Mechanistic Elucidation of the Anti-Ageing Effects of Dendrobium officinale via Network Pharmacology and Experimental Validation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
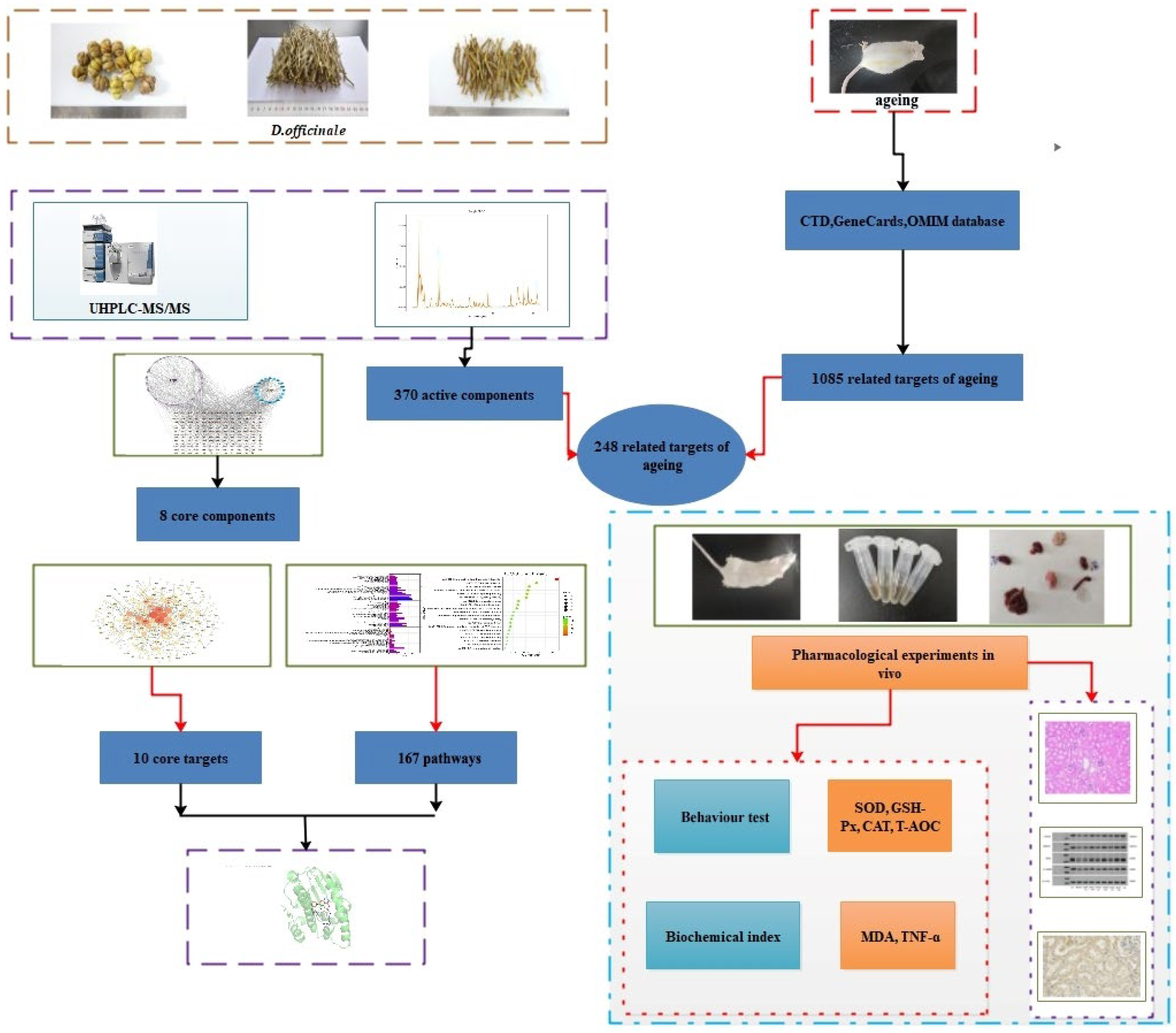
2.2. Network Pharmacology Analysis
2.2.1. Collection and Screening of Chemical Components in D. officinale
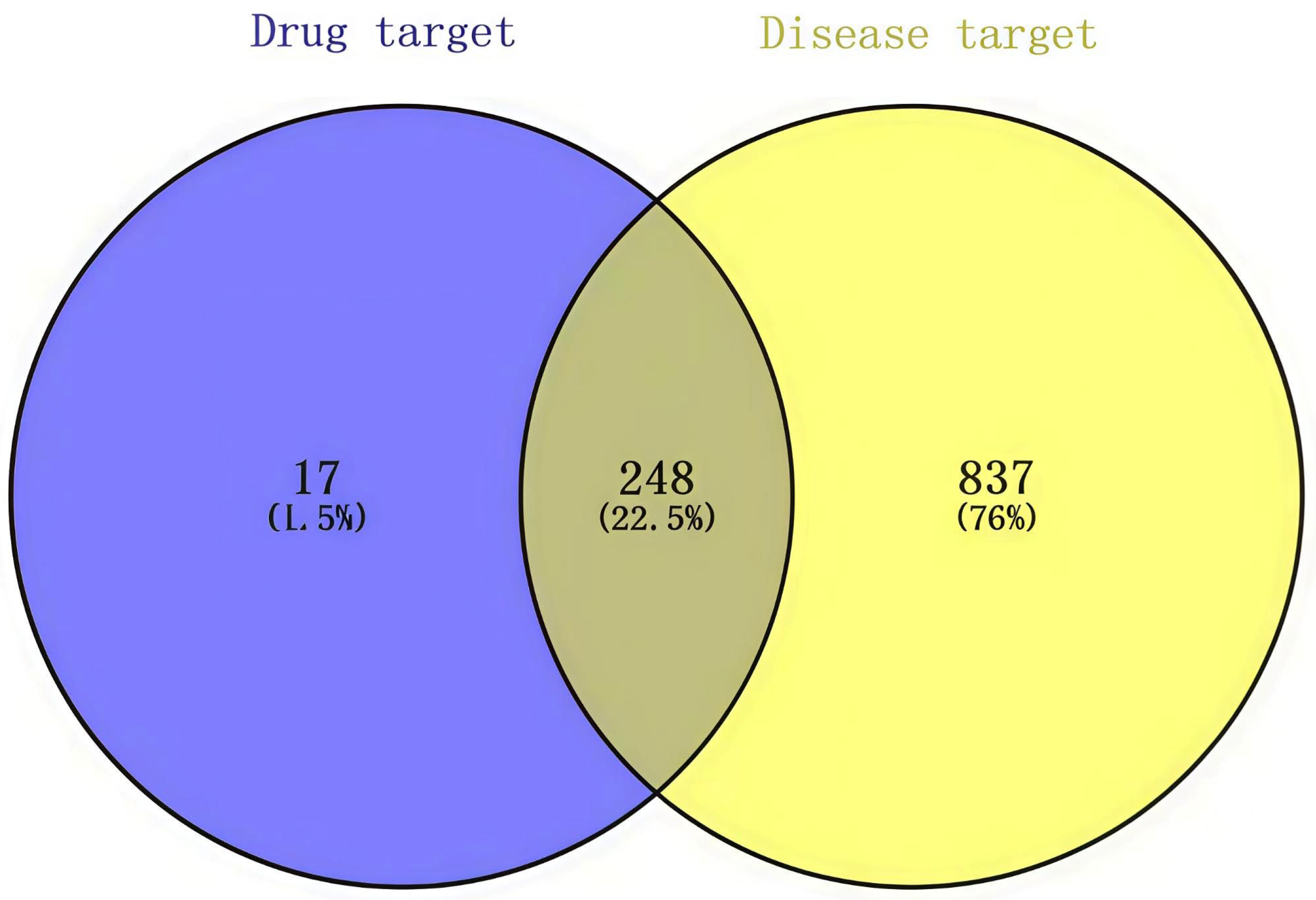
2.2.2. Screening of Ageing-Related Targets
2.2.3. Protein–Protein Interaction Network Construction
2.2.4. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Analyses
2.2.5. Molecular Docking Methodology
2.3. Experimental Verification
2.3.1. Samples and Sample Preparation
