Application Potential of Lion’s Mane Mushroom in Soy-Based Meat Analogues by High Moisture Extrusion: Physicochemical, Structural and Flavor Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Extrusion of Meat Analogue
2.3. Rheological Analysis
2.4. Color Properties Analysis
2.5. Texture Profile Analysis (TPA)
2.6. Fibrous Degree Analysis
2.7. Microstructure Observation
2.8. Thermal Properties Analysis
2.9. Particle Size Distribution (PSD)
2.10. Protein Secondary Structure Determination
2.11. Protein Tertiary Structure Determination
2.12. HS-SPME-GC–MS Analysis
2.12.1. HS-SPME-GC–MS Conditions
2.12.2. Volatile Components Qualitative and Quantitative Analysis
2.12.3. Analysis of Relative Odor Activity Values
2.13. Statistical Analysis
3. Results and Discussion
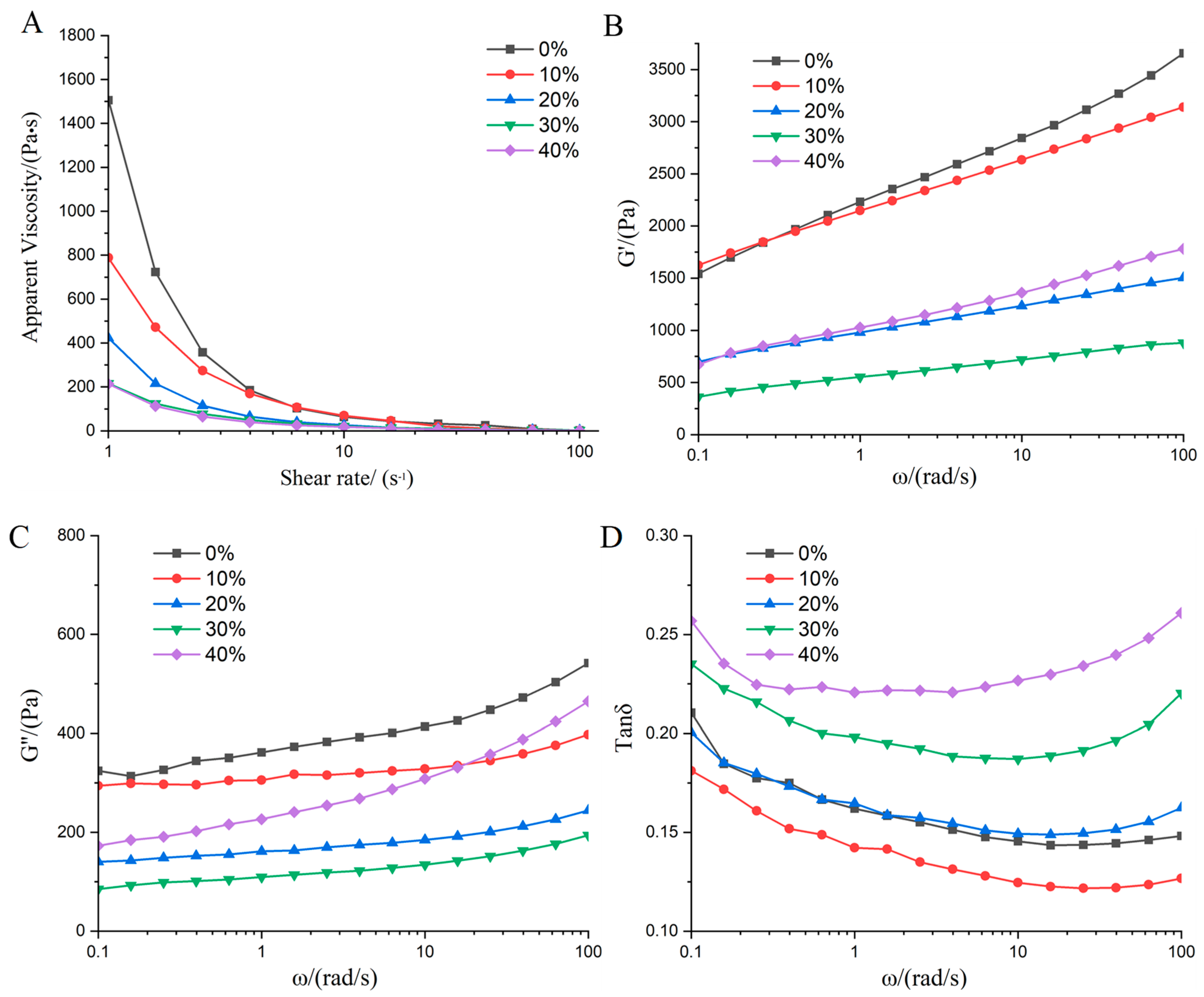
3.1. Rheological Properties Analysis
3.2. Effects of LMM Content on the Apparent Properties
3.2.1. Texture Properties Analysis
3.2.2. Color Analysis
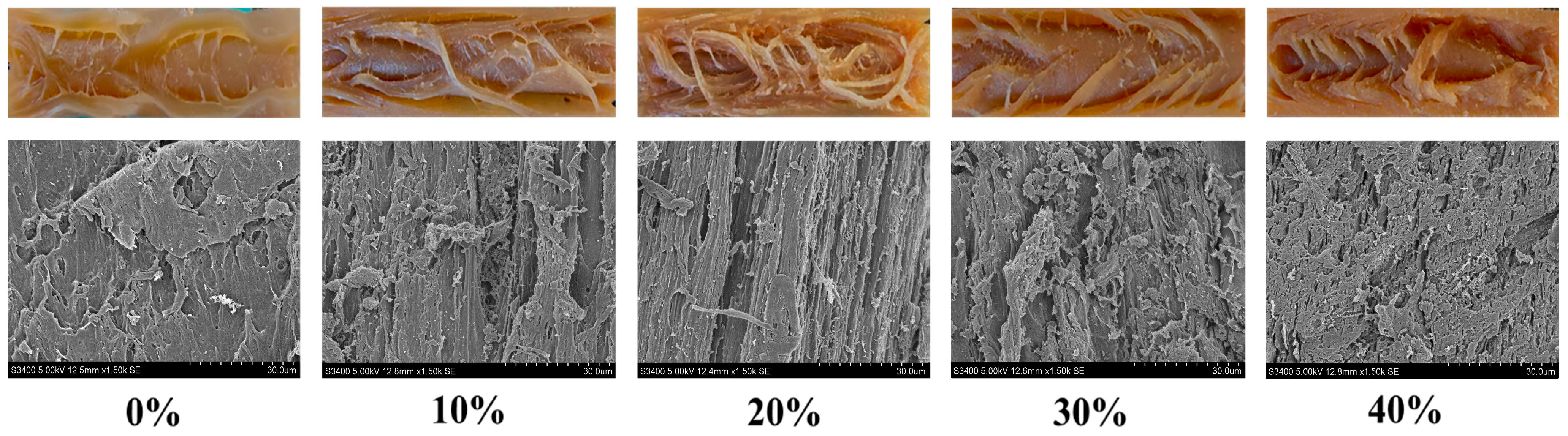
3.3. Microstructure and Visual Observation
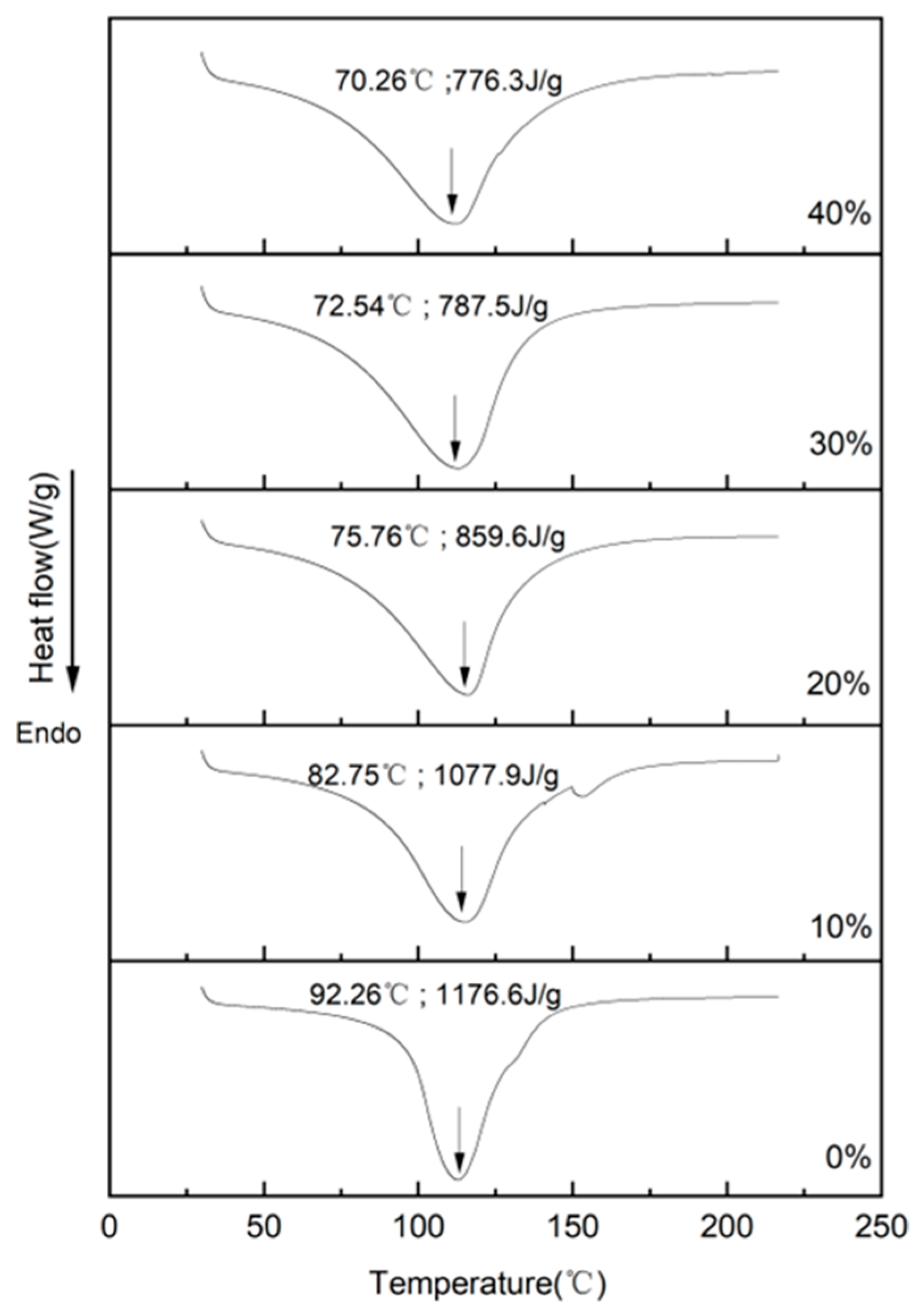
3.4. Thermal Behavior Analysis
3.5. Particle Size Analysis
3.6. Protein Secondary Structure Analysis
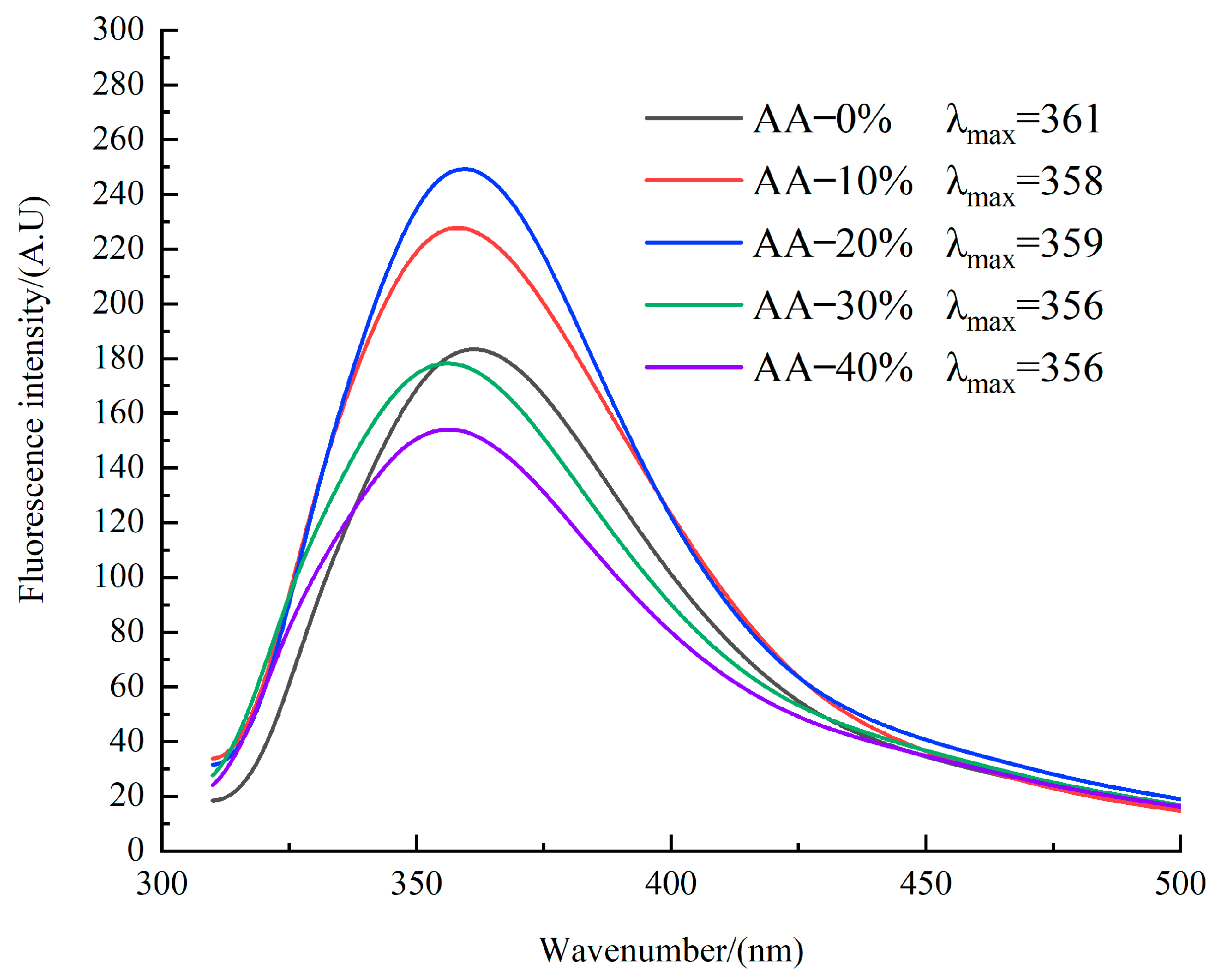
3.7. Protein Tertiary Structure Analysis
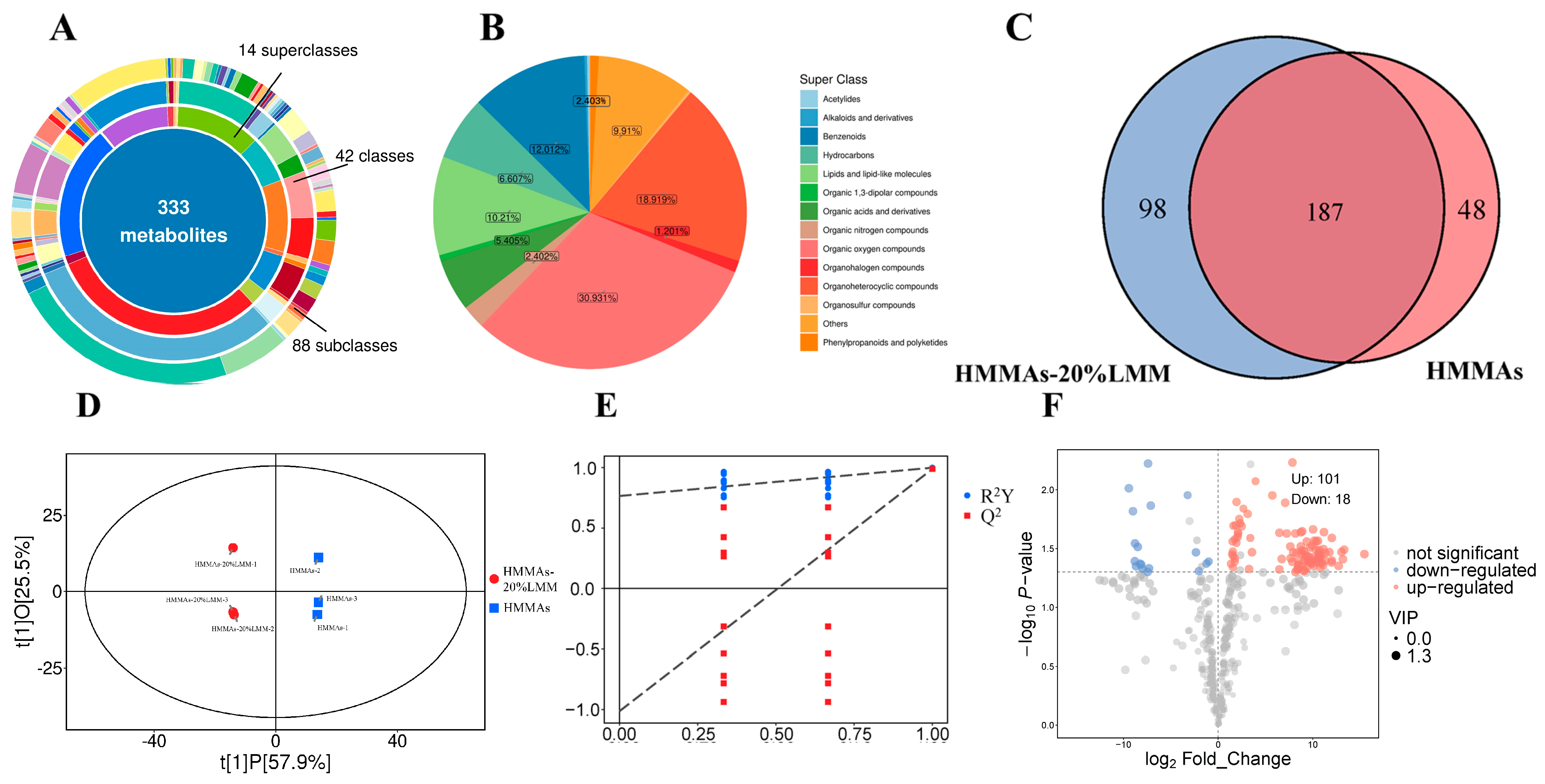
3.8. Volatile Flavor Components Analysis
3.9. Relative Odor Activity Values (ROAV) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scholtmeijer, K.; Van Den Broek, L.A.M.; Fischer, A.R.H.; Van Peer, A. Potential Protein Production from Lignocellulosic Materials Using Edible Mushroom Forming Fungi. J. Agric. Food Chem. 2023, 71, 4450–4457. [Google Scholar] [CrossRef]
- Ishaq, A.; Irfan, S.; Sameen, A.; Khalid, N. Plant-Based Meat Analogs: A Review with Reference to Formulation and Gastrointestinal Fate. Curr. Res. Food Sci. 2022, 5, 973–983. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, T.; Zhang, Y.; Jiang, L.; Sui, X. Potential of Hydrolyzed Wheat Protein in Soy-Based Meat Analogues: Rheological, Textural and Functional Properties. Food Chem. X 2023, 20, 100921. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mehta, N.; Abubakar, A.A.; Verma, A.K.; Kaka, U.; Sharma, N.; Sazili, A.Q.; Pateiro, M.; Kumar, M.; Lorenzo, J.M. Potential Alternatives of Animal Proteins for Sustainability in the Food Sector. Food Rev. Int. 2023, 39, 5703–5728. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; He, S.; Li, X.; Jin, R.; Liu, Q.; Chen, S.; Sun, H. High-Moisture Extrusion Technology Application in the Processing of Textured Plant Protein Meat Analogues: A Review. Food Rev. Int. 2023, 39, 4873–4908. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; Van Der Goot, A.J. Plant-Based Meat Analogues. In Sustainable Meat Production and Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–126. ISBN 978-0-12-814874-7. [Google Scholar]
- Ma, X.; Ryu, G. Effects of Green Tea Contents on the Quality and Antioxidant Properties of Textured Vegetable Protein by Extrusion-Cooking. Food Sci. Biotechnol. 2019, 28, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gopal, J.; Sivanesan, I.; Muthu, M.; Oh, J.-W. Scrutinizing the Nutritional Aspects of Asian Mushrooms, Its Commercialization and Scope for Value-Added Products. Nutrients 2022, 14, 3700. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, X.; Chen, J.; Zhou, J. Novel Fungal Alternative Proteins from Penicillium Limosum for Enhancing Structural and Functional Properties of Plant-Based Meat Analogues. Food Chem. 2024, 444, 138627. [Google Scholar] [CrossRef]
- Pérez-Montes, A.; Rangel-Vargas, E.; Lorenzo, J.M.; Romero, L.; Santos, E.M. Edible Mushrooms as a Novel Trend in the Development of Healthier Meat Products. Curr. Opin. Food Sci. 2021, 37, 118–124. [Google Scholar] [CrossRef]
- Yuan, X.; Jiang, W.; Zhang, D.; Liu, H.; Sun, B. Textural, Sensory and Volatile Compounds Analyses in Formulations of Sausages Analogue Elaborated with Edible Mushrooms and Soy Protein Isolate as Meat Substitute. Foods 2021, 11, 52. [Google Scholar] [CrossRef]
