Production Systems and Feeding Strategies in the Aromatic Fingerprinting of Animal-Derived Foods: Invited Review
Abstract
1. Introduction
2. Methodological Approach
- The effects of conventional, organic, and sustainable farming systems on animal metabolism and the generation of aroma precursors.
- The interaction between animal breeds and environmental conditions influencing aromatic profiles.
- The application of advanced analytical technologies (e.g., GC–MS, GC–IMS, electronic nose, FTIR spectroscopy) in VOC profiling and fingerprinting.
3. Volatile Organic Compounds: Sources and Biogenesis
3.1. Thermal Pathways
3.1.1. Maillard Reaction
3.1.2. Strecker Degradation
3.1.3. Lipid Oxidation
3.1.4. Thiamine Degradation
3.2. Non-Thermal Pathways
3.2.1. Microbial Metabolism
3.2.2. Aging and Storage
3.2.3. Packaging
4. Farming Practices and Aromatic Profiling of Animal-Derived Food
4.1. VOCs in Milk
4.2. VOCs in Muscle Foods
4.2.1. VOCs in Beef
4.2.2. VOCs in Sheep and Lamb Meat
4.2.3. VOCs in Poultry Meat
4.2.4. VOCs in Rabbit Meat
4.3. VOCs in Eggs
5. Aromatic Finger Printing
5.1. Species-Specific Biomarkers
5.2. Processing-Derived Biomarkers
5.3. Diet-Metabolic Biomarker
5.4. Food Quality and Shelf-Life Indicators
5.5. Authenticity/Traceability Markers
6. Role of Aroma-Active VOCs in Consumer Perception and Market Behavior
6.1. Influence of Aroma and Flavor on Sensory Evaluation and Purchasing Decisions
6.2. Role of Authenticity and Quality Claims in Consumer Behavior and Market Segmentation
6.3. Market Trends and Sociocultural Factors Shaping Preferences
7. Aromatic Fingerprinting Techniques
7.1. Analytical Techniques for Aromatic Fingerprinting
7.1.1. Gas Chromatography–Mass Spectrometry (GC–MS)
7.1.2. Electronic Noses (E-Nose)
7.2. Spectroscopic and Emerging Sensor Methods
7.2.1. FTIR Spectroscopy
7.2.2. Ion Mobility Spectrometry (IMS)
8. Conclusions
- Standardize VOC biomarkers and analytical protocols so results are comparable across systems and studies.
- Advance rapid, on-site detection tools (e.g., portable instruments, sensor platforms) to enable real-time monitoring in production chains.
- Validate multi-omics flavor models (linking VOCs with genomics and metabolomics) through robust statistical approaches for practical application.
- Connect farm-level feeding practices with advanced omics to ensure that fundamental nutritional effects on VOC development are not overlooked in high-tech models.
9. Future Perspectives and Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VOCs | Volatile Organic Compounds |
| HS–GC–MS–O | Headspace–Gas Chromatography–Mass Spectrometry–Olfactometry |
| GC–MS | Gas Chromatography–Mass Spectrometry |
| GC–IMS | Gas Chromatography–Ion Mobility Spectrometry |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| E-nose | Electronic Nose |
| OAV | Odor Activity Value |
| PCA | Principal Component Analysis |
| PLSR | Partial Least Squares Regression |
| LDA | Linear Discriminant Analysis |
| ANN | Artificial Neural Network |
| QCM | Quartz Crystal Microbalance |
| MOS | Metal Oxide Semiconductor (sensor) |
| CP | Conducting Polymer (sensor) |
| SFA | Saturated Fatty Acids |
| PUFA | Polyunsaturated Fatty Acids |
| MUFA | Monounsaturated Fatty Acids |
| BCFA | Branched-Chain Fatty Acids |
| IMF | Intramuscular Fat |
| DM | Dry Matter |
| CLA | Conjugated Linoleic Acid |
| ALA | α-Linolenic Acid |
| EPA | Eicosapentaenoic Acid |
| DHA | Docosahexaenoic Acid |
| PDO | Protected Designation of Origin |
| SIRA | Stable Isotope Ratio Analysis |
| DNA | Deoxyribonucleic Acid |
| PCR-RFLP | Polymerase Chain Reaction–Restriction Fragment Length Polymorphism |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| TBARS | Thiobarbituric Acid Reactive Substances |
| MAP | Modified Atmosphere Packaging |
| VP | Vacuum Packaging |
| CP (packaging) | Cling-Wrapped Packaging |
| GP | Genetic Programming |
| SVM | Support Vector Machine |
| AFLS | Adaptive Fuzzy Logic System |
References
- Al-Khalili, M.; Pathare, P.B.; Rahman, S.; Al-Habsi, N. Aroma Compounds in Food: Analysis, Characterization and Flavor Perception. Meas. Food 2025, 18, 100220. [Google Scholar] [CrossRef]
- Flores, M. The Eating Quality of Meat: III—Flavor. In Lawrie’s Meat Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 421–455. ISBN 978-0-323-85408-5. [Google Scholar]
- Ni, Q.; Amalfitano, N.; Biasioli, F.; Gallo, L.; Tagliapietra, F.; Bittante, G. Bibliometric Review on the Volatile Organic Compounds in Meat. Foods 2022, 11, 3574. [Google Scholar] [CrossRef]
- Kosowska, M.; Majcher, A.M.; Fortuna, T. Volatile Compounds in Meat and Meat Products. Food Sci. Technol 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Li, C.; Al-Dalali, S.; Wang, Z.; Xu, B.; Zhou, H. Investigation of Volatile Flavor Compounds and Characterization of Aroma-Active Compounds of Water-Boiled Salted Duck Using GC–MS–O, GC–IMS, and E-Nose. Food Chem. 2022, 386, 132728. [Google Scholar] [CrossRef]
- Davis, H.; Magistrali, A.; Butler, G.; Stergiadis, S. Nutritional Benefits from Fatty Acids in Organic and Grass-Fed Beef. Foods 2022, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.S.; Sohaib, M.; Ahmad, R.S.; Nadeem, M.T.; Imran, A.; Arshad, M.U.; Kwon, J.-H.; Amjad, Z. Ruminant Meat Flavor Influenced by Different Factors with Special Reference to Fatty Acids. Lipids Health Dis. 2018, 17, 223. [Google Scholar] [CrossRef]
- Yuan, N.; Chi, X.; Ye, Q.; Liu, H.; Zheng, N. Analysis of Volatile Organic Compounds in Milk during Heat Treatment Based on E-Nose, E-Tongue and HS-SPME-GC-MS. Foods 2023, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Biçer, Y.; Telli, A.E.; Sönmez, G.; Telli, N.; Uçar, G. Comparison of Microbiota and Volatile Organic Compounds in Milk from Different Sheep Breeds. J. Dairy Sci. 2021, 104, 12303–12311. [Google Scholar] [CrossRef]
- Moscovici Joubran, A.; Pierce, K.M.; Garvey, N.; Shalloo, L.; O’Callaghan, T.F. Invited Review: A 2020 Perspective on Pasture-Based Dairy Systems and Products. J. Dairy Sci. 2021, 104, 7364–7382. [Google Scholar] [CrossRef]
- Prache, S.; Lebret, B.; Baéza, E.; Martin, B.; Gautron, J.; Feidt, C.; Médale, F.; Corraze, G.; Raulet, M.; Lefèvre, F.; et al. Review: Quality and Authentication of Organic Animal Products in Europe. Animal 2022, 16, 100405. [Google Scholar] [CrossRef]
- Cuchillo-Hilario, M.; Fournier-Ramírez, M.-I.; Díaz Martínez, M.; Montaño Benavides, S.; Calvo-Carrillo, M.-C.; Carrillo Domínguez, S.; Carranco-Jáuregui, M.-E.; Hernández-Rodríguez, E.; Mora-Pérez, P.; Cruz-Martínez, Y.R.; et al. Animal Food Products to Support Human Nutrition and to Boost Human Health: The Potential of Feedstuffs Resources and Their Metabolites as Health-Promoters. Metabolites 2024, 14, 496. [Google Scholar] [CrossRef]
- Wanapat, M.; Cherdthong, A.; Phesatcha, K.; Kang, S. Dietary Sources and Their Effects on Animal Production and Environmental Sustainability. Anim. Nutr. 2015, 1, 96–103. [Google Scholar] [CrossRef]
- Stampa, E.; Schipmann-Schwarze, C.; Hamm, U. Consumer Perceptions, Preferences, and Behavior Regarding Pasture-Raised Livestock Products: A Review. Food Qual. Prefer. 2020, 82, 103872. [Google Scholar] [CrossRef]
- Font-i-Furnols, M.; Guerrero, L. Consumer Preference, Behavior and Perception about Meat and Meat Products: An Overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef]
- Wojtasik-Kalinowska, I.; Szpicer, A.; Binkowska, W.; Hanula, M.; Marcinkowska-Lesiak, M.; Poltorak, A. Effect of Processing on Volatile Organic Compounds Formation of Meat—Review. Appl. Sci. 2023, 13, 705. [Google Scholar] [CrossRef]
- Shakoor, A.; Zhang, C.; Xie, J.; Yang, X. Maillard Reaction Chemistry in Formation of Critical Intermediates and Flavour Compounds and Their Antioxidant Properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, H.; Ma, G.; Zhang, T.; Wang, L.; Pei, H.; Li, X.; Gao, L. Insights into Flavor and Key Influencing Factors of Maillard Reaction Products: A Recent Update. Front. Nutr. 2022, 9, 973677. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Pan, T.; Hui, T.; Ma, J. Characterization of Key Aroma Compounds in Beijing Roasted Duck by Gas Chromatography–Olfactometry–Mass Spectrometry, Odor-Activity Values, and Aroma-Recombination Experiments. J. Agric. Food Chem. 2019, 67, 5847–5856. [Google Scholar] [CrossRef]
