Supplementation with Lentil (Lens culinaris) Hull Soluble Dietary Fiber Ameliorates Sodium Dextran Sulfate-Induced Colitis and Behavioral Deficits via the Gut-Brain Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Structural Characterizations and Functional Properties of SDFs
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Fourier Transfer-Infrared Spectrometry (FT-IR)
2.2.3. Particle Size
2.2.4. X-Ray Diffraction (XRD)
2.2.5. Adsorption Capacity of Glucose and Cholesterol
2.2.6. Flow Behavior
Static Rheology of SDFs
Dynamic Rheology of SDFs
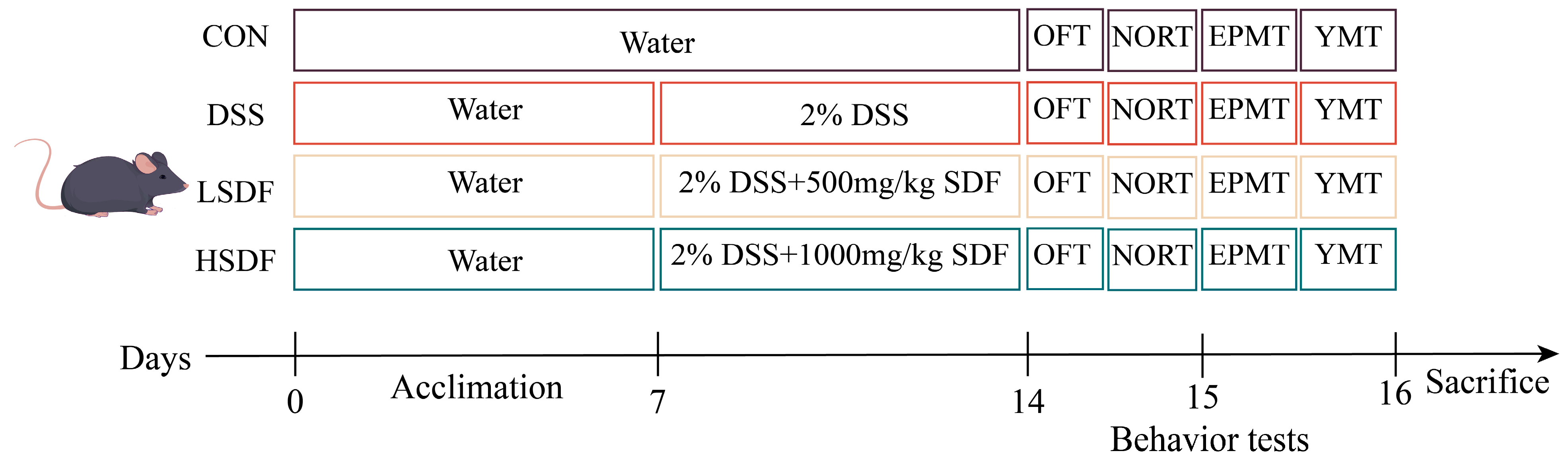
2.3. Animals, DSS-Induced Colitis Model, and SDF Intervention
2.3.1. Behavioral Tests
OFT
NORT
EPMT
YMT
2.3.2. Disease Activity Index (DAI) Assessment
2.3.3. Experimental Records and Sample Collection
2.3.4. Serum LPS and BDNF Level Detection
2.3.5. Hematoxylin and Eosin (H&E) Staining of Colon and Brain
2.3.6. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.3.7. Gut Microbiota Analysis
2.3.8. Measurements of Fecal SCFAs
2.3.9. Untargeted Metabolomics Analysis
2.4. Statistical Analysis
3. Results
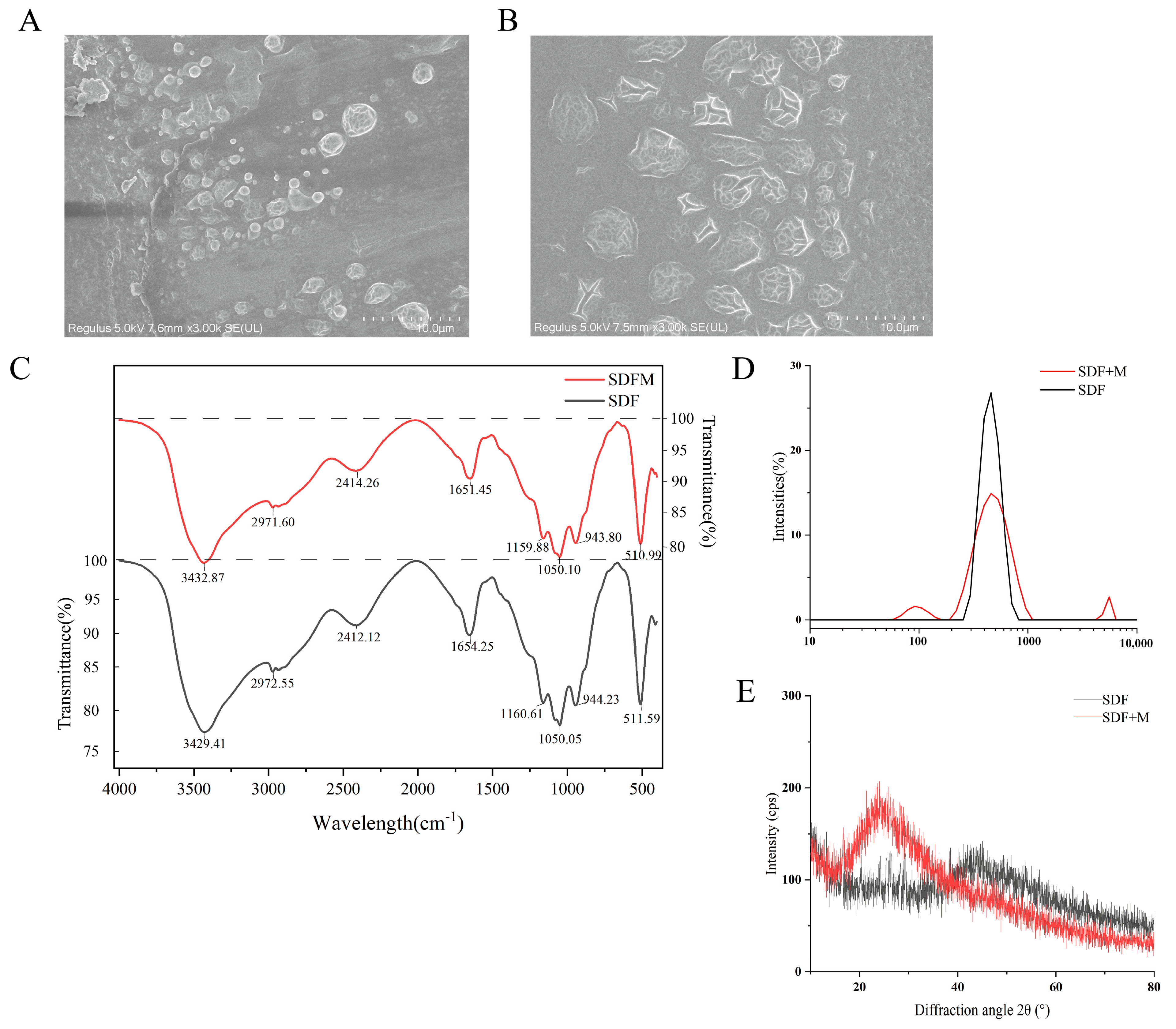
3.1. Scanning Electron Microscopy Observation
3.2. Fourier Transform Infrared Spectroscopy (FT-IR) of SDFs
3.3. Particle Size Analysis Results of SDFs
3.4. X-Ray Diffraction (XRD) Analysis Results of SDFs
3.5. Glucose and Cholesterol Adsorption Capacity
3.6. The Rheological Characteristic
3.6.1. The Steady State Rheology of SDFs
3.6.2. The Dynamic Rheology of SDFs
3.7. Effects of SDFs on DSS-Induced Anxiety and Depression-like Behavior in Mice
3.8. Effects of SDFs on the Disease Parameters of DSS-Induced IBD Mice
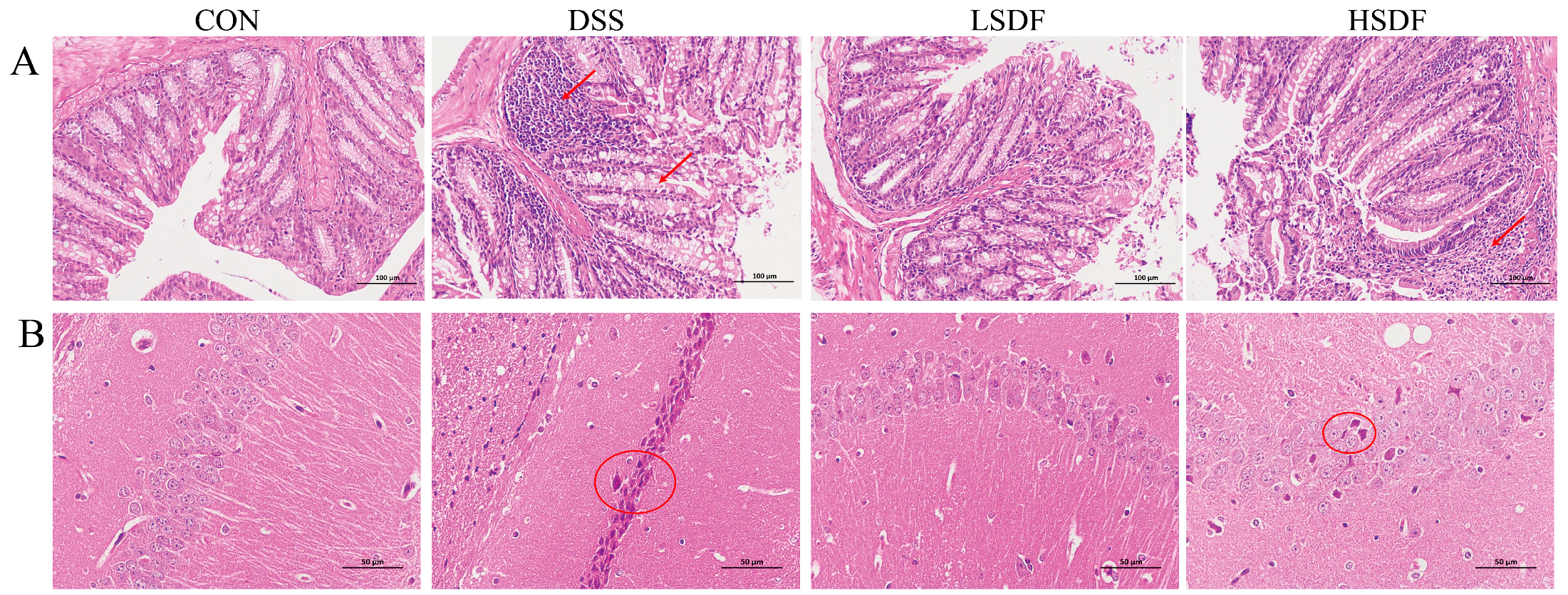
3.9. Effect of SDFs on DSS-Induced Inflammation of the Gut and the Cerebral Cortex
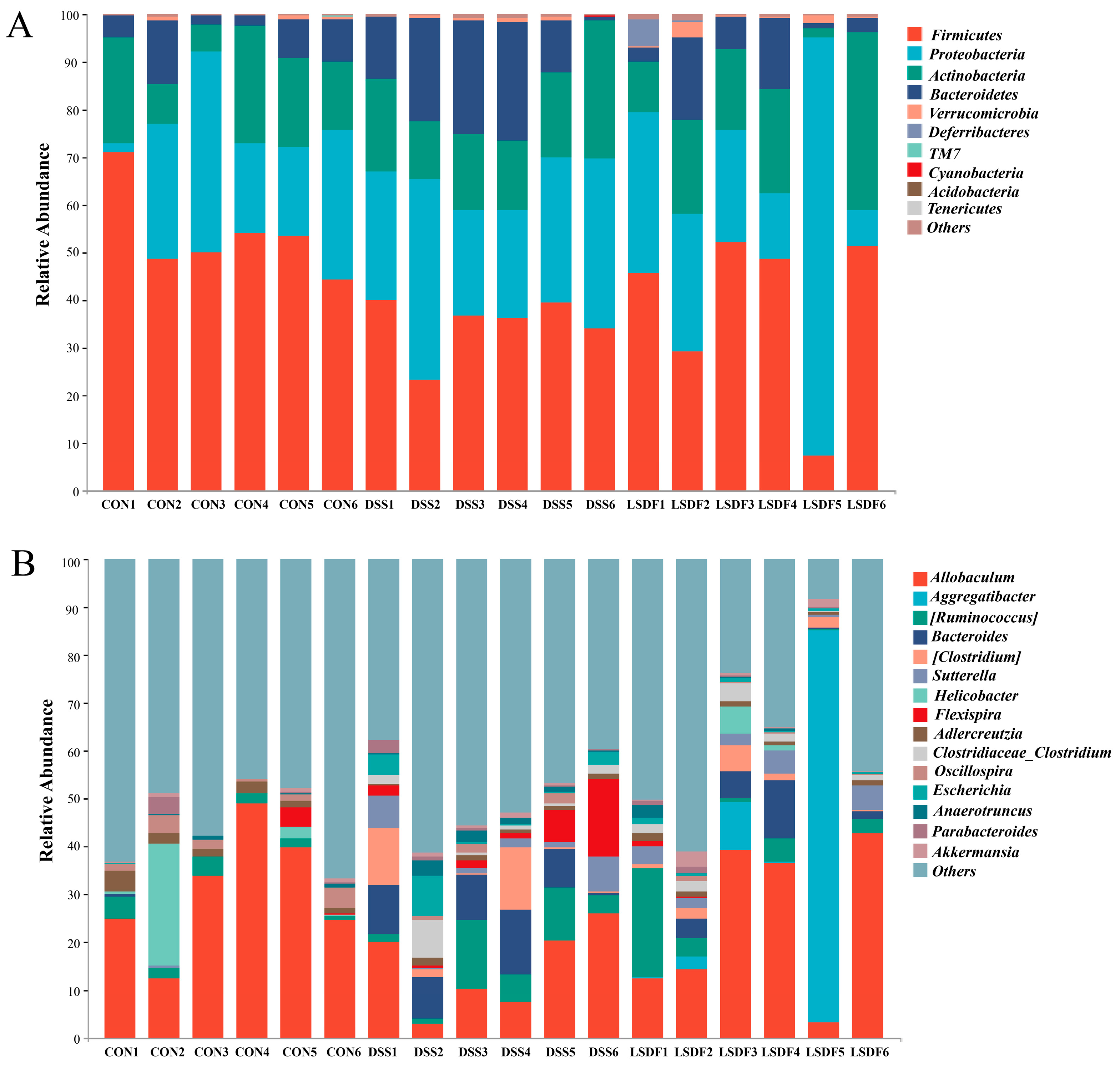
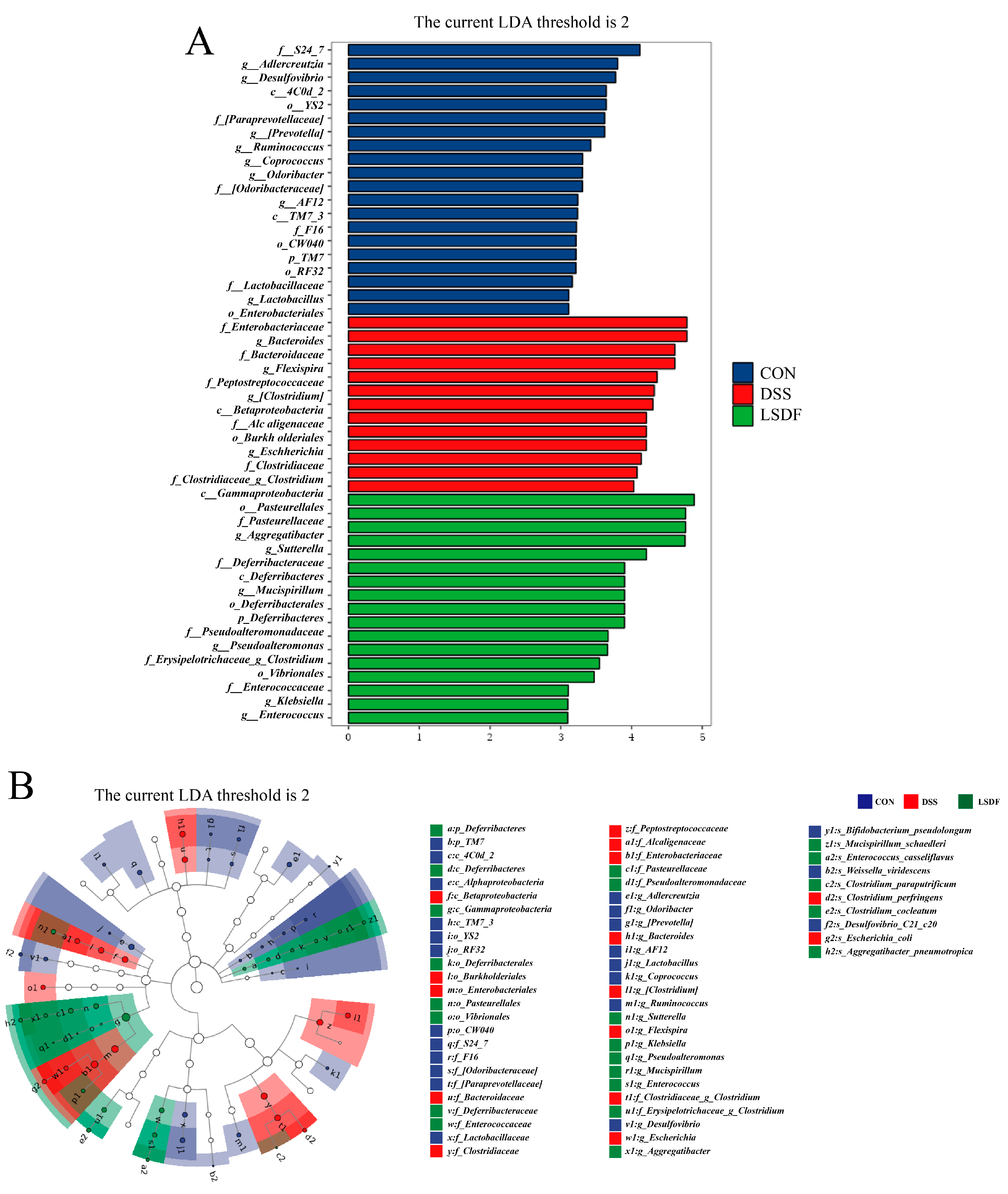
3.10. Effect of LSDF on DSS-Induced Changes in Gut Microbiological Composition
3.11. Effect of SDFs on DSS-Induced Changes in Fecal SCFAs Levels
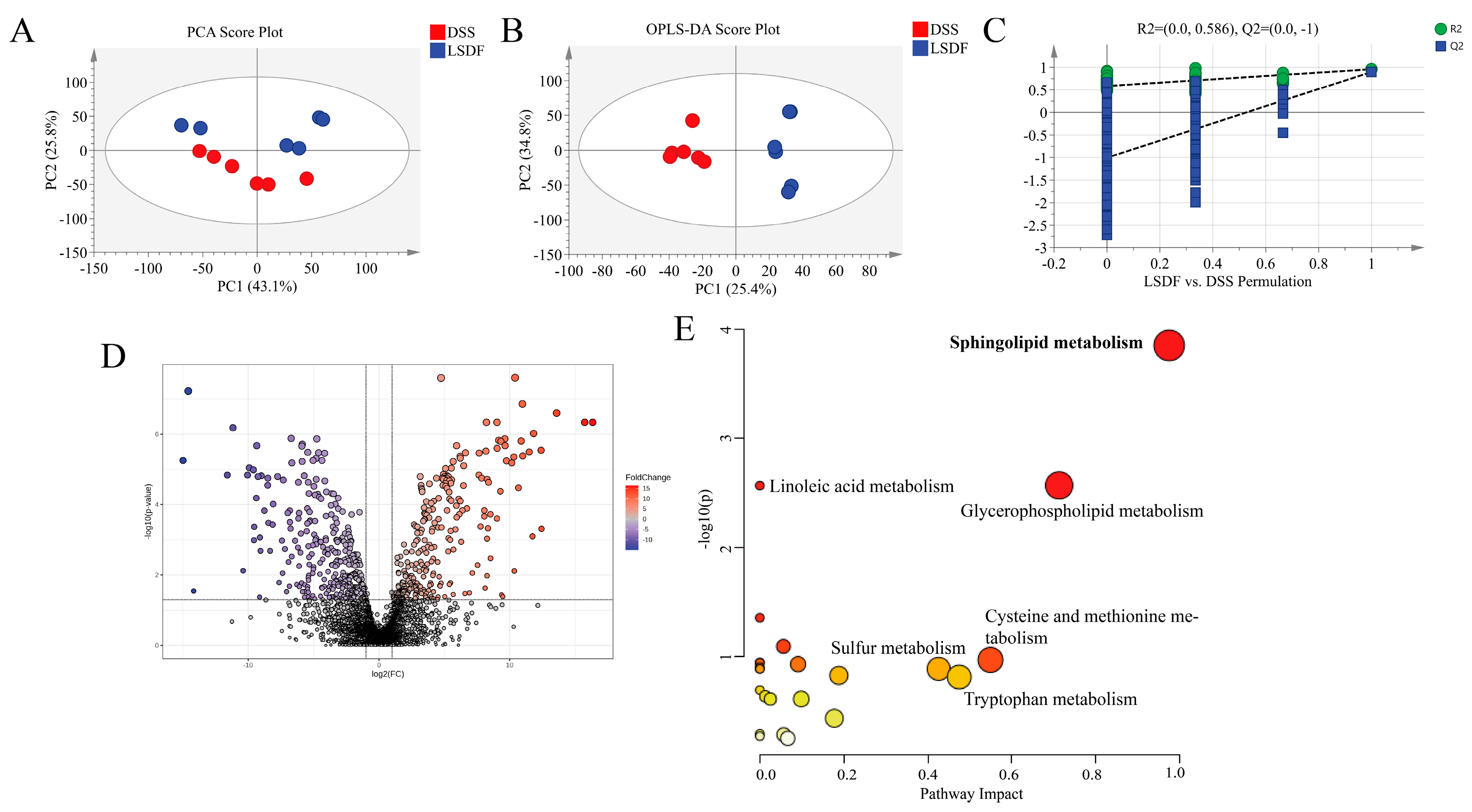
3.12. LSDF Altered the Metabolic Profile of DSS-Induced Mice
3.13. Spearman Correlation Analysis Between Biochemical Indices, Behavioral Parameters, Brain Metabolites, and the Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thavarajah, D.; Thavarajah, P.; Sarker, A.; Vandenberg, A. Lentils (Lens culinaris Medikus Subspecies culinaris): A whole food for increased iron and zinc intake. J. Agric. Food. Chem. 2009, 57, 5413–5419. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Fang, Z.; Wahlqvist, M.L.; Wu, G.; Hodgson, J.M.; Johnson, S.K. Seed coats of pulses as a food ingredient: Characterization, processing, and applications. Trends Food Sci. Technol. 2018, 80, 35–42. [Google Scholar] [CrossRef]
- Tiwari, U.; Cummins, E. Chapter 7—Legume fiber characterization, functionality, and process effects. In Pulse Foods, 2nd ed.; Tiwari, B.K., Gowen, A., McKenna, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 147–175. ISBN 978-0-12-818184-3. [Google Scholar]
- Armstrong, H.; Mander, I.; Zhang, Z.; Armstrong, D.; Wine, E. Not All Fibers Are Born Equal; Variable Response to Dietary Fiber Subtypes in IBD. Front. Pediatr. 2021, 8, 620189. [Google Scholar] [CrossRef] [PubMed]
- Gidley, M.J.; Yakubov, G.E. Functional categorisation of dietary fibre in foods: Beyond ‘soluble’ vs ‘insoluble’. Trends Food Sci. Technol. 2019, 86, 563–568. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Wong, C.; Harris, P.J.; Ferguson, L.R. Potential Benefits of Dietary Fibre Intervention in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2016, 17, 919. [Google Scholar] [CrossRef]
- Andoh, A.; Tsujikawa, T.; Fujiyama, Y. Role of dietary fiber and short-chain fatty acids in the colon. Curr. Pharm. Des. 2003, 9, 347–358. [Google Scholar] [CrossRef]
- Sokol, H.; Lay, C.; Seksik, P.; Tannock, G.W. Analysis of bacterial bowel communities of IBD patients: What has it revealed? Inflamm. Bowel Dis. 2008, 14, 858–867. [Google Scholar] [CrossRef]
