Metabolic Remodeling and Flavor Enhancement of Mulberry Juice Through Lactic Acid Bacteria Fermentation: A GC-IMS and Untargeted Metabolomics Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Strain Sources and Preparation of Fermented Mulberry Juice
2.3. Color and Sensory Assessment
2.3.1. Sensory Evaluation
2.3.2. Color Assessment
2.4. Determination of Physical and Chemical Parameters
2.5. Bioactive Substances Determination
2.6. Volatile Compound Analysis
2.7. Untargeted Metabolomics Product Analysis
2.8. Data Statistical Analysis
3. Results and Discussion
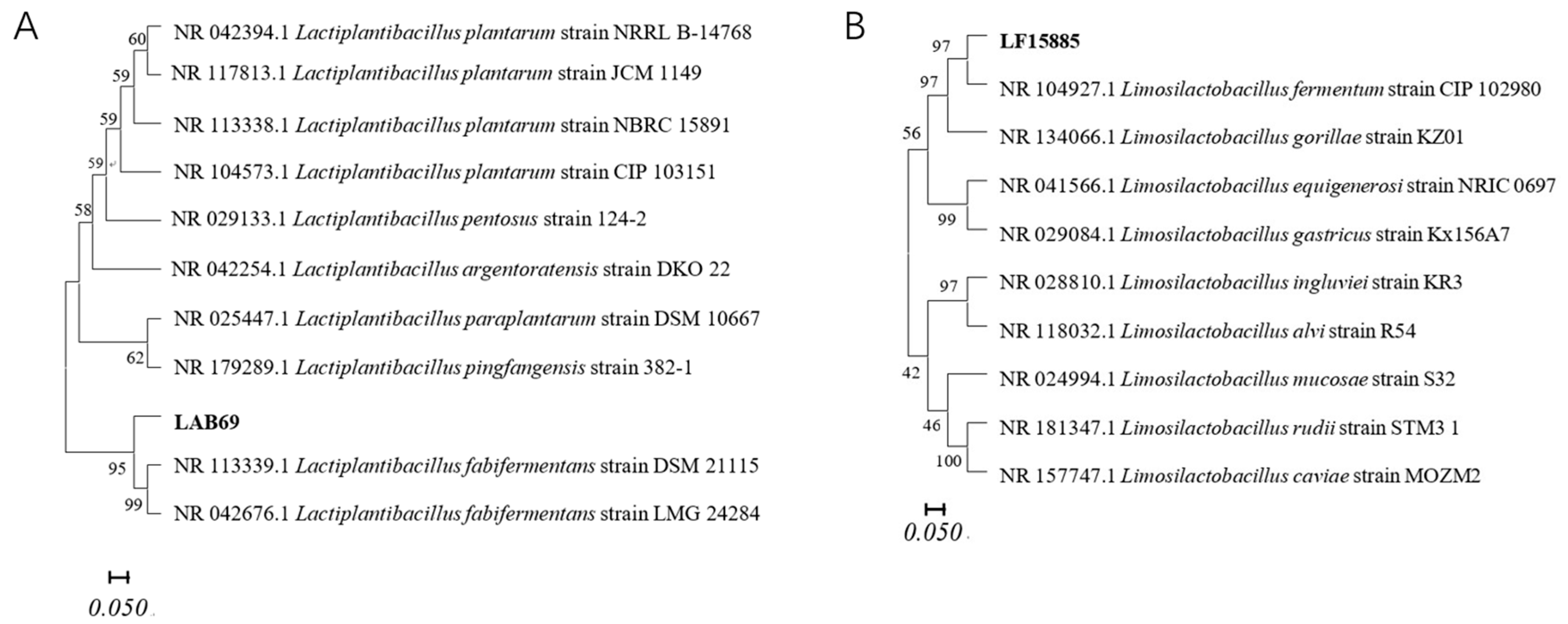
3.1. Identification of Fermentation Strains
3.2. FMJ’s Color Difference and Sensory Evaluation
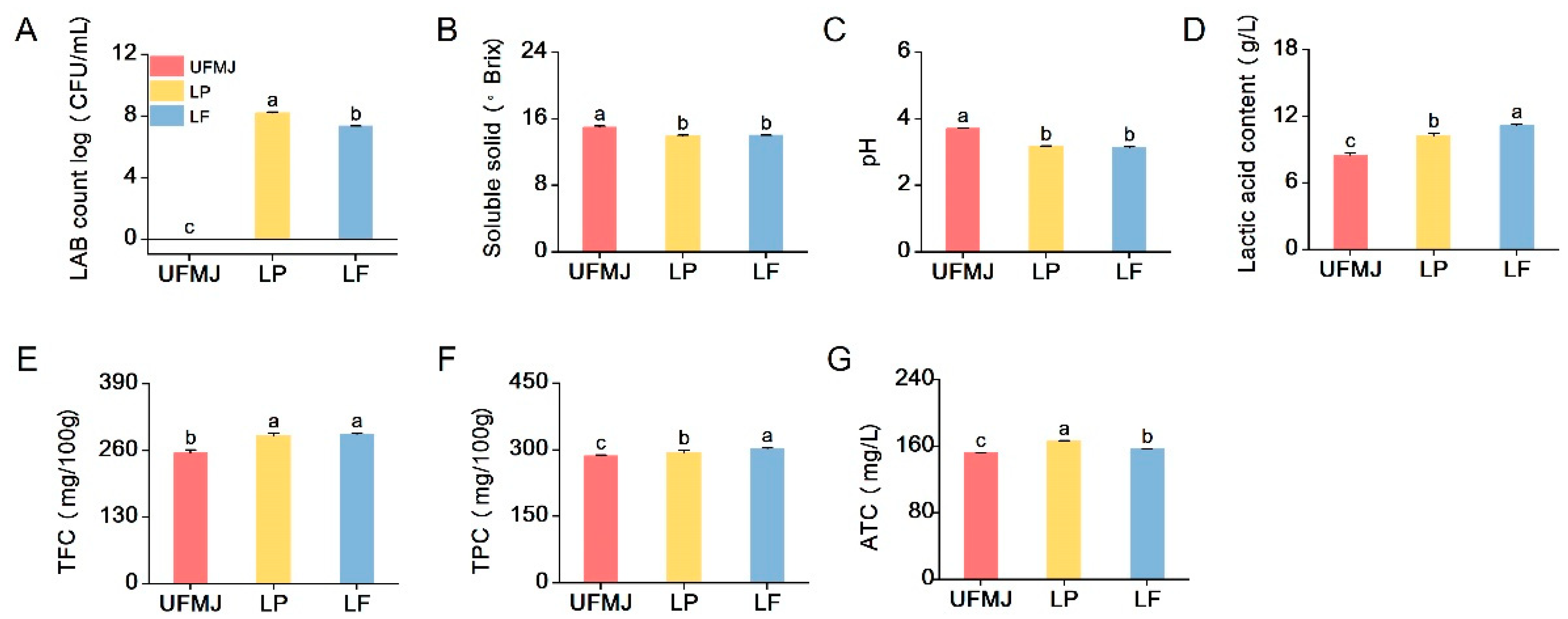
3.3. Physicochemical Properties of FMJ
3.4. Analysis of Bioactive Components in FMJ
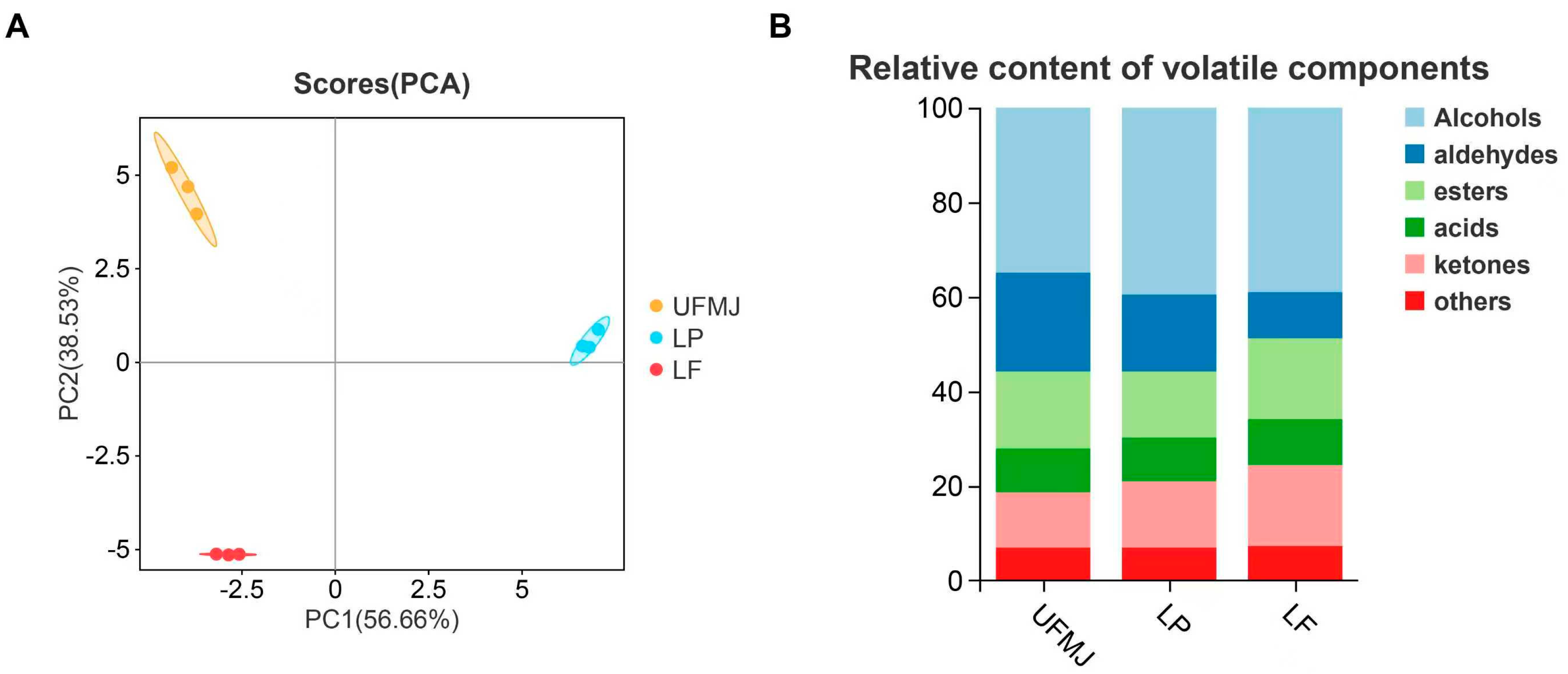
3.5. Changes in Volatile Compounds
3.6. Metabolic Analysis of FMJ
3.7. Analysis of Key Functional Metabolites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Liu, Y.; Wang, X. Effects of bioactive compounds and pharmacological activities in medicinal fruits and vegetables by thermal processing. J. Future Foods 2023, 3, 252–262. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Dai, F.; Luo, G.; Xiao, G.; Tang, C. Genetic diversity among mulberry genotypes from seven countries. Physiol. Mol. Biol. Plants 2017, 23, 421–427. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Huang, H.; Mbanyele, W.; Wei, Z.; Li, X. How does green industrial policy affect corporate green innovation? Evidence from the green factory identification in China. Energy Econ. 2025, 141, 108047. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, L.; Wan, J.; Liu, J.; Wei, Y.; Ouyang, Z.; Yu, X. Molecular cloning, expression, and functional analysis of copper amine oxidase gene from mulberry (Morus alba L.). Protein Expr. Purif. 2023, 201, 106166. [Google Scholar] [CrossRef]