- Tamanna, N.; Mahmood, N. Food Processing and Maillard Reaction Products: Effect on Human Health and Nutrition. Int. J. Food Sci. 2015, 2015, 526762. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, F.; Wang, B.; Chen, L.; Liu, W.; Tan, S. A Literature Review on Maillard Reaction Based on Milk Proteins and Carbohydrates in Food and Pharmaceutical Products: Advantages, Disadvantages, and Avoidance Strategies. Foods 2021, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.S.; Campo, M.M.; Enser, M.; Mottram, D.S. Effect of Lipid Composition on Meat-like Model Systems Containing Cysteine, Ribose, and Polyunsaturated Fatty Acids. J. Agric. Food Chem. 2002, 50, 1126–1132. [Google Scholar] [CrossRef]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Shah, H.; Patil, P. Aroma Compounds Identified in Cooked Meat: A Review. Food Res. Int. 2022, 157, 111385. [Google Scholar] [CrossRef]
- Geng, L.; Liu, K.; Zhang, H. Lipid Oxidation in Foods and Its Implications on Proteins. Front. Nutr. 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, J.; Ebner, E.E.; Bak, K.H. Formation and Analysis of Volatile and Odor Compounds in Meat—A Review. Molecules 2022, 27, 6703. [Google Scholar] [CrossRef] [PubMed]
- Flores, M. Understanding the Implications of Current Health Trends on the Aroma of Wet and Dry Cured Meat Products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I. Thiamin: Physiology. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2013; pp. 274–279. ISBN 978-0-12-384885-7. [Google Scholar]
- Thomas, C.; Mercier, F.; Tournayre, P.; Martin, J.-L.; Berdagué, J.-L. Effect of Added Thiamine on the Key Odorant Compounds and Aroma of Cooked Ham. Food Chem. 2015, 173, 790–795. [Google Scholar] [CrossRef]
- Brehm, L.; Frank, O.; Jünger, M.; Wimmer, M.; Ranner, J.; Hofmann, T. Novel Taste-Enhancing 4-Amino-2-Methyl-5-Heteroalkypyrimidines Formed from Thiamine by Maillard-Type Reactions. J. Agric. Food. Chem. 2019, 67, 13986–13997. [Google Scholar] [CrossRef]
- Huang, G.; Li, N.; Liu, K.; Yang, J.; Zhao, S.; Zheng, N.; Zhou, J.; Zhang, Y.; Wang, J. Effect of Flaxseed Supplementation in Diet of Dairy Cow on the Volatile Organic Compounds of Raw Milk by HS-GC–IMS. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef]
- Flores, M.; Perea-Sanz, L.; López-Díez, J.J.; Belloch, C. Meaty Aroma Notes from Free Amino Acids and Thiamine in Nitrite-Reduced, Dry-Fermented, Yeast-Inoculated Sausages. Food Chem. 2021, 361, 129997. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.J.; Yoon, J.W.; Kim, M.; Jo, C. Effect of Different Aging Methods on the Formation of Aroma Volatiles in Beef Strip Loins. Foods 2021, 10, 146. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.J.; Kim, M.; Yoon, J.W.; Shin, D.J.; Jo, C. Storage Stability of Vacuum-Packaged Dry-Aged Beef during Refrigeration at 4 °C. Food Sci. Anim. Resour. 2019, 39, 266–275. [Google Scholar] [CrossRef]
- Iida, F.; Miyazaki, Y.; Tsuyuki, R.; Kato, K.; Egusa, A.; Ogoshi, H.; Nishimura, T. Changes in Taste Compounds, Breaking Properties, and Sensory Attributes during Dry Aging of Beef from Japanese Black Cattle. Meat Sci. 2016, 112, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Gkarane, V.; Brunton, N.P.; Allen, P.; Gravador, R.S.; Claffey, N.A.; Diskin, M.G.; Fahey, A.G.; Farmer, L.J.; Moloney, A.P.; Alcalde, M.J.; et al. Effect of Finishing Diet and Duration on the Sensory Quality and Volatile Profile of Lamb Meat. Food Res. Int. 2019, 115, 54–64. [Google Scholar] [CrossRef]
- Bhadury, D.; Nolvachai, Y.; Marriott, P.J.; Tanner, J.; Tuck, K.L. Detection of Volatiles from Raw Beef Meat from Different Packaging Systems Using Solid-Phase Microextraction GC–Accurate Mass Spectrometry. Foods 2021, 10, 2018. [Google Scholar] [CrossRef]
- Scollan, N.D.; Price, E.M.; Morgan, S.A.; Huws, S.A.; Shingfield, K.J. Can We Improve the Nutritional Quality of Meat? Proc. Nutr. Soc. 2017, 76, 603–618. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat Deposition, Fatty Acid Composition and Meat Quality: A Review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G. The Composition of Bovine Milk Lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef]
- Fraeye, I.; Bruneel, C.; Lemahieu, C.; Buyse, J.; Muylaert, K.; Foubert, I. Dietary Enrichment of Eggs with Omega-3 Fatty Acids: A Review. Food Res. Int. 2012, 48, 961–969. [Google Scholar] [CrossRef]
- Li, J.; Yang, C.; Ran, J.; Yu, C.; Yin, L.; Li, Z.; Liu, Y. The Age-Dependent Variations for Fatty Acid Composition and Sensory Quality of Chicken Meat and Associations between Gene Expression Patterns and Meat Quality. Livest. Sci. 2021, 254, 104736. [Google Scholar] [CrossRef]
- Xiang, X.; Jin, G.; Gouda, M.; Jin, Y.; Ma, M. Characterization and Classification of Volatiles from Different Breeds of Eggs by SPME-GC–MS and Chemometrics. Food Res. Int. 2019, 116, 767–777. [Google Scholar] [CrossRef]
- Villeneuve, M.-P.; Lebeuf, Y.; Gervais, R.; Tremblay, G.F.; Vuillemard, J.C.; Fortin, J.; Chouinard, P.Y. Milk Volatile Organic Compounds and Fatty Acid Profile in Cows Fed Timothy as Hay, Pasture, or Silage. J. Dairy Sci. 2013, 96, 7181–7194. [Google Scholar] [CrossRef]
- Duman, M.; Özpolat, E. Effects of Water Extract of Propolis on Fresh Shibuta (Barbus grypus) Fillets during Chilled Storage. Food Chem. 2015, 189, 80–85. [Google Scholar] [CrossRef]
- Yang, W.; Jia, Y.; Yang, Y.; Chen, H.; Zhou, L.; Wang, L.; Lv, X.; Zhao, Q.; Qin, Y.; Zhang, J.; et al. Sacha Inchi Oil Addition to Hen Diets and the Effects on Egg Yolk Flavor Based on Multiomics and Flavoromics Analysis. Food Chem. 2025, 475, 143251. [Google Scholar] [CrossRef]
- Bergamaschi, M.; Aprea, E.; Betta, E.; Biasioli, F.; Cipolat-Gotet, C.; Cecchinato, A.; Bittante, G.; Gasperi, F. Effects of Dairy System, Herd within Dairy System, and Individual Cow Characteristics on the Volatile Organic Compound Profile of Ripened Model Cheeses. J. Dairy Sci. 2015, 98, 2183–2196. [Google Scholar] [CrossRef]
- Bendall, J.G. Aroma Compounds of Fresh Milk from New Zealand Cows Fed Different Diets. J. Agric. Food Chem. 2001, 49, 4825–4832. [Google Scholar] [CrossRef]
- Sacchi, R.; Marrazzo, A.; Masucci, F.; Di Francia, A.; Serrapica, F.; Genovese, A. Effects of Inclusion of Fresh Forage in the Diet for Lactating Buffaloes on Volatile Organic Compounds of Milk and Mozzarella Cheese. Molecules 2020, 25, 1332. [Google Scholar] [CrossRef]
- Borge, G.I.A.; Sandberg, E.; Øyaas, J.; Abrahamsen, R.K. Variation of Terpenes in Milk and Cultured Cream from Norwegian Alpine Rangeland-Fed and in-Door Fed Cows. Food Chem. 2016, 199, 195–202. [Google Scholar] [CrossRef]
- Cheng, Z.; O’Sullivan, M.G.; Miao, S.; Kerry, J.P.; Kilcawley, K.N. Sensorial, Cultural and Volatile Properties of Milk, Dairy Powders, Yoghurt and Butter: A Review. Int. J. Dairy Technol. 2022, 75, 761–790. [Google Scholar] [CrossRef]
- Wójtowski, J.A.; Majcher, M.; Danków, R.; Pikul, J.; Mikołajczak, P.; Molińska-Glura, M.; Foksowicz-Flaczyk, J.; Gryszczyńska, A.; Łowicki, Z.; Zajączek, K.; et al. Effect of Herbal Feed Additives on Goat Milk Volatile Flavor Compounds. Foods 2023, 12, 2963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, P.; Guo, H.; Zhang, X.; You, M.; He, X.; Zhao, X.; Ma, N. Alterations of Meat Quality, Lipid Composition and Flavor in Breast Meat of Laying Hens with Fatty Liver Hemorrhagic Syndrome. Poult. Sci. 2024, 103, 104360. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.C.; Tullio, R.R.; Marcondes, C.R.; Pflanzer, S.B.; Nassu, R.T. Meat Quality, Aroma Profile and Consumer Preference of Dry-Aged Beef. Meat Muscle Biol. 2019, 3. [Google Scholar] [CrossRef]
- Wang, L.M.; Bohrer, B.M. Effects of Replacing Antibiotics in Finishing Cattle Diets with Plant-Based Additives on Meat Quality and Sensory Attributes. Meat Muscle Biol. 2019, 3. [Google Scholar] [CrossRef]