- Cuervo-Zanatta, D.S.T.S. Dietary Fiber Modulates the Release of Gut Bacterial Products Preventing Cognitive Decline in an Alzheimer’s Mouse Model. Cell. Mol. Neurobiol. 2023, 43, 1595–1618. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Mai, P.; Hao, Y.; Wang, Z.; Wang, J. Quinoa bran soluble dietary fiber ameliorates dextran sodium sulfate induced ulcerative colitis in BALB/c mice by maintaining intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2022, 216, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Qin, L.; Zhang, L.; Wang, K.; Yao, M.; Qu, C.; Miao, J. Protective effect of cellulose and soluble dietary fiber from Saccharina japonica by-products on regulating inflammatory responses, gut microbiota, and SCFAs production in colitis mice. Int. J. Biol. Macromol. 2024, 267, 131214. [Google Scholar] [CrossRef]
- Girolamo, F.; Coppola, C.; Ribatti, D. Immunoregulatory effect of mast cells influenced by microbes in neurodegenerative diseases. Brain Behav. Immun. 2017, 65, 68–89. [Google Scholar] [CrossRef]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef]
- Geerlings, S.Y.; Kostopoulos, I.; de Vos, W.M.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Kim, H.J.; Leeds, P.; Chuang, D. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009, 110, 1226–1240. [Google Scholar] [CrossRef]
- Wei, Y.; Melas, P.A.; Wegener, G.; Mathé, A.A.; Lavebratt, C. Antidepressant-like effect of sodium butyrate is associated with an increase in TET1 and in 5-hydroxymethylation levels in the Bdnf gene. Int. J. Neuropsychopharmacol. 2014, 18, pyu032. [Google Scholar] [CrossRef]
- MacFabe, D.F. Enteric short-chain fatty acids: Microbial messengers of metabolism, mitochondria, and mind: Implications in autism spectrum disorders. Microb Ecol Health Dis. 2015, 26, 28177. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Roy Sarkar, S.; Banerjee, S. Gut microbiota in neurodegenerative disorders. J. Neuroimmunol. 2019, 328, 98–104. [Google Scholar] [CrossRef]
- Neuendorf, R.; Harding, A.; Stello, N.; Hanes, D.; Wahbeh, H. Depression and anxiety in patients with Inflammatory Bowel Disease: A systematic review. J. Psychosom. Res. 2016, 87, 70–80. [Google Scholar] [CrossRef]
- Carloni, S.; Bertocchi, A.; Mancinelli, S.; Bellini, M.; Erreni, M.; Borreca, A.; Braga, D.; Giugliano, S.; Mozzarelli, A.M.; Manganaro, D.; et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 2021, 374, 439–448. [Google Scholar] [CrossRef]
- Horvath, T.D.; Haidacher, S.J.; Engevik, M.A.; Luck, B.; Ruan, W.; Ihekweazu, F.; Bajaj, M.; Hoch, K.M.; Oezguen, N.; Spinler, J.K.; et al. Interrogation of the mammalian gut-brain axis using LC-MS/MS-based targeted metabolomics with in vitro bacterial and organoid cultures and in vivo gnotobiotic mouse models. Nat. Protoc. 2023, 18, 490–529. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut-Brain Axis Balance. J. Agric. Food. Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef]
- Qian, Q.; Qiu, D.; Wu, Z.; Yang, H.; Xie, Y.; Li, S.; Yin, Y.; Li, X. Apple polyphenol extract alleviates DSS-induced ulcerative colitis and linked behavioral disorders via regulating the gut-brain axis. Food Biosci. 2023, 53, 102720. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Y.; Jia, X.; Lin, J.; Xiao, L.; Liu, D.; Liang, M. Lactiplantibacillus plantarum DMDL 9010 alleviates dextran sodium sulfate (DSS)-induced colitis and behavioral disorders by facilitating microbiota-gut-brain axis balance. Food Funct. 2022, 13, 411–424. [Google Scholar] [CrossRef]
- Mehdi, S.M.A.; Costa, A.P.; Svob, C.; Pan, L.; Dartora, W.J.; Talati, A.; Gameroff, M.J.; Wickramaratne, P.J.; Weissman, M.M.; McIntire, L.B.J. Depression and cognition are associated with lipid dysregulation in both a multigenerational study of depression and the National Health and Nutrition Examination Survey. Transl. Psychiatry 2024, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Ai, Z.; Zeng, S.; Song, Y.; Song, J.; Zeng, Q.; Liao, Z.; Wang, T.; Huang, C.; Su, D. Gut microbiome-derived lactate promotes to anxiety-like behaviors through GPR81 receptor-mediated lipid metabolism pathway. Psychoneuroendocrinology 2020, 117, 104699. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hatcher, D.W.; Toews, R.; Gawalko, E.J. Influence of cooking and dehulling on nutritional composition of several varieties of lentils (Lens culinaris). LWT—Food Sci. Technol. 2009, 42, 842–848. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Q.; Gao, Y.; Xiang, H.; Zhang, C.; Ding, P.; Wu, T.; Ji, G. Metabolomics window into the diagnosis and treatment of inflammatory bowel disease in recent 5 years. Int. Immunopharmacol. 2022, 113, 109472. [Google Scholar] [CrossRef]
