Post-Fermentation Application of Pea Protein-Based Fining Agents: Effects on Aromatic White Wine from Tămâioasa Românească
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials: Wine and Fining Agents
2.2. Experiments for Wine Fining
2.3. Wine Analysis
2.3.1. Main Wine Parameters Determinations
2.3.2. Wine Colour Determination
2.3.3. Wine Volatile Compounds Evaluation
2.4. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of the Experimental Wines
3.2. Volatile Profile of the Experimental Wines
4. Discussion
4.1. Influence of the Fining Treatments on the Main Wine Parameters
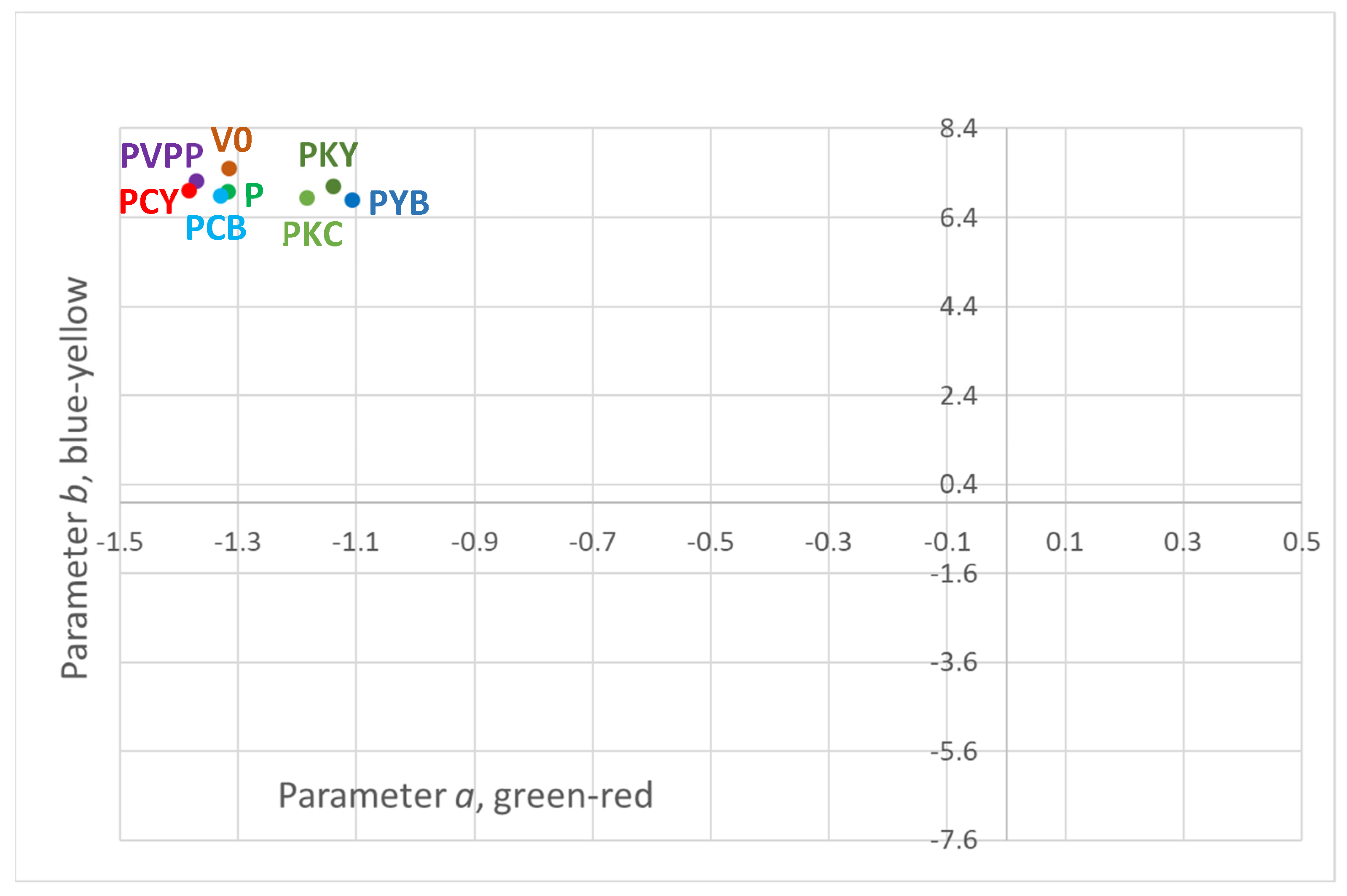
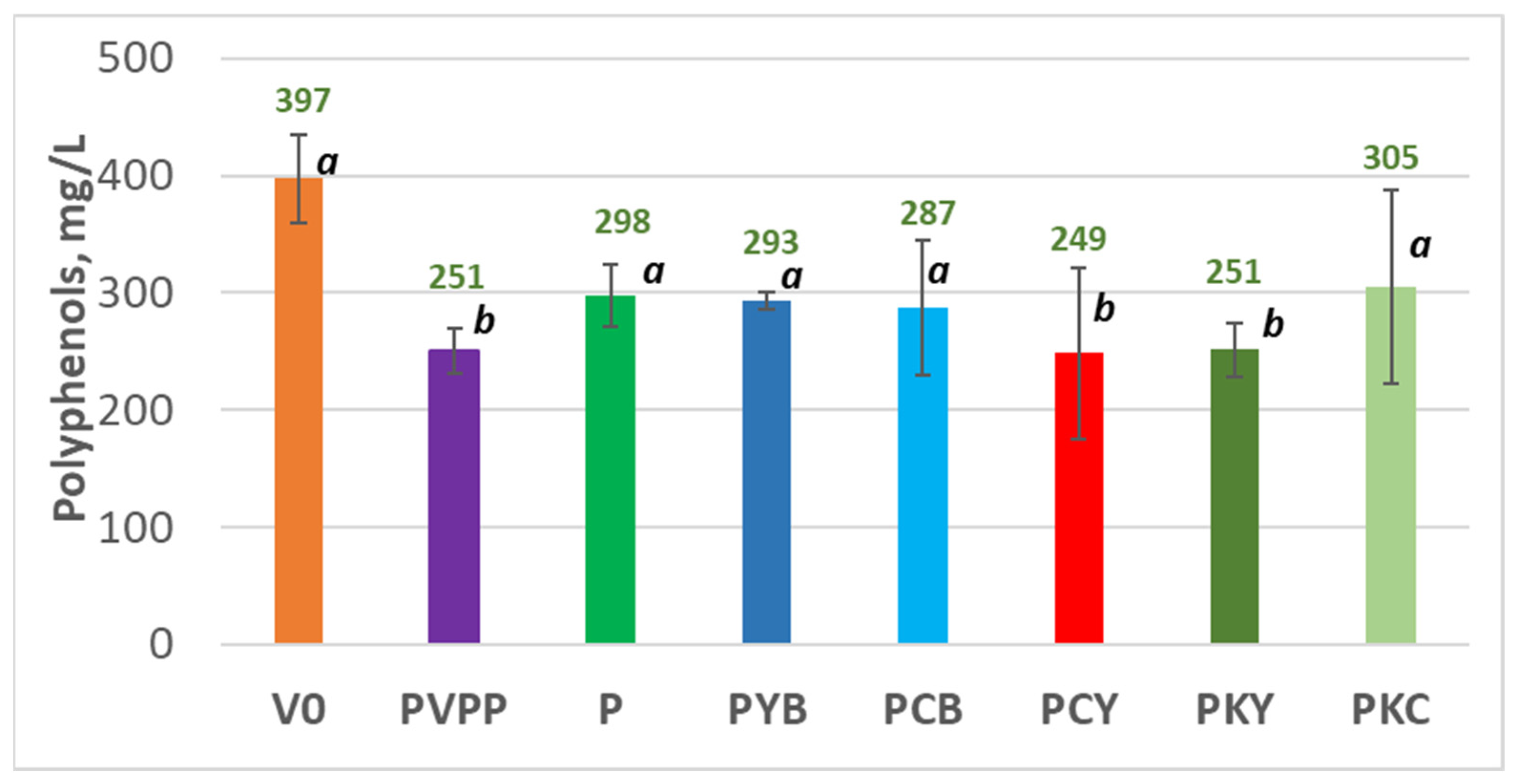
4.2. Influence of the Fining Treatments on the Wine Colour and Polypehnol Content
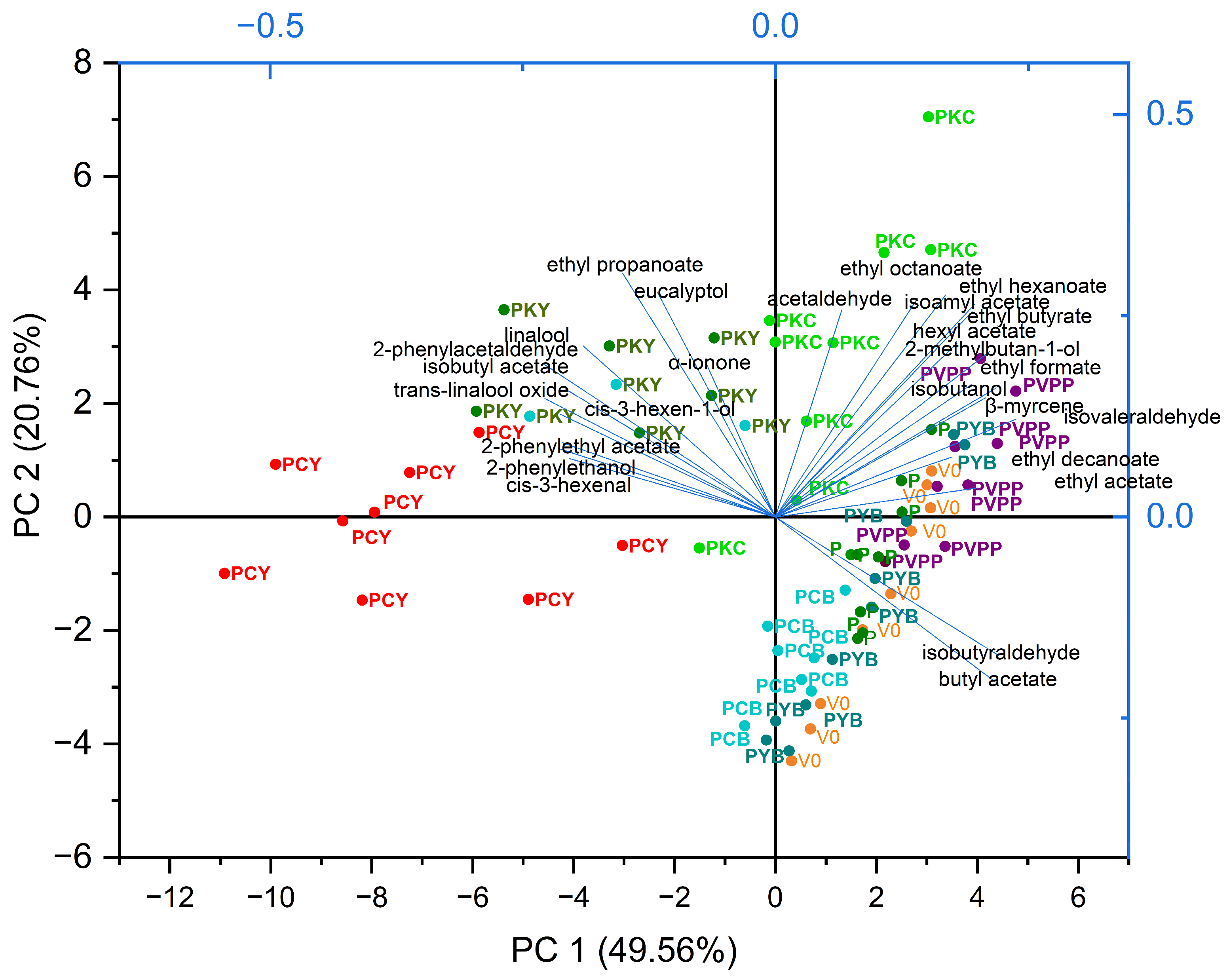
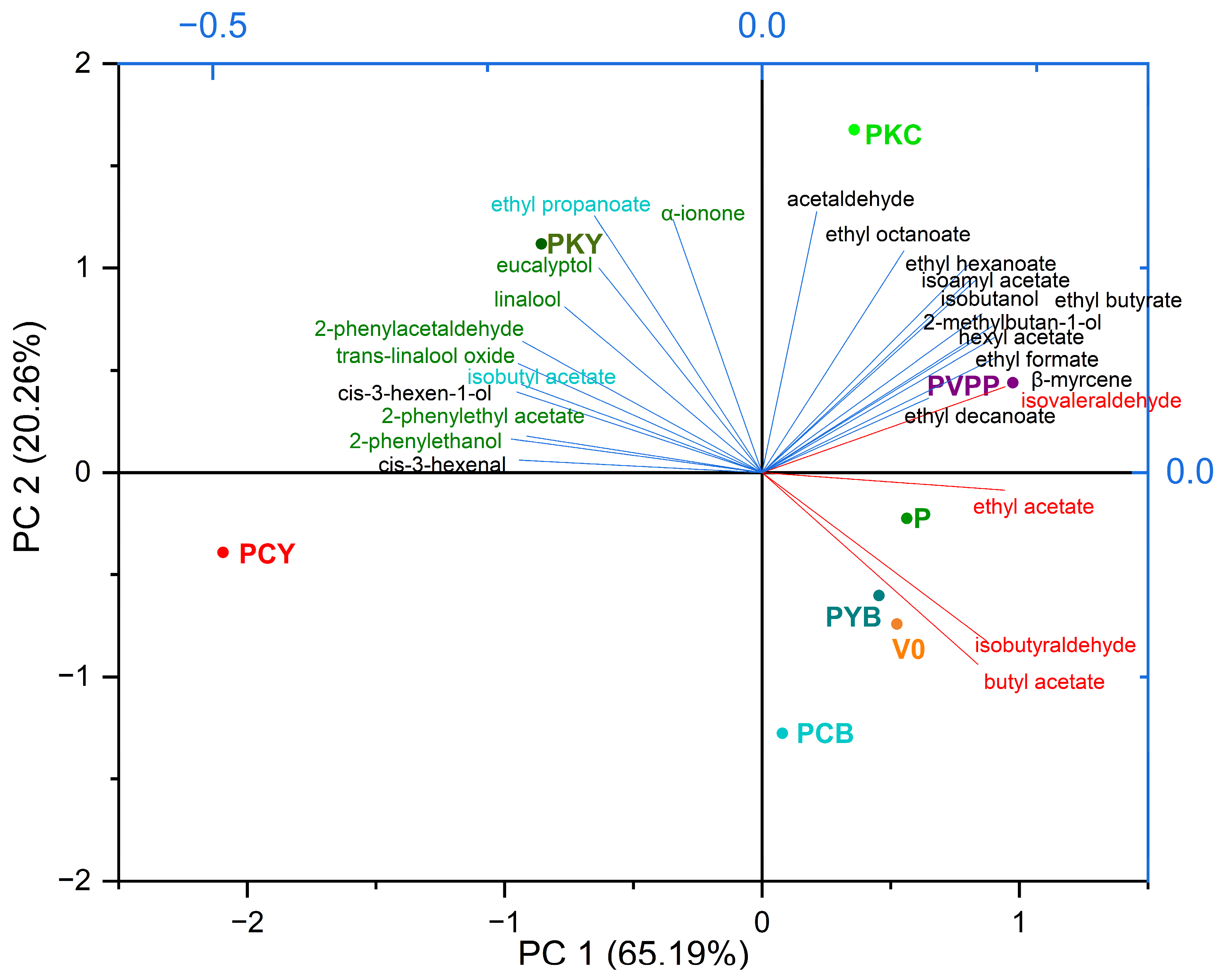
4.3. Influence of the Fining Treatments on the Volatile and Aromatic Profile of Wine
5. Conclusions
- ▪
- PKY (Pea protein + Chitosan + Yeast hulls): Most expressive wines; high terpenes and esters; floral, fruity, herbal notes.