- Park, M.K.; Choi, Y.-S. Effective Strategies for Understanding Meat Flavor: A Review. Food Sci. Anim. Resour. 2025, 45, 165–184. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.; Moloney, A.P.; O’Riordan, E.G.; Regan, M.; Lenehan, C.; Kelly, A.K.; Crosson, P. Pasture-Finishing of Late-Maturing Bulls or Steers in a Suckler Calf-to-Beef System: Animal Production, Meat Quality, Economics, Greenhouse Gas Emissions and Human-Edible Food-Feed Efficiency. Agric. Syst. 2023, 209, 103672. [Google Scholar] [CrossRef]
- Pogorzelska-Przybyłek, P.; Nogalski, Z.; Sobczuk-Szul, M.; Momot, M. The Effect of Gender Status on the Growth Performance, Carcass and Meat Quality Traits of Young Crossbred Holstein-Friesian × Limousin Cattle. Anim. Biosci. 2021, 34, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Herrera, N.J.; Calkins, C.R. Developments in Meat Flavor. In New Aspects of Meat Quality; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–235. ISBN 978-0-323-85879-3. [Google Scholar]
- Soulat, J.; Picard, B.; Monteils, V. Does the Rearing Management Following by Charolais Cull Cows Influence the Qualities of Carcass and Beef Meat? Foods 2022, 11, 2889. [Google Scholar] [CrossRef]
- Belhaj, K.; Mansouri, F.; Tikent, A.; Taaifi, Y.; Boukharta, M.; Serghini, H.C.; Elamrani, A. Effect of Age and Breed on Carcass and Meat Quality Characteristics of Beni-Guil and Ouled-Djellal Sheep Breeds. Sci. World J. 2021, 5536793. [Google Scholar] [CrossRef]
- Watkins, P.J.; Jaborek, J.R.; Teng, F.; Day, L.; Castada, H.Z.; Baringer, S.; Wick, M. Branched Chain Fatty Acids in the Flavour of Sheep and Goat Milk and Meat: A Review. Small Rumin. Res. 2021, 200, 106398. [Google Scholar] [CrossRef]
- Jia, R.; He, Y.; Liao, G.; Yang, Z.; Gu, D.; Pu, Y.; Huang, M.; Wang, G. Identification of Umami Peptides from Wuding Chicken by Nano-HPLC-MS/MS and Insights into the Umami Taste Mechanisms. Food Res. Int. 2023, 172, 113208. [Google Scholar] [CrossRef]
- Echegaray, N.; Domínguez, R.; Cadavez, V.A.P.; Bermúdez, R.; Pateiro, M.; Gonzales-Barron, U.; Lorenzo, J.M. Influence of Feeding System on Longissimus thoracis et lumborum Volatile Compounds of an Iberian Local Lamb Breed. Small Rumin. Res. 2021, 201, 106417. [Google Scholar] [CrossRef]
- Zhao, X.; Zuo, S.; Guo, Y.; Zhang, C.; Wang, Y.; Peng, S.; Liu, M.; Wang, B.; Zhang, H.; Luo, H. Carcass Meat Quality, Volatile Compound Profile, and Gene Expression in Tan Sheep under Different Feeding Regimes. Food Biosci. 2023, 56, 103213. [Google Scholar] [CrossRef]
- Silva, L.A.S.; Lima, C.L.S.; Pina, D.d.S.; Alba, H.D.R.; de Araújo, M.L.G.M.L.; Cirne, L.G.A.; Azevêdo, J.A.G.; Rodrigues, C.S.; Borges, L.M.; Chaves, M.L.O.; et al. Carcass Traits and Meat Quality of Lambs Fed with Rehydrated Ground Corn Silage. Small Rumin. Res. 2024, 231, 107193. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Liu, G.; Shi, B.; Zhu, B.; Gao, L.; Zhong, K.; Zhang, Y.; Zhao, L.; Li, R.; et al. An Assessment of the Sensory Drivers Influencing Consumer Preference in Infant Formula, Assessed via Sensory Evaluation and GC-O-MS. Food Chem. 2024, 455, 139881. [Google Scholar] [CrossRef]
- Gadzama, I.U.; Hoffman, L.C.; Holman, B.W.B.; Chaves, A.V.; Meale, S.J. Effects of Supplementing a Feedlot Diet with Microalgae (Chlorella vulgaris) on the Performance, Carcass Traits and Meat Quality of Lambs. Livest. Sci. 2024, 288, 105552. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Chen, B.; Zhou, W.; Zhang, N.; Tu, Y.; Diao, Q.; Ma, T.; Chen, H.; Chen, K.; et al. Evaluation of Ensiled Protein Grass as a Novel Feed Ingredient in Diets for Lambs: Effects on Fattening Performance, Meat Quality and Flavor. Food Chem. 2025, 482, 144220. [Google Scholar] [CrossRef]
- Fathi, M.; Hosayni, M.; Alizadeh, S.; Zandi, R.; Rahmati, S.; Rezaee, V. Effects of Black Cumin (Nigella sativa) Seed Meal on Growth Performance, Blood and Biochemical Indices, Meat Quality and Cecal Microbial Load in Broiler Chickens. Livest. Sci. 2023, 274, 105272. [Google Scholar] [CrossRef]
- Radulović, S.; Pavlović, M.; Šefer, D.; Katoch, S.; Hadži-Milić, M.; Jovanović, D.; Grdović, S.; Marković, R. Effects of Housefly Larvae (Musca domestica) Dehydrated Meal on Production Performances and Sensory Properties of Broiler Meat. Thai J. Vet. Med. 2018, 48, 63–70. [Google Scholar] [CrossRef]
- Shan, L.; He, J.; Yang, R.; Dong, J.; Du, Z.; Duan, S.; Li, Y.; Lu, X.; Shen, Y.; Fu, J.; et al. Exploring Effects of Dietary Coffee Pericarp Addition on Growth, Meat Quality, Gut Flora in White-Feather Broilers. Poult. Sci. 2025, 104, 105077. [Google Scholar] [CrossRef] [PubMed]
- Rasinska, E.; Rutkowska, J.; Czarniecka-Skubina, E.; Tambor, K. Effects of Cooking Methods on Changes in Fatty Acids Contents, Lipid Oxidation and Volatile Compounds of Rabbit Meat. LWT 2019, 110, 64–70. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Liu, X.; Wang, Y.; Yang, W.; Zhao, W.; Zhao, G.; Cui, H.; Wen, J. Identification of Characteristic Aroma Compounds in Chicken Meat and Their Metabolic Mechanisms Using Gas Chromatography–Olfactometry, Odor Activity Values, and Metabolomics. Food Res. Int. 2024, 175, 113782. [Google Scholar] [CrossRef]
- Gao, L.; Liu, C.; Wu, J.; Cui, Y.; Zhang, M.; Bi, C.; Shan, A.; Dou, X. EGCG Improve Meat Quality, Restore Lipid Metabolism Disorder and Regulate Intestinal Flora in High-Fat Fed Broilers. Poul. Sci. 2025, 104, 104875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Mei, J.; Wang, Y.; Yu, B.; Liu, H. Functional Properties and Flavor Characteristics of Milk from Cows Supplemented with Jujube Powder. J. Dairy Sci. 2024, 107, 3492–3501. [Google Scholar] [CrossRef]
- Mikulski, D.; Zduńczyk, Z.; Juśkiewicz, J.; Rogiewicz, A.; Jankowski, J. The Effect of Different Blue Lupine (L. angustifolius) Inclusion Levels on Gastrointestinal Function, Growth Performance and Meat Quality in Growing-Finishing Turkeys. Anim. Feed Sci. Technol. 2014, 198, 347–352. [Google Scholar] [CrossRef]
- Geldenhuys, G.; Muller, N.; Hoffman, L.C. The Influence of Season on the Sensory Profile of Egyptian Goose (Alopochen aegyptiacus) Meat. Poult. Sci. 2016, 95, 2174–2185. [Google Scholar] [CrossRef]
- Jalal, H.; Doğan, S.C.; Giammarco, M.; Cavallini, D.; Lanzoni, L.; Pezzi, P.; Akram, M.Z.; Fusaro, I. Evaluation of Dietary Supplementation of Garlic Powder (Allium sativum) on the Growth Performance, Carcass Traits and Meat Quality of Japanese Quails (Coturnix coturnix Japonica). Poult. Sci. 2024, 103, 104231. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Li, X.; Zhao, D.; Qin, S.; Shi, Z.; Wang, Z. Dietary Zinc Supplementation in Breeding Pigeons Improves the Carcass Traits of Squabs through Regulating Antioxidant Capacity and Myogenic Regulatory Factor Expression. Poult. Sci. 2023, 102, 102809. [Google Scholar] [CrossRef]
- Al-Soufi, S.; García, J.; Nicodemus, N.; Lorenzo, J.M.; Cegarra, E.; Muíños, A.; Losada, A.P.; Miranda, M.; López-Alonso, M. Marine Macroalgae in Rabbit Feed–Effects on Meat Quality. Meat Sci. 2024, 216, 109584. [Google Scholar] [CrossRef] [PubMed]
- Miciński, J.; Viliene, V.; Racevičiūtė-Stupelienė, A.; Klementavičiūtė, J.; Sasyte, V.; Bliznikas, S.; Matusevicius, P.; Nutautaitė, M. Impact of Forms of Selenium and Supplemental Vitamin E on Rabbits’ Growth, Slaughter Performance and Muscle Quality. J. Elem. 2021, 26, 383–405. [Google Scholar] [CrossRef]
- Dutra, D.R.; Villegas-Cayllahua, E.A.; Baptista, G.G.; Ferreira, L.E.; Cavalcanti, É.N.F.; Carneiro, N.M.G.M.; Dias, A.V.L.; Giampietro-Ganeco, A.; Pereira, M.R.; Castilha, L.D.; et al. Influence of Carcass Chilling Time on the Progression of Rigor Mortis, Carcass Characteristics and Physicochemical Properties Related to the Colour and Tenderness of Longissimus thoracis et lumborum and Biceps Femoris Muscles in Botucatu Rabbits. Meat Sci. 2025, 222, 109739. [Google Scholar] [CrossRef]
- Hernández, P.; dalle Zotte, A. Influence of Diet on Rabbit Meat Quality. In Nutrition of the Rabbit; CABI Books; CABI Digital Library: Wallingford, UK, 2020; pp. 172–192. ISBN 978-1-78924-127-3. [Google Scholar]
- Foti, F.; Scerra, M.; Caparra, P.; Bognanno, M.; Cilione, C.; Fortugno, P.; De Caria, P.; Chinè, V.; Mangione, G.; Gagliano, S.; et al. Effect of Coffee Silverskin on Meat Quality of Growing Rabbits. Foods 2025, 14, 812. [Google Scholar] [CrossRef] [PubMed]
