Moraes, C.C.; Morais, M.M.; Rosa, G.S. da Promising New Material for Food Packaging: An Active and Intelligent Carrageenan Film with Natural Jaboticaba Additive. Foods 2022, 11, 792. [Google Scholar] [CrossRef]
- Milea, A.Ș.; Vasile, A.M.; Cîrciumaru, A.; Dumitrașcu, L.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Valorizations of Sweet Cherries Skins Phytochemicals by Extraction, Microencapsulation and Development of Value-Added Food Products. Foods 2019, 8, 188. [Google Scholar] [CrossRef]
- da Fonseca Machado, A.P.; Alves Rezende, C.; Alexandre Rodrigues, R.; Fernández Barbero, G.; de Tarso Vieira e Rosa, P.; Martínez, J. Encapsulation of Anthocyanin-Rich Extract from Blackberry Residues by Spray-Drying, Freeze-Drying and Supercritical Antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Sharifi, A.; Khoshnoudi-Nia, S. Ranking Novel Extraction Systems of Seedless Barberry (Berberis vulgaris) Bioactive Compounds with Fuzzy Logic-Based Term Weighting Scheme. Sustain. Chem. Pharm. 2022, 25, 100561. [Google Scholar] [CrossRef]
- Gil-Martínez, L.; Mut-Salud, N.; Ruiz-García, J.A.; Falcón-Piñeiro, A.; Maijó-Ferré, M.; Baños, A.; De la Torre-Ramírez, J.M.; Guillamón, E.; Verardo, V.; Gómez-Caravaca, A.M. Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruits. Foods 2023, 12, 1505. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; Oleszek, W.; Braca, A. Quali-Quantitative Analyses of Flavonoids of Morus nigra L. and Morus alba L. (Moraceae) Fruits. J. Agric. Food Chem. 2008, 56, 3377–3380. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Tomas, M.; Tekin-Çakmak, Z.H.; Karusu, S. Berberis crataegina DC. as a Novel Natural Food Colorant Source: Ultrasound-Assisted Extraction Optimization Using Response Surface Methodology and Thermal Stability Studies. Food Sci. Technol. 2022, 42, e13421. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of Anthocyanins from Grape By-Products Assisted by Ultrasonics, High Hydrostatic Pressure or Pulsed Electric Fields: A Comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Moldovan, C.; Frumuzachi, O.; Babotă, M.; Menghini, L.; Cesa, S.; Gavan, A.; Sisea, C.R.; Tanase, C.; Dias, M.I.; Pereira, C.; et al. Development of an Optimized Drying Process for the Recovery of Bioactive Compounds from the Autumn Fruits of Berberis vulgaris L. and Crataegus monogyna Jacq. Antioxidants 2021, 10, 1579. [Google Scholar] [CrossRef]