2.3.2. Animal Experiments
2.3.3. Measurement of Physiological Indicators
Body Weight Measurement
Organ Index Measurement
2.4. Determination of Biochemical Parameters
2.5. Western Blotting Analysis
2.6. Histologic Examination
2.7. Basic Methodology Description
2.8. Statistical Analysis
3. Results
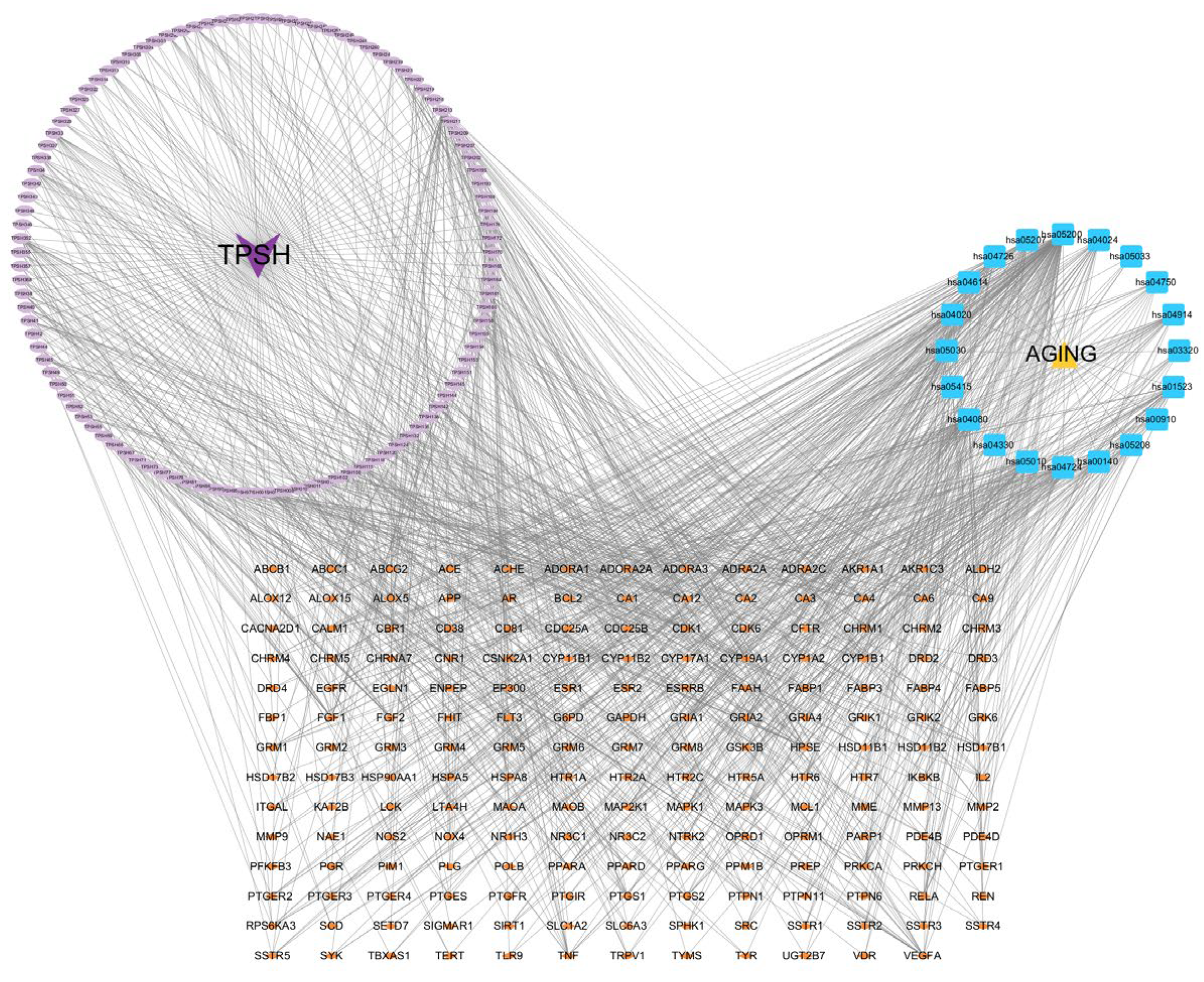
3.1. Active Compounds and Target Screening
3.2. PPI Network Analysis and Key Target Identification
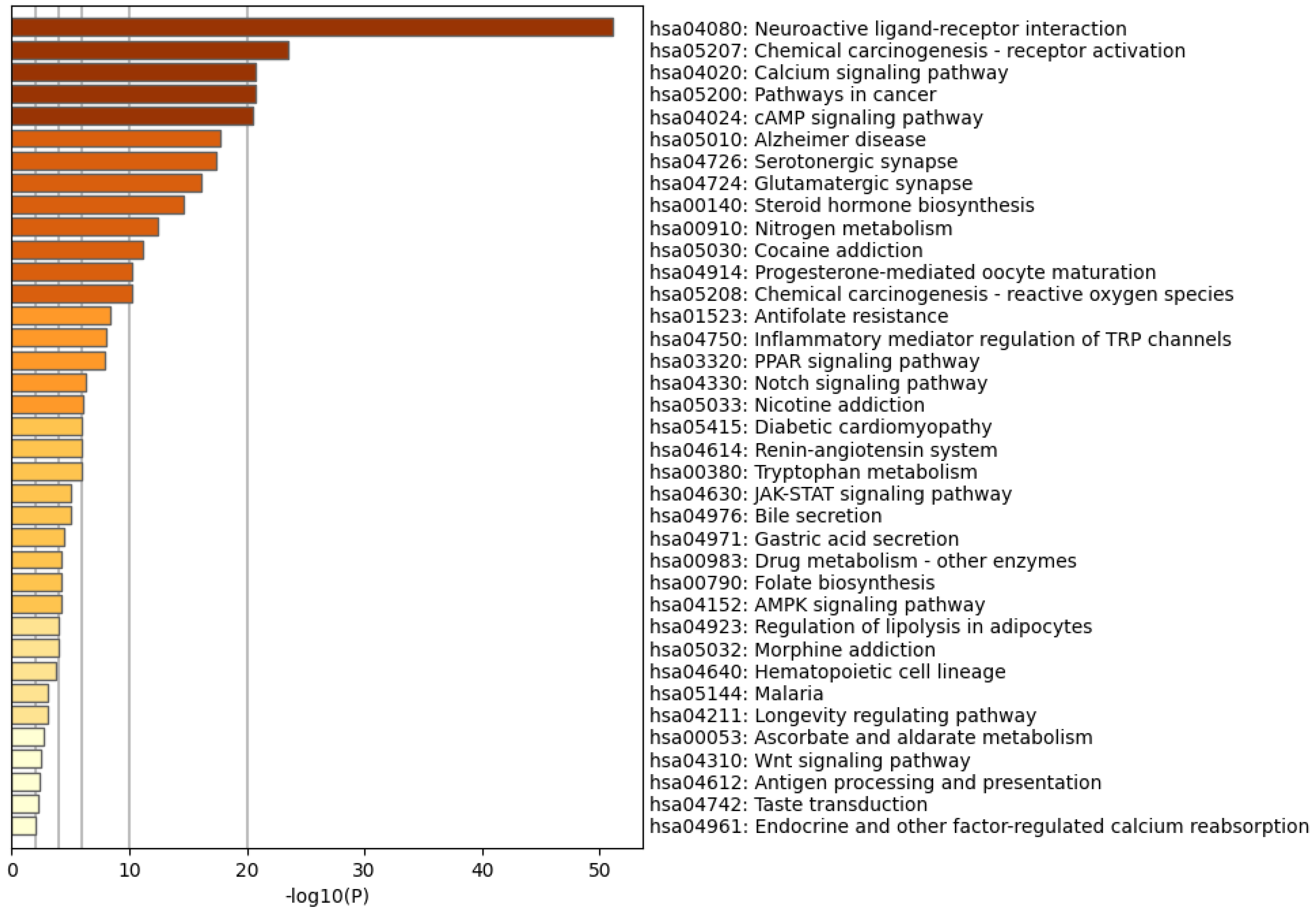
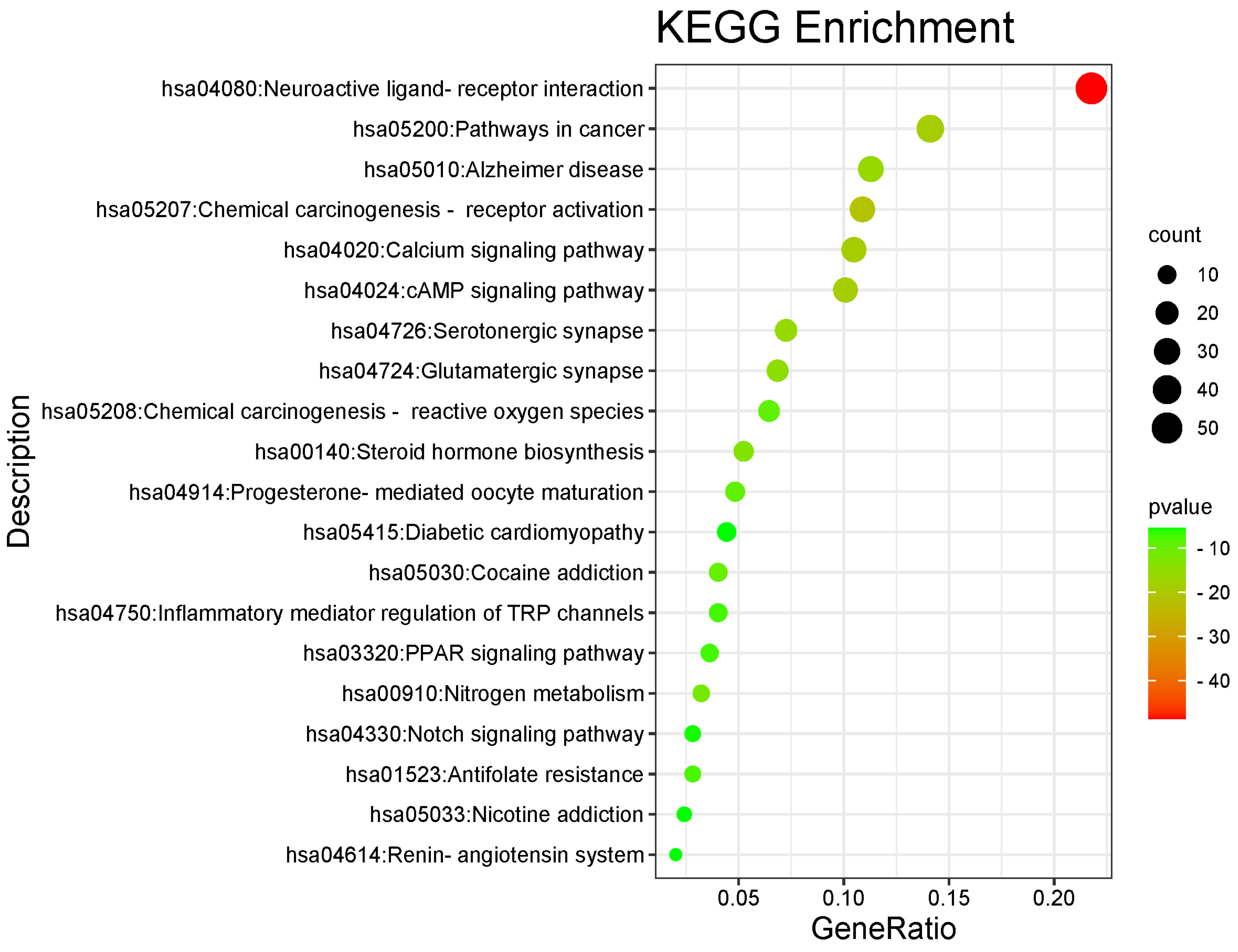
3.3. GO and KEGG Analyses
3.4. Identification of Core Active Ingredients
3.5. Molecular Docking
3.6. Effect of D. officinale on Body Weight and Organ Index