- El-Gindy, Y.M. The Impact of Enriching Heat-Stressed Rabbit Diets with Flaxseed Oil with/ without Allicin, Lycopene, or Punicalagin on Antioxidative Status, Physiological Response and Meat Omega-3. BMC Vet. Res. 2025, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Dutra, D.R.; Villegas-Cayllahua, E.A.; Baptista, G.G.; Ferreira, L.E.; Castilha, L.D.; Borba, H. Characterization of Post-Mortem pH Evolution and Rigor Mortis Process in Botucatu Rabbit Carcasses of Different Categories. Animals 2024, 14, 2502. [Google Scholar] [CrossRef]
- Badr, H.M. Use of Irradiation to Control Foodborne Pathogens and Extend the Refrigerated Market Life of Rabbit Meat. Meat Sci. 2004, 67, 541–548. [Google Scholar] [CrossRef]
- Ping, C.; Zhao, X.; He, C.; Zheng, Y.; Zhang, H. Comparing Effects of Tangerine-Peel (Citrus reticulata Blanco) Age and Concentration on Deep-Fried Rabbit Meat: Impact on Heterocyclic Aromatic Amines, Amino Acids, and Flavor Compound Formation. Food Chem. X 2024, 24, 101902. [Google Scholar] [CrossRef]
- Bianospino, E.; Moura, A.S.A.M.T.; Wechsler, F.S.; Fernandes, S.; Dal-Pai-Silva, M. Age-Related Changes in Muscle Fiber Type Frequencies and Cross-Sectional Areas in Straightbred and Crossbred Rabbits. Animal 2008, 2, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, C.; Qi, Y.; Lu, W.; Xu, G.; Wang, B.; Zhang, D.; Zhao, S.; Ding, M. Identification of Volatile Organic Compounds in Muscle Tissues of Different Species Based on Headspace-Gas-Chromatography Ion-Mobility Spectrometry. Leg. Med. 2022, 59, 102132. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, L.; Li, J.; Xu, C.; Xiong, Z.; Xu, X.; Han, M. The Effect of Raw Egg Storage Time on the Quality, Fatty Acid Composition and Volatile Organic Compounds of Salt-Baked Marinated Eggs. Int. J. Gastron. Food Sci. 2025, 40, 101146. [Google Scholar] [CrossRef]
- Yenilmez, F. Characterization and Comparison of Volatile Compounds of Cage, Organic and Free-Range Systems Eggs. Braz. J. Poult. Sci. 2024, 26, eRBCA-2023-1872. [Google Scholar] [CrossRef]
- Plagemann, I.; Zelena, K.; Krings, U.; Berger, R.G. Volatile Flavours in Raw Egg Yolk of Hens Fed on Different Diets. J. Sci. Food Agric. 2011, 91, 2061–2065. [Google Scholar] [CrossRef]
- Borras, E.; Wang, Y.; Shah, P.; Bellido, K.; Hamera, K.L.; Arlen, R.A.; McCartney, M.M.; Portillo, K.; Zhou, H.; Davis, C.E.; et al. Active Sampling of Volatile Chemicals for Non-Invasive Classification of Chicken Eggs by Sex Early in Incubation. PLoS ONE 2023, 18, e0285726. [Google Scholar] [CrossRef] [PubMed]
- Kalus, K.; Konkol, D.; Korczyński, M.; Koziel, J.A.; Opaliński, S. Laying Hens Biochar Diet Supplementation—Effect on Performance, Excreta N Content, NH3 and VOCs Emissions, Egg Traits and Egg Consumers Acceptance. Agriculture 2020, 10, 237. [Google Scholar] [CrossRef]
- Nasiru, M.M.; Umair, M.; Boateng, E.F.; Alnadari, F.; Khan, K.R.; Wang, Z.; Luo, J.; Yan, W.; Zhuang, H.; Majrashi, A.; et al. Characterisation of Flavour Attributes in Egg White Protein Using HS-GC-IMS Combined with E-Nose and E-Tongue: Effect of High-Voltage Cold Plasma Treatment Time. Molecules 2022, 27, 601. [Google Scholar] [CrossRef]
- Cumeras, R.; Aksenov, A.A.; Pasamontes, A.; Fung, A.G.; Cianchetta, A.N.; Doan, H.; Davis, R.M.; Davis, C.E. Identification of Fungal Metabolites from inside Gallus Gallus domesticus Eggshells by Non-Invasively Detecting Volatile Organic Compounds (VOCs). Anal. Bioanal. Chem. 2016, 408, 6649–6658. [Google Scholar] [CrossRef]
- Man, L.; Ren, W.; Qin, H.; Sun, M.; Yuan, S.; Zhu, M.; Liu, G.; Wang, C.; Li, M. Characterization of the Relationship between Lipids and Volatile Compounds in Donkey, Bovine, and Sheep Meat by UHPLC–ESI–MS and SPME–GC–MS. LWT 2023, 175, 114426. [Google Scholar] [CrossRef]
- Kerler, J.; Grosch, W. Character Impact Odorants of Boiled Chicken: Changes during Refrigerated Storage and Reheating. Z. Lebensm. Unters Forsch. 1997, 205, 232–238. [Google Scholar] [CrossRef]
- Park, M.K.; Kim, B.-G.; Kang, M.-C.; Kim, T.-K.; Choi, Y.-S. Distinctive Volatile Compound Profile of Different Raw Meats, Including Beef, Pork, Chicken, and Duck, Based on Flavor Map. Appl. Food Res. 2025, 5, 100655. [Google Scholar] [CrossRef]
- García, Y.; Rufini, J.; Campos, M.; Guedes, M.; Augusti, R.; Melo, J. SPME Fiber Evaluation for Volatile Organic Compounds Extraction from Acerola. J. Braz. Chem. Soc. 2018, 30, 247–255. [Google Scholar] [CrossRef]
- Hough, R.; Archer, D.; Probert, C. A Comparison of Sample Preparation Methods for Extracting Volatile Organic Compounds (VOCs) from Equine Faeces Using HS-SPME. Metabolomics 2018, 14, 19. [Google Scholar] [CrossRef]
- Ahamed, Z.; Seo, J.; Eom, J.-U.; Yang, H.-S. Optimization of Volatile Compound Extraction on Cooked Meat Using HS-SPME-GC-MS, and Evaluation of Diagnosis to Meat Species Using Volatile Compound by Multivariate Data Analysis. LWT 2023, 188, 115374. [Google Scholar] [CrossRef]
- Yin, X.; Wen, R.; Sun, F.; Wang, Y.; Kong, B.; Chen, Q. Collaborative Analysis on Differences in Volatile Compounds of Harbin Red Sausages Smoked with Different Types of Woodchips Based on Gas Chromatography–Mass Spectrometry Combined with Electronic Nose. LWT 2021, 143, 111144. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, X.; Hong, P.; Liu, M.; Li, Z.; Zhou, C.; Zhong, S.; Liu, S. GC-MS, GC-IMS, and E-Nose Analysis of Volatile Aroma Compounds in Wet-Marinated Fermented Golden Pomfret Prepared Using Different Cooking Methods. Foods 2024, 13, 390. [Google Scholar] [CrossRef]
- García-González, D.L.; Tena, N.; Aparicio-Ruiz, R.; Morales, M.T. Relationship between Sensory Attributes and Volatile Compounds Qualifying Dry-Cured Hams. Meat Sci. 2008, 80, 315–325. [Google Scholar] [CrossRef]
- Vossen, E.; Goethals, S.; De Vrieze, J.; Boon, N.; Van Hecke, T.; De Smet, S. Red and Processed Meat Consumption within Two Different Dietary Patterns: Effect on the Colon Microbial Community and Volatile Metabolites in Pigs. Food Res. Int. 2020, 129, 108793. [Google Scholar] [CrossRef]
- Alothman, M.; Hogan, S.A.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; O’Donovan, M.; Tobin, J.; Fenelon, M.A.; O’Callaghan, T.F. The “Grass-Fed” Milk Story: Understanding the Impact of Pasture Feeding on the Composition and Quality of Bovine Milk. Foods. 2019, 8, 350. [Google Scholar] [CrossRef]
- Timlin, M.; Fitzpatrick, E.; McCarthy, K.; Tobin, J.T.; Murphy, E.G.; Pierce, K.M.; Murphy, J.P.; Hennessy, D.; O’Donovan, M.; Harbourne, N.; et al. Impact of Varying Levels of Pasture Allowance on the Nutritional Quality and Functionality of Milk throughout Lactation. J. Dairy Sci. 2023, 106, 6597–6622. [Google Scholar] [CrossRef]
- Faulkner, H.; O’Callaghan, T.F.; McAuliffe, S.; Hennessy, D.; Stanton, C.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Effect of Different Forage Types on the Volatile and Sensory Properties of Bovine Milk. J. Dairy Sci. 2018, 101, 1034–1047. [Google Scholar] [CrossRef]
- Genovese, A.; Marrazzo, A.; De Luca, L.; Romano, R.; Manzo, N.; Masucci, F.; Di Francia, A.; Sacchi, R. Volatile Organic Compound and Fatty Acid Profile of Milk from Cows and Buffaloes Fed Mycorrhizal or Nonmycorrhizal Ensiled Forage. Molecules 2019, 24, 1616. [Google Scholar] [CrossRef]
- Davis, H.; Stergiadis, S.; Chatzidimitriou, E.; Sanderson, R.; Leifert, C.; Butler, G. Meeting Breeding Potential in Organic and Low-Input Dairy Farming. Front. Vet. Sci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, R.S.; Hernandez, B.C.; Hernandez, E.L. Identification of Meat and Poultry Species in Food Products Using DNA Barcoding. Food Control 2017, 80, 23–28. [Google Scholar] [CrossRef]
- Conter, M. Recent Advancements in Meat Traceability, Authenticity Verification, and Voluntary Certification Systems. Ital. J. Food Saf. 2024, 14, 12971. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Zhao, S.; Zhang, Z.; Bai, L.; Zhaxi, P.; Qu, S.; Zhao, Y. Effects of Sampling Time and Location on the Geographical Origin Traceability of Protected Geographical Indication (PGI) Hongyuan Yak Milk: Based on Stable Isotope Ratios. Food Chem. 2024, 441, 138283. [Google Scholar] [CrossRef] [PubMed]