- Gıdık, B. Antioxidant, Antimicrobial Activities and Fatty Acid Compositions of Wild Berberis Spp. by Different Techniques Combined with Chemometrics (PCA and HCA). Molecules 2021, 26, 7448. [Google Scholar] [CrossRef]
- Motalleb, G.; Hanachi, P.; Kua, P.; Fauziah, S.H.; Asmah, R. Evaluation of Phenolic Content and Total Antioxidant Activity in Berberis vulgaris Fruit Extract. Asian Netw. Sci. Inf. 2005, 5, 648–653. [Google Scholar]
- Drăghici-Popa, A.-M.; Boscornea, A.C.; Brezoiu, A.-M.; Tomas, Ș.T.; Pârvulescu, O.C.; Stan, R. Effects of Extraction Process Factors on the Composition and Antioxidant Activity of Blackthorn (Prunus spinosa L.) Fruit Extracts. Antioxidants 2023, 12, 1897. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry Phenolics and Their Antioxidant Activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef]
- Rocha, R.; Pinela, J.; Abreu, R.M.V.; Añibarro-Ortega, M.; Pires, T.C.S.P.; Saldanha, A.L.; Alves, M.J.; Nogueira, A.; Ferreira, I.C.F.R.; Barros, L. Extraction of Anthocyanins from Red Raspberry for Natural Food Colorants Development: Processes Optimization and In Vitro Bioactivity. Processes 2020, 8, 1447. [Google Scholar] [CrossRef]
- Kazemipoor, M.; Fadaei Tehrani, P.; Zandi, H.; Golvardi Yazdi, R. Chemical Composition and Antibacterial Activity of Berberis vulgaris (Barberry) against Bacteria Associated with Caries. Clin. Exp. Dent. Res. 2021, 7, 601–608. [Google Scholar] [CrossRef]
- Ghada, B.; Pereira, E.; Pinela, J.; Prieto, M.A.; Pereira, C.; Calhelha, R.C.; Stojković, D.; Sokóvić, M.; Zaghdoudi, K.; Barros, L.; et al. Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties. Molecules 2020, 25, 3203. [Google Scholar] [CrossRef]

| Location 1 | Location 2 | |
|---|---|---|
 |  | |
| Location | Dehesa de Carrascosa, Cuenca (Spain) | Laguna de El Tobar Beteta, Cuenca (Spain) |
| Latitude | 40°35′14.8″ N | 40°32′21.1″ N |
| Longitude | 2°08′49.9″ W | 2°03′19.5″ W |
| Code | pH | P (W) | t (min) | S/L (mg/mL) | Code | pH | P (W) | t (min) | S/L (mg/mL) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 250 | 2.5 | 33.33 | 31 | 6 | 250 | 2.5 | 33.33 |
| 2 | 3 | 250 | 5 | 33.33 | 32 | 6 | 250 | 5 | 33.33 |
| 3 | 3 | 250 | 10 | 33.33 | 33 | 6 | 250 | 10 | 33.33 |
| 4 | 3 | 250 | 15 | 33.33 | 34 | 6 | 250 | 15 | 33.33 |