- Filimoniuk, A.; Blachnio-Zabielska, A.; Imierska, M.; Lebensztejn, D.M.; Daniluk, U. Sphingolipid Analysis Indicate Lactosylceramide as a Potential Biomarker of Inflammatory Bowel Disease in Children. Biomolecules 2020, 10, 1083. [Google Scholar] [CrossRef]
- Farooqui, A.A. Sphingosine and Sphingosine 1 Phosphate in the Brain. In Lipid Mediators and Their Metabolism in the Brain; Farooqui, A.A., Ed.; Springer: New York, NY, USA, 2011; pp. 245–266. ISBN 978-1-4419-9940-5. [Google Scholar]
- Karunakaran, I.; van Echten-Deckert, G. Sphingosine 1-phosphate-A double edged sword in the brain. Biochim. Biophys. Acta (BBA)—Biomembr. 2017, 1859, 1573–1582. [Google Scholar] [CrossRef]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Guan, Z.; Yu, E.; Feng, Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef]
- Gupta, M.; Pandey, S.; Rumman, M.; Singh, B.; Mahdi, A.A. Molecular mechanisms underlying hyperglycemia associated cognitive decline. IBRO Neurosci. Rep. 2023, 14, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Daou, C.; Hui, Z. Study on Functional Properties of Physically Modified Dietary Fibres Derived from Defatted Rice Bran. J. Agric. Sci. 2012, 4, 85. [Google Scholar] [CrossRef]
- Herbert, K.E.; Erridge, C. Regulation of low-density lipoprotein cholesterol by intestinal inflammation and the acute phase response. Cardiovasc. Res. 2018, 114, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Morozova, A.; Zorkina, Y.; Abramova, O.; Pavlova, O.; Pavlov, K.; Soloveva, K.; Volkova, M.; Alekseeva, P.; Andryshchenko, A.; Kostyuk, G.; et al. Neurobiological Highlights of Cognitive Impairment in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 1217. [Google Scholar] [CrossRef]
- Guo, F.; Tsao, R.; Li, C.; Wang, X.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Green Pea (Pisum sativum L.) Hull Polyphenol Extracts Ameliorate DSS-Induced Colitis through Keap1/Nrf2 Pathway and Gut Microbiota Modulation. Foods 2021, 10, 2765. [Google Scholar] [CrossRef]
- Wang, X.; Xue, H.; Li, Y.; Li, P.; Liu, Z.; Piao, C. Characterization of physicochemical and functional properties of soluble dietary fiber from separate and co-fermented okara by lactic acid bacteria and Kluyveromyces marxianus C21. Lwt 2024, 205, 116476. [Google Scholar] [CrossRef]
- Shi, X.; Yin, J.; Cui, S.W.; Wang, Q.; Huang, X.; Nie, S. Studies on O-acetyl-glucomannans from Amorphophallus species: Comparison of fine structure. Food Hydrocoll. 2020, 100, 105391. [Google Scholar] [CrossRef]
- Alba, K.; MacNaughtan, W.; Laws, A.P.; Foster, T.J.; Campbell, G.M.; Kontogiorgos, V. Fractionation and characterisation of dietary fibre from blackcurrant pomace. Food Hydrocoll. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- Vlaic Marc, R.A. Dietary Fibers and Their Importance in the Diet. In New Insights in Dietary Fibers; Vlaic Marc, R.A., Muresan, C.C., Eds.; IntechOpen: Rijeka, Croatia, 2024; ISBN 978-0-85014-502-1. [Google Scholar]
- Grassino, A.N.H.J. Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll. 2016, 52, 265–274. [Google Scholar] [CrossRef]
- Al Faruq, A.; Farahnaky, A.; Torley, P.J.; Buckow, R.; Eri, R.; Majzoobi, M. Sustainable approaches to boost soluble dietary fibre in foods: A path to healthier foods. Food Hydrocoll. 2025, 162, 110880. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.; Xu, F.; Bian, J.; Peng, P.; Sun, R. Comparative Study of Hemicelluloses Obtained by Graded Ethanol Precipitation from Sugarcane Bagasse. J. Agric. Food. Chem. 2009, 57, 6305–6317. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wen, J.; Ma, M.; Sun, R. Successive alkali extraction and structural characterization of hemicelluloses from sweet sorghum stem. Carbohydr. Polym. 2013, 92, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.M.; Cummings, J.H. Water-holding by dietary fibre in vitro and its relationship to faecal output in man. Gut 1979, 20, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Hanau, S.; Almugadam, S.H.; Sapienza, E.; Cacciari, B.; Manfrinato, M.C.; Trentini, A.; Kennedy, J.F. Schematic overview of oligosaccharides, with survey on their major physiological effects and a focus on milk ones. Carbohydr. Polym. Technol. Appl. 2020, 1, 100013. [Google Scholar] [CrossRef]