- Xu, X.; Huang, Y.; Xu, J.; He, X.; Wang, Y. Anti-neuroinflammatory and antioxidant phenols from mulberry fruit (Morus alba L.). J. Funct. Foods 2020, 68, 103914. [Google Scholar] [CrossRef]
- Yin, X.; Xiao, W.; Zhang, S.; Yu, Z.; Ai, W.; Fu, S.; Liu, J.; Huang, D. Impacts of Five Different Drying Methods on Volatile Organic Compounds in Mulberry Fruits. Foods 2024, 13, 3514. [Google Scholar] [CrossRef]
- Yuasa, M.; Shimada, A.; Matsuzaki, A.; Eguchi, A.; Tominaga, M. Chemical composition and sensory properties of fermented citrus juice using probiotic lactic acid bacteria. Food Biosci. 2021, 39, 100810. [Google Scholar] [CrossRef]
- Chen, T.; Shuang, F.F.; Fu, Q.Y.; Ju, Y.X.; Zong, C.M.; Zhao, W.G.; Zhang, D.Y.; Yao, X.H.; Cao, F.L. Evaluation of the Chemical Composition and Antioxidant Activity of Mulberry (Morus alba L.) Fruits from Different Varieties in China. Molecules 2022, 27, 2688. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, X.; Li, J.; Wu, J.; Jiang, S.; Xue, H.; Zhang, J.; Jha, R.; Wang, R. Lactic acid bacteria fermentation improves physicochemical properties, bioactivity, and metabolic profiles of Opuntia ficus-indica fruit juice. Food Chem. 2024, 453, 139646. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, S.; Wang, X.; Mao, K.; Liu, Y.; Gao, J.; Sang, Y. Characterization and discrimination of fermented sweet melon juice by different microbial strains via GC-IMS-based volatile profiling and chemometrics. Food Sci. Hum. Wellness 2023, 12, 1241–1247. [Google Scholar] [CrossRef]
- Lan, T.; Lv, X.; Zhao, Q.; Lei, Y.; Gao, C.; Yuan, Q.; Sun, X.; Liu, X.; Ma, T. Optimization of strains for fermentation of kiwifruit juice and effects of mono- and mixed culture fermentation on its sensory and aroma profiles. Food Chem. X 2023, 17, 100595. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Liu, G.; Li, B.; Tan, H.; Zheng, R.; Sun, X.; He, F. Influence on the aroma substances and functional ingredients of apple juice by lactic acid bacteria fermentation. Food Biosci. 2023, 51, 102337. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Gao, T.; Wu, Y.; Sun, H.; Zhu, Q.; Liu, C.; Zhou, C.; Han, Y.; Tao, Y. Fermentation and Storage Characteristics of “Fuji” Apple Juice Using Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus plantarum: Microbial Growth, Metabolism of Bioactives and in vitro Bioactivities. Front. Nutr. 2022, 9, 833906. [Google Scholar] [CrossRef]
- Li, M.; Xu, X.; Jia, Y.; Yuan, Y.; Na, G.; Zhu, L.; Xiao, X.; Zhang, Y.; Ye, H. Transformation of mulberry polyphenols by Lactobacillus plantarum SC-5: Increasing phenolic acids and enhancement of anti-aging effect. Food Res. Int. 2024, 192, 114778. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Z.; Sun, Z.; Xu, J.; Meng, X.; Wang, H.; Wang, R.; Chen, F.; Hu, X.; Zhang, J. The fermentation of carambola juice with lactic acid bacteria improves its flavor, bioactive properties, and metabolic composition. Food Biosci. 2025, 66, 106307. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Q.; Inostroza, A.C.; Kierszniowska, S.; Liu, L.; Li, Y.; Ruan, J. Application of metabolic fingerprinting in tea quality evaluation. Food Control 2024, 160, 110361. [Google Scholar] [CrossRef]
- Küçükgöz, K.; Venema, K.; Trząskowska, M. Beetroot ketchup as a stable carrier of potential probiotic Lacticaseibacillus rhamnosus K3 and Lactobacillus johnsonii K4: A study on sensory attributes, storage viability, and in vitro gastrointestinal survival. Food Bioprod. Process. 2024, 148, 519–526. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, W.; Ma, W.; Yuan, A.; Ma, F.; Xue, Y.; Zhang, J. Effects of Dioscorea opposita mucilage addition on the physicochemical properties, texture, and stability of set-type yogurt. LWT 2024, 206, 116586. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Yuan, T.; Xie, J.; Yu, Q.; Chen, Y. Polyphenol compounds contributing to the improved bioactivities of fermented Rubus chingii Hu. Food Res. Int. 2024, 197, 115218. [Google Scholar] [CrossRef] [PubMed]