- ▪
- PKC (Pea protein + Chitosan + Bentonite): Preserves ester-dominated profile; moderate terpene increase; clean and complex wines.
- ▪
- PCY (Pea protein + Chitosan + Yeast hulls): Enhances Muscat-type aromas; slightly reduces some esters; high floral notes.
- ▪
- P (Pea protein alone): Maintains aroma close to untreated; minimal impact on volatiles; PVPP alternative.
- ▪
- PVPP: Effective polyphenol reduction; greener tones; minor aroma changes; less expressive in terpenes.
- ▪
- PCB (Pea protein + Bentonite + Chitosan) & PYB (Pea protein + Yeast hulls + Bentonite): Simplified aromas; reduced esters and terpenes; diminished varietal character.
6. Patents: Deposit
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosme, F.; Capão, I.; Filipe-Ribeiro, L.; Bennett, R.N.; Mendes-Faia, A. Evaluating Potential Alternatives to Potassium Caseinate for White Wine Fining: Effects on Physicochemical and Sensory Characteristics. LWT—Food Sci. Technol. 2012, 46, 382–387. [Google Scholar] [CrossRef]
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine Fining with Plant Proteins. Molecules 2019, 24, 2186. [Google Scholar] [CrossRef]
- Peñas, E.; di Lorenzo, C.; Uberti, F.; Restani, P. Allergenic Proteins in Enology: A Review on Technological Applications and Safety Aspects. Molecules 2015, 20, 13144–13164. [Google Scholar] [CrossRef]
- Regulation (EU) No. 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers; Article 21. Official Journal of the European Union, L 304, 22 November 2011; pp. 18–63. Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj/eng (accessed on 16 September 2025).
- Commission Delegated Regulation (EU) 2019/33 of 17 October 2018 Supplementing Regulation (EU) No. 1308/2013 of the European Parliament and of the Council as Regards Applications for Protection of Designations of Origin, Geographical Indications and Traditional Terms in the Wine Sector, the Objection Procedure, Restrictions of Use, Amendments to Product Specifications, Cancellation of Protection, and Labelling and Presentation; Article 41. Official Journal of the European Union, L 9, 10 January 2019; pp. 2–45. Available online: https://eur-lex.europa.eu/eli/reg_del/2019/33/oj/eng (accessed on 16 September 2025).
- International Organisation of Vine and Wine (OIV). Wine—Fining Using Proteins of Plant Origin (Resolution OENO 8/2004); OIV: Paris, France, 2004; Available online: https://www.oiv.int/node/3642 (accessed on 22 August 2025).
- Lárez Velásquez, C. Chitosan and Its Applications in Oenology. OENO One 2023, 57, 121–132. [Google Scholar] [CrossRef]
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The Use of Chitosan as Alternative to Bentonite for Wine Fining: Effects on Heat-Stability, Proteins, Organic Acids, Colour, and Volatile Compounds in an Aromatic White Wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). Cellular Yeast Hulls—Codex (Yeast Walls) (OENO 497/2013); OIV: Paris, France, 2013; Available online: https://www.oiv.int/standards/international-oenological-codex/part-i-monographs/monographs/yeast-hulls (accessed on 22 August 2025).
- Antoce, O.A.; Cojocaru, G.A. Effects of Pre-Fermentative Treatments with Non-Synthetic Ternary Component Fining Agents Based on Pea Protein on the Volatile Profiles of Aromatic Wines of Tămâioasă Românească. Beverages 2024, 10, 81. [Google Scholar] [CrossRef]
- Kang, W.; Niimi, J.; Bastian, S.E.P. Reduction of Red Wine Astringency Perception Using Vegetable Protein Fining Agents. Am. J. Enol. Vitic. 2018, 69, 22–31. [Google Scholar] [CrossRef]
- Kokkinomagoulos, E.; Kandylis, P. Sustainable Wine Fining: Evaluating Grape Pomace as a Natural Alternative to Commercial Agents. Beverages 2025, 11, 31. [Google Scholar] [CrossRef]
- Costa, E.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Algae Protein: Fining Agent for White Wine, Sustainable, Non-Allergenic and Animal-Free. In IVES Conference Series: OIV 2024; IVES Open Science: Villenave d’Ornon, France, 2024; p. 48464. Available online: https://ives-openscience.eu/48464 (accessed on 22 August 2025).
- Kumar, Y.; Suhag, R. Impact of Fining Agents on Color, Phenolics, Aroma, and Sensory Properties of Wine: A Review. Beverages 2024, 10, 71. [Google Scholar] [CrossRef]
- Gambuti, A.; Rinaldi, A.; Moio, L. Use of Patatin, a Protein Extracted from Potato, as an Alternative to Animal Proteins in Fining of Red Wine. Eur. Food Res. Technol. 2012, 235, 753–765. [Google Scholar] [CrossRef]
- Gordillo, B.; Chamizo-González, F.; Lourdes González-Miret, M.; Heredia, F.J. Impact of Alternative Protein Fining Agents on the Phenolic Composition and Color of Syrah Red Wines from Warm Climate. Food Chem. 2020, 342, 128297. [Google Scholar] [CrossRef]
- Popp, J.; Trendelenburg, V.; Niggemann, B.; Randow, S.; Völker, E.; Vogel, L.; Reuter, A.; Spiric, J.; Schiller, D.; Beyer, K.; et al. Pea (Pisum sativum) Allergy in Children: Pis s1 Is an Immunodominant Major Pea Allergen and Presents IgE Binding Sites with Potential Diagnostic Value. Clin. Exp. Allergy 2020, 50, 625–635. [Google Scholar] [CrossRef]
- Cojocaru, G.A.; Antoce, A.O. Use of Vegetable Proteins as Alternatives to PVPP and Caseinate for Removing Polyphenols from White Grape Musts. Sci. Pap. Ser. B Hortic. 2022, 66, 237–244. Available online: https://horticulturejournal.usamv.ro/pdf/2022/issue_1/Art38.pdf (accessed on 22 August 2025).
- Antoce, A.O. Pea Proteins as an Alternative to Proteins of Animal Origin for Wine Clarification—A Minireview. Sci. Pap. Ser. A Agron. 2025, LXVIII. (accepted for publication). [Google Scholar]
- Antoce, A.O.; Cojocaru, G.A. Testing the Potential of Innovative Treatments of White Grape Must with Vegetal Proteins—Sensory Impact on Wine. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2024, 24, 91–98. Available online: https://managementjournal.usamv.ro/pdf/vol.24_2/Art8.pdf (accessed on 22 August 2025).
- Order No. 273 of 17 September 2020 for the Approval of the List of Wine Grape Varieties That Can Be Planted, Replanted, or Grafted on the Territory of Romania for the Purpose of Wine Production. ONVPV. Available online: https://www.onvpv.ro/sites/default/files/ord_273_2020_lista_soiuri.pdf (accessed on 2 February 2025).
- Wine Grape Varieties Authorised for Production and for Labelling and Presentation in the Wine Sector, in Application of Article 81 and Article 120(2)(b) of Regulation (EU) No 1308/2013 (Article 50(1)(g) and 51(2) of R. (EU) 2018/273). European Commission Agriculture. Available online: https://agriculture.ec.europa.eu/document/download/9727a371-11b1-470b-a3c9-4af45962dd86_en?filename=wine-list-08a-grape-varieties-by-variety_en.pdf (accessed on 2 February 2025).
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and Their Role in Wine Flavour: Recent Advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine (OIV). International Code of Oenological Practices; OIV: Paris, France, 2021; Available online: https://www.oiv.int/public/medias/7713/en-oiv-code-2021.pdf (accessed on 15 August 2025).