| 5 | 3 | 250 | 20 | 33.33 | 35 | 6 | 250 | 20 | 33.33 |
| 6 | 3 | 400 | 2.5 | 33.33 | 36 | 6 | 400 | 2.5 | 33.33 |
| 7 | 3 | 400 | 5 | 33.33 | 37 | 6 | 400 | 5 | 33.33 |
| 8 | 3 | 400 | 10 | 33.33 | 38 | 6 | 400 | 10 | 33.33 |
| 9 | 3 | 400 | 15 | 33.33 | 39 | 6 | 400 | 15 | 33.33 |
| 10 | 3 | 400 | 20 | 33.33 | 40 | 6 | 400 | 20 | 33.33 |
| 11 | 3 | 500 | 2.5 | 33.33 | 41 | 6 | 500 | 2.5 | 33.33 |
| 12 | 3 | 500 | 5 | 33.33 | 42 | 6 | 500 | 5 | 33.33 |
| 13 | 3 | 500 | 10 | 33.33 | 43 | 6 | 500 | 10 | 33.33 |
| 14 | 3 | 500 | 15 | 33.33 | 44 | 6 | 500 | 15 | 33.33 |
| 15 | 3 | 500 | 20 | 33.33 | 45 | 6 | 500 | 20 | 33.33 |
| 16 | 3 | 250 | 2.5 | 16.66 | 46 | 6 | 250 | 2.5 | 16.66 |
| 17 | 3 | 250 | 5 | 16.66 | 47 | 6 | 250 | 5 | 16.66 |
| 18 | 3 | 250 | 10 | 16.66 | 48 | 6 | 250 | 10 | 16.66 |
| 19 | 3 | 250 | 15 | 16.66 | 49 | 6 | 250 | 15 | 16.66 |
| 20 | 3 | 250 | 20 | 16.66 | 50 | 6 | 250 | 20 | 16.66 |
| 21 | 3 | 400 | 2.5 | 16.66 | 51 | 6 | 400 | 2.5 | 16.66 |
| 22 | 3 | 400 | 5 | 16.66 | 52 | 6 | 400 | 5 | 16.66 |
| 23 | 3 | 400 | 10 | 16.66 | 53 | 6 | 400 | 10 | 16.66 |
| 24 | 3 | 400 | 15 | 16.66 | 54 | 6 | 400 | 15 | 16.66 |
| 25 | 3 | 400 | 20 | 16.66 | 55 | 6 | 400 | 20 | 16.66 |
| 26 | 3 | 500 | 2.5 | 16.66 | 56 | 6 | 500 | 2.5 | 16.66 |
| 27 | 3 | 500 | 5 | 16.66 | 57 | 6 | 500 | 5 | 16.66 |
| 28 | 3 | 500 | 10 | 16.66 | 58 | 6 | 500 | 10 | 16.66 |
| 29 | 3 | 500 | 15 | 16.66 | 59 | 6 | 500 | 15 | 16.66 |
| 30 | 3 | 500 | 20 | 16.66 | 60 | 6 | 500 | 20 | 16.66 |
| Independent Variables Evaluated | |||||||
|---|---|---|---|---|---|---|---|
| Code | TAC (mg cya-3-glu/g) | L* | a* | b* | C* | h | RGB |
| 1 | 10.58 ± 0.04 | 22.79 ± 0.93 | 47.32 ± 0.96 | 3.72 ± 0.44 | 47.48 ± 0.68 | 4.50 ± 0.69 | |
| 2 | 10.07 ± 0.12 | 22.16 ± 1.13 | 46.96 ± 1.42 | 4.81 ± 0.63 | 47.21 ± 1.01 | 5.87 ± 1.08 | |
| 3 | 10.79 ± 0.02 | 22.17 ± 1.69 | 46.89 ± 0.89 | 4.76 ± 1.36 | 47.16 ± 0.52 | 5.81 ± 1.62 | |
| 4 | 9.87 ± 0.36 | 22.70 ± 0.01 | 46.77 ± 0.95 | 4.35 ± 0.46 | 46.98 ± 0.80 | 5.30 ± 0.90 | |
| 5 | 10.76 ± 0.38 | 22.93 ± 0.22 | 46.35 ± 1.05 | 3.91 ± 0.20 | 46.51 ± 0.82 | 4.82 ± 0.59 | |
| 6 | 11.03 ± 0.09 | 22.15 ± 1.16 | 47.21 ± 1.08 | 4.83 ± 0.43 | 47.46 ± 0.76 | 5.85 ± 0.66 | |
| 7 | 10.96 ± 0.01 | 21.83 ± 1.72 | 46.71 ± 1.56 | 5.20 ± 1.23 | 47.03 ± 1.04 | 6.39 ± 1.70 | |
| 8 | 11.25 ± 0.04 | 22.28 ± 1.29 | 47.57 ± 1.03 | 4.32 ± 0.61 | 47.79 ± 0.70 | 5.21 ± 0.78 | |