3.7. Alcoholic Extracts of D. officinale Alleviate D-Galactose-Induced Oxidative Stress and Inflammation by Modulating Antioxidant Enzyme Activities and Suppressing TNF-α Levels
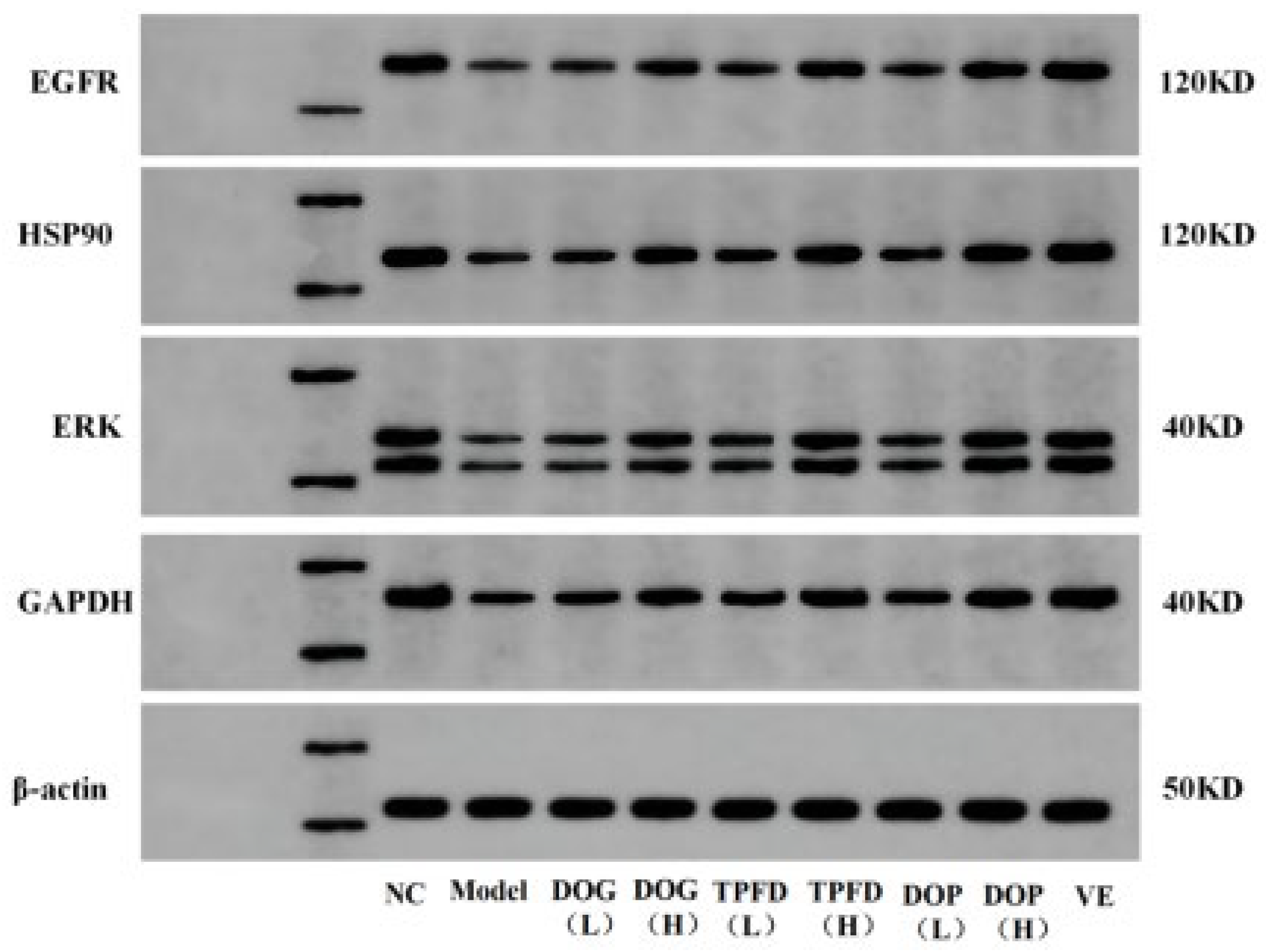
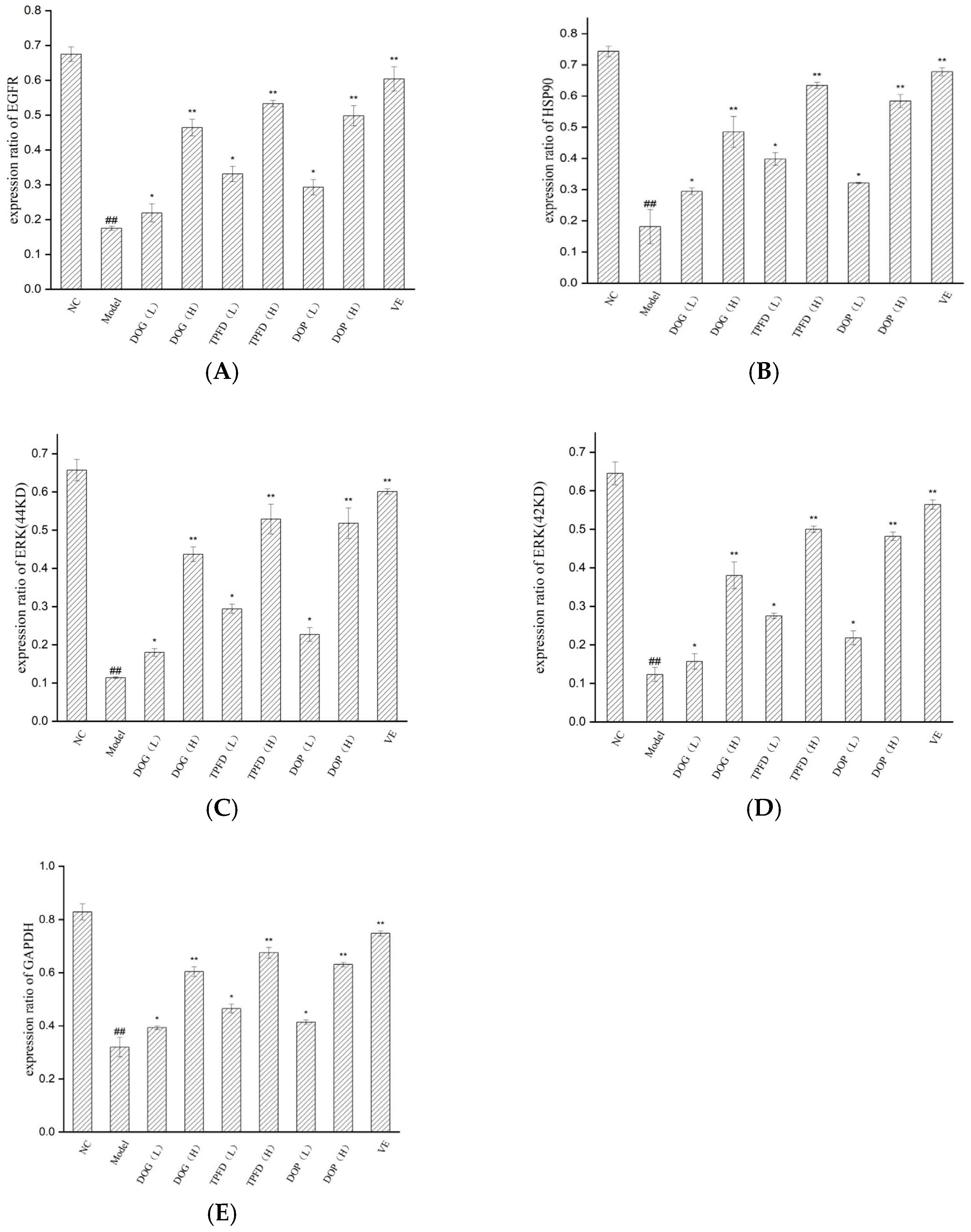
3.8. Effects of Different D. officinale Extracts on the Expression of Key Proteins in D-Galactose-Induced Ageing Kidney
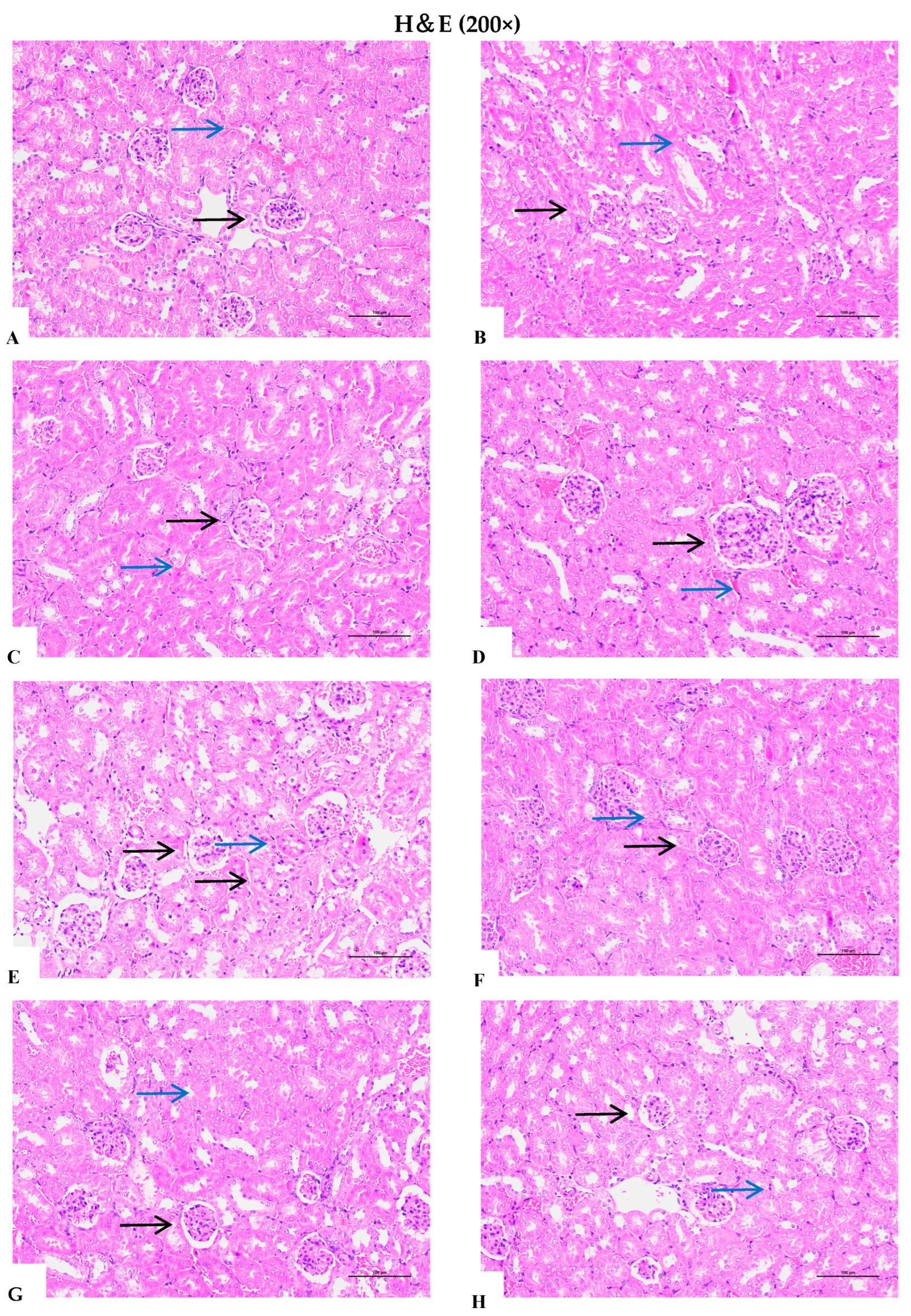
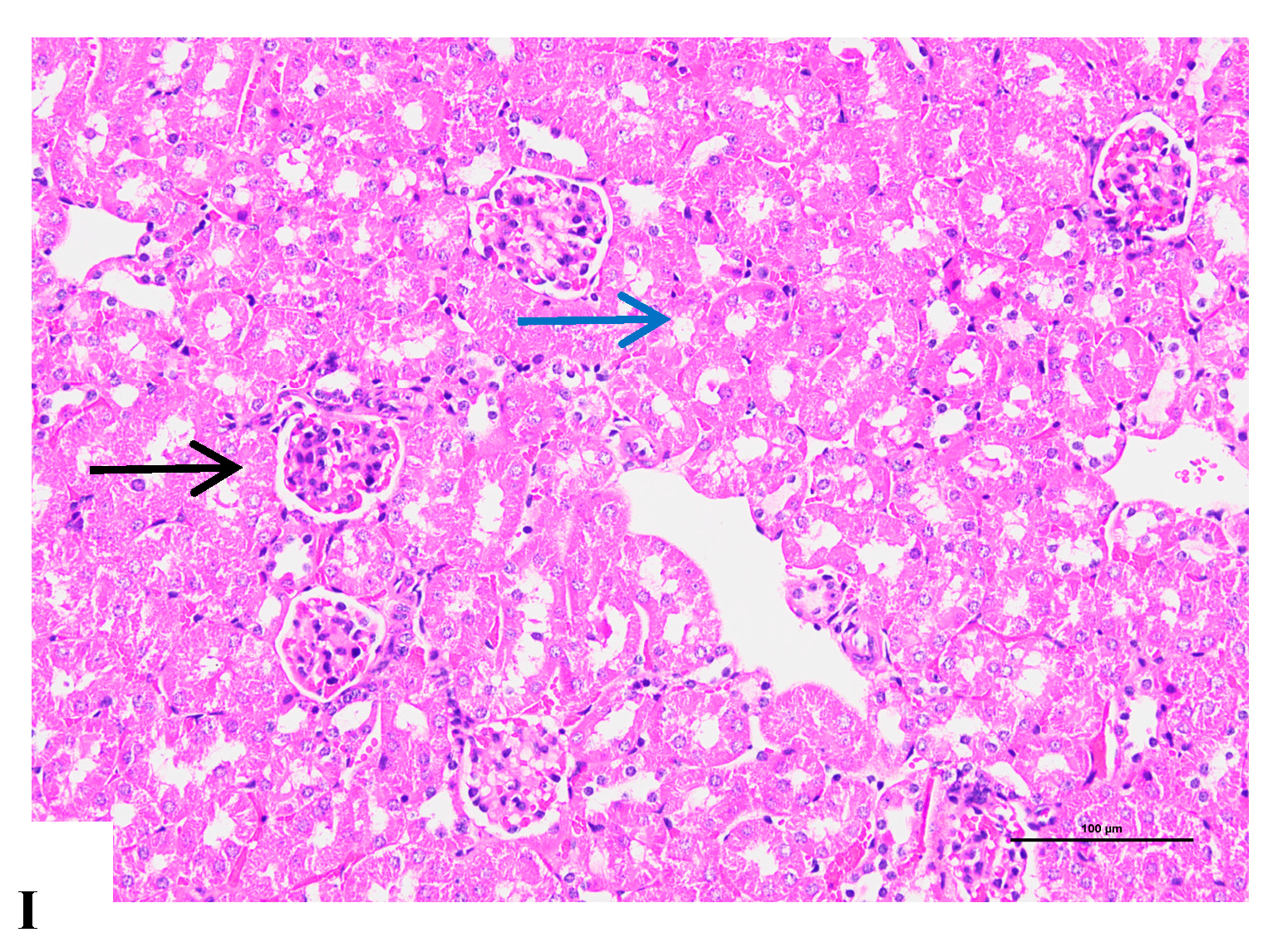
3.9. Effects of Different D. officinale Extracts on Alleviating Morphological Damage to the Kidneys of D-Gal-Induced Ageing Rats
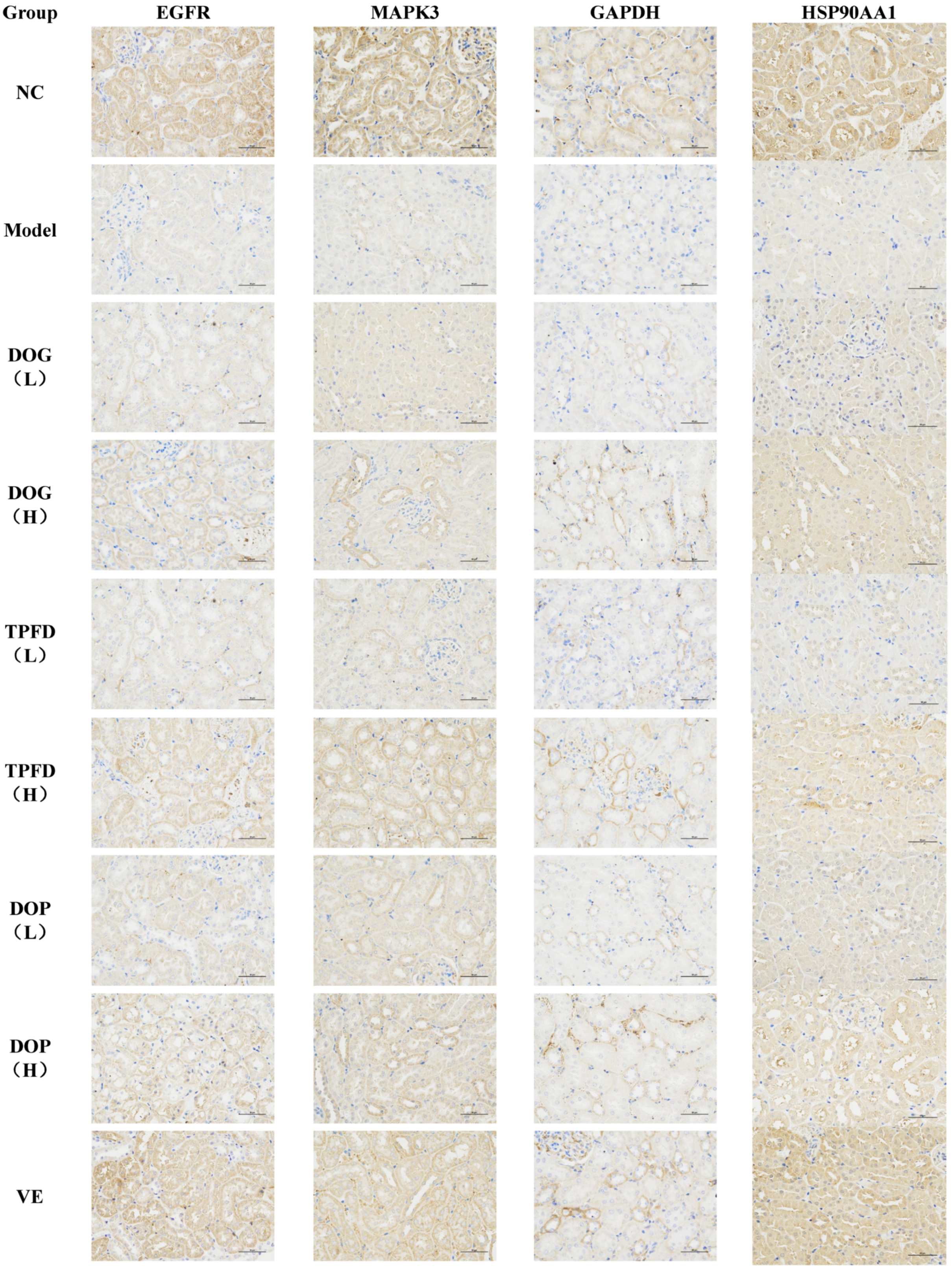