- Antoce, A.O.; Cojocaru, G.A. Tri-Component Plant-Based Products Based on Pea Protein for Fining White Wines. Patent Application A/00118/2025; OSIM (State Office for Inventions and Trademarks, Romania), 28 March 2025. [Google Scholar]
- International Organisation of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis; OIV: Dijon, France, 2024; Available online: https://www.oiv.int/standards/compendium-of-international-methods-of-wine-and-must-analysis (accessed on 11 February 2025).
- Campo-Martínez, J.F.; Enseñat-Berea, M.L.; Fernández-Paz, J.; González-Castro, M.J. Validation of a Fast Automated Photometric Method for the Analysis of Sulfur Dioxide in Wines. Eur. Food Res. Technol. 2024, 250, 1611–1618. [Google Scholar] [CrossRef]
- González-González, M.; Flores-Dela Toba, R.; Ortiz-Martínez, M.; Rito-Palomares, M. Development and Evaluation of Colorimetric pH Determination Methods as a Potential Tool for Biomarkers Monitoring. J. Chem. Technol. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 30, 144–158. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin–Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- BioSystems Enology Catalog. Available online: https://www.metrohm.com/content/dam/metrohm/en_au/documents/BioSystems_-_Enology_Catalogue.pdf (accessed on 16 September 2025).
- BioSystems [Barcelona, Spain]. Available online: https://einfo.bio (accessed on 25 July 2025).
- Vermerris, W.; Nicholson, R. Isolation and Identification of Phenolic Compounds. In Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2006; pp. 235–255. [Google Scholar] [CrossRef]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in Wood: Comparison of Different Estimation Methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Bate-Smith, E.C. Astringent Tannins of Acer Species. Phytochemistry 1977, 16, 1421–1426. [Google Scholar] [CrossRef]
- Antoce, A.O.; Artem, V.; Cojocaru, G.A. Chitosan Treatments in Organic Vineyard and Their Impact on the Colour and Sensory Parameters of Feteasca Neagra Wines. Sci. Pap. Ser. A Agron. 2022, 65, 635–642. Available online: https://agronomyjournal.usamv.ro/pdf/2022/issue_1/Art90.pdf (accessed on 22 August 2025).
- Hensel, M.; Di Nonno, S.; Mayer, Y.; Scheiermann, M.; Fahrer, J.; Durner, D.; Ulber, R. Specification and Simplification of Analytical Methods to Determine Wine Color. Processes 2022, 10, 2707. [Google Scholar] [CrossRef]
- Khashayar, G.; Bain, P.A.; Salari, S.; Dozic, A.; Kleverlaan, C.J.; Feilzer, A.J. Perceptibility and Acceptability Thresholds for Colour Differences in Dentistry. J. Dent. 2014, 42, 637–644. [Google Scholar] [CrossRef]
- Hensel, M.; Scheiermann, M.; Fahrer, J.; Durner, D. New Insights into Wine Color Analysis: A Comparison of Analytical Methods to Sensory Perception for Red and White Varietal Wines. J. Agric. Food Chem. 2024, 72, 2008–2017. [Google Scholar] [CrossRef]
- Antoce, A.O.; Cojocaru, G.A. Evaluation by Flash GC Electronic Nose of the Effect of Combinations of Yeasts and Nutrients on the Aromatic Profiles of Feteasca Regala Wines after Two Years of Storage. Fermentation 2021, 7, 223. [Google Scholar] [CrossRef]
- Cojocaru, G.A.; Antoce, A.O. Influence of Glutathione and Ascorbic Acid Treatments during Vinification of Feteasca Regala Variety and Their Antioxidant Effect on Volatile Profile. Biosensors 2019, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Pherobase. Pherobase Database: Database of Pheromones and Semiochemicals. Available online: https://www.pherobase.com (accessed on 14 June 2025).
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: https://www.flavornet.org/index.html (accessed on 14 June 2025).
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database, Version 5.12; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2024. [CrossRef]
- Banită, C.; Antoce, O.A.; Cojocaru, G.A. Evaluation by a GC Electronic Nose of the Differences in Volatile Profile Induced by Stopping Fermentation with Octanoic and Decanoic Acid to Produce Sweet Wines. Chemosensors 2023, 11, 98. [Google Scholar] [CrossRef]
- Antoce, O.A.; Namolosanu, C.I. Rapid and Precise Discrimination of Wines by Means of an Electronic Nose Based on Gas-Chromatography. Rev. Chim. 2011, 62, 593–595. [Google Scholar]
- Antoce, O.A.; Stroe, M.V.; Cojocaru, G.A. Tentative Application of an Electronic Nose to the Study of the Parentage of Romanian Grape Varieties Sarba and Alb Aromat. Agric. Agric. Sci. Procedia 2015, 6, 110–117. [Google Scholar] [CrossRef]
- Pambianchi, D. Understanding and Measuring Residual Sugars. WineMaker Magazine. Available online: https://winemakermag.com/article/residual-sugars (accessed on 22 August 2025).
- Payan, C.; Gancel, A.-L.; Jourdes, M.; Christmann, M.; Teissedre, P.-L. Wine Acidification Methods: A Review. OENO One 2023, 57, 113–126. [Google Scholar] [CrossRef]
- Stockley, C.; Paschke-Kratzin, A.; Teissedre, P.-L.; Restani, P.; Garcia Tejedor, N.; Quini, C. SO2 and Wine: A Review, 1st ed.; OIV Collective Expertise Document; OIV Publications: Paris, France, 2021; ISBN 978-2-85038-022-8. [Google Scholar]
- Bonilla, F.; Mayen, M.; Merida, J.; Medina, M. Yeasts Used as Fining Treatment to Correct Browning in White Wines. J. Agric. Food Chem. 2001, 49, 1928–1933. [Google Scholar] [CrossRef]
- Tița, O.; Onache, P.A.; Geana, E.-I.; Ciucure, C.T.; Sumedrea, D.I.; Florea, A. Phytochemical Screening and Antioxidant Activities of White and Red Wines from Different Varieties and Wine Regions in Romania. Antioxidants 2025, 14, 564. [Google Scholar] [CrossRef] [PubMed]
- Symoneaux, R.; Baron, A.; Marnet, N.; Bauduin, R.; Chollet, S. Impact of Apple Procyanidins on Sensory Perception in Model Cider (Part 1): Polymerisation Degree and Concentration. LWT—Food Sci. Technol. 2014, 57, 22–27. [Google Scholar] [CrossRef]
- de-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Ferreira, V. On the Effects of Higher Alcohols on Red Wine Aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Marrufo-Curtido, A.; de-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Ferreira, V.; Bueno, M.; Escudero, A. Sensory Relevance of Strecker Aldehydes in Wines. Preliminary Studies of Its Removal with Different Type of Resins. Foods 2021, 10, 1711. [Google Scholar] [CrossRef]
- Tian, H.Y.; Zhang, J.; Sun, B.G.; Huang, M.Q.; Li, J.R.; Han, X.X. Preparation of Natural Isovaleraldehyde by the Maillard Reaction. Chin. Chem. Lett. 2007, 18, 1049–1052. [Google Scholar] [CrossRef]
- Qian, M. Aroma Compounds in Wine: A Review. Flavour Fragr. J. 2003, 18, 252. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape Contribution to Wine Aroma: Production of Hexyl Acetate, Octyl Acetate, and Benzyl Acetate during Yeast Fermentation Is Dependent upon Precursors in the Must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the Aroma of Grapes and Wines: A Review. South Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Chigo-Hernandez, M.M.; DuBois, A.; Tomasino, E. Aroma Perception of Rose Oxide, Linalool and α-Terpineol Combinations in Gewürztraminer Wine. Fermentation 2022, 8, 30. [Google Scholar] [CrossRef]
- Luzzini, G.; Slaghenaufi, D.; Ugliano, M. Approaches to the Classification of Wine Aroma Ageing Potential: Applications to the Case of Terpenoids in Valpolicella Red Wines. OENO One 2022, 56, 231–242. [Google Scholar] [CrossRef]
- Langen, J.; Wegmann-Herr, P.; Schmarr, H.G. Quantitative Determination of α-Ionone, β-Ionone, and β-Damascenone and Enantio Differentiation of α-Ionone in Wine for Authenticity Control Using Multidimensional Gas Chromatography with Tandem Mass Spectrometric Detection. Anal. Bioanal. Chem. 2016, 408, 6483–6496. [Google Scholar] [CrossRef]
- Li, C.; Chen, H.; Li, Y.; Du, T.; Jia, J.; Xi, Z. The Expression of Aroma Components and Related Genes in Merlot and Marselan Scion-Rootstock Grape and Wine. Foods 2022, 11, 2777. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- ChemicalBook. 2-Methyl-1-Butanol. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB5697005.htm (accessed on 5 August 2025).