| 9 | 11.00 ± 0.01 | 22.00 ± 1.77 | 46.80 ± 0.82 | 4.83 ± 1.50 | 47.08 ± 0.44 | 5.91 ± 1.90 | |
| 10 | 10.89 ± 0.09 | 22.17 ± 1.25 | 46.41 ± 0.05 | 5.06 ± 0.63 | 46.70 ± 0.19 | 6.22 ± 0.87 | |
| 11 | 12.17 ± 0.75 | 21.63 ± 1.79 | 47.18 ± 1.48 | 5.41 ± 1.10 | 47.53 ± 0.95 | 6.58 ± 1.50 | |
| 12 | 11.96 ± 0.63 | 21.46 ± 1.86 | 46.93 ± 1.20 | 5.79 ± 1.37 | 47.33 ± 0.68 | 7.07 ± 1.81 | |
| 13 | 11.44 ± 0.17 | 22.22 ± 1.60 | 46.88 ± 0.42 | 4.58 ± 1.07 | 47.12 ± 0.21 | 5.59 ± 1.34 | |
| 14 | 11.35 ± 0.40 | 21.79 ± 2.01 | 47.04 ± 1.29 | 5.66 ± 2.03 | 47.43 ± 0.73 | 6.90 ± 0.63 | |
| 15 | 11.56 ± 0.81 | 21.23 ± 1.50 | 46.67 ± 1.03 | 7.15 ± 0.69 | 47.22 ± 0.63 | 8.72 ± 1.01 | |
| 16 | 12.15 ± 0.31 | 29.04 ± 1.76 | 46.10 ± 0.03 | −5.75 ± 0.64 | 46.47 ± 0.31 | −7.10 ± 0.76 | |
| 17 | 11.69 ± 0.06 | 29.01 ± 1.73 | 46.44 ± 0.51 | −5.76 ± 0.84 | 46.81 ± 0.58 | −7.06 ± 1.02 | |
| 18 | 11.58 ± 0.38 | 28.95 ± 2.49 | 46.31 ± 0.72 | −5.45 ± 0.95 | 46.65 ± 0.51 | −6.72 ± 1.33 | |
| 19 | 10.68 ± 0.07 | 29.73 ± 1.18 | 45.52 ± 0.20 | −6.53 ± 0.41 | 45.99 ± 0.32 | −8.16 ± 0.74 | |
| 20 | 10.09 ± 0.47 | 30.13 ± 1.64 | 45.06 ± 0.48 | −6.70 ± 0.86 | 45.56 ± 0.48 | −8.46 ± 1.04 | |
| 21 | 11.35 ± 0.31 | 28.28 ± 1.02 | 46.90 ± 0.20 | −5.55 ± 0.75 | 47.23 ± 0.27 | −6.74 ± 0.96 | |
| 22 | 10.60 ± 0.01 | 29.29 ± 1.55 | 45.88 ± 0.13 | −6.31 ± 0.80 | 46.31 ± 0.34 | −7.83 ± 0.93 | |
| 23 | 11.21 ± 0.60 | 29.55 ± 1.52 | 45.29 ± 0.23 | −6.16 ± 0.50 | 45.71 ± 0.24 | −7.75 ± 0.66 | |
| 24 | 10.28 ± 0.52 | 30.25 ± 1.23 | 44.42 ± 0.14 | −6.23 ± 0.45 | 44.86 ± 0.29 | −7.98 ± 0.56 | |
| 25 | 10.43 ± 0.90 | 29.35 ± 0.45 | 45.64 ± 1.33 | −6.16 ± 0.36 | 46.06 ± 1.11 | −7.68 ± 0.50 | |
| 26 | 10.65 ± 0.18 | 28.40 ± 1.15 | 46.89 ± 0.54 | −5.42 ± 0.70 | 47.20 ± 0.53 | −6.59 ± 0.62 | |
| 27 | 10.58 ± 0.05 | 28.68 ± 1.31 | 46.37 ± 0.53 | −5.60 ± 0.44 | 46.71 ± 0.55 | −6.88 ± 0.56 | |
| 28 | 10.87 ± 0.53 | 28.81 ± 1.56 | 46.40 ± 0.03 | −5.80 ± 0.71 | 46.77 ± 0.34 | −7.12 ± 0.97 | |
| 29 | 10.15 ± 0.36 | 29.31 ± 1.24 | 45.48 ± 0.11 | −5.47 ± 0.45 | 45.82 ± 0.51 | −6.87 ± 0.66 | |
| 30 | 10.73 ± 0.40 | 28.65 ± 1.41 | 46.21 ± 0.02 | −4.97 ± 0.53 | 46.48 ± 0.37 | −6.14 ± 0.54 | |
| 31 | 10.76 ± 0.28 | 25.68 ± 1.59 | 40.33 ± 0.37 | −7.11 ± 0.96 | 40.96 ± 0.53 | −9.99 ± 0.36 | |
| 32 | 10.44 ± 0.03 | 26.84 ± 2.60 | 38.46 ± 1.27 | −6.28 ± 0.36 | 38.97 ± 0.99 | −9.28 ± 0.63 | |