- Chen, Y.; Sui, X.; Wang, Y.; Zhao, Z.; Han, T.; Liu, Y.; Zhang, J.; Zhou, P.; Yang, K.; Ye, Z. Preparation, structural characterization, biological activity, and nutritional applications of oligosaccharides. Food Chem. X 2024, 22, 101289. [Google Scholar] [CrossRef]
- Han, X.; Yang, D.; Zhang, S.; Liu, X.; Zhao, Y.; Song, C.; Sun, Q. Characterization of insoluble dietary fiber from Pleurotus eryngii and evaluation of its effects on obesity-preventing or relieving effects via modulation of gut microbiota. J. Future Foods 2023, 3, 55–66. [Google Scholar] [CrossRef]
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments. Lwt 2017, 75, 344–351. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, H. Study on crystal structures of enzyme-hydrolyzed cellulosic materials by X-ray diffraction. Enzym. Microb. Technol. 2005, 36, 314–317. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Napolitano, A.; Lanzuise, S.; Ruocco, M.; Arlotti, G.; Ranieri, R.; Knutsen, S.H.; Lorito, M.; Fogliano, V. Treatment of cereal products with a tailored preparation of trichoderma enzymes increases the amount of soluble dietary fiber. J. Agric. Food. Chem. 2006, 54, 7863–7869. [Google Scholar] [CrossRef] [PubMed]
- Opperman, C.; Majzoobi, M.; Farahnaky, A.; Shah, R.; Van, T.T.H.; Ratanpaul, V.; Blanch, E.W.; Brennan, C.; Eri, R. Beyond soluble and insoluble: A comprehensive framework for classifying dietary fibre’s health effects. Food Res. Int. 2025, 115843, in press. [Google Scholar] [CrossRef]
- Gaenssle, A.L.O.; Satyawan, C.A.; Xiang, G.; van der Maarel, M.J.E.C.; Jurak, E. Long chains and crystallinity govern the enzymatic degradability of gelatinized starches from conventional and new sources. Carbohydr. Polym. 2021, 260, 117801. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhu, K.; Hu, F.; Hu, R.; Dong, W. Effects of ultrasonic treatment on the physicochemical, structural, and functional properties of soluble dietary fiber from coffee peels. Ultrason. Sonochem. 2025, 114, 107247. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, K.M.; White, M.J.; Kemps, E.; Moore, H.; Dymond, A.; Bartlett, S.E. The Impact of Free and Added Sugars on Cognitive Function: A Systematic Review and Meta-Analysis. Nutrients 2023, 16, 75. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Zacherl, C.; Eisner, P.; Engel, K. In vitro model to correlate viscosity and bile acid-binding capacity of digested water-soluble and insoluble dietary fibres. Food Chem. 2011, 126, 423–428. [Google Scholar] [CrossRef]
- Gunness, P.; Gidley, M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010, 1, 149–155. [Google Scholar] [CrossRef]
- Boytsov, S.A.; Samorodskaya, I.V. Cardiovascular Diseases and Cognitive Impairments. Neurosci. Behav. Physiol. 2023, 53, 186–192. [Google Scholar] [CrossRef]
- Meriem-Benziane, M.; Abdul-Wahab, S.A.; Benaicha, M.; Belhadri, M. Investigating the rheological properties of light crude oil and the characteristics of its emulsions in order to improve pipeline flow. Fuel 2012, 95, 97–107. [Google Scholar] [CrossRef]
- Ge, Z.; Yin, D.; Li, Z.; Chen, X.; Dong, M. Effects of Commercial Polysaccharides Stabilizers with Different Charges on Textural, Rheological, and Microstructural Characteristics of Set Yoghurts. Foods 2022, 11, 1764. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, Y.; Chudan, S.; Ishibashi, R.; Ohue-Kitano, R.; Nishikawa, M.; Tabuchi, Y.; Kimura, I.; Nagai, Y.; Ikushiro, S.; Furusawa, Y. The Impact of Low-Viscosity Soluble Dietary Fibers on Intestinal Microenvironment and Experimental Colitis: A Possible Preventive Application of Alpha-Cyclodextrin in Intestinal Inflammation. Mol. Nutr. Food Res. 2022, 66, e2200063. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wei, H.; Zhao, X.; Xu, C.; Peng, J. Effects of dietary fibers with high water-binding capacity and swelling capacity on gastrointestinal functions, food intake and body weight in male rats. Food Nutr. Res. 2017, 61, 1308118. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Hong, F.; Xie, Y.; Xie, Y.; Shi, F.; Wang, R.; Wang, L.; Chen, Z.; Liu, X. Dose-dependent action of cordycepin on the microbiome-gut-brain-adipose axis in mice exposed to stress. Biomed. Pharmacother. 2023, 168, 115796. [Google Scholar] [CrossRef]