- Kalpage, M.; Dissanayake, C.; Diyabalanage, S.; Chandrajith, R.; Frew, R.; Fernando, R. Stable Isotope and Element Profiling for Determining the Agroclimatic Origin of Cow Milk within a Tropical Country. Foods 2022, 11, 275. [Google Scholar] [CrossRef]
- Potočnik, D.; Nečemer, M.; Perišić, I.; Jagodic, M.; Mazej, D.; Camin, F.; Eftimov, T.; Strojnik, L.; Ogrinc, N. Geographical Verification of Slovenian Milk Using Stable Isotope Ratio, Multi-Element and Multivariate Modelling Approaches. Food Chem. 2020, 326, 126958. [Google Scholar] [CrossRef]
- Li, H.; Mo, H.; Song, Y.-C.; Chen, G.; Wu, C.-E.; Zhu, F.-Y. The Integration of Machine Learning into Proteomics Advances Food Authentication and Adulteration Control. Trends Food Sci. Technol. 2025, 161, 105029. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Priyashantha, H.; Vidanarachchi, J.K.; Kiani, A.; Holman, B.W.B. Effects of Nutritional Factors on Fat Content, Fatty Acid Composition, and Sensorial Properties of Meat and Milk from Domesticated Ruminants: An Overview. Animals 2024, 14, 840. [Google Scholar] [CrossRef]
- Stanton, C.; Mills, S.; Ryan, A.; Di Gioia, D.; Ross, R.P. Influence of Pasture Feeding on Milk and Meat Products in Terms of Human Health and Product Quality. Ir. J. Agric. Food Res. 2021, 59, 292–302. [Google Scholar] [CrossRef]
- Hossain, M.J.; Alam, A.N.; Kim, S.-H.; Kim, C.-J.; Joo, S.-T.; Hwang, Y.-H. Techniques and Emerging Trends in Flavor and Taste Development in Meat. Food Sci. Anim. Resour. 2025, 45, 266–281. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Dong, X.; Gao, Z.; Gibney, E.R.; Yang, S.; McGuinness, L.; Noronha, N.; Feeney, E.L. Food Labeling and Chinese Consumer Preference for Naturalness: A New Way to Differentiate Grass-Fed Dairy Products. J. Dairy Sci. 2025, 108, 2340–2353. [Google Scholar] [CrossRef]
- De La Torre-Santos, S.; Royo, L.J.; Martínez-Fernández, A.; Chocarro, C.; Vicente, F. The Mode of Grass Supply to Dairy Cows Impacts on Fatty Acid and Antioxidant Profile of Milk. Foods 2020, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Jiang, X.; Han, C.; Wei, S.; Yu, D.; Tong, Y. The Effect of Credence Attributes on Willingness to Pay a Premium for Organic Food: A Moderated Mediation Model of Attitudes and Uncertainty. Front. Psychol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Bouhaddane, M.; Halawany-Darson, R.; Rochette, C.; Amblard, C. Legitimate or Not, Does It Really Matter? A Reading of the PDO Label’s Legitimacy through Consumers’ Perception. Foods 2023, 12, 2365. [Google Scholar] [CrossRef]
- Cubero Dudinskaya, E.; Naspetti, S.; Arsenos, G.; Caramelle-Holtz, E.; Latvala, T.; Martin-Collado, D.; Orsini, S.; Ozturk, E.; Zanoli, R. European Consumers’ Willingness to Pay for Red Meat Labelling Attributes. Animals 2021, 11, 556. [Google Scholar] [CrossRef]
- Thøgersen, J. How Does Origin Labelling on Food Packaging Influence Consumer Product Evaluation and Choices? A Systematic Literature Review. Food Policy 2023, 119, 102503. [Google Scholar] [CrossRef]
- Glogovețan, A.-I.; Pocol, C.B. The Role of Promoting Agricultural and Food Products Certified with European Union Quality Schemes. Foods 2024, 13, 970. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Tacken, G.M.L.; Liu, Y.; Sijtsema, S.J. Consumer Trust in the Dairy Value Chain in China: The Role of Trustworthiness, the Melamine Scandal, and the Media. J. Dairy Sci. 2021, 104, 8554–8567. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Furtwaengler, P.; Siegrist, M. Consumers’ Evaluation of the Environmental Friendliness, Healthiness and Naturalness of Meat, Meat Substitutes, and Other Protein-Rich Foods. Food Qual. Prefer. 2022, 97, 104486. [Google Scholar] [CrossRef]
- Narvhus, J.A.; Abrahamsen, R.K. Traditional and Modern Nordic Fermented Milk Products: A Review. Int. Dairy J. 2023, 142, 105641. [Google Scholar] [CrossRef]
- Priyashantha, H.; Ranadheera, C.S.; Rasika, D.M.D.; Vidanarachchi, J.K. Traditional Sri Lankan Fermented Buffalo (Bubalus bubalis) Milk Gel (Meekiri): Technology, Microbiology and Quality Characteristics. J. Ethn. Foods 2021, 8, 27. [Google Scholar] [CrossRef]
- Knychala, M.M.; Boing, L.A.; Ienczak, J.L.; Trichez, D.; Stambuk, B.U. Precision Fermentation as an Alternative to Animal Protein: A Review. Fermentation 2024, 10, 315. [Google Scholar] [CrossRef]
- Magano, N.N.; Tuorila, H.; De Kock, H.L. Food Choice Drivers at Varying Income Levels in an Emerging Economy. Appetite 2023, 189, 107001. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Herrero, A.M.; Pintado, T.; Delgado-Pando, G. Sensory Analysis and Consumer Research in New Meat Products Development. Foods 2021, 10, 429. [Google Scholar] [CrossRef]
- Hassoun, A.; Dankar, I.; Bhat, Z.; Bouzembrak, Y. Unveiling the Relationship between Food Unit Operations and Food Industry 4.0: A Short Review. Heliyon 2024, 10, e39388. [Google Scholar] [CrossRef]
- Van Ba, H.; Amna, T.; Hwang, I. Significant Influence of Particular Unsaturated Fatty Acids and pH on the Volatile Compounds in Meat-like Model Systems. Meat Sci. 2013, 94, 480–488. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent Progress in Food Flavor Analysis Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Posudin, Y. Methods of Analysis of Volatile Organic Compounds. In Methods of Measuring Environmental Parameters; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 303–316. [Google Scholar]
- Nie, S.; Li, L.; Wang, Y.; Wu, Y.; Li, C.; Chen, S.; Zhao, Y.; Wang, D.; Xiang, H.; Wei, Y. Discrimination and Characterization of Volatile Organic Compound Fingerprints during Sea Bass (Lateolabrax japonicas) Fermentation by Combining GC-IMS and GC-MS. Food Biosci. 2022, 50, 102048. [Google Scholar] [CrossRef]
- Grob, R.L.; Barry, E.F. (Eds.) Modern Practice of Gas Chromatography, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Yoshida, T.; Matsunaga, I.; Oda, H. Simultaneous Determination of Semivolatile Organic Compounds in Indoor Air by Gas Chromatography-Mass Spectrometry after Solid-Phase Extraction. J. Chromat. A 2004, 1023, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Piao, F.; Zhong, L.; Asakawa, F.; Jitsunari, F. Investigation of Trends and Risk of Indoor Air Pollution by VOCs and HCHO. Dalian Yike Daxue Xuebao 2005, 27, 337–340. [Google Scholar]
- Hodgson, A.T. A Review and a Limited Comparison of Methods for Measuring Total Volatile Organic Compounds in Indoor Air. Indoor Air 1995, 5, 247–257. [Google Scholar] [CrossRef][Green Version]
- Stachowiak-Wencek, A.; Pradzynski, W. Investigations of Volatile Organic Compounds during Finishing Furniture Surfaces as Well as from Furniture Coating. Acta Sci. Pol. Silv. 2005, 57, 220–224. [Google Scholar][Green Version]
- Zhu, J.P.; Cao, X.L. A Simple Method to Determine VOC Emissions from Constant Emission Sources and Its Application in Indoor Air Quality Studies. In Proceedings of the Air Conditioning in High Rise Buildings 2000, International Symposium, Shanghai, China, 24 October 2000; pp. 204–209. [Google Scholar][Green Version]
- Ivanović, S.; Pavlović, M.; Pavlović, M.; Tasić, A.; Janjić, J.; Baltić, M.Ž. Influence of Breed on Selected Quality Parameters of Fresh Goat Meat. Arch. Anim. Breed. 2020, 14, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the Key Aroma Compounds in Laoshan Green Teas by Application of Odour Activity Value (OAV), Gas Chromatography-Mass Spectrometry-Olfactometry (GC-MS-O) and Comprehensive Two-Dimensional Gas Chromatography Mass Spectrometry (GC × GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef]
- O’Sullivan, M.G.; Kerry, J.P. Sensory Evaluation of Fresh Meat. In Improving the Sensory and Nutritional Quality of Fresh Meat; Kerry, J.P., Ledward, D.A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 178–196. [Google Scholar]
- Górska-Horczyczak, E.; Guzek, D.; Molęda, Z.; Wojtasik-Kalinowska, I.; Brodowska, M.; Wierzbicka, A. Applications of Electronic Noses in Meat Analysis. Food Sci. Technol. 2016, 36, 389–395. [Google Scholar] [CrossRef]
- Acevedo, C.A.; Creixell, W.; Pavez-Barra, C.; Sánchez, E.; Albornoz, F.; Young, M.E. Modeling Volatile Organic Compounds Released by Bovine Fresh Meat Using an Integration of Solid Phase Microextraction and Databases. Food Bioprocess Technol. 2012, 5, 2557–2567. [Google Scholar] [CrossRef]