- Wine Enthusiast. The Science Behind the Main Wine Aromas, Explained. Available online: https://www.wineenthusiast.com/culture/wine/aromas-wine-describe-guide/ (accessed on 5 August 2025).
- Oenobrands. Key Positive Aroma Compounds. Available online: https://oenobrands.com/solution/wine-aroma/key-positive-aroma-compounds/ (accessed on 5 August 2025).
- Scentspiracy. Phenylethyl Acetate. Available online: https://www.scentspiracy.com/fragrance-ingredients/p/phenylethyl-acetate (accessed on 5 August 2025).
- PubChem. 1,8-Cineole. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2758 (accessed on 5 August 2025).
- Lira, E.; Salazar, F.N.; Rodríguez-Bencomo, J.J.; Vincenzi, S.; Curioni, A.; López, F. Effect of Using Bentonite during Fermentation on Protein Stabilisation and Sensory Properties of White Wine. Int. J. Food Sci. Technol. 2014, 49, 1074–1081. [Google Scholar] [CrossRef]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of Combined Effect of Proteins and Bentonite Fining on the Wine Aroma Loss. J. Agric. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Lambri, M.; Dordoni, R.; Silva, A.; De Faveri, D.M. Effect of Bentonite Fining on Odor-Active Compounds in Two Different White Wine Styles. Am. J. Enol. Vitic. 2010, 61, 225–233. [Google Scholar] [CrossRef]

| Sample Code (Tanks) | PVPP | P/Pea Protein | B/Bentonite | C/Carbon | Y/Yeast Hulls | K/Chitosan |
|---|---|---|---|---|---|---|
| Dose, g/hL | ||||||
| V0 (V0_1, V0_2, V0_3) | 0 | 0 | 0 | 0 | 0 | 0 |
| PVPP (PV_1, PV_2, PV_3) | 20 | 0 | 0 | 0 | 0 | 0 |
| P (P_1, P_2, P_3) | 0 | 20 | 0 | 0 | 0 | 0 |
| PYB (PYB_1, PYB_2, PYB_3) | 0 | 10 | 5 | 0 | 5 | 0 |
| PCB | 0 | 10 | 5 | 5 | 0 | 0 |
| PCY | 0 | 10 | 0 | 5 | 5 | 0 |
| PKY | 0 | 10 | 0 | 0 | 5 | 5 |
| PKC | 0 | 10 | 0 | 5 | 0 | 5 |
| Sample Code | Free Sugar (Glucose + Fructose), g/L | Ethanol, % v/v | Titratable Acidity, g/L | pH | Malic Acid, g/L | Acetic Acid, g/L | Total SO2, mg/L |
|---|---|---|---|---|---|---|---|
| V0 | 2.22 ± 0.27 a | 14.32 ± 0.04 a | 4.79 ± 0.06 c | 3.6 ± 0.0 b | 0.93 ± 0.01 a | 0.20 ± 0.07 a | 115.67 ± 3.51 a |
| PVPP | 2.03 ± 0.08 ab | 14.20 ± 0.11 a | 4.84 ± 0.03 cb | 3.7 ± 0.1 ab | 0.91 ± 0.09 a | 0.23 ± 0.01 a | 114.67 ± 5.69 a |
| P | 1.76 ± 0.17 b | 14.30 ± 0.07 a | 4.77 ± 0.02 c | 3.7 ± 0.1 a | 0.91 ± 0.07 a | 0.18 ± 0.08 a | 93.00 ± 10.44 bc |
| PYB | 1.75 ± 0.05 b | 14.33 ± 0.02 a | 4.82 ± 0.01 c | 3.7 ± 0.1 a | 0.89 ± 0.08 a | 0.17 ± 0.08 a | 86.00 ± 2.65 c |
| PCB | 1.68 ± 0.09 b | 14.22 ± 0.10 a | 4.92 ± 0.01 ab | 3.7 ± 0.1 a | 0.80 ± 0.11 a | 0.19 ± 0.10 a | 101.00 ± 2.59 abc |
| PCY | 1.77 ± 0.09 b | 14.24 ± 0.08 a | 4.92 ± 0.02 ab | 3.7 ± 0.1 ab | 0.73 ± 0.01 a | 0.22 ± 0.01 a | 99.67 ± 6.03 abc |
| PKY | 1.96 ± 0.08 ab | 14.24 ± 0.08 a | 4.98 ± 0.02 a | 3.6 ± 0.1 b | 0.81 ± 0.09 a | 0.22 ± 0.01 a | 101.67 ± 3.21 abc |
| PKC | 1.89 ± 0.09 ab | 14.31 ± 0.01 a | 4.97 ± 0.04 a | 3.6 ± 0.1 b | 0.73 ± 0.06 a | 0.29 ± 0.03 a | 109.33 ± 10.97 ab |
| Sample Code | L | a | b | cab | hab | ΔE |
|---|---|---|---|---|---|---|
| V0 | 98.18 ± 0.22 ab | −1.32 ± 0.08 abc | 7.50 ± 0.04 a | 7.62 ± 0.04 a | 100.04 ± 0.01 abc | |
| PVPP | 97.80 ± 0.15 b | −1.37 ± 0.03 a | 7.22 ± 0.03 ab | 7.35 ± 0.03 ab | 100.86 ± 0.00 abc | 0.52 ± 0.20 a |
| P | 98.41 ± 0.08 a | −1.32 ± 0.07 ab | 6.98 ± 0.24 bcd | 7.11 ± 0.25 bcd | 100.82 ± 0.00 abc | 0.62 ± 0.15 a |
| PYB | 98.25 ± 0.18 ab | −1.11 ± 0.15 c | 6.78 ± 0.10 d | 6.87 ± 0.07 d | 99.22 ± 0.02 bc | 0.82 ± 0.03 a |
| PCB | 98.51 ± 0.17 a | −1.33 ± 0.04 ab | 6.88 ± 0.09 cd | 7.01 ± 0.09 cd | 101.09 ± 0.00 ab | 0.76 ± 0.08 a |
| PCY | 98.40 ± 0.18 a | −1.38 ± 0.02 a | 7.00 ± 0.03 bcd | 7.13 ± 0.03 abc | 101.24 ± 0.00 a | 0.59 ± 0.05 a |
| PKY | 98.44 ± 0.09 a | −1.14 ± 0.02 bc | 7.11 ± 0.06 bc | 7.20 ± 0.06 bc | 99.09 ± 0.00 c | 0.52 ± 0.05 a |
| PKC | 98.60 ± 0.14 a | −1.18 ± 0.07 abc | 6.83 ± 0.01 cd | 6.93 ± 0.02 cd | 99.87 ± 0.01 abc | 0.83 ± 0.06 a |
| Sample Code | Proanthocyanidins | Flavan-3,4-diols | Sum Proanthocyanidins+Flavan-3,4-diols | Total Polyphenols, mg/L | Total Polyphenols, % |
|---|---|---|---|---|---|
| mg/L Cyanidin Equivalents | |||||
| V0 | 15.12 ± 0.57 a | 3.70 ± 0.51 c | 18.82 ± 0.08 ab | 397.33 ± 37.50 a | 100.00 |
| PVPP | 12.27 ± 0.16 a | 2.59 ± 0.33 b | 14.87 ± 0.03 b | 251.00 ± 19.16 b | 63.17 |
| P | 15.30 ± 1.49 a | 3.94 ± 0.12 ac | 19.24 ± 1.48 ab | 297.67 ± 26.10 ab | 74.92 |
| PYB | 15.63 ± 0.29 a | 3.87 ± 0.53 ac | 19.50 ± 0.39 ab | 293.00 ±7.21 ab | 73.74 |
| PCB | 16.65 ± 1.15 a | 3.76 ± 0.19 ac | 20.41 ± 1.17 a | 286.67 ± 57.55 ab | 72.15 |
| PCY | 15.54 ± 0.24 a | 3.84 ± 0.33 ac | 19.38 ± 0.51 ab | 248.67 ± 73.22 b | 62.59 |
| PKY | 15.99 ± 4.61 a | 3.94 ± 0.18 ac | 19.92 ± 4.47 a | 251.33 ± 22.37 b | 63.26 |
| PKC | 14.86 ± 1.21 a | 4.59 ± 0.05 a | 19.45 ± 0.1.23 ab | 305.33 ± 82.25 ab | 76.85 |
| Adj. R2 | Column | Identified Compounds | Weighted Kovats Index | Control | PVPP | P | PYB | PCB | PKY | PKC | PCY |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | |||||||||||