- Mohamad Mazlan, M.; Talib, R.A.; Chin, N.L.; Shukri, R.; Taip, F.S.; Mohd Nor, M.Z.; Abdullah, N. Physical and Microstructure Properties of Oyster Mushroom-Soy Protein Meat Analog via Single-Screw Extrusion. Foods 2020, 9, 1023. [Google Scholar] [CrossRef]
- Durmus, A.; Durmus, I.; Bender, O.; Karatepe, O. The Effect of Hericium Erinaceum on the Prevention of Chemically Induced Experimental Colitis in Rats. Korean J. Intern. Med. 2021, 36, S44–S52. [Google Scholar] [CrossRef]
- Cho, S.Y.; Ryu, G.H. Effects of Oyster Mushroom Addition on Quality Characteristics of Full Fat Soy-based Analog Burger Patty by Extrusion Process. J. Food Process Eng. 2023, 46, e14128. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Y.; Liu, F.; Chen, Y.; Li, B.; Feng, Y.; Wang, H.; Xia, G.; He, H. Structural and Functional Analysis of Soy Protein-Skipjack Tuna Protein Gels Prepared by Two-Screw Extrusion and Their Effects on Metabolites and Gut Microbiota in Vitro. Food Chem. X 2025, 27, 102466. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lian, W.; Zhang, H.; Zhu, Y.; Huang, Y.; Liu, L.; Zhu, X. Mechanism of L-Cysteine-Induced Fibrous Structural Changes of Soybean Protein at Different High-Moisture Extrusion Zones. Int. J. Biol. Macromol. 2024, 268, 131621. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, J.; Wang, S.; Qi, M.; Yue, M.; Zhang, S.; Song, J.; Wang, C.; Zhang, D.; Wang, X.; et al. High Moisture Extrusion of Pea Protein: Effect of l-Cysteine on Product Properties and the Process Forming a Fibrous Structure. Food Hydrocoll. 2022, 129, 107633. [Google Scholar] [CrossRef]
- Yu, X.; Wang, H.; Yuan, Y.; Shi, J.; Duan, Y.; Wang, L.; Wang, P.; Xiao, Z. Changes in Physicochemical and Structural Properties of Pea Protein during the High Moisture Extrusion Process: Effects of Carboxymethylcellulose Sodium and Different Extrusion Zones. Int. J. Biol. Macromol. 2023, 251, 126350. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lian, W.; Gu, X.; Zhang, H.; Gao, Y.; Lü, M.; Zhu, Y.; Huang, Y.; Sun, Y.; Zhu, X. Effect of Polysaccharides on the Rheological Behaviour of Soy–Wheat Protein Aggregation and Conformational Changes during High-moisture Extrusion. J. Sci. Food Agric. 2023, 103, 5992–6004. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Su, X.; Jiang, R.; Zhou, H.; Xie, T. Low Moisture Extrusion of Soybean Protein Isolate: Effect of β-Glucan on the Physicochemical Properties of the Product. LWT 2023, 179, 114660. [Google Scholar] [CrossRef]
- Song, L.; Zhao, Q.; Shao, X.; Lv, X.; Lu, J.; Luo, R.; Liu, Y.; Zhou, X.; Li, Q.; Gui, M. Identification of Characteristic Flavor Compounds and Quality Evaluation of Butyriboletus Roseoflavus from Different Regions in Yunnan. Foods 2025, 14, 1676. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, Q.; Shao, J.; Yang, L.; Zhang, X. Flavoromics Analysis of Passion Fruit-Roasted Chicken. Foods 2024, 13, 2221. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, R.; Mu, Y.; Lv, M.; Xing, J.; Zheng, L.; Aihaiti, A.; Wang, L. Study on the Mechanisms of Flavor Compound Changes During the Lactic Fermentation Process of Peach and Apricot Mixed Juice. Foods 2024, 13, 3835. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, H.; Zhang, J.; Liu, S.; Tian, Y.; Cheng, T.; Guo, Z.; Wang, Z. Improve the Fiber Structure and Texture Properties of Plant-Based Meat Analogues by Adjusting the Ratio of Soy Protein Isolate (SPI) to Wheat Gluten (WG). Food Chem. X 2024, 24, 101962. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Feng, X.; Fan, X.; Liu, Y.; Xu, X.; Zhou, G.; Zhu, B.; Ullah, N.; Chen, L. Effects of Low-Frequency and High-Intensity Ultrasonic Treatment Combined with Curdlan Gels on the Thermal Gelling Properties and Structural Properties of Soy Protein Isolate. Food Hydrocoll. 2022, 127, 107506. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, X.; Scholten, E. Rheological Behavior of Emulsion Gels Stabilized by Zein/Tannic Acid Complex Particles. Food Hydrocoll. 2018, 77, 363–371. [Google Scholar] [CrossRef]