- Fujioka, K. Comparison of Cheese Aroma Intensity Measured Using an Electronic Nose (E-Nose) Non-Destructively with the Aroma Intensity Scores of a Sensory Evaluation: A Pilot Study. Sensors 2021, 21, 8368. [Google Scholar] [CrossRef]
- Wojtasik-Kalinowska, I.; Guzek, D.; Górska-Horczyczak, E.; Głąbska, D.; Brodowska, M.; Sun, D.-W.; Wierzbicka, A. Volatile Compounds and Fatty Acids Profile in Longissimus Dorsi Muscle from Pigs Fed with Feed Containing Bioactive Components. LWT–Food Sci. Technol. 2016, 67, 112–117. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.-J.; Villani, F.; Ercolini, D. Bacterial Populations and the Volatilome Associated to Meat Spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef]
- Del Olmo, A.; Calzada, J.; Nuñez, M. Effect of High-Pressure-Processing and Modified-Atmosphere-Packaging on the Volatile Compounds and Odour Characteristics of Sliced Ready-to-Eat “Lacón”, a Cured–Cooked Pork Meat Product. Innov. Food Sci. Emerg. Technol. 2014, 26, 134–142. [Google Scholar] [CrossRef]
- Hong, X.; Wang, J.; Hai, Z. Discrimination and Prediction of Multiple Beef Freshness Indexes Based on Electronic Nose. Sens. Actuators B Chem. 2012, 161, 381–389. [Google Scholar] [CrossRef]
- Ferrier, P.; Spethmann, Y.; Claussen, B.; Nsubuga, L.; Marcondes, T.L.; Høegh, S.; Heptaskin, T.; Wiechmann, C.; Rubahn, H.-G.; De Oliveira Hansen, R. Application of a Handheld Electronic Nose for Real-Time Poultry Freshness Assessment. Sens. Biosens. Res. 2024, 45, 100685. [Google Scholar] [CrossRef]
- Nurjuliana, M.; Che Man, Y.B.; Mat Hashim, D.; Mohamed, A.K.S. Rapid Identification of Pork for Halal Authentication Using the Electronic Nose and Gas Chromatography Mass Spectrometer with Headspace Analyzer. Meat Sci. 2011, 88, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, J.; Cui, S. Analysis of Pork Adulteration in Minced Mutton Using Electronic Nose of Metal Oxide Sensors. J. Food Eng. 2013, 119, 744–749. [Google Scholar] [CrossRef]
- Putri, L.A.; Rahman, I.; Puspita, M.; Hidayat, S.N.; Dharmawan, A.B.; Rianjanu, A.; Wibirama, S.; Roto, R.; Triyana, K.; Wasisto, H.S. Rapid Analysis of Meat Floss Origin Using a Supervised Machine Learning-Based Electronic Nose towards Food Authentication. npj Sci. Food 2023, 7, 31. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Rossetti, L.; Grigioni, G.; Irurueta, M.; Sancho, A.M.; Carrete, J.; Pensel, N.A. Antioxidant Status and Odour Profile in Fresh Beef from Pasture or Grain-Fed Cattle. Meat Sci. 2007, 75, 299–307. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Li, J.; Lim, N.-R.; Kang, B.-S.; Park, H.-J. Prediction of Warmed-over Flavour Development in Cooked Chicken by Colorimetric Sensor Array. Food Chem. 2016, 211, 440–447. [Google Scholar] [CrossRef]
- Vestergaard, J.S.; Haugen, J.-E.; Byrne, D.V. Application of an Electronic Nose for Measurements of Boar Taint in Entire Male Pigs. Meat Sci. 2006, 74, 564–577. [Google Scholar] [CrossRef]
- Bougrini, M.; Tahri, K.; Haddi, Z.; El Bari, N.; Llobet, E.; Jaffrezic-Renault, N.; Bouchikhi, B. Aging Time and Brand Determination of Pasteurized Milk Using a Multisensor E-Nose Combined with a Voltammetric e-Tongue. Mater. Sci. Eng. C 2014, 45, 348–358. [Google Scholar] [CrossRef]
- Damdam, A.N.; Ozay, L.O.; Ozcan, C.K.; Alzahrani, A.; Helabi, R.; Salama, K.N. IoT-Enabled Electronic Nose System for Beef Quality Monitoring and Spoilage Detection. Foods 2023, 12, 2227. [Google Scholar] [CrossRef] [PubMed]
- Argyri, A.A.; Jarvis, R.M.; Wedge, D.; Xu, Y.; Panagou, E.Z.; Goodacre, R.; Nychas, G.J.E. A Comparison of Raman and FT-IR Spectroscopy for the Prediction of Meat Spoilage. Food Control 2013, 29, 461–470. [Google Scholar] [CrossRef]
- Davis, R.; Mauer, L.J. Fourier Transform Infrared (FT-IR) Spectroscopy: A Rapid Tool for Detection and Analysis of Foodborne Pathogenic Bacteria. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2010. [Google Scholar]
- Candoğan, K.; Altuntas, E.G.; İğci, N. Authentication and Quality Assessment of Meat Products by Fourier-Transform Infrared (FTIR) Spectroscopy. Food Eng. Rev. 2021, 13, 66–91. [Google Scholar] [CrossRef]
- Ellis, D.I.; Broadhurst, D.; Kell, D.B.; Rowland, J.J.; Goodacre, R. Rapid and Quantitative Detection of the Microbial Spoilage of Meat by Fourier Transform Infrared Spectroscopy and Machine Learning. Appl. Environ. Microbiol. 2002, 68, 2822–2828. [Google Scholar] [CrossRef] [PubMed]
- Amamcharla, J.K.; Panigrahi, S.; Logue, C.M.; Marchello, M.; Sherwood, J.S. Fourier Transform Infrared Spectroscopy (FTIR) as a Tool for Discriminating Salmonella Typhimurium Contaminated Beef. Sens. Instrum. Food Qual. 2010, 4, 1–12. [Google Scholar] [CrossRef]
- Zajac, A.; Dyminska, L.; Lorenc, J.; Hanuza, J. Fourier Transform Infrared and Raman Spectroscopy Studies of the Time-Dependent Changes in Chicken Meat as a Tool for Recording Spoilage Processes. Food Anal. Methods 2017, 10, 640–648. [Google Scholar] [CrossRef]
- Pavli, F.G.; Argyri, A.A.; Chorianopoulos, N.G.; Nychas, G.J.E.; Tassou, C.C. Effect of Lactobacillus Plantarum L125 Strain with Probiotic Potential on Physicochemical, Microbiological and Sensorial Characteristics of Dry Fermented Sausages. LWT-Food Sci. Technol. 2020, 118, 108810. [Google Scholar] [CrossRef]
- Shen, C.; Cai, Y.; Wu, X.; Gai, S.; Wang, B.; Liu, D. Characterization of Selected Commercially Available Grilled Lamb Shashliks Based on Flavor Profiles Using GC-MS, GC × GC-TOF-MS, GC-IMS, E-Nose and E-Tongue Combined with Chemometrics. Food Chem. 2023, 423, 136257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Sun, L.; Li, M.; Zhu, Y.; Deng, W.; Yu, J.; Zhang, W.; Song, Z. Investigating Flavor and Quality Characteristics in Chinese Bacon from Different Regions Using Integrated GC-IMS, Electronic Sensory Assessment, and Sensory Analysis. Meat Sci. 2025, 220, 109709. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Meng, Q.; Li, N.; Ren, L. Evaluation of Beef by Electronic Tongue System TS-5000Z: Flavor Assessment, Recognition and Chemical Compositions According to Its Correlation with Flavor. PLoS ONE 2015, 10, e0137807. [Google Scholar] [CrossRef]
- Surányi, J.; Zaukuu, J.-L.Z.; Friedrich, L.; Kovacs, Z.; Horváth, F.; Németh, C.; Kókai, Z. Electronic Tongue as a Correlative Technique for Modeling Cattle Meat Quality and Classification of Breeds. Foods 2021, 10, 2283. [Google Scholar] [CrossRef]
- Cho, S.; Moazzem, M.S. Recent Applications of Potentiometric Electronic Tongue and Electronic Nose in Sensory Evaluation. Prev. Nutr. Food Sci. 2022, 27, 354–364. [Google Scholar] [CrossRef]
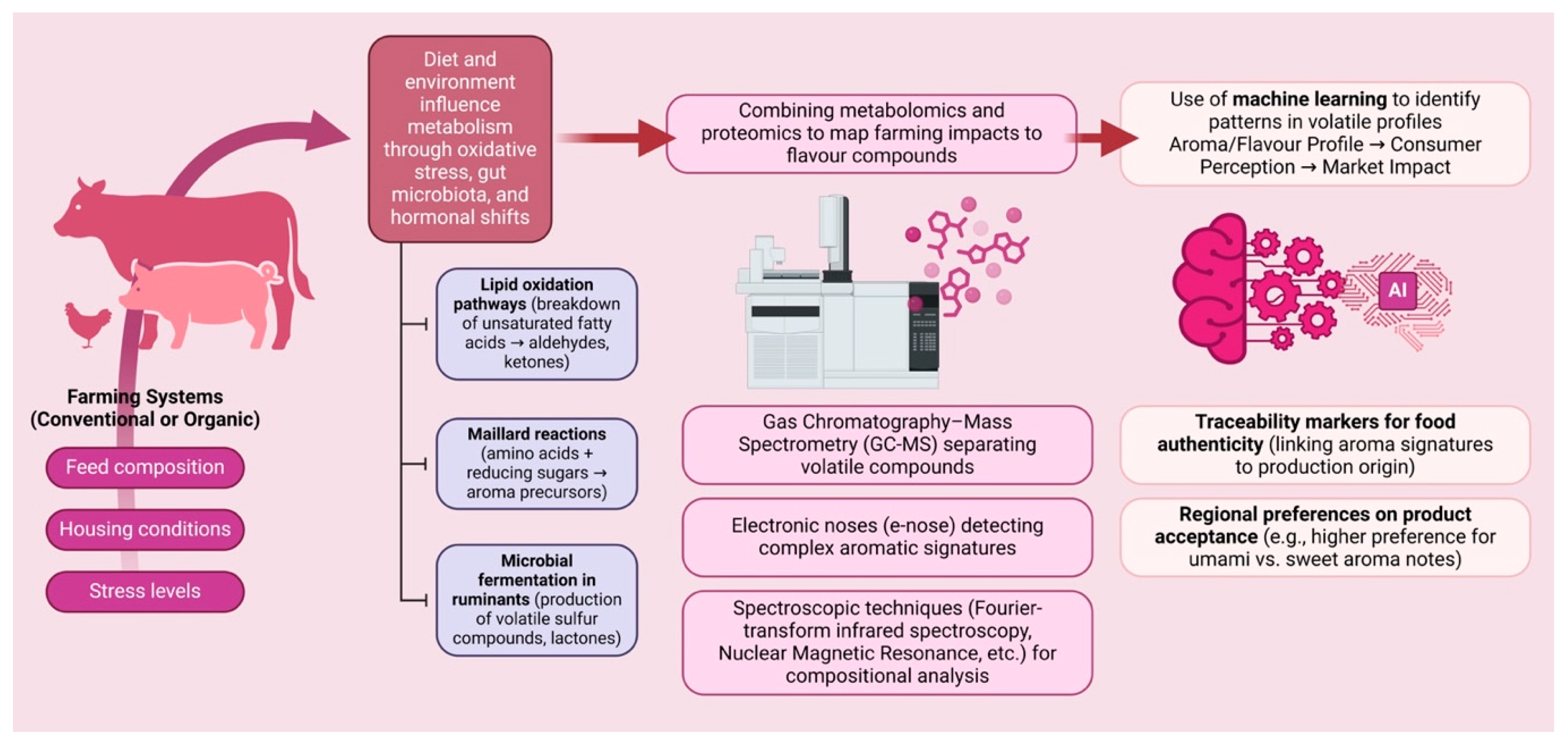
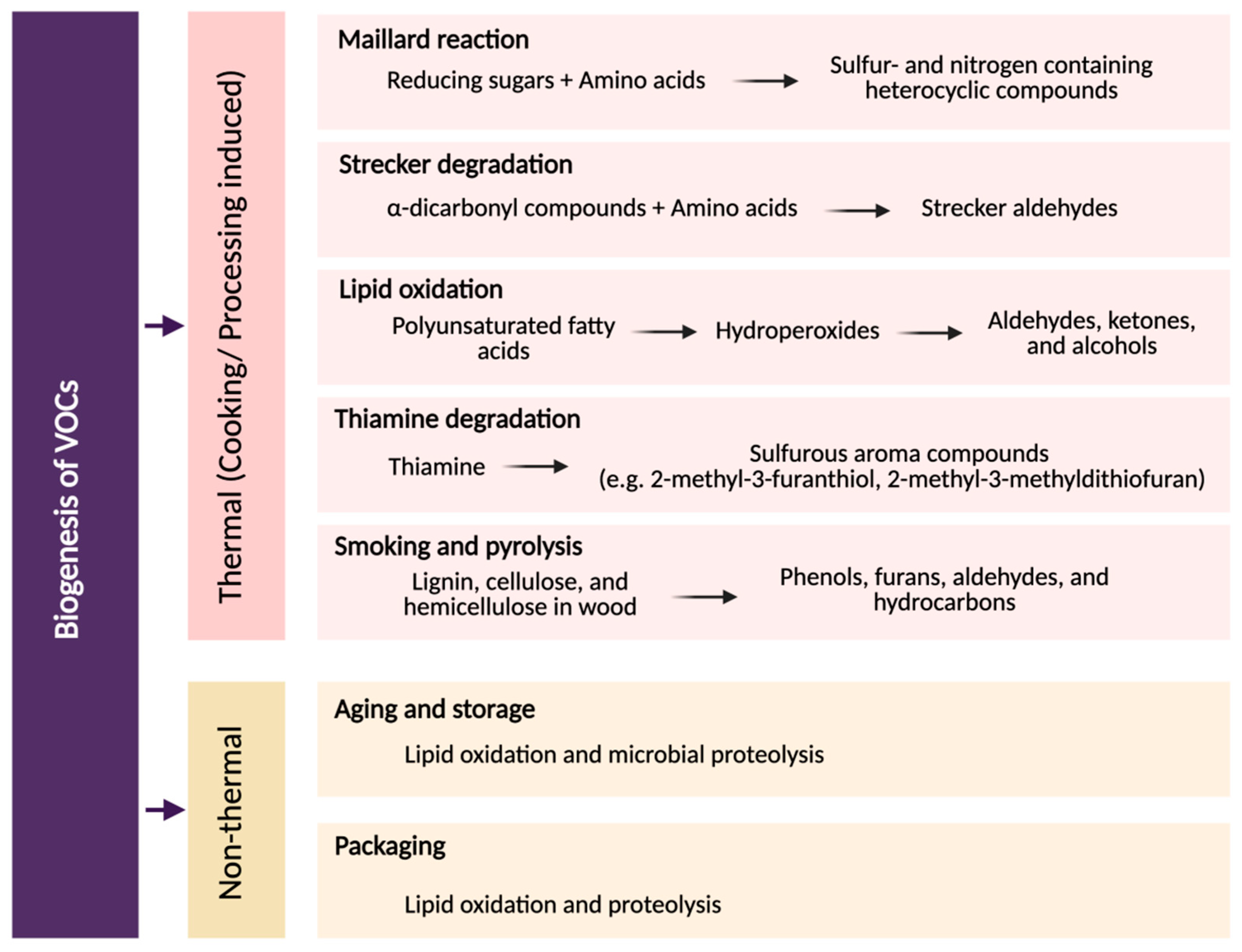
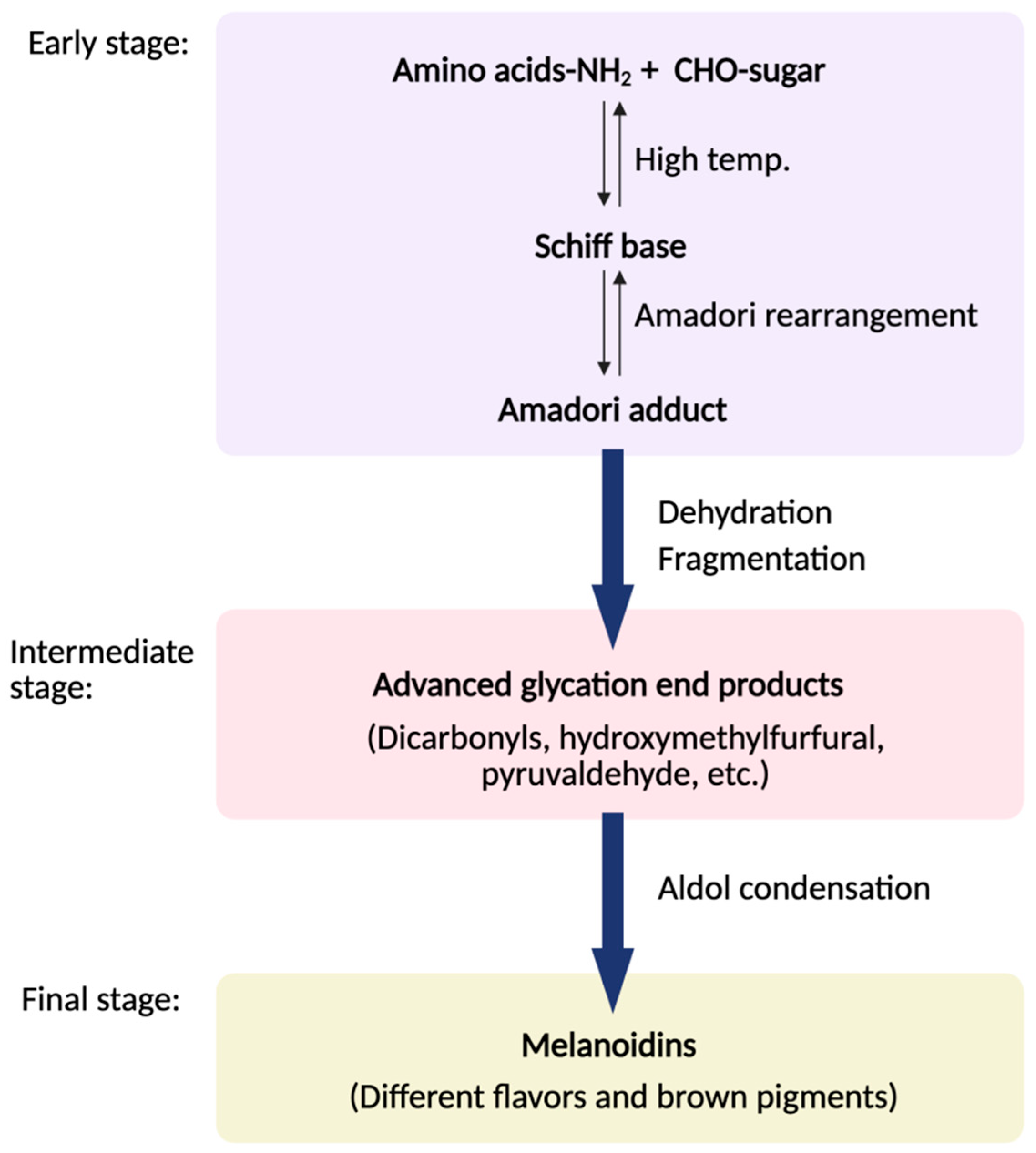

| Factor | Food Matrix | Key VOCs | Effect on VOC Profile | Reference |
|---|---|---|---|---|
| Dairy system (TMR vs. separate feeds; herd, lactation stage) | Cheese, Milk | Alcohols, esters ↑; Acetic acid ↓ | TMR increased fruity notes; silage-based TMR reduced overall volatiles; days in milk significantly influenced VOC patterns | [46] |
| Feeding system (Rangeland vs. Indoor) | Milk | Terpenes (unspecified) | Rangeland milk showed terpene variations, though specific compounds were not detailed | [49] |
| Grazing vs. Indoor feeding | Milk | Aldehydes, terpenes, sulfur ↑ (grazing); Ketones (acetone), acids (hexanoic, octanoic) ↑ (indoor) | Clear diet-dependent segregation of VOC classes | [48] |
| Fresh forage inclusion (Sorghum vs. silage) | Milk, Cheese | Aldehydes (green notes) ↑; Ketones, acids, esters (fruity/cheesy notes) ↑ | Forage enhanced green notes; silage increased fruity/fermented notes | [48] |
| Breed (Merino, Lacaune, Assaf) | Milk | Ketones (Merino: 71.8%); Hydrocarbons (Lacaune: 37.2%; Assaf: 55.4%); Acetone correlated with Salinicoccus, Psychrobacter | Breed-specific microbial–VOC associations evident | [9] |
| Diet (Whole vs. ground flaxseed) | Milk | Aldehydes (nonanal ↑); Fruity/sweet VOCs ↓ | Whole flaxseed altered 22 VOCs; ground flaxseed only 5 VOCs altered | [30] |