| 0.573 | DB5 | isobutanol | 624.8 | 7378.55 ± 448.64 bcd | 8135.19 ± 275.00 a | 7818.19 ± 320.37 abc | 7574.70 ± 103.32 bc | 7324.14 ± 325.61 cd | 7364.14 ± 138.10 d | 7932.53 ± 273.83 ab | 6831.46 ± 500.72 d |
| 0.473 | DB5 | 2-methylbutan-1-ol | 739.8 | 166,103.08 ± 7539.16 abc | 176,903.50 ± 6346.12 a | 170,950.69 ± 4625.53 abc | 168,064.87 ± 8315.51 abc | 163,699.88 ± 7197.66 abc | 163,016.53 ± 6167.99 bcd | 174,563.15 ± 6840.33 cd | 154,644.89 ± 5796.10 d |
| 0.505 | DB1701 | 849.9 | 132,524.24 ± 1991.44 bcd | 138,097.00 ± 2234.83 a | 135,731.87 ± 4073.72 abc | 131,498.57 ± 5558.89 abcde | 129,153.50 ± 4543.37 cde | 128,651.14 ± 4499.21 de | 136,622.50 ± 4080.49 ab | 123,949.93 ± 5349.94 e | |
| 0.475 | DB1701 | cis-3-hexen-1-ol | 969.2 | 2821.27 ± 188.96 c | 3065.27 ± 204.18 abc | 2952.47 ± 160.75 abc | 2952.90 ± 140.50 abc | 2912.74 ± 219.22 bc | 3870.18 ± 740.59 ab | 3425.84 ± 356.78 a | 5020.75 ± 1766.82 abc |
| 0.518 | DB5 | 2-phenylethanol | 1107.2 | 779.39 ± 85.31 abc | 707.29 ± 28.53 bc | 698.57 ± 50.90 bc | 691.49 ± 50.06 c | 665.01 ± 91.54 bc | 1195.85 ± 495.17 abc | 788.23 ± 65.89 ab | 1800.71 ± 824.65 a |
| 0.741 | DB1701 | 1276.9 | 682.14 ± 28.65 cde | 714.95 ± 28.74 bcd | 717.33 ± 66.10 bcde | 655.70 ± 34.12 e | 658.19 ± 77.50 de | 1685.11 ± 766.25 abc | 809.01 ± 74.45 b | 1879.85 ± 37.11 a | |
| Aldehydes | |||||||||||
| 0.550 | DB1701 | acetaldehyde | 547.1 | 4673.70 ± 145.63 d | 5107.07 ± 275.02 bc | 5252.23 ± 361.86 abc | 5519.60 ± 490.73 abc | 4859.69 ± 227.12 cd | 5305.19 ± 148.01 ab | 6258.78 ± 849.25 a | 4886.67 ± 254.63 cd |
| 0.905 | DB1701 | isobutyraldehyde | 819.8 | 779.33 ± 71.96 ab | 799.49 ± 33.53 a | 707.73 ± 40.38 b | 770.81 ± 52.92 ab | 739.47 ± 61.98 ab | 425.40 ± 42.97 d | 563.13 ± 36.36 c | 418.40 ± 28.66 d |
| 0.694 | DB5 | isovaleraldehyde (3-methylbutanal) | 653.9 | 641.18 ± 40.79 a | 637.63 ± 69.62 abc | 603.98 ± 10.38 ab | 578.78 ± 20.30 bc | 550.23 ± 33.34 cd | 472.15 ± 68.43 de | 576.99 ± 17.55 c | 422.94 ± 69.28 e |
| 0.646 | DB1701 | 731.1 | 11,267.74 ± 504.46 abc | 11,966.26 ± 470.64 a | 11,468.16 ± 260.32 ab | 11,090.87 ± 322.54 bc | 10,748.97 ± 209.38 c | 10,516.14 ± 540.93 cd | 11,500.81 ± 444.31 ab | 9979.41 ± 538.42 d | |
| 0.419 | DB5 | cis-3-hexenal | 786 | 276.83 ± 59.87 ab | 337.12 ± 67.01 ab | 295.34 ± 18.87 b | 329.43 ± 21.10 a | 261.76 ± 141.59 ab | 451.86 ± 197.96 ab | 328.37 ± 112.86 ab | 804.59 ± 441.34 ab |
| 0.914 | DB5 | 2-phenylacetaldehyde | 1042.4 | 56.92 ± 2.19 d | 76.12 ± 8.58 c | 81.27 ± 13.01 c | 70.04 ± 17.90 cd | 72.36 ± 11.33 c | 248.53 ± 50.88 a | 128.59 ± 28.26 b | 272.52 ± 28.67 a |
| Acetate Esters * | |||||||||||
| 0.954 | DB5 | butyl acetate | 817.6 | 644.55 ± 25.26 a | 656.06 ± 59.97 a | 587.40 ± 75.67 a | 643.49 ± 13.78 a | 617.83 ± 36.75 a | 188.82 ± 53.73 c | 297.86 ± 44.82 b | 152.83 ± 10.49 c |
| 0.622 | DB5 | isobutyl acetate (isobutyl ethanoate) | 771.0 | 1518.54 ± 112.94 b | 1681.06 ± 104.44 b | 1579.96 ± 64.75 b | 1618.38 ± 103.56 b | 1613.96 ± 116.34 b | 1956.59 ± 173.35 a | 1833.73 ± 280.31 ab | 2440.52 ± 460.95 a |
| 0.461 | DB5 | isoamyl acetate (3-methylbutyl ethanoate) | 870.5 | 25,778.37 ± 1262.69 abc | 27,037.34 ± 1042.26 a | 25,934.23 ± 780.43 abc | 25,572.72 ± 1546.96 abcd | 24,959.43 ± 374.18 c | 25,239.64 ± 665.52 bc | 27,204.52 ± 1483.81 ab | 23,777.46 ± 806.27 d |
| 0.422 | DB1701 | 938.5 | 17,870.12 ± 845.94 ab | 18,508.53 ± 329.44 a | 17,974.11 ± 439.96 ab | 17,650.99 ± 1085.55 abc | 17,509.82 ± 460.71 b | 17,552.60 ± 393.41 b | 18,697.83 ± 942.16 ab | 16,569.84 ± 494.68 c | |
| 0.270 | DB5 | hexyl acetate | 1003.7 | 473.02 ± 42.44 a | 477.93 ± 40.07 a | 455.35 ± 35.82 a | 449.43 ± 57.84 ab | 401.10 ± 17.73 b | 433.48 ± 51.74 ab | 465.70 ± 66.39 ab | 384.87 ± 44.50 b |
| 0.347 | DB1701 | 1075.5 | 675.59 ± 35.74 ab | 716.75 ± 24.42 a | 675.36 ± 40.44 ab | 672.13 ± 67.95 abc | 645.55 ± 12.23 b | 653.91 ± 33.96 b | 691.22 ± 79.29 abc | 587.44 ± 25.17 c | |
| 0.599 | DB1701 | 2-phenylethyl acetate | 1338.4 | 132.24 ± 10.86 cd | 140.49 ± 3.78 bc | 147.87 ± 19.92 bc | 119.28 ± 12.30 de | 108.54 ± 6.53 e | 186.93 ± 20.31 a | 133.96 ± 21.76 cde | 286.70 ± 114.11 ab |
| Ethyl esters | |||||||||||
| 0.677 | DB5 | ethyl formate | 541.5 | 76,684.69 ± 2167.09 a | 79,766.50 ± 2428.92 a | 76,971.01 ± 2406.17 a | 75,683.30 ± 4391.43 ab | 72,918.82 ± 989.62 b | 72,132.80 ± 3047.97 bc | 80,351.78 ± 2682.08 a | 68,099.56 ± 1157.63 c |
| 0.631 | DB1701 | 582.5 | 58,655.02 ± 3193.07 abc | 60,749.90 ± 1630.88 a | 58,176.74 ± 1584.48 ac | 57,264.03 ± 3041.25 abc | 55,389.34 ± 1079.34 b | 54,742.72 ± 2623.20 bcd | 60,528.22 ± 1318.58 a | 52,082.11 ± 720.40 d | |
| 0.423 | DB5 | ethyl acetate | 614.5 | 79,097.53 ± 3293.11 a | 79,651.64 ± 3565.31 a | 77,027.24 ± 3212.75 a | 75,415.68 ± 3322.98 a | 78,270.62 ± 3842.38 a | 73,778.59 ± 4243.77 ab | 77,349.69 ± 3676.11 a | 69,544.97 ± 1779.45 b |
| 0.411 | DB1701 | 677 | 58,400.27 ± 2495.85 a | 59,381.77 ± 2666.82 a | 57,276.20 ± 2006.80 a | 56,178.44 ± 2147.81 a | 57,882.72 ± 2899.98 a | 54,326.62 ± 3466.29 ab | 56,922.87 ± 2582.70 a | 51,635.60 ± 2427.58 b | |