- Saldanha Do Carmo, C.; Knutsen, S.H.; Malizia, G.; Dessev, T.; Geny, A.; Zobel, H.; Myhrer, K.S.; Varela, P.; Sahlstrøm, S. Meat Analogues from a Faba Bean Concentrate Can Be Generated by High Moisture Extrusion. Future Foods 2021, 3, 100014. [Google Scholar] [CrossRef]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of Soy Protein to Wheat Gluten Ratio on the Physicochemical Properties of Extruded Meat Analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Wang, H.; Van Den Berg, F.W.J.; Zhang, W.; Czaja, T.P.; Zhang, L.; Jespersen, B.M.; Lametsch, R. Differences in Physicochemical Properties of High-Moisture Extrudates Prepared from Soy and Pea Protein Isolates. Food Hydrocoll. 2022, 128, 107540. [Google Scholar] [CrossRef]
- Li, J.; Janssen, F.; Verfaillie, D.; Brijs, K.; Delcour, J.A.; Gunes, D.Z.; Cardinaels, R.; Van Royen, G.; Wouters, A.G.B. The Interplay between Soy Proteins and Dietary Fiber in Determining the Structure and Texture of High Moisture Extrudates. Food Hydrocoll. 2024, 156, 110256. [Google Scholar] [CrossRef]
- Chiang, J.H.; Tay, W.; Ong, D.S.M.; Liebl, D.; Ng, C.P.; Henry, C.J. Physicochemical, Textural and Structural Characteristics of Wheat Gluten-Soy Protein Composited Meat Analogues Prepared with the Mechanical Elongation Method. Food Struct. 2021, 28, 100183. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Liu, H.; Li, T.; Wang, Q.; Kaplan, D.L. Microscopic Insight into the Interactions between Pea Protein and Fatty Acids during High-Moisture Extrusion Processing. Food Chem. 2023, 404, 134176. [Google Scholar] [CrossRef]
- Zhao, Y.-R.; Peng, N.; Li, Y.-Q.; Liang, Y.; Guo, Z.-W.; Wang, C.-Y.; Wang, Z.-Y.; Wang, C.; Ren, X. Physicochemical Properties, Structural Characteristics and Protein Digestibility of Pea Protein-Wheat Gluten Composited Meat Analogues Prepared via High-Moisture Extrusion. Food Hydrocoll. 2024, 156, 110283. [Google Scholar] [CrossRef]