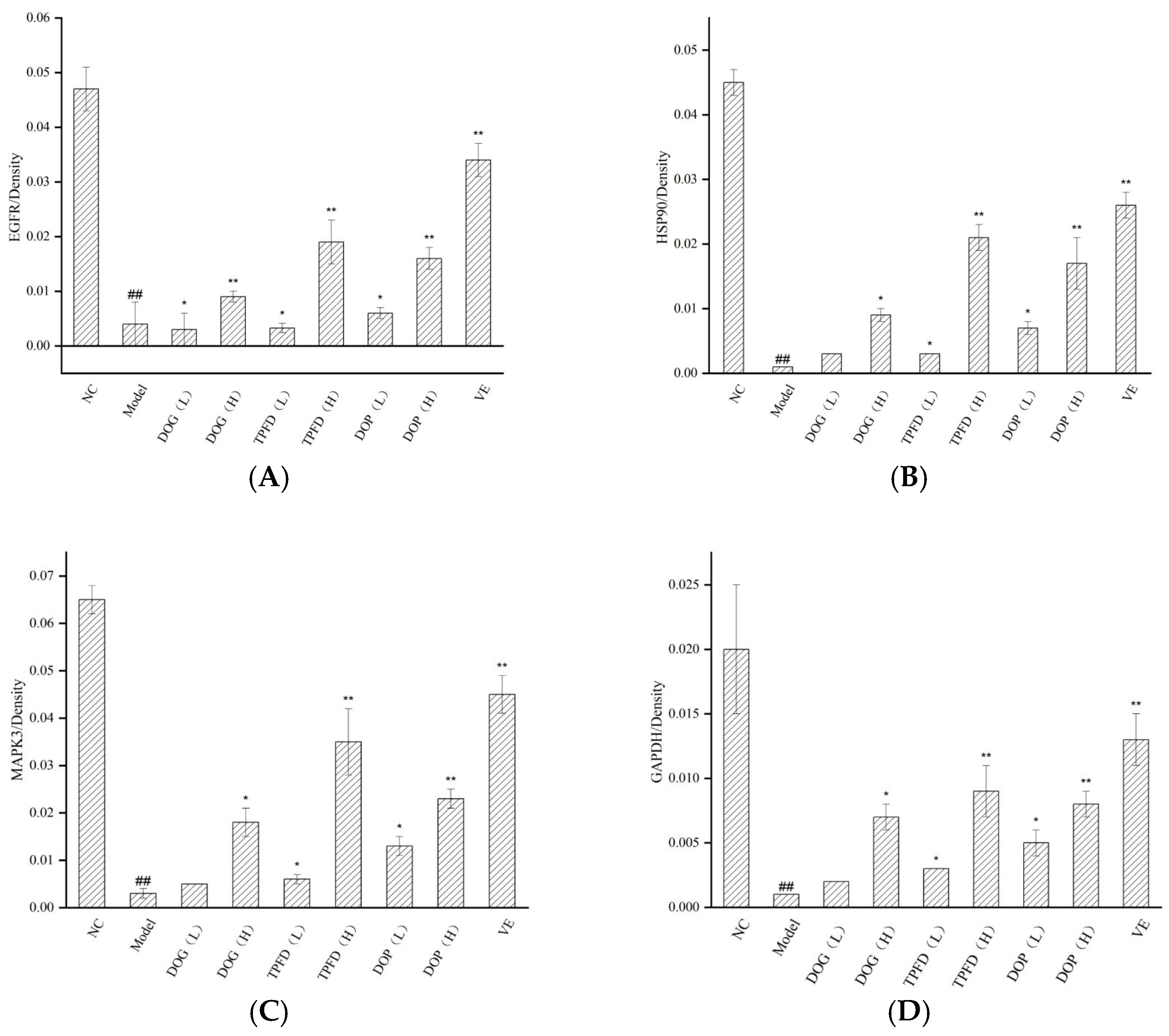
3.10. Immunohistochemical Analysis of the Renal Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Use of Artificial Intelligence
Acknowledgments
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| D-Gal | D-Galactose |
| DOG | Dried D. officinale |
| DOP | Dried strips processed by stir-frying at 150 °C for 10 min, shaping at 60 °C, and drying at 90 °C |
| EGFR | Epidermal growth factor receptor |
| ERK | Extracellular regulated protein kinases |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GSH-Px | Glutathione peroxidase |
| HSP90AA1 | Recombinant human heat shock protein hsp 90-alpha (hsp90aa1) |
| MDA | Malondialdehyde |
| NC | Normal control |
| SOD | Superoxide dismutase |
| T-AOC | Total antioxidant capacity |
| TNF-α | Tumour necrosis factor-a |
| TPFD | Tiepi Fengdou |
| VE | Vitamin E |
References
- Bao, Q.; Pan, J.; Qi, H.; Wang, L.; Qian, H.; Jiang, F.; Shao, Z.; Xu, F.; Tao, Z.; Ma, Q.; et al. Aging and age-related diseases—From endocrine therapy to target therapy. Mol. Cell. Endocrinol. 2014, 394, 115–118. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- UNDESA. World Population Prospects the 2015 Revision, United Nations Department of Economic and Social Affairs: New York, NY, USA, 2015.