| 0.794 | DB1701 | ethyl propanoate | 769.5 | 2049.62 ± 86.81 bc | 2086.04 ± 63.24 b | 2076.47 ± 93.43 bc | 1975.80 ± 46.58 c | 2049.46 ± 88.74 bc | 2404.57 ± 139.56 a | 2396.40 ± 52.80 a | 2352.71 ± 66.34 a |
| 0.510 | DB5 | ethyl butyrate | 796.4 | 5587.00 ± 267.24 ab | 5867.52 ± 204.89 a | 5662.63 ± 133.37 ab | 5508.65 ± 237.58 ab | 5520.06 ± 188.29 b | 5545.20 ± 53.43 b | 5919.55 ± 299.50 ab | 5160.94 ± 171.00 c |
| 0.308 | DB5 | ethyl hexanoate | 991.3 | 15,758.77 ± 870.79 abc | 16,456.57 ± 531.86 a | 16,059.06 ± 716.57 ab | 15,814.54 ± 1255.01 abcd | 15,001.87 ± 224.11 cd | 15,585.15 ± 304.65 b | 16,668.46 ± 1810.23 abcd | 14,469.61 ± 545.79 d |
| 0.345 | DB1701 | 1056.9 | 11,805.61 ± 623.82 abc | 12,394.14 ± 452.99 a | 12,102.01 ± 526.59 ab | 11,809.79 ± 842.76 abcd | 11,271.09 ± 140.59 cd | 11,767.17 ± 117.67 b | 12,672.19 ± 1374.69 abcd | 10,908.74 ± 412.80 d | |
| 0.620 | DB5 | ethyl octanoate | 1189.9 | 15,540.08 ± 912.89 ab | 16,305.46 ± 1133.47 ab | 15,331.32 ± 726.07 b | 15,492.52 ± 1453.57 ab | 13,403.70 ± 520.27 c | 16,477.86 ± 313.67 a | 15,774.70 ± 976.63 ab | 13,157.18 ± 496.45 c |
| 0.453 | DB1701 | 1257.6 | 20,435.58 ± 1186.61 a | 21,190.73 ± 951.65 a | 20,243.18 ± 982.92 ab | 20,510.42 ± 1978.66 ab | 17,791.17 ± 528.09 c | 21,437.83 ± 603.11 a | 20,334.01 ± 1803.20 ab | 18,354.10 ± 1300.93 bc | |
| 0.953 | DB5 | ethyl decanoate | 1385 | 5277.23 ± 130.54 b | 5728.74 ± 183.03 a | 4740.93 ± 28.39 c | 4927.96 ± 370.01 bc | 3535.42 ± 132.35 e | 5114.56 ± 107.39 b | 4045.32 ± 179.04 d | 3140.32 ± 182.13 f |
| 0.933 | DB1701 | 1453.6 | 3736.40 ± 191.63 ab | 3906.41 ± 133.60 a | 3114.83 ± 111.58 c | 3312.50 ± 325.79 bc | 2222.63 ± 124.49 e | 3642.46 ± 100.24 b | 2665.27 ± 125.57 d | 2120.16 ± 156.77 e | |
| Terpenes and derivates | |||||||||||
| 0.615 | DB5 | eucalyptol (1,8-cineole) | 1026.5 | 310.71 ± 32.62 bcde | 325.28 ± 18.70 bc | 296.45 ± 15.63 de | 269.23 ± 27.41 e | 292.73 ± 34.44 cde | 382.85 ± 9.17 a | 325.12 ± 31.25 bcd | 351.40 ± 26.15 ab |
| 0.805 | DB1701 | linalool | 1191.7 | 309.29 ± 19.41 b | 282.48 ± 9.95 cd | 266.77 ± 15.08 de | 256.28 ± 8.73 e | 252.20 ± 4.05 e | 368.90 ± 37.28 a | 309.55 ± 22.30 bc | 361.04 ± 26.98 a |
| 0.633 | DB5 | trans-linalool oxide | 1091.3 | 196.46 ± 20.73 c | 190.06 ± 37.54 cd | 217.28 ± 56.55 cd | 152.92 ± 10.44 d | 151.82 ± 5.58 d | 867.30 ± 475.25 ab | 359.18 ± 38.19 b | 1038.75 ± 500.89 a |
| 0.590 | DB1701 | β-myrcene | 1015.1 | 198.73 ± 13.08 b | 228.77 ± 8.62 a | 216.83 ± 17.27 ab | 206.03 ± 15.88 b | 210.73 ± 2.44 b | 192.04 ± 19.83 b | 231.39 ± 34.00 ab | 161.02 ± 1.01 c |
| 0.668 | DB5 | α-ionone | 1426.3 | 362.51 ± 137.05 e | 578.37 ± 173.33 cde | 546.38 ± 66.89 de | 698.28 ± 97.53 c | 775.69 ± 77.08 bc | 1316.03 ± 451.36 ab | 1194.48 ± 234.66 a | 721.93 ± 165.33 bcd |
| Identified Volatile Compounds | Aroma Descriptors * | Impact on Wine Aroma | |
|---|---|---|---|
| Lower Concentrations | Higher Concentrations | ||
| Alcohols | |||
| isobutanol | solvent, bitter, glue, alcohol, leek, licorice | + enhances the overall complexity | − − − chemical off-odors, adding a solvent-like or alcoholic note |
| 2-methylbutan-1-ol | malty, cooked, roasted aroma with fruity or alcoholic undernotes, buttery | + enhances complexity or body | − − − solvent-like, nail polish remover notes, considered a fault |
| cis-3-hexen-1-ol | fresh, green, grassy, leafy | + + fresh, herbaceous complexity | − − overly grassy, green, overpowering more delicate aromas |
| 2-phenylethanol | floral, rose-like aroma, llilac, herbal spicy, honey-like, sweet, yeast | + + + elegant rose and floral notes adds complexity, enhances the perceived sweetness | |
| Aldehydes | |||
| acetaldehyde | green apple, cut grass, nutty, sherry-like, bruised apple flavour, paint | + green apple notes, nutty-oxidative aroma | − − − paint, pungent, oxidized, considered a fault |
| isobutyraldehyde | green, pungent, burnt, malty, toasted, fruity | +/− contributes to perceived graininess or maltiness, may even be perceived as a positive character | − − − undesirable aromas, associated with oxidation or spoilage. In young wines can reduce fruitiness. |
| Isovaleraldehyde (3-methylbutanal) | fruity, almond, toasted, malty, green, herbal | + pleasant, complex aromas, contributes to wine’s bouquet | − − unpleasant aromas described as “cardboard”, “rancid”, “ cheesy” or “sweaty” |
| cis-3-hexenal | freshly cut grass and leaves | + + pleasant freshly cut green apple | − − raw, vegetal, or unripe |
| 2-phenylacetaldehyde | floral, rose, lilac, hyacinth, sweet, honey-like and slightly fruity | + + + pleasant, key aroma compound in many floral wines | − heavy, perfumy character |
| Acetate Esters * | |||
| butyl acetate | fruity, banana, pear, pineapple, bitter, green, sweaty, strong | + reminiscent of red delicious apples | − − solvent-like, artificial, or nail-polish remover-like, fault |
| isobutyl acetate (isobutyl ethanoate) | fruity, pear, banana | + fruity pear-banana scent reminiscent of raspberry | − − solvent-like, artificial, glue-like, fault |