- Hossain Brishti, F.; Chay, S.Y.; Muhammad, K.; Rashedi Ismail-Fitry, M.; Zarei, M.; Karthikeyan, S.; Caballero-Briones, F.; Saari, N. Structural and Rheological Changes of Texturized Mung Bean Protein Induced by Feed Moisture during Extrusion. Food Chem. 2021, 344, 128643. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Yang, R.; Liu, G.; Sun, C.; Fang, Y. Bean Powders Improved the Quality Characteristics of High-Moisture Extrudate Prepared from Pea Protein Isolate: Texture, Structure and Sensory. Food Hydrocoll. 2025, 165, 111232. [Google Scholar] [CrossRef]
- Sun, D.; Wu, M.; Zhang, T.; Wei, D.; Zhou, C.; Shang, N. Conformational Changes and Physicochemical Attributes of Texturized Pea Protein Isolate-Konjac Gum: With a New Perspective of Residence Time during Extrusion. Food Res. Int. 2023, 165, 112500. [Google Scholar] [CrossRef] [PubMed]
- Vivian, J.T.; Callis, P.R. Mechanisms of Tryptophan Fluorescence Shifts in Proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef]
- Sun, D.; Wu, M.; Zhou, C.; Wang, B. Transformation of High Moisture Extrusion on Pea Protein Isolate in Melting Zone during: From the Aspects of the Rheological Property, Physicochemical Attributes and Modification Mechanism. Food Hydrocoll. 2022, 133, 108016. [Google Scholar] [CrossRef]
- Yun, J.; Cui, C.; Zhang, S.; Zhu, J.; Peng, C.; Cai, H.; Yang, X.; Hou, R. Use of Headspace GC/MS Combined with Chemometric Analysis to Identify the Geographic Origins of Black Tea. Food Chem. 2021, 360, 130033. [Google Scholar] [CrossRef]
- Hou, Z.; Xia, R.; Li, Y.; Xu, H.; Wang, Y.; Feng, Y.; Pan, S.; Wang, Z.; Ren, H.; Qian, G.; et al. Key Components, Formation Pathways, Affecting Factors, and Emerging Analytical Strategies for Edible Mushrooms Aroma: A Review. Food Chem. 2024, 438, 137993. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Wu, Q.; Zheng, W.; Pan, D.; Benjakul, S.; Liu, Z.; Lin, H.; Zhang, B. Effects of DBD-CP Pre-Treatment on Muscle Quality, Volatile Flavor Compounds, and Microbial Community Composition of Ready-to-Eat Drunken Shrimp (Solenocera crassicornis) during Frozen Storage. Food Chem. X 2025, 29, 102723. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.S.; Chen, Q.; Edleman, D.; Annor, G.A.; Dias, F.F.G. Unraveling the Impacts of Germination on the Volatile and Fatty Acid Profile of Intermediate Wheatgrass (Thinopyrum intermedium) Seeds. Molecules 2024, 29, 4268. [Google Scholar] [CrossRef]
- Kumazawa, K.; Baba, R.; Nishimura, O. Automated analysis of 4-mercapto-4-methyl-2-pentanone in Japanese green tea (Sen-cha) by headspace solid-phase microextraction and gas chromatography with mass spectrometric determination. Food Sci. Technol. Res. 2010, 16, 59–64. [Google Scholar] [CrossRef]
- Jin, Y.; Cui, H.; Yuan, X.; Liu, L.; Liu, X.; Wang, Y.; Ding, J.; Xiang, H.; Zhang, X.; Liu, J.; et al. Identification of the main aroma compounds in Chinese local chicken high-quality meat. Food Chem. 2021, 359, 129930. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Kim, S.Y.; Choi, H.K.; Kim, Y.-S. Characterization of aroma-active compounds in raw and cooked pine-mushrooms (Tricholoma matsutake Sing.). J. Agric. Food Chem. 2006, 54, 6332–6335. [Google Scholar] [CrossRef] [PubMed]


| LMM Content (%) | Fibrous Degree | Hardness (g) | Springiness | Chewiness (g) | L* | A* | B* | Browning Index (BI) |
|---|---|---|---|---|---|---|---|---|
| 0 | 1.05 ± 0.03 d | 7044.55 ± 144.10 a | 0.96 ± 0.02 a | 5394.12 ± 298.25 a | 45.54 ± 0.61 a | 3.46 ± 0.20 c | 16.23 ± 0.30 d | 48.77 ± 1.43 d |
| 10 | 1.28 ± 0.01 b | 6501.09 ± 314.92 b | 0.94 ± 0.01 a | 4750.47 ± 211.31 b | 45.05 ± 0.33 ab | 4.94 ± 0.26 b | 18.75 ± 0.60 c | 61.75 ± 1.25 c |
| 20 | 1.54 ± 0.01 a | 5574.34 ± 506.08 c | 0.95 ± 0.03 a | 4092.60 ± 294.57 c | 44.52 ± 0.68 b | 7.15 ± 0.53 a | 22.09 ± 0.62 b | 80.83 ± 0.42 b |
| 30 | 1.26 ± 0.05 b | 4691.55 ± 383.83 d | 0.92 ± 0.04 a | 3192.07 ± 242.24 d | 45.61 ± 0.08 a | 7.73 ± 0.22 a | 23.18 ± 0.16 a | 81.20 ± 0.50 b |