| 33 | 9.82 ± 0.25 | 28.09 ± 2.43 | 37.37 ± 1.15 | −6.01 ± 0.32 | 37.85 ± 0.90 | −9.15 ± 0.72 | |
| 34 | 9.51 ± 0.10 | 27.38 ± 2.14 | 37.44 ± 0.44 | −5.65 ± 1.07 | 37.88 ± 0.54 | −8.56 ± 1.25 | |
| 35 | 9.15 ± 0.20 | 27.68 ± 0.65 | 37.75 ± 1.07 | −5.70 ± 0.93 | 38.18 ± 1.02 | −8.57 ± 0.89 | |
| 36 | 9.89 ± 0.50 | 26.68 ± 0.31 | 40.12 ± 0.00 | −7.25 ± 0.59 | 40.77 ± 0.18 | −10.25 ± 0.74 | |
| 37 | 9.95 ± 0.51 | 27.08 ± 0.67 | 40.35 ± 0.31 | −7.94 ± 0.15 | 41.12 ± 0.31 | −11.13 ± 0.40 | |
| 38 | 9.98 ± 0.10 | 28.13 ± 1.27 | 38.35 ± 0.55 | −6.85 ± 0.74 | 38.96 ± 0.59 | −10.11 ± 0.76 | |
| 39 | 9.85 ± 0.32 | 27.99 ± 1.07 | 38.12 ± 2.19 | −6.25 ± 0.30 | 38.63 ± 1.72 | −9.31 ± 0.21 | |
| 40 | 9.71 ± 0.39 | 28.13 ± 0.19 | 37.95 ± 1.47 | −6.00 ± 0.87 | 38.43 ± 1.25 | −8.96 ± 0.77 | |
| 41 | 9.27 ± 0.31 | 27.53 ± 1.03 | 38.83 ± 0.08 | −6.98 ± 0.41 | 39.46 ± 0.29 | −10.19 ± 0.55 | |
| 42 | 8.85 ± 0.38 | 28.53 ± 0.51 | 37.87 ± 0.18 | −6.53 ± 0.40 | 38.43 ± 0.31 | −9.78 ± 0.55 | |
| 43 | 9.56 ± 0.13 | 27.51 ± 0.53 | 39.22 ± 0.59 | −7.07 ± 0.31 | 39.85 ± 0.51 | −10.22 ± 0.53 | |
| 44 | 9.48 ± 0.72 | 27.36 ± 2.37 | 38.96 ± 1.77 | −6.49 ± 0.91 | 39.50 ± 1.50 | −9.44 ± 0.73 | |
| 45 | 9.23 ± 0.50 | 27.26 ± 0.55 | 39.14 ± 2.21 | −6.49 ± 0.78 | 39.68 ± 1.81 | −9.40 ± 0.59 | |
| 46 | 9.79 ± 0.60 | 39.82 ± 1.09 | 25.81 ± 0.06 | −7.85 ± 0.04 | 26.98 ± 0.56 | −16.93 ± 0.50 | |
| 47 | 9.98 ± 0.26 | 39.78 ± 0.82 | 25.28 ± 0.46 | −7.62 ± 0.10 | 26.40 ± 0.50 | −16.77 ± 0.12 | |
| 48 | 11.25 ± 2.25 | 36.97 ± 6.06 | 28.12 ± 5.77 | −7.12 ± 0.57 | 29.02 ± 4.45 | −14.39 ± 1.36 | |
| 49 | 9.89 ± 0.64 | 40.19 ± 0.90 | 24.37 ± 0.13 | −7.13 ± 0.12 | 25.39 ± 0.21 | −16.30 ± 0.28 | |
| 50 | 9.48 ± 0.02 | 41.02 ± 1.31 | 23.17 ± 0.16 | −6.44 ± 0.08 | 24.05 ± 0.33 | −15.52 ± 0.12 | |
| 51 | 12.55 ± 0.31 | 35.47 ± 0.24 | 31.29 ± 0.85 | −9.10 ± 0.02 | 32.59 ± 0.70 | −16.23 ± 0.36 | |
| 52 | 12.50 ± 0.32 | 34.28 ± 0.47 | 32.36 ± 1.60 | −8.94 ± 0.25 | 33.58 ± 1.16 | −15.47 ± 0.89 | |
| 53 | 9.96 ± 0.02 | 39.83 ± 0.18 | 24.79 ± 1.54 | −7.13 ± 0.63 | 25.79 ± 1.33 | −16.05 ± 0.34 | |
| 54 | 10.00 ± 0.00 | 40.60 ± 0.93 | 23.43 ± 0.00 | −5.90 ± 0.25 | 24.16 ± 0.36 | −14.13 ± 0.46 | |
| 55 | 9.50 ± 0.70 | 40.38 ± 0.08 | 24.18 ± 2.26 | −6.46 ± 0.96 | 25.03 ± 1.91 | −14.93 ± 0.61 | |
| 56 | 10.38 ± 0.05 | 38.89 ± 0.57 | 28.49 ± 0.55 | −9.01 ± 0.10 | 29.88 ± 0.52 | −17.55 ± 0.40 | |