- Koraqi, H.; Trajkovska Petkoska, A.; Khalid, W.; Kumar, N.; Pareek, S. Optimization of experimental conditions for bioactive compounds recovery from raspberry fruits (Rubus idaeus L.) by using combinations of ultrasound-assisted extraction and deep eutectic solvents. Appl. Food Res. 2023, 3, 100346. [Google Scholar] [CrossRef]
- Yan, S.; Li, Y.; Liu, J.; Si, D.; Zhang, X. Guideline for extraction, qualitative, quantitative, and stability analysis of anthocyanins. eFood 2023, 4, e59. [Google Scholar] [CrossRef]
- Van Meulebroek, L.; Vanden Bussche, J.; Steppe, K.; Vanhaecke, L. High-resolution Orbitrap mass spectrometry for the analysis of carotenoids in tomato fruit: Validation and comparative evaluation towards UV-VIS and tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2613–2626. [Google Scholar] [CrossRef]
- Yang, C.; Fan, X.; Lao, F.; Huang, J.; Giusti, M.M.; Wu, J.; Lu, H. A Comparative Study of Physicochemical, Aroma, and Color Profiles Affecting the Sensory Properties of Grape Juice from Four Chinese Vitis vinifera x Vitis labrusca and Vitis vinifera Grapes. Foods 2024, 13, 3889. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, J.; Lu, Y.; Wu, Q.; Liu, Y.; Liu, Y.; Kumar, S.; Zhu, G.; Zhu, Z. Pre-Treatment, Extraction Solvent, and Color Stability of Anthocyanins from Purple Sweetpotato. Foods 2024, 13, 833. [Google Scholar] [CrossRef]
- Clydesdale, F.M. Color as a factor in food choice. Crit. Rev. Food Sci. Nutr. 1993, 33, 83–101. [Google Scholar] [CrossRef]
- Mandha, J.; Shumoy, H.; Devaere, J.; Schouteten, J.J.; Gellynck, X.; De Winne, A.; Matemu, A.O.; Raes, K. Effect of Lactic Acid Fermentation on Volatile Compounds and Sensory Characteristics of Mango (Mangifera indica) Juices. Foods 2022, 11, 383. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, F.; Hohn, K.; Vadlani, P.V. Metabolic flux analysis of carbon balance in Lactobacillus strains. Biotechnol. Prog. 2016, 32, 1397–1403. [Google Scholar] [CrossRef]