| Diet (Soybean meal, Yellow wine lees, Fermented lees) | Milk | PCA-based VOC differences (specific compounds not detailed) | Distinct diet-dependent VOC patterns identified | [30] |
| Diet (Grass, Grass/clover, TMR) | Milk powder | 1-Pentanol, 1-Hexanol | Levels varied significantly across diets, influencing sensory attributes | [50] |
| Herbal feed additives | Milk | Caproic (C6:0), Caprylic (C8:0), Capric (C10:0) acids ↓; Methyl ketones (2-heptanone, 2-nonanone) ↑; Esters ↑ | Reduced “goaty” smell; enhanced fruity/creamy notes | [51] |
| Diet (Jujube supplementation) | Milk | PCA correlations with VOCs (specific VOCs not detailed) | Jujube supplementation altered serum–VOC correlations | [52] |
| Breed | Feed/Duration | Key Flavor and Aroma Compounds | Sensory Qualities | Key Findings | References |
|---|---|---|---|---|---|
| Late maturing suckler steers | Barley-based concentrate (97 days) | Increased Maillard-derived compounds | ↑ Tenderness, IMF ↑ cooking loss | Grain-finishing enhances marbling but may reduce juiciness. | [56] |
| Crossbred steers | Benzoic acid (0.5% DM, 98 days) | Enhanced beefy, roasted notes | Stronger beef flavor, no texture differences | No impact on shear force or oxidation. | [54] |
| Holstein-Friesian × Limousin | Grass silage + concentrate (18 months) | Higher aldehydes (hexanal, nonanal) | Bulls: Leaner, less tender; Steers: Juicier | Gender affects tenderness more than diet. | [57] |
| Grass-fed vs. grain-fed | Pasture vs. concentrate (~100 days) | Grass-fed: Grassy (hexanal); Grain-fed: Roasted (nonanal) | Grass-fed: ↓ Tenderness, ↑ oxidation stability | Grain-fed preferred for “beefy” flavor. | [58] |
| Canchim steers (5/8 Charolais × 3/8 Zebu) | Pellet diet (peanut shell, corn, soybean meal), dry-aged 28 days | Methional (cheddar cheese), furan (roasted beef) | Enhanced tenderness, preferred flavor | Dry aging increased tenderness and unique volatile compounds. | [53] |
| Charolais cull cows | RM-1: Mostly pasture-fed, low concentrate | - | ↓ Flavor intensity, ↓ fat aroma | Yellower fat, smoother meat grain. | [59] |
| Charolais cull cows | RM-3: High concentrate, mainly housed | - | ↑ Flavor intensity, ↑ fat aroma | Stronger but sometimes atypical flavors. | [59] |
| Breed | Feed/Duration | Key Flavor and Aroma Compounds | Sensory Qualities | Key Findings | Reference |
|---|---|---|---|---|---|
| Texel × Scottish Blackface lambs | Silage vs. concentrate finishing | ↑ Lamb aroma (concentrate); manure/fecal notes (silage) | Silage: off-notes reduced by mixed diets | Mixed diets reduce negative sensory traits | [35] |
| Gallega Iberian lambs | Silage vs. concentrate (4–4.5 months) | ↑ Hydrocarbons and aldehydes (concentrate) | Grass-fed: benzyl alcohol marker | Concentrate increases aldehydes; grass-fed retains pasture markers | [63] |
| Tan sheep | Mixed grazing + indoor (90 days) | ↑ Pleasant volatiles (ketones) | ↑ IMF, juiciness | Mixed systems optimize flavor and tenderness | [64] |
| Santa Inês lambs | Rehydrated corn silage | Not specified | Improved tenderness and balanced aroma | Complete corn replacement feasible with no carcass penalty | [65] |
| Crossbred lambs | Yeast culture (1.0%, 60 days) | ↑ 2-decenal (E), nonanal | Higher IMF, reduced cooking loss | Increased oleic acid and redness (a*) | [66] |
| Merino × Dorper lambs | Microalgae (0.5–1% DM, 98 days) | ↑ ALA and omega-3 LC-FAs | ↑ Drip loss at 0.5% | 1% DM reduced IMF; no impact on growth | [67] |
| Small-Tailed Han sheep | Ensiled protein grass (8 weeks) | Citrus-like aldehydes | ↑ Omega-3, diversified aroma | Improved fatty acid profile and aroma complexity | [68] |
| Species/Breed | Factor (Feed/Age/Environment) | Key VOCs (Examples) | Positive Attributes | Negative Attributes | Reference |
|---|---|---|---|---|---|
| Ross 308 broilers | Black cumin seed meal (20–60 g/kg) | Pyrazines, aldehydes ↑ | Improved aroma, reduced drip loss, better protein and color | – | [69] |
| Daheng broilers | Age (60–180 days) | Hexanal, 1-octen-3-ol | Higher IMF, richer flavor at 150 days | Slightly higher oxidative products with age | [41] |
| Native Chinese chickens | L-glutamine supplementation | Nonanal, hexanal ↑ | Enhanced umami and Maillard aromas | – | [73] |
| Arbor Acres broilers | Epigallocatechin gallate (750 mg/kg) | Flavor amino acids ↑ | Improved antioxidant capacity, reduced drip loss, lighter color | – | [74] |
| White-Feather broiler | Fermented coffee pericarp (2.5%) | Aldehydes, ketones, alcohols, esters ↑ | Enhanced aroma, reduced drip loss, higher protein | – | [71] |
| Broiler chickens | Housefly larva meal (5%) | Sulfurous thiols | Higher flavor desirability, sustainable protein source | – | [70] |
| Jingfen laying hens | HELP diet (model group) | Fruity, waxy, tropical VOCs | – | Reduced tenderness, higher cooking loss, lower pH | [75] |
| Turkeys | Blue lupine meal (180 g/kg) | Not specified | Improved weight gain | Increased breast hardness | [76] |
| Egyptian goose | Seasonal diet (winter vs. summer) | PUFA volatiles (winter), MUFA volatiles (summer) | Summer diet: sweet-oily mild aroma | Winter diet: strong gamey aroma | [77] |
| Japanese quail | Garlic powder (1%) | Reduced oxidation (TBA/peroxides) | Improved stability, best sensory score at 1% | – | [78] |
| Laying hens | Sacha inchi oil (0.5%) | ω-3 PUFA ↑, improved ω-6/ω-3 | Healthier fatty acid profile, stronger desirable flavor | Potential oxidative susceptibility at high ω-3 | [45] |
| Pigeon (squabs) | DL-methionine (30–120 mg/kg) | Not specified | Improved tenderness, higher yield | – | [79] |
| Factor Investigated | Key Findings on VOCs and Meat Quality | Reference |
|---|---|---|
| Diet | ||
| Marine macroalgae (Ulva spp.) | Increased fat content (0.96% vs. 0.33% control) and MUFA by 22%. No effect on moisture, protein, or ash. No negative sensory impact. | [80] |
| Coffee silverskin (CSS) | Reduced ω-3 fatty acids but improved oxidative stability (lower TBARS). No change in total SFA/MUFA/PUFA. | [84] |
| Flaxseed oil (FSO) + antioxidants (ALC, LCO, PCA) | Increased ω-3 content but required antioxidants to prevent oxidation. Punicalagin showed the strongest antioxidant effect. | [85] |
| Selenium (Se) + Vitamin E | Organic Se + Vitamin E improved PUFA content and oxidative stability (lower MDA). Higher Se deposition in muscles than inorganic Se. | [81] |
| Processing and storage | ||
| Chilling time (18–24 h) | Reduced thawing losses, improved tenderness, and stabilized pH. Rigor mortis resolved by 18 h, enhancing meat quality. | [82] |
| Freezing vs. chilling | Freezing pre-rigor meat increased exudate loss and toughness. Optimal chilling (18 h at 4 °C) before freezing improved quality. | [86] |
| Irradiation (up to 3 kGy) | Reduced microbial load but increased lipid oxidation (TBARS). No significant sensory changes. | [87] |
| Cooking methods (roasting, boiling, sous-vide) | Roasting produced the highest aldehydes (hexanal, 13-fold increase). Sous-vide had lower oxidation but generated sulfur-containing VOCs. Boiling increased furans. | [72] |
| Tangerine peel (TP) in frying | Reduced carcinogenic HAAs (94% inhibition with 5-year TP). Unique VOCs (d-limonene, thymol) decreased with TP aging. | [88] |
| Biological factors | ||
| Age at slaughter | Younger rabbits (63 days) had lower intramuscular fat (0.53%) vs. older rabbits (70–80 days; ~1.4–2%). | [83] |
| Sex differences | Males had higher redness (a*) and shear force (tougher meat) but improved water-holding capacity (WHC) with longer chilling. | [82] |
| Breed differences | Botucatu rabbits showed different muscle fiber composition vs. hybrids, affecting rigor mortis and tenderness. | [89] |
| VOC profiles | ||
| VOC diversity | Rabbit meat has fewer VOCs (6) than chicken (29) or beef (28). Profiles stable in fresh meat but diversify during decomposition. | [90] |
| Factor Investigated | Key Findings on VOCs | Key Findings on Egg Quality | Reference |
|---|---|---|---|
| Dietary Sapindus saponaria oil (SIO: 0%, 0.5%, 1%) | 38 VOCs detected (aldehydes and aromatic hydrocarbons dominant). Flavor compounds varied with SIO levels. PUFAs linked to flavor formation. | Higher sensory scores (nutty, roasted potato) in 0.5% SIO group. Increased PUFAs (ALA, DHA) with SIO. Lower ω-6:ω-3 ratio. | [45] |