- Barton, M.; Husmann, M.; Meyer, M.R. Accelerated vascular aging as a paradigm for hypertensive vascular disease: Prevention and therapy. Can. J. Cardiol. 2016, 32, 680–686.e4. [Google Scholar] [CrossRef]
- Buford, T.W. Hypertension and aging. Ageing Res. Rev. 2016, 26, 96–111. [Google Scholar] [CrossRef]
- Harvey, A.; Montezano, A.C.; Lopes, R.A.; Rios, F.; Touyz, R.M. Vascular fibrosis in aging and hypertension: Molecular mechanisms and clinical implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef]
- Palmer, A.K.; Kirkland, J.L. Aging and adipose tissue: Potential interventions for diabetes and regenerative medicine. Exp. Gerontol. 2016, 86, 97–105. [Google Scholar] [CrossRef]
- Domingues-Faria, C.; Vasson, M.P.; Goncalves-Mendes, N.; Boirie, Y.; Walrand, S. Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res. Rev. 2016, 26, 22–36. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular smooth muscle cells and arterial stiffening: Relevance in development, aging, and disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef]
- Ma, W.; Wong, W.T. Aging changes in retinal microglia and their relevance to age-related retinal disease. In Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology; Bowes Rickman, C., LaVail, M., Anderson, R., Grimm, C., Hollyfield, J., Ash, J., Eds.; Springer: Cham, Switzerland, 2015; pp. 73–78. [Google Scholar]
- Cleeland, C.; Pipingas, A.; Scholey, A.; White, D. Neurochemical changes in the aging brain: A systematic review. Neurosci. Biobehav. Rev. 2019, 98, 306–319. [Google Scholar] [CrossRef]
- Maldonado-Lasuncion, I.; Atienza, M.; Sanchez-Espinosa, M.P.; Cantero, J.L. Aging-related changes in cognition and cortical integrity are associated with serum expression of candidate microRNAs for Alzheimer disease. Cereb. Cortex 2019, 29, 4426–4437. [Google Scholar] [CrossRef]
- Armenta, A.M.; Henkel, E.D.; Ahmed, A.M. Pigmentation disorders in the elderly. Drugs Aging 2019, 36, 235–245. [Google Scholar] [CrossRef]
- Elliott, J.E.; Mantilla, C.B.; Pabelick, C.M.; Roden, A.C.; Sieck, G.C. Aging-related changes in respiratory system mechanics and morphometry in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L167–L176. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, V.M.; Souza, A.S. An age-related deficit in preserving the benefits of attention in working memory. Psychol. Aging 2019, 34, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Maeso-Díaz, R.; Ortega-Ribera, M.; Fernández-Iglesias, A.; Hide, D.; Muñoz, L.; Hessheimer, A.J.; Vila, S.; Francés, R.; Fondevila, C.; Albillos, A.; et al. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell 2018, 17, e12829. [Google Scholar] [CrossRef] [PubMed]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and human aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Vicini, C.; de Vito, A.; Iannella, G.; Gobbi, R.; Corso, R.M.; Montevecchi, F.; Polimeni, A.; De Vincentiis, M.; Meccariello, G.; D’agostino, G.; et al. The aging effect on upper airways collapse of patients with obstructive sleep apnea syndrome. Eur. Arch. Otorhinolaryngol. 2018, 275, 2983–2990. [Google Scholar] [CrossRef]
- Kooman, J.P.; Kotanko, P.; Schols, A.M.W.J.; Shiels, P.G.; Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 2014, 10, 732–742. [Google Scholar] [CrossRef]
- Schmitt, R.; Melk, A. Molecular mechanisms of renal aging. Kidney Int. 2017, 92, 569–579. [Google Scholar] [CrossRef]
- Schmitt, R.; Susnik, N.; Melk, A. Molecular aspects of renal senescence. Curr. Opin. Organ Transplant. 2015, 20, 412–416. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Khan, S.A.; Ali, N.; Sharif, N.; Ghafar, R.; Daglia, M.; Nazir, S. Nephroprotective effects of Datura metel extract in gentamicin induced mice model: Biochemical and histological evidences. Cell. Mol. Biol. 2020, 66, 208–213. [Google Scholar] [CrossRef]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Current understanding of modes of action of multicomponent bioactive phytochemicals: Potential for nutraceuticals and antimicrobials. Annu. Rev. Food Sci. Technol. 2022, 13, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D.E. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020, 64, 101194. [Google Scholar] [CrossRef]
- Kong, S.Z.; Li, J.C.; Li, S.D.; Liao, M.N.; Li, C.P.; Zheng, P.J.; Guo, M.H.; Tan, W.X.; Zheng, Z.H.; Hu, Z. Anti-aging effect of chitosan oligosaccharide on d-galactose-induced subacute aging in mice. Mar. Drugs 2018, 16, 181. [Google Scholar] [CrossRef]
- Yang, H.; Qu, Z.; Zhang, J.; Huo, L.; Gao, J.; Gao, W. Ferulic acid ameliorates memory impairment in d-galactose-induced aging mouse model. Int. J. Food Sci. Nutr. 2016, 67, 806–817. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Kornatowski, M.; Krzywińska, O.; Kędziora-Kornatowska, K. Changes in the blood antioxidant defense of advanced age people. Clin. Interv. Aging 2019, 14, 763–771. [Google Scholar] [CrossRef]
- Sidorova, Y.; Domanskyi, A. Detecting oxidative stress biomarkers in neurodegenerative disease models and patients. Methods Protoc. 2020, 3, 66. [Google Scholar] [CrossRef]
- Alberro, A.; Iribarren-Lopez, A.; Sáenz-Cuesta, M.; Matheu, A.; Vergara, I.; Otaegui, D. Inflammaging markers characteristic of advanced age show similar levels with frailty and dependency. Sci. Rep. 2021, 11, 4358. [Google Scholar] [CrossRef]
- Ebert, T.; Pawelzik, S.-C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Bäck, M.; Stenvinkel, P. Inflammation and premature ageing in chronic kidney disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, X.; Zhu, X.; Hua, Y. Chemical constituents, bioactivities, and pharmacological mechanisms of Dendrobium officinale: A review of the past decade. J. Agric. Food Chem. 2023, 71, 14870–14889. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Ratanji, S.; Cidade, H.; Sousa, E.; Cruz, M.T.; Oliveira, R.; Almeida, I.F. Phenolics as active ingredients in skincare products: A myth or reality? Molecules 2025, 30, 1423. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Li, X.H.; Zeng, L.H.; Li, Z.; Hu, Y.; Wen, H.M.; Cao, F.J.; Wan, G.X. Mechanistic insights into flavonoid subclasses as cardioprotective agents against doxorubicin-induced cardiotoxicity: A comprehensive review. Drug Des. Dev. Ther. 2025, 19, 5553–5596. [Google Scholar] [CrossRef]