- He, Y.; Tian, Y.; Xiong, H.; Deng, Z.; Zhang, H.; Guo, F.; Sun, Y. Rice Protein Peptides Ameliorate DSS-Induced Cognitive Impairment and Depressive Behavior in Mice by Modulating Phenylalanine Metabolism and the BDNF/TRKB/CREB Pathway. J. Agric. Food. Chem. 2024, 72, 19812–19825. [Google Scholar] [CrossRef]
- Kogan, S.; Ospina, L.H.; Mittal, V.A.; Kimhy, D. The impact of inflammation on neurocognition and risk for psychosis: A critical review. Eur. Arch. Psychiatry. Clin. Neurosci. 2020, 270, 793–802. [Google Scholar] [CrossRef]
- Liang, N.; Nho, K.; Newman, J.W.; Arnold, M.; Huynh, K.; Meikle, P.J.; Borkowski, K.; Kaddurah-Daouk, R. Peripheral inflammation is associated with brain atrophy and cognitive decline linked to mild cognitive impairment and Alzheimer’s disease. Sci. Rep. 2024, 14, 17423. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, T.; Gong, G.; Li, Y.; Zhang, J.; Wu, B.; Bi, K.; Jia, Y. Antidepressant-like effects of Schisandrin on lipopolysaccharide-induced mice: Gut microbiota, short chain fatty acid and TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2020, 89, 107029. [Google Scholar] [CrossRef]
- Yu, Z.; Xie, Y.; Huang, Z.; Yang, K.; Wang, Z.; Hu, H. Study of the therapeutic effect of raw and processed Vladimiriae Radix on ulcerative colitis based on intestinal flora, metabolomics and tissue distribution analysis. Phytomedicine 2021, 85, 153538. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Wrzosek, L.; Miquel, S.; Noordine, M.; Bouet, S.; Joncquel Chevalier-Curt, M.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef]
- Gracie, D.J.; Hamlin, P.J.; Ford, A.C. The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol. Hepatol. 2019, 4, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jia, M.; Zhao, Y.; Hui, Y.; Pan, J.; Yu, H.; Yan, S.; Dai, X.; Liu, X.; Liu, Z. Supplementation of Sesamin Alleviates Stress-Induced Behavioral and Psychological Disorders via Reshaping the Gut Microbiota Structure. J. Agric. Food. Chem. 2019, 67, 12441–12451. [Google Scholar] [CrossRef] [PubMed]
- Gracie, D.J.; Guthrie, E.A.; Hamlin, P.J.; Ford, A.C. Bi-directionality of Brain-Gut Interactions in Patients with Inflammatory Bowel Disease. Gastroenterology 2018, 154, 1635–1646. [Google Scholar] [CrossRef]
- Mikocka-Walus, A.; Pittet, V.; Rossel, J.; von Känel, R. Symptoms of Depression and Anxiety Are Independently Associated with Clinical Recurrence of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 829–835. [Google Scholar] [CrossRef]
- Barberio, B.; Zamani, M.; Black, C.J.; Savarino, E.V.; Ford, A.C. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 359–370. [Google Scholar] [CrossRef]
- Do, J.; Woo, J. From Gut to Brain: Alteration in Inflammation Markers in the Brain of Dextran Sodium Sulfate-induced Colitis Model Mice. Clin. Psychopharmacol. Neurosci. 2018, 16, 422–433. [Google Scholar] [CrossRef]
- Nakagawasai, O.; Yamada, K.; Takahashi, K.; Odaira, T.; Sakuma, W.; Ishizawa, D.; Takahashi, N.; Onuma, K.; Hozumi, C.; Nemoto, W.; et al. Liver hydrolysate prevents depressive-like behavior in an animal model of colitis: Involvement of hippocampal neurogenesis via the AMPK/BDNF pathway. Behav. Brain Res. 2020, 390, 112640. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakagawasai, O.; Nemoto, W.; Odaira, T.; Sakuma, W.; Onogi, H.; Nishijima, H.; Furihata, R.; Nemoto, Y.; Iwasa, H.; et al. Effect of Enterococcus faecalis 2001 on colitis and depressive-like behavior in dextran sulfate sodium-treated mice: Involvement of the brain-gut axis. J. Neuroinflamm. 2019, 16, 201. [Google Scholar] [CrossRef]