| 57 | 10.27 ± 0.22 | 38.68 ± 1.49 | 27.37 ± 0.52 | −8.73 ± 0.38 | 28.73 ± 0.57 | −17.69 ± 0.32 | |
| 58 | 10.39 ± 0.30 | 38.93 ± 1.33 | 26.17 ± 0.46 | −7.59 ± 0.68 | 27.25 ± 0.63 | −16.15 ± 0.86 | |
| 59 | 9.43 ± 0.22 | 41.35 ± 3.14 | 22.38 ± 3.00 | −5.63 ± 1.40 | 23.08 ± 2.56 | −13.98 ± 1.39 | |
| 60 | 11.13 ± 0.02 | 39.06 ± 1.07 | 26.50 ± 0.49 | −8.13 ± 0.08 | 27.72 ± 0.50 | −17.05 ± 0.22 | |
| Peak | Rt | UV | [M]+ | MS2 | Tentative Identification | Quantification (mg/g, dw) | |
|---|---|---|---|---|---|---|---|
| BVFF | BVE | ||||||
| 1 A | 5.58 | 522 | 465 | 303(100) | Delphinidin-3-O-glucoside | 3.54 ± 0.01 b | 5.27 ± 0.02 a |
| 2 B | 7.60 | 516 | 449 | 287 (100) | Cyanidin-3-O-glucoside | 2.69 ± 0.004 b | 4.00 ± 0.01 a |
| 3 C | 8.98 | 524 | 479 | 317 (100) | Petunidin-3-O-glucoside | 1.48 ± 0.001 b | 2.20 ± 0.002 a |
| 4 D | 10.35 | 507 | 433 | 271 (100) | Pelargonidin-3-O-glucoside | 0.67 ± 0.001 b | 1.00 ± 0.002 a |
| 5 E | 14.05 | 526 | 493 | 331 (100) | Malvidin-3-O-glucoside | 3.01 ± 0.004 b | 4.47 ± 0.01 a |
| CHEMICAL COMPOSITION | ||||||||||
| Phenolic compounds | ||||||||||
| Total polyphenols (mg GAE/g, dw) | Hydroxybenzoic acids (mg GAE/g, dw) | Hydroxycinnamic acids (mg FAE/g, dw) | Flavonols (mg QE/g, dw) | |||||||
| BVFF | 159.90 ± 12.45 b | 47.30 ± 4.24 b | 31.09 ± 1.11 b | 7.41 ± 0.65 b | ||||||
| BVE | 290.72 ± 22.64 a | 81.74 ± 0.76 a | 46.52 ± 4.13 a | 10.00 ± 0.73 a | ||||||
| BIOACTIVE PROPERTIES | ||||||||||
| Antioxidant activity | ||||||||||
| in vitro chemical | in vitro biological | |||||||||
| DPPH (mg TE/g, dw) | Folin–Ciocalteu (mg GAE/g, dw) | OxHLIA (IC50 values, µg/mL) | ||||||||
| BVFF | 76.30 ± 4.00 b | 60.99 ± 3.83 b | 60 ± 3 b | |||||||
| BVE | 111.37 ± 9.65 a | 88.03 ± 7.84 a | 125 ± 4 a | |||||||
| Antibacterial activity (mg/mL hydroethanolic extract) | ||||||||||
| BVFF | BVE | Streptomycin | Methicillin | Ampicillin | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Gram-negative bacteria | ||||||||||
| Enterobacter Cloacae | >10 | >10 | 5 | 10 | 0.007 | 0.007 | n.t. | n.t | 0.15 | 0.15 |
| Escherichia coli | 5 | >10 | 2.5 | 10 | 0.01 | 0.01 | n.t. | n.t. | 0.15 | 0.15 |
| Pseudomonas aeruginosa | 10 | >10 | 5 | 10 | 0.06 | 0.06 | n.t. | n.t. | 0.63 | 0.63 |
| Salmonella enterica | 2.5 | >10 | 1.25 | 20 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 |