- Chomchoei, N.; Leelapornpisid, P.; Tipduangta, P.; Sangthong, P.; Papan, P.; Sirithunyalug, B.; Samutrtai, P. Potential of electro-sprayed purified mangiferin nanoparticles for anti-aging cosmetic applications. RSC Adv. 2023, 13, 34987–35002. [Google Scholar] [CrossRef]
- Chen, W.; Lu, J.; Zhang, J.; Wu, J.; Yu, L.; Qin, L.; Zhu, B. Traditional uses, phytochemistry, pharmacology, and quality control of Dendrobium officinale Kimura et. Migo. Front. Pharmacol. 2021, 12, 726528. [Google Scholar] [CrossRef]
- Chu, W.; Wang, P.; Ma, Z.; Peng, L.; Wang, Z.; Chen, Z. Ultrasonic treatment of Dendrobium officinale polysaccharide enhances antioxidant and anti-inflammatory activity in a mouse D-galactose-induced aging model. Food Sci. Nutr. 2022, 10, 2620–2630. [Google Scholar] [CrossRef]
- Liang, C.Y.; Liang, Y.M.; Liu, H.Z.; Zhu, D.M.; Hou, S.Z.; Wu, Y.Y.; Huang, S.; Lai, X.P. Effect of Dendrobium officinale on D-galactose-induced aging mice. Chin. J. Integr. Med. 2017, 23, 1–9. [Google Scholar] [CrossRef]
- Wei, W.; Li, Z.-P.; Zhu, T.; Fung, H.-Y.; Wong, T.-L.; Wen, X.; Ma, D.-L.; Leung, C.-H.; Han, Q.-B. Anti-fatigue effects of the unique polysaccharide marker of Dendrobium officinale on BALB/c mice. Molecules 2017, 22, 155. [Google Scholar] [CrossRef]
- Okoro, N.O.; Odiba, A.S.; Yu, Q.; He, B.; Liao, G.; Jin, C.; Fang, W.; Wang, B. Polysaccharides extracted from Dendrobium officinale grown in different environments elicit varying health benefits in Caenorhabditis elegans. Nutrients 2023, 15, 2641. [Google Scholar] [CrossRef]
- Hao, D.C.; Xiao, P.G. Network pharmacology: A Rosetta Stone for traditional Chinese medicine. Drug Dev. Res. 2014, 75, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China: Part I, 11th ed.; China Medical Science Press: Beijing, China, 2020; p. 295. [Google Scholar]
- Zhong, X.; Di, S.; Wang, X.; Liu, R.; Gao, Y.; Wang, H.; An, Y.; Zhu, X. Exploration about clinical application and dose-eff ect relationship of Dendrobium. Jilin J. Chin. Med. 2020, 40, 104–107. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, Y.; Chen, Z.; Yan, X.; Zhao, Y.; Gao, L.; Yang, L. Traditional processing increases biological activities of Dendrobium offificinale Kimura et. Migo in Southeast Yunnan, China. Sci. Rep. 2022, 12, 14814. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Zhai, Y.Y.; Yao, W.F.; Xu, J.; Feng, L.; Bao, B.H.; Cao, Y.D.; Zhang, L.; Ding, A.W. The mechanism study of protecting kidney of Erzhi pill based on network pharmacology. Acta Pharm. Sin. 2019, 54, 877–885. [Google Scholar] [CrossRef]
- Hsin, K.Y.; Ghosh, S.; Kitano, H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS ONE 2013, 8, e83922. [Google Scholar] [CrossRef]
- Liu, F.H.; Chen, S.J.; Ni, W.J. Study on the computer virtual screening of antithrombotic active ingredients in Chuanxiong Rhizoma. China Pharm. 2017, 28, 2182–2186. [Google Scholar]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and functional changes with the aging kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef]
- Lee, R.; Mason, A. Cost of aging. Fin. Dev. 2017, 54, 7–9. [Google Scholar]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network pharmacology databases for traditional Chinese medicine: Review and assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Rehman, S.U.; Shah, S.A.; Ali, T.; Chung, J.I.; Kim, M.O. Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol. Neurobiol. 2017, 54, 255–271. [Google Scholar] [CrossRef]
- Chu, S.-H.; Yang, D.; Wang, Y.-P.; Yang, R.; Qu, L.; Zeng, H.-J. Effect of resveratrol on the repair of kidney and brain injuries and its regulation on klotho gene in d-galactose-induced aging mice. Bioorg. Med. Chem. Lett. 2021, 40, 127913. [Google Scholar] [CrossRef] [PubMed]
- Franco, N.H. Animal experiments in biomedical research: A historical perspective. Animals 2013, 3, 238–273. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, M.; Zhang, C.; Tian, C.; Wang, X.; Song, X.; Jing, H.; Gao, Z.; Ren, Z.; Liu, W.; et al. Purification, in vitro antioxidant and in vivo anti-aging activities of soluble polysaccharides by enzyme-assisted extraction from Agaricus bisporus. Int. J. Biol. Macromol. 2018, 109, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Chen, H.Z.; Zhong, C.F.; Zhu, S.Y.; Li, P.; Du, B. Anti-aging effect of polysaccharide from Dendrobium officinale leaves in Caenorhabditis elegans. Food Sci. 2022, 43, 203–208. [Google Scholar] [CrossRef]
- Alarcón De La Lastra, C.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Cho, H.; Yun, C.-W.; Park, W.-K.; Kong, J.-Y.; Kim, K.S.; Park, Y.; Lee, S.; Kim, B.K. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Kim, D.-C.; Quang, T.H.; Oh, H.; Kim, Y.-C. Steppogenin isolated from Cudrania tricuspidata shows antineuroinflammatory effects via NF-κB and MAPK pathways in LPS-stimulated BV2 and primary rat microglial cells. Molecules 2017, 22, 2130. [Google Scholar] [CrossRef]
- Patel, K.; Singh, G.K.; Patel, D.K. A review on pharmacological and analytical aspects of naringenin. Chin. J. Integr. Med. 2018, 24, 551–560. [Google Scholar] [CrossRef]
- Nautiyal, J.; Singh Kanwar, S.S.; Majumdar, A.P.N. EGFR(s) in aging and carcinogenesis of the gastrointestinal tract. Curr. Protein Pept. Sci. 2010, 11, 436–450. [Google Scholar] [CrossRef]
- Alexander, P.B.; Yuan, L.; Yang, P.; Sun, T.; Chen, R.; Xiang, H.; Chen, J.; Wu, H.; Radiloff, D.R.; Wang, X.F. EGF promotes mammalian cell growth by suppressing cellular senescence. Cell Res. 2015, 25, 135–138. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Song, J.; Zhou, L.; Chen, X.; Ge, W.; Dong, T.; Luo, Y.; Mao, T.; Li, Z.; et al. Salidroside promotes healthy longevity by interfering with HSP90 activity. GeroScience 2024, 46, 1641–1655. [Google Scholar] [CrossRef]