| Management (cage, organic, free-range) | Free-range: 8 VOCs; cage: 15; organic: 11. D-limonene dominant. | Diet/foraging altered aroma/flavor | [92] |
| Breed (White Leghorn, Hy-line Brown, Jing Fen) | Nonanal, decanal key VOCs. Aldehydes (~80% of profile). Breed influenced VOC distinctions. | — | [42] |
| Diets (cabbage/onion/rapeseed oil, free-range) | Raw yolks had low VOCs; sulfur compounds increased with rapeseed oil. Free-range eggs had fewer VOCs. Aldehydes formed during cooking. | No impact on shell stiffness/sensory quality. Feed influenced carotenoids, ω-3 fatty acids. | [93] |
| Embryo sex, fertility, and development | VOCs encode embryo sex/fertility info. Non-invasive detection possible. | — | [94] |
| Dietary biochar (BC) and biochar-based mixture (BCM) | No significant VOC differences in excreta. | Improved shell resistance (6–10%), egg mass (2–4%). No sensory differences in boiled eggs. | [95] |
| Raw egg storage time (0–28 days) for salt-baked marinated eggs (SBMEs) | Aldehydes (benzaldehyde, hexanal) dominant. VOC changes faster in yolk than white. Storage time significantly altered profiles. | PUFAs and MUFAs decreased after 28-day storage. Best sensory score at 7 days. Moisture content shifted after 21 days. | [91] |
| High-voltage cold plasma (HVCP) treatment time (0–300 s) | 65 VOCs identified (aldehydes highest). Fluctuating aldehyde concentrations with treatment time. | No change in protein/reducing sugars; mineral content varied. | [96] |
| Fungal contamination (storage time) | 2-Pentanone, 1-Pentanol linked to microbial growth. | Pathogen risk increased with storage. | [97] |
| Species | Class of VOC | VOC | Concentration (µg/g) | Characteristic Odor |
|---|---|---|---|---|
| Beef | Aldehydes | Hexadecanal | 81.41 | Cardboard |
| Aldehydes | Nonanal | 5.39 | Fat, citrus | |
| Aldehydes | Hexanal | 2.08 | Grass, fat | |
| Aldehydes | Benzaldehyde | 0.12 | Almond, burnt sugar | |
| Alcohols | Z-9-octadecen-1-ol | 0.34 | Fatty, animal | |
| Alcohols | 1-octen-3-ol | 0.16 | Mushroom | |
| Ketones | 3-Hydroxy-2-butanone | 0.7 | Buttery, creamy, fatty, sweet | |
| Ketones | 2-Octadecanone | 0.55 | Green | |
| Carboxylic acids | Hexanoic acid | 0.89 | Sweat | |
| Carboxylic acids | 2,4-Hexadienoic acid | 0.21 | Acrid | |
| Esters | Ethyl acetate | 50.58 | Pineapple | |
| Esters | Ethyl 9-hexadecenoate | 0.18 | Fruity | |
| Furans | 5-Methyl-2-acetylfuran | 0.71 | Nutty | |
| Furans | Tetrahydrofuran | 0.66 | Butter, caramel | |
| Heterocyclic | 3,5-Diethyl-1,2,4-trithiocyclopentane | 2.85 | Beef aroma | |
| Pork | Aldehydes | Nonanal | 2.86 | Fatty, floral, wax |
| Aldehydes | Benzaldehyde | 2.53 | Bitter almond | |
| Aldehydes | Octanal | 1.97 | Fatty, pungent | |
| Aldehydes | Trans-2-nonenal | 1.47 | Cucumber, farinaceous, greasy, grassy | |
| Aldehydes | Heptanal | 1.25 | Fatty, putty | |
| Aldehydes | Hexanal | 0.95 | Green, grass | |
| Alcohols | 3-Methyl-1-butanol | 3.1 | Pungent | |
| Alcohols | Hexanol | 1.11 | Woody, grassy, fruity, metallic | |
| Alcohols | 1-Octen-3-ol | 0.83 | Mushroom | |
| Alcohols | 3-Methyl-3-buten-1-ol | 0.34 | Sweet fruity | |
| Ketones | 2-Butanone | 0.83 | Burnt, chocolate | |
| Ketones | 2-Heptanone | 0.8 | Citrus, spicy | |
| Esters | γ-Butyrolactone | 0.96 | Creamy, sweet | |
| Esters | Ethyl 2-methylbutanoate | 0.35 | Fruity, strawberry | |
| Carboxylic acids | Hexanoic acid | 0.81 | Goaty | |
| Carboxylic acids | Nonanoic acid | 0.25 | Fatty, cheese | |
| Sulfur compounds | Methional | 1.74 | Cooked potato, roasted | |
| Sulfur compounds | Dimethyl disulfide | 1.24 | Moldy, onion-like | |
| Pyrazines | 2,5-Dimethyl pyrazine | 0.24 | Nutty, roasted | |
| Furans | 2-Pentylfuran | 1.29 | Green bean, butter | |
| Chicken | Aldehydes | P-methoxybenzaldehyde | 20.9 | Anisic, hawthorn-like |
| Aldehydes | Benzaldehyde | 9.88 | Almond, burnt sugar | |
| Aldehydes | Nonanal | 0.73 | Fatty, citrus, wax | |
| Alcohols | 1-Octen-3-ol | 0.06 | Shiitake mushroom | |
| Ketones | P-methoxypropiophenone | 0.39 | Musty, anisic | |
| Esters | Trans vinyl cinnamate | 0.92 | NR | |
| Furans | 2-Pentylfuran | 0.81 | Green bean, butter | |
| Furans | 2-Acetylfuran | 0.21 | Butter, meaty | |
| Lamb | Aldehydes | Hexanal | 109.23 | Apple, leaf, delicate |
| Aldehydes | Heptanal | 31.32 | Nutty, fruity green | |
| Aldehydes | (E)-2-nonenal | 30.09 | Fatty, paper | |
| Aldehydes | Nonanal | 18.25 | Fatty, rancid | |
| Aldehydes | Benzaldehyde | 13.09 | Almond, burnt sugar | |
| Alcohols | Hexanol | 12.42 | Woody, fruity, winey | |
| Carboxylic acids | 4-Methylnonanoic acid | 316.73 | Sweet muttony | |
| Carboxylic acids | 4-Ethyloctanoic acid | 186.22 | Sweet muttony | |
| Carboxylic acids | Acetic acid | 5.09 | Vinegar | |
| Esters | Ethyl dodecanoate | 6.18 | Fatty | |
| Furans | 2-Methyl-5-(methylthio)furan | 36.09 | Meat, onion | |
| Furans | 2-Pentylfuran | 24.21 | Green bean, butter | |
| Pyrazines | 2,3,5,6-Tetramethylpyrazine | 15.52 | Chocolate-like | |
| Sulfur compounds | Benzyl methyl sulfide | 4.88 | Roasted, muttony |
| Technique | Detection Principle | Detection/Quantification Limits | Advantages | Limitations | References |
|---|---|---|---|---|---|
| GC–MS | Separation of VOCs on a GC column followed by mass spectral identification | Down to low ppb levels for many volatiles | Gold standard; compound-specific; structural info; quantitative | Time-consuming; costly; requires expertise; limited for highly volatile/reactive compounds | [141,142,143,144,145,146,147] |
| E-nose | Arrays of semi-selective sensors (MOS, CP, QCM) responding to headspace VOCs | µg/L to mg/L (compound-dependent; not absolute) | Rapid, non-destructive; pattern recognition; spoilage/authenticity detection | No compound-specific info; sensor drift; humidity-sensitive | [149,150,151,152,153,158,159,160] |
| FTIR spectroscopy | Absorbance of IR radiation by functional groups, generating spectral fingerprints | Typically, ppm range; sensitive to functional group classes | Fast, reagentless, minimal prep; chemometric integration | Overlapping peaks; indirect compound identification; matrix effects | [166,167,168,169,170,171,172] |
| IMS/GC–IMS | Separation of ionized volatiles by drift time in electric field (±GC pre-sep) | Low ppb detection; quantitative with calibration | High sensitivity; rapid (10–15 min); on-site analysis; 2D fingerprints | Lower resolution than GC–MS; compound identification less robust | [72,105,173,174] |
| E-tongue | Sensor arrays mimicking taste receptor responses (potentiometric, voltammetric, impedance) | mg/L for salts/organic acids; µM–mM for many tastants | Complementary to E-nose; detects non-volatile taste-active compounds; combined use gives full flavor profile | Less specific than chromatography; cross-sensitivity; needs calibration | [164,175,176,177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponnampalam, E.N.; Jairath, G.; Gadzama, I.U.; Li, L.; Santhiravel, S.; Ma, C.; Flores, M.; Priyashantha, H. Production Systems and Feeding Strategies in the Aromatic Fingerprinting of Animal-Derived Foods: Invited Review. Foods 2025, 14, 3400. https://doi.org/10.3390/foods14193400
Ponnampalam EN, Jairath G, Gadzama IU, Li L, Santhiravel S, Ma C, Flores M, Priyashantha H. Production Systems and Feeding Strategies in the Aromatic Fingerprinting of Animal-Derived Foods: Invited Review. Foods. 2025; 14(19):3400. https://doi.org/10.3390/foods14193400
Chicago/Turabian StylePonnampalam, Eric N., Gauri Jairath, Ishaya U. Gadzama, Long Li, Sarusha Santhiravel, Chunhui Ma, Mónica Flores, and Hasitha Priyashantha. 2025. "Production Systems and Feeding Strategies in the Aromatic Fingerprinting of Animal-Derived Foods: Invited Review" Foods 14, no. 19: 3400. https://doi.org/10.3390/foods14193400
APA StylePonnampalam, E. N., Jairath, G., Gadzama, I. U., Li, L., Santhiravel, S., Ma, C., Flores, M., & Priyashantha, H. (2025). Production Systems and Feeding Strategies in the Aromatic Fingerprinting of Animal-Derived Foods: Invited Review. Foods, 14(19), 3400. https://doi.org/10.3390/foods14193400