- Gareau, M.G. Microbiota-gut-brain axis and cognitive function. Adv. Exp. Med. Biol. 2014, 817, 357–371. [Google Scholar] [PubMed]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020–2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr. Today 2021, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-Chain Fatty Acids Regulate Cytokines and Th17/Treg Cells in Human Peripheral Blood Mononuclear Cells in vitro. Immunol. Investig. 2016, 45, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Cavaglieri, C.R.; Nishiyama, A.; Fernandes, L.C.; Curi, R.; Miles, E.A.; Calder, P.C. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003, 73, 1683–1690. [Google Scholar] [CrossRef]
- De Preter, V.; Joossens, M.; Ballet, V.; Shkedy, Z.; Rutgeerts, P.; Vermeire, S.; Verbeke Phd, K. Metabolic profiling of the impact of oligofructose-enriched inulin in Crohn’s disease patients: A double-blinded randomized controlled trial. Clin. Transl. Gastroenterol. 2013, 4, e30. [Google Scholar] [CrossRef]
- Wedlake, L.; Slack, N.; Andreyev, H.J.N.; Whelan, K. Fiber in the treatment and maintenance of inflammatory bowel disease: A systematic review of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 576–586. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Wang, W.; Zhang, D. Consumption of vegetables and fruit and the risk of inflammatory bowel disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 623–630. [Google Scholar] [CrossRef]
- Wang, H.; Huang, X.; Xia, S.; Chen, C.; Chen, X.; Zhang, Y.; Farag, M.A.; Xiao, J.; Nie, S. Celery soluble dietary fiber antagonizes flavonoids ameliorative effect on dextran-sodium-sulfate-induced colitis in mice. J. Adv. Res. 2023, 52, 73–88. [Google Scholar] [CrossRef]
- Wang, W.; Kou, F.; Wang, J.; Quan, Z.; Zhao, S.; Wang, Y.; Hu, X.; Sun, H.; Cao, L. Pretreatment with millet-derived selenylated soluble dietary fiber ameliorates dextran sulfate sodium-induced colitis in mice by regulating inflammation and maintaining gut microbiota balance. Front. Nutr. 2022, 9, 928601. [Google Scholar] [CrossRef]
- Kang, Y.; Park, H.; Choe, B.; Kang, B. The Role and Function of Mucins and Its Relationship to Inflammatory Bowel Disease. Front. Med. 2022, 9, 848344. [Google Scholar] [CrossRef]
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.V.; Hansson, G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Burcelin, R.; Garidou, L.; Pomié, C. Immuno-microbiota cross and talk: The new paradigm of metabolic diseases. Semin. Immunol. 2012, 24, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Peluzio, M.D.C.G.; Alfenas, R.D.C.G. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef]
- Khlevner, J.; Park, Y.; Margolis, K.G. Brain-Gut Axis: Clinical Implications. Gastroenterol. Clin. N. Am. 2018, 47, 727–739. [Google Scholar] [CrossRef]
- Yusufov, M.; Weyandt, L.L.; Piryatinsky, I. Alzheimer’s disease and diet: A systematic review. Int. J. Neurosci. 2017, 127, 161–175. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zheng, X.; Fang, T.; Yang, X.; Luo, X.; Guo, A.; Newell, K.A.; Huang, X.; Yu, Y. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J. Neuroinflamm. 2018, 15, 112. [Google Scholar] [CrossRef]
- Podbielska, M.; Das, A.; Smith, A.W.; Chauhan, A.; Ray, S.K.; Inoue, J.; Azuma, M.; Nozaki, K.; Hogan, E.L.; Banik, N.L. Neuron-microglia interaction induced bi-directional cytotoxicity associated with calpain activation. J. Neurochem. 2016, 139, 440–455. [Google Scholar] [CrossRef]
- Zhang, J.; Rong, P.; Zhang, L.; He, H.; Zhou, T.; Fan, Y.; Mo, L.; Zhao, Q.; Han, Y.; Li, S.; et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci. Adv. 2021, 7, eabb9888. [Google Scholar] [CrossRef]
- Miller, A.H. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav. Immun. 2009, 23, 149–158. [Google Scholar] [CrossRef]
- Brebner, K.; Hayley, S.; Zacharko, R.; Merali, Z.; Anisman, H. Synergistic Effects of Interleukin-1β, Interleukin-6, and Tumor Necrosis Factor-α: Central Monoamine, Corticosterone, and Behavioral Variations. Neuropsychopharmacology 2000, 22, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.J.; Davis, S.; Morris, C.; Jackson, E.; Harrison, R.; O’Brien, J.T. Increase in interleukin-1beta in late-life depression. Am. J. Psychiatry 2005, 162, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Corwin, E.J.; Johnston, N.; Pugh, L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol. Res. Nurs. 2008, 10, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Manfro, P.H.; Anselmi, L.; Barros, F.; Gonçalves, H.; Murray, J.; Oliveira, I.O.; Tovo-Rodrigues, L.; Wehrmeister, F.C.; Menezes, A.M.B.; Mondelli, V.; et al. Youth depression and inflammation: Cross-sectional network analyses of C-Reactive protein, interleukin-6 and symptoms in a population-based sample. J. Psychiatr. Res. 2022, 150, 197–201. [Google Scholar] [CrossRef]