- Nakajima, H.; Itakura, M.; Kubo, T.; Kaneshige, A.; Harada, N.; Izawa, T.; Azuma, Y.T.; Kuwamura, M.; Yamaji, R.; Takeuchi, T. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) aggregation causes mitochondrial dysfunction during oxidative stress-induced cell death. J. Biol. Chem. 2017, 292, 4727–4742. [Google Scholar] [CrossRef]
- Bisht, A.; Tewari, D.; Kumar, S.; Chandra, S. Network pharmacology, molecular docking, and molecular dynamics simulation to elucidate the mechanism of anti-aging action of Tinospora cordifolia. Mol. Divers. 2024, 28, 1743–1763. [Google Scholar] [CrossRef] [PubMed]
- Ureshino, R.P.; Rocha, K.K.; Lopes, G.S.; Bincoletto, C.; Smaili, S.S. Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid. Redox Signal. 2014, 21, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. NF-κB signaling in the aging process. J. Clin. Immunol. 2009, 29, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Djordjevic, A.; Spasic, S.; Jovanovic-Galovic, A.; Djordjevic, R.; Grubor-Lajsic, G. Oxidative stress in diabetic pregnancy: SOD, CAT and GSH-Px activity and lipid peroxidation products. J. Matern. Fetal Neonatal Med. 2004, 16, 367–372. [Google Scholar] [CrossRef]
- Valen, G.; Starkopf, J.; Takeshima, S.; Kullisaar, T.; Vihalemm, T.; Kengsepp, A.T.; Löwbeer, C.; Vaage, J.; Zilmer, M. Preconditioning with hydrogen peroxide (H2O2) or ischemia in H2O2-induced cardiac dysfunction. Free Radic. Res. 1998, 29, 235–245. [Google Scholar] [CrossRef]
- Jadoon, S.; Malik, A. A review article on the formation, mechanism and biochemistry of MDA and MDA as a biomarker of oxidative stress. Int. J. Adv. Res. 2017, 5, 811–818. [Google Scholar] [CrossRef]
- Bai, Z.-L.; Li, X.-S.; Chen, G.-Y.; Du, Y.; Wei, Z.-X.; Chen, X.; Zheng, G.-E.; Deng, W.; Cheng, Y. Serum oxidative stress marker levels in unmedicated and medicated patients with schizophrenia. J. Mol. Neurosci. 2018, 66, 428–436. [Google Scholar] [CrossRef]
- De Gonzalo-Calvo, D.; Neitzert, K.; Fernández, M.; Vega-Naredo, I.; Caballero, B.; García-Macía, M.; Suárez, F.M.; Rodríguez-Colunga, M.J.; Solano, J.J.; Coto-Montes, A. Differential inflammatory responses in aging and disease: TNF-α and IL-6 as possible biomarkers. Free Radic. Biol. Med. 2010, 49, 733–737. [Google Scholar] [CrossRef]



| Experimental Group | Kidney Index (Left) | Kidney Index (Right) | Heart Index | Liver Index | Spleen Index | Lung Index | Brain Index |
|---|---|---|---|---|---|---|---|
| NC | 0.78 ± 0.038 | 0.79 ± 0.05 | 0.54 ± 0.02 | 4.45 ± 0.18 | 0.31 ± 0.02 | 0.60 ± 0.02 | 1.18 ± 0.03 |
| Model | 0.50 ± 0.12 ## | 0.52 ± 0.03 ## | 0.38 ± 0.01 ## | 3.21 ± 0.01 ## | 0.19 ± 0.01 ## | 0.44 ± 0.02 ## | 0.82 ± 0.03 ## |
| DOG (L) | 0.65 ± 0.028 ** | 0.64 ± 0.09 * | 0.49 ± 0.02 ** | 3.72 ± 0.13 | 0.31 ± 0.04 | 0.55 ± 0.02 ** | 0.96 ± 0.04 ** |
| DOG (H) | 0.66 ± 0.026 ** | 0.68 ± 0.015 * | 0.50 ± 0.04 ** | 3.88 ± 0.17 * | 0.32 ± 0.04 * | 0.59 ± 0.03 ** | 0.90 ± 0.02 ** |
| TPFD (L) | 0.62 ± 0.029 * | 0.65 ± 0.02 * | 0.48 ± 0.018 ** | 3.77 ± 0.12 | 0.28 ± 0.03 | 0.51 ± 0.02 * | 0.88 ± 0.03 |
| TPFD (H) | 0.66 ± 0.025 ** | 0.67 ± 0.02 * | 0.46 ± 0.04 ** | 3.63 ± 0.15 | 0.35 ± 0.07 * | 0.60 ± 0.07 ** | 0.93 ± 0.03 ** |
| DOP (L) | 0.70 ± 0.04 ** | 0.71 ± 0.05 ** | 0.47 ± 0.02 ** | 4.03 ± 0.24 * | 0.31 ± 0.05 | 0.53 ± 0.03 * | 0.93 ± 0.03 ** |
| DOP (H) | 0.54 ± 0.02 | 0.61 ± 0.03 * | 0.49 ± 0.03 ** | 3.84 ± 0.14 * | 0.33 ± 0.08 * | 0.53 ± 0.03 * | 0.91 ± 0.04 ** |
| VE | 0.64 ± 0.02 ** | 0.67 ± 0.036 ** | 0.48 ± 0.02 ** | 4.48 ± 0.15 ** | 0.23 ± 0.02 | 0.56 ± 0.05 * | 0.87 ± 0.02 * |
| Group | T-AOC (mM) | TNF-α (ng/L) |
|---|---|---|
| NC | 1.167 ± 0.069 | 133.576 ± 13.224 |
| DOG (L) | 0.312 ± 0.026 * | 400.281 ± 10.392 * |
| Model | 0.156 ± 0.055 ## | 463.94 ± 19.443 ## |
| DOG (H) | 0.722 ± 0.050 ** | 292.954 ± 10.291 ** |
| TPFD (L) | 0.548 ± 0.034 * | 315.680 ± 21.696 * |
| TPFD (H) | 0.940 ± 0.047 ** | 221.043 ± 19.267 ** |
| DOP (L) | 0.452 ± 0.041 * | 359.179 ± 19.867 * |
| DOP (H) | 0.849 ± 0.048 ** | 268.982 ± 7.093 ** |
| VE | 1.046 ± 0.054 ** | 173.581 ± 17.287 ** |
| Group | MDA (nmol/mL) | GSH-Px (U/gprot) | CAT (U/mgprot) | SOD (U/mgprot) |
|---|---|---|---|---|
| NC | 0.772 ± 0.192 | 30.465 ± 1.952 | 20.256 ± 1.190 | 37.943 ± 1.461 |
| Model | 3.987 ± 0.225 ## | 7.317 ± 1.276 ## | 3.913 ± 0.616 ## | 10.054 ± 1.610 ## |
| DOG (L) | 3.427 ± 0.229 * | 11.353 ± 1.046 * | 6.403 ± 0.880 * | 14.262 ± 1.258 * |
| DOG (H) | 2.215 ± 0.117 ** | 18.055 ± 1.290 ** | 12.091 ± 0.704 ** | 22.040 ± 1.429 ** |
| TPFD (L) | 2.864 ± 0.093 * | 15.607 ± 1.131 * | 9.875 ± 0.792 ** | 20.929 ± 1.890 * |
| TPFD (H) | 1.526 ± 0.117 ** | 24.138 ± 1.730 ** | 15.795 ± 0.774 ** | 30.685 ± 0.904 ** |
| DOP (L) | 3.263 ± 0.149 * | 15.988 ± 0.706 * | 9.440 ± 0.719 * | 16.518 ± 1.308 * |
| DOP (H) | 1.803 ± 0.125 ** | 21.625 ± 0.565 ** | 13.638 ± 0.660 ** | 23.333 ± 1.593 ** |
| VE | 1.298 ± 0.094 ** | 27.054 ± 1.141 ** | 16.717 ± 1.031 ** | 32.935 ± 1.353 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Yang, Z.; Liang, S.; Ze, W.; Lin, Z.; Cai, Y.; Yang, L.; Feng, T. Mechanistic Elucidation of the Anti-Ageing Effects of Dendrobium officinale via Network Pharmacology and Experimental Validation. Foods 2025, 14, 3418. https://doi.org/10.3390/foods14193418
Chen Z, Yang Z, Liang S, Ze W, Lin Z, Cai Y, Yang L, Feng T. Mechanistic Elucidation of the Anti-Ageing Effects of Dendrobium officinale via Network Pharmacology and Experimental Validation. Foods. 2025; 14(19):3418. https://doi.org/10.3390/foods14193418
Chicago/Turabian StyleChen, Zhilin, Zhoujie Yang, Shanshan Liang, Weiwei Ze, Zhou Lin, Yuexin Cai, Lixin Yang, and Tingting Feng. 2025. "Mechanistic Elucidation of the Anti-Ageing Effects of Dendrobium officinale via Network Pharmacology and Experimental Validation" Foods 14, no. 19: 3418. https://doi.org/10.3390/foods14193418
APA StyleChen, Z., Yang, Z., Liang, S., Ze, W., Lin, Z., Cai, Y., Yang, L., & Feng, T. (2025). Mechanistic Elucidation of the Anti-Ageing Effects of Dendrobium officinale via Network Pharmacology and Experimental Validation. Foods, 14(19), 3418. https://doi.org/10.3390/foods14193418