| 40 | 1.19 ± 0.02 c | 3665.08 ± 417.57 e | 0.94 ± 0.06 a | 2536.08 ± 395.64 e | 45.06 ± 0.48 ab | 7.76 ± 0.23 a | 23.06 ± 0.20 a | 82.07 ± 1.66 a |
| No. | Compound | Threshold (μg/g) | Odor Description | ROVA | |
|---|---|---|---|---|---|
| HMMAs-20%LMM | HMMAs | ||||
| 1 | 4-mercapto-4-methyl-2-pentanone | 0.000005 | black currant flavor | 100.000 ** | 100.000 ** |
| 2 | Hexanal | 0.0075 | grass flavor | 30.748 ** | 7.779 ** |
| 3 | 1-Octen-3-ol | 0.007 | mushroom flavor | 18.043 ** | 2.874 ** |
| 4 | Benz aldehyde | 0.00075 | bitter almond flavor and caramel flavor | 4.568 ** | 0.725 * |
| 5 | 2-Heptanone | 0.2 | blue Cheese flavor and nuts flavor | 4.456 ** | 0.756 * |
| 6 | Heptanal | 0.01 | fatty flavor | 1.967 ** | 1.034 ** |
| 7 | Nonanal | 0.015 | oily flavor | 1.910 ** | 2.521 ** |
| 8 | 2-Methylbutyraldehyde | 0.003 | almond flavor and malt flavor | 0.367 * | 1.105 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Yan, S.; Chen, K.; Chen, Q.; Li, B.; Li, J. Application Potential of Lion’s Mane Mushroom in Soy-Based Meat Analogues by High Moisture Extrusion: Physicochemical, Structural and Flavor Characteristics. Foods 2025, 14, 3402. https://doi.org/10.3390/foods14193402
Gao Y, Yan S, Chen K, Chen Q, Li B, Li J. Application Potential of Lion’s Mane Mushroom in Soy-Based Meat Analogues by High Moisture Extrusion: Physicochemical, Structural and Flavor Characteristics. Foods. 2025; 14(19):3402. https://doi.org/10.3390/foods14193402
Chicago/Turabian StyleGao, Yang, Song Yan, Kaixin Chen, Qing Chen, Bo Li, and Jialei Li. 2025. "Application Potential of Lion’s Mane Mushroom in Soy-Based Meat Analogues by High Moisture Extrusion: Physicochemical, Structural and Flavor Characteristics" Foods 14, no. 19: 3402. https://doi.org/10.3390/foods14193402
APA StyleGao, Y., Yan, S., Chen, K., Chen, Q., Li, B., & Li, J. (2025). Application Potential of Lion’s Mane Mushroom in Soy-Based Meat Analogues by High Moisture Extrusion: Physicochemical, Structural and Flavor Characteristics. Foods, 14(19), 3402. https://doi.org/10.3390/foods14193402