- Zadka, A.; Dzięgiel, P.; Kulus, M.; Olajossy, M. Clinical Phenotype of Depression Affects Interleukin-6 Synthesis. J. Interferon Cytokine Res. 2017, 37, 231–245. [Google Scholar] [CrossRef]
- Hodes, G.E.; Ménard, C.; Russo, S.J. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol. Stress 2016, 4, 15–22. [Google Scholar] [CrossRef]
- Das, R.; Emon, M.P.Z.; Shahriar, M.; Nahar, Z.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, S.N.; Islam, M.R. Higher levels of serum IL-1β and TNF-α are associated with an increased probability of major depressive disorder. Psychiatry Res. 2021, 295, 113568. [Google Scholar] [CrossRef]
- Church, J.S.; Bannish, J.A.M.; Adrian, L.A.; Rojas Martinez, K.; Henshaw, A.; Schwartzer, J.J. Serum short chain fatty acids mediate hippocampal BDNF and correlate with decreasing neuroinflammation following high pectin fiber diet in mice. Front. Neurosci. 2023, 17, 1134080. [Google Scholar] [CrossRef]
- Molteni, M.; Gemma, S.; Rossetti, C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016, 2016, 6978936. [Google Scholar] [CrossRef]
- Stierschneider, A.; Wiesner, C. Shedding light on the molecular and regulatory mechanisms of TLR4 signaling in endothelial cells under physiological and inflamed conditions. Front. Immunol. 2023, 14, 1264889. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-kB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev. Neurother. 2019, 19, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xie, W.; Wan, X.; Deng, T. Clostridium butyricum protects intestinal barrier function via upregulation of tight junction proteins and activation of the Akt/mTOR signaling pathway in a mouse model of dextran sodium sulfate-induced colitis. Exp. Ther. Med. 2020, 20, 10. [Google Scholar] [CrossRef]
- Ohno, K.; Bernier, F.; Katsumata, N.; Shimizu, T.; Xiao, J. Anti-inflammation as one of the mechanisms in the improvement of cognitive function by probiotic Bifidobacterium breve strain. Alzheimer’s Dement. 2021, 17, e051966. [Google Scholar] [CrossRef]
- Ghavami, S.B.; Rostami, E.; Sephay, A.A.; Shahrokh, S.; Balaii, H.; Aghdaei, H.A.; Zali, M.R. Alterations of the human gut Methanobrevibacter smithii as a biomarker for inflammatory bowel diseases. Microb. Pathog. 2018, 117, 285–289. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; De Castro, C.; Silipo, A.; Molinaro, A. Lipopolysaccharide structures of Gram-negative populations in the gut microbiota and effects on host interactions. FEMS Microbiol. Rev. 2019, 43, 257–272. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl. Microbiol. Biotechnol. 2021, 105, 5785–5794. [Google Scholar] [CrossRef]
- Alvarado, D.M.; Chen, B.; Iticovici, M.; Thaker, A.I.; Dai, N.; VanDussen, K.L.; Shaikh, N.; Lim, C.K.; Guillemin, G.J.; Tarr, P.I.; et al. Epithelial Indoleamine 2,3-Dioxygenase 1 Modulates Aryl Hydrocarbon Receptor and Notch Signaling to Increase Differentiation of Secretory Cells and Alter Mucus-Associated Microbiota. Gastroenterology 2019, 157, 1093–1108. [Google Scholar] [CrossRef]
- Zhai, R.; Xue, X.; Zhang, L.; Yang, X.; Zhao, L.; Zhang, C. Strain-Specific Anti-inflammatory Properties of Two Akkermansia muciniphila Strains on Chronic Colitis in Mice. Front. Cell. Infect. Microbiol. 2019, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Li, X.; Ji, H. Regulation of gut microbiota in Alzheimer’s disease mice by silibinin and silymarin and their pharmacological implications. Appl. Microbiol. Biotechnol. 2019, 103, 7141–7149. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, G.; Yang, Q.; Ye, J.; Cai, X.; Tsering, P.; Cheng, X.; Hu, C.; Zhang, S.; Cao, P. Gut microbiota drives the attenuation of dextran sulphate sodium-induced colitis by Huangqin decoction. Oncotarget 2017, 8, 48863–48874. [Google Scholar] [CrossRef] [PubMed]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L.J. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Varela, E.; Manichanh, C.; Gallart, M.; Torrejón, A.; Borruel, N.; Casellas, F.; Guarner, F.; Antolin, M. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2013, 38, 151–161. [Google Scholar] [CrossRef]