| Yersinia enterocolitica | >10 | >10 | 10 | 20 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 |
| Gram-positive bacteria | ||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Bacillus cereus | 10 | >10 | 1.25 | 10 | 0.007 | 0.007 | n.t. | n.t. | n.t. | n.t. |
| Listeria monocytogenes | 10 | >10 | 5 | >20 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 |
| Staphylococcus aureus | 10 | >10 | 2.5 | 20 | 0.007 | 0.007 | 0.007 | 0.007 | 0.15 | 0.15 |
| Antifungal activity (mg/mL hydroethanolic extract) | ||||||||||
| BVFF | BVE | Ketoconazole | ||||||||
| MIC | MFC | MIC | MFC | MIC | MFC | |||||
| Aspergillus brasiliensis | 2.5 | >10 | 5 | >10 | 0.06 | 0.125 | ||||
| Aspergillus fumigatus | 10 | >10 | 10 | >10 | 0.5 | 1.0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega, E.N.; González-Zamorano, L.; Cebadera, E.; Barros, L.; Pires, T.C.S.P.; Molina, A.K.; da Silveira, T.F.F.; Vidal-Diez de Ulzurrun, G.; Tardío, J.; Cámara, M.; et al. Natural Food Colorant Obtained from Wild Berberis vulgaris L. by Ultrasound-Assisted Extraction: Optimization and Characterization. Foods 2025, 14, 183. https://doi.org/10.3390/foods14020183
Vega EN, González-Zamorano L, Cebadera E, Barros L, Pires TCSP, Molina AK, da Silveira TFF, Vidal-Diez de Ulzurrun G, Tardío J, Cámara M, et al. Natural Food Colorant Obtained from Wild Berberis vulgaris L. by Ultrasound-Assisted Extraction: Optimization and Characterization. Foods. 2025; 14(2):183. https://doi.org/10.3390/foods14020183
Chicago/Turabian StyleVega, Erika N., Lorena González-Zamorano, Elena Cebadera, Lillian Barros, Tânia C. S. P. Pires, Adriana K. Molina, Tayse F. F. da Silveira, Guillermo Vidal-Diez de Ulzurrun, Javier Tardío, Montaña Cámara, and et al. 2025. "Natural Food Colorant Obtained from Wild Berberis vulgaris L. by Ultrasound-Assisted Extraction: Optimization and Characterization" Foods 14, no. 2: 183. https://doi.org/10.3390/foods14020183
APA StyleVega, E. N., González-Zamorano, L., Cebadera, E., Barros, L., Pires, T. C. S. P., Molina, A. K., da Silveira, T. F. F., Vidal-Diez de Ulzurrun, G., Tardío, J., Cámara, M., Fernández-Ruiz, V., & Morales, P. (2025). Natural Food Colorant Obtained from Wild Berberis vulgaris L. by Ultrasound-Assisted Extraction: Optimization and Characterization. Foods, 14(2), 183. https://doi.org/10.3390/foods14020183