| isoamyl acetate (3-methylbutyl ethanoate) | banana-like or fruity aroma, fresh, sweet, fruity, pear | + + pleasant banana, pear and bubblegum aroma | − − synthetic or chemical odor |
| hexyl acetate | fruity, green, grassy aroma, spicy, herbal, rubbery, tobacco, citrus | + + described as pear-like with floral notes | − overly green or herbaceous |
| 2-phenylethyl acetate | rose, floral, fruity, sweet, sometimes rasppery/peach aroma | + + + desirable pleasant floral and fruity aroma | |
| Ethyl esters | |||
| ethyl formate | rum-like and sometimes fruity aroma, particularly resembling raspberries, sweet | + + ethereal-fruity | − solvent-like, artificial, or nail-polish remover-like, fault |
| ethyl acetate | caramel, sweet, solvent, fruity, acid, buttery, pungent | + adds fruity, fresh notes | − − overpowering, solvent-like, a sign of acetic spoilage, fault |
| ethyl propanoate | fruity, apple, pear, pineapples, sweet, solvent, acetone | + + sweet aroma reminiscent of pineapples and pears | − rarely overly sweet or artificial |
| ethyl butyrate | tropical fruit, overripe bananas, pineapple, caramel | + + sweet fruity notes | − rarely overly sweet or artificial |
| ethyl hexanoate | apples, pineapple, fruity, strawberry, anise, sweet, green apple, slightly floral | + + fresh, fruity notes, enhances overall aroma complexit | − rarely overly sweet, heavy, or slightly solvent-like |
| ethyl octanoate | fruity, pear, apricot, banana, pineapple, fresh, floral, fatty, green, leafy, anise, baked fruit, sweet, soapy | + + fruity, pleasant, enhances fruity complexity | − overly sweet, heavy, or slightly fatty |
| ethyl decanoate | sweet, apple, pear, and brandy-like aroma, grape, fruity | + + contributes to roundness, complexity, and subtle fruity notes | − fatty or soapy |
| Terpenes and derivates | |||
| eucalyptol (1,8-cineole) | herbal, camphor-like, eucalyptus spicy, mint, cooling | + + adds complexity, freshness, and herbal/spicy nuance | − medicinal, camphor-like |
| linalool | floral and slightly citrus aroma, flowery, lavender, orange blossom | + + + enhances floral and aromatic intensity | − overly perfumed or artificial |
| trans-linalool oxide | pleasant floral and herbal scent, sweet, floral, creamy, leafy, earthy, green | + + + adds complexity and soft floral nuances | rarely a problem |
| β-myrcene | herbal, resinous, anise, grape, fruity, herbaceous, vanilla, wine-like, vegetable, woody, green, metallic, musty, geranium, sweet, ethereal, soapy, lemon, spicy | + + subtle herbal, resinous, citrusy aroma, enhancing complexity | − too green, resinous or medicinal |
| α-ionone | violet, floral, and sometimes woody aroma | + + + adds subtle floral and violet notes, enhancing complexity | |
| Volatile Compounds | Loading on PC1 65.19% | Loadings on PC2 20.26% |
|---|---|---|
| Positive aroma varietal compounds | ||
| cis-3-hexen-1-ol | 0.24351 | 0.1515 |
| 2-phenylethanol | 0.24576 | −0.01736 |
| 2-phenylacetaldehyde | 0.28406 | 0.07289 |
| 2-phenylethyl acetate | 0.22382 | 0.04395 |
| eucalyptol | 0.19756 | 0.15424 |
| linalool | 0.25785 | 0.0217 |
| trans-linalool oxide | 0.27522 | 0.01049 |
| β-myrcene | −0.12992 | 0.27925 |
| α-ionone | 0.18613 | 0.11434 |
| Positive aroma fermentation compounds | ||
| isobutanol | −0.11392 | 0.24584 |
| 2-methylbutan-1-ol | −0.1528 | 0.28321 |
| acetaldehyde | 0.06346 | 0.25489 |
| cis-3-hexenal | 0.19369 | 0.10026 |
| isoamyl acetate | −0.11493 | 0.32901 |
| hexyl acetate | −0.12212 | 0.23973 |
| ethyl formate | −0.18579 | 0.21936 |
| ethyl butyrate | −0.08848 | 0.33594 |
| ethyl hexanoate | −0.06248 | 0.31162 |
| ethyl octanoate | 0.06949 | 0.233 |
| ethyl decanoate | −0.07229 | −0.07601 |
| Negative or dual aroma compounds | ||
| isobutyl acetate | 0.21601 | 0.18456 |
| ethyl propanoate | 0.22727 | 0.18809 |
| ethyl acetate | −0.18322 | 0.0738 |
| isobutyraldehyde | −0.26687 | −0.03865 |
| isovaleraldehyde | −0.22244 | 0.20702 |
| butyl acetate | −0.26549 | −0.07573 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoce, O.A.; Cojocaru, G.A. Post-Fermentation Application of Pea Protein-Based Fining Agents: Effects on Aromatic White Wine from Tămâioasa Românească. Foods 2025, 14, 3448. https://doi.org/10.3390/foods14193448
Antoce OA, Cojocaru GA. Post-Fermentation Application of Pea Protein-Based Fining Agents: Effects on Aromatic White Wine from Tămâioasa Românească. Foods. 2025; 14(19):3448. https://doi.org/10.3390/foods14193448
Chicago/Turabian StyleAntoce, Oana Arina, and George Adrian Cojocaru. 2025. "Post-Fermentation Application of Pea Protein-Based Fining Agents: Effects on Aromatic White Wine from Tămâioasa Românească" Foods 14, no. 19: 3448. https://doi.org/10.3390/foods14193448
APA StyleAntoce, O. A., & Cojocaru, G. A. (2025). Post-Fermentation Application of Pea Protein-Based Fining Agents: Effects on Aromatic White Wine from Tămâioasa Românească. Foods, 14(19), 3448. https://doi.org/10.3390/foods14193448