- Song, Y.; Lai, M.; Liao, Z.; Zhang, Z.; Zhu, G.; Yang, M.; Ai, Z.; Zheng, Q.; Su, D. Saikosaponin antidepressant mechanism: Improving the sphingolipid metabolism in the cortex via Apolipoprotein E and triggering neurovascular coupling. Phytomedicine 2024, 132, 155829. [Google Scholar] [CrossRef]
- Brunkhorst-Kanaan, N.; Klatt-Schreiner, K.; Hackel, J.; Schröter, K.; Trautmann, S.; Hahnefeld, L.; Wicker, S.; Reif, A.; Thomas, D.; Geisslinger, G.; et al. Targeted lipidomics reveal derangement of ceramides in major depression and bipolar disorder. Metabolism 2019, 95, 65–76. [Google Scholar] [CrossRef]
- Demirkan, A.; Isaacs, A.; Ugocsai, P.; Liebisch, G.; Struchalin, M.; Rudan, I.; Wilson, J.F.; Pramstaller, P.P.; Gyllensten, U.; Campbell, H.; et al. Plasma phosphatidylcholine and sphingomyelin concentrations are associated with depression and anxiety symptoms in a Dutch family-based lipidomics study. J. Psychiatr. Res. 2013, 47, 357–362. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Lanctôt, K.L. Ceramides and depression: A systematic review. J. Affect. Disord. 2017, 213, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Suarez, M.D.; Yadav, P.K.; Walsh, M.T.; Li, Y.; Wu, Y.; Huang, Z.; James, A.W.; Escobar, V.; Mokbe, A.; et al. ATP-binding cassette protein ABCA7 deficiency impairs sphingomyelin synthesis, cognitive discrimination, and synaptic plasticity in the entorhinal cortex. J. Biol. Chem. 2022, 298, 102411. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Walter, S.; Becker, K.A.; Halmer, R.; Liu, Y.; Reichel, M.; Edwards, M.J.; Muller, C.P.; Fassbender, K.; Kornhuber, J. A central role for the acid sphingomyelinase/ceramide system in neurogenesis and major depression. J. Neurochem. 2015, 134, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Pujol-Lereis, L.M. Alteration of Sphingolipids in Biofluids: Implications for Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3564. [Google Scholar] [CrossRef]
- Henriques, A.; Croixmarie, V.; Bouscary, A.; Mosbach, A.; Keime, C.; Boursier-Neyret, C.; Walter, B.; Spedding, M.; Loeffler, J. Sphingolipid Metabolism Is Dysregulated at Transcriptomic and Metabolic Levels in the Spinal Cord of an Animal Model of Amyotrophic Lateral Sclerosis. Front. Molec. Neurosci. 2017, 10, 433. [Google Scholar] [CrossRef]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019, 31, 617–625. [Google Scholar] [CrossRef]
- Mao, C.; Obeid, L.M. Ceramidases: Regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim. Biophys. Acta 2008, 1781, 424–434. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Oh, S.Y.; Cho, K.; Kang, J.L.; Kim, K.H.; Woo, S. Comparison of experimental mouse models of inflammatory bowel disease. Int. J. Mol. Med. 2014, 33, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Merlin, D. Unveiling Colitis: A Journey through the Dextran Sodium Sulfate-induced Model. Inflamm. Bowel Dis. 2024, 30, 844–853. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Bi, X.; Feng, Q.; Sun, Y. Supplementation with Lentil (Lens culinaris) Hull Soluble Dietary Fiber Ameliorates Sodium Dextran Sulfate-Induced Colitis and Behavioral Deficits via the Gut-Brain Axis. Foods 2025, 14, 870. https://doi.org/10.3390/foods14050870
Chen D, Bi X, Feng Q, Sun Y. Supplementation with Lentil (Lens culinaris) Hull Soluble Dietary Fiber Ameliorates Sodium Dextran Sulfate-Induced Colitis and Behavioral Deficits via the Gut-Brain Axis. Foods. 2025; 14(5):870. https://doi.org/10.3390/foods14050870
Chicago/Turabian StyleChen, Dongying, Xin Bi, Qian Feng, and Yong Sun. 2025. "Supplementation with Lentil (Lens culinaris) Hull Soluble Dietary Fiber Ameliorates Sodium Dextran Sulfate-Induced Colitis and Behavioral Deficits via the Gut-Brain Axis" Foods 14, no. 5: 870. https://doi.org/10.3390/foods14050870
APA StyleChen, D., Bi, X., Feng, Q., & Sun, Y. (2025). Supplementation with Lentil (Lens culinaris) Hull Soluble Dietary Fiber Ameliorates Sodium Dextran Sulfate-Induced Colitis and Behavioral Deficits via the Gut-Brain Axis. Foods, 14(5), 870. https://doi.org/10.3390/foods14050870