- Buron-Moles, G.; Chailyan, A.; Dolejs, I.; Forster, J.; Miks, M.H. Uncovering carbohydrate metabolism through a genotype-phenotype association study of 56 lactic acid bacteria genomes. Appl. Microbiol. Biotechnol. 2019, 103, 3135–3152. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Goya, L.; Mateos, R. Antioxidant and Anti-inflammatory Effects of Marine Phlorotannins and Bromophenols Supportive of Their Anticancer Potential. Nutr. Rev. 2025, 83, e1225–e1242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tang, F.; Cai, W.; Peng, B.; Zhang, P.; Shan, C. Effect of fermentation by lactic acid bacteria on the phenolic composition, antioxidant activity, and flavor substances of jujube–wolfberry composite juice. LWT 2023, 184, 114884. [Google Scholar] [CrossRef]
- Yaqoob, S.; Imtiaz, A.; Awan, K.A.; Murtaza, M.S.; Mubeen, B.; Yinka, A.A.; Boasiako, T.A.; Alsulami, T.; Rehman, A.; Khalifa, I.; et al. Impact of fermentation through synergistic effect of different lactic acid bacteria (mono and co-cultures) on metabolic and sensorial profile of mulberry juice. J. Food Meas. Charact. 2024, 18, 9364–9384. [Google Scholar] [CrossRef]
- Memete, A.R.; Timar, A.V.; Vuscan, A.N.; Miere, F.; Venter, A.C.; Vicas, S.I. Phytochemical Composition of Different Botanical Parts of Morus Species, Health Benefits and Application in Food Industry. Plants 2022, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in color expression and antioxidant activity of strawberry juice fermented with lactic acid bacteria: A phenolic-based research. Food Chem. X 2023, 17, 100535. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, J.; Gu, Y.; He, Y.; Chen, J.; Ye, X.; Zhou, Z. Mixed fermentation of Chinese bayberry pomace using yeast, lactic acid bacteria and acetic acid bacteria: Effects on color, phenolics and antioxidant ingredients. LWT 2022, 163, 113503. [Google Scholar] [CrossRef]
- Tian, H.; Xiong, J.; Yu, H.; Chen, C.; Lou, X. Flavor optimization in dairy fermentation: From strain screening and metabolic diversity to aroma regulation. Trends Food Sci. Technol. 2023, 141, 104194. [Google Scholar] [CrossRef]
- Xiang, L.; Zhu, W.; Jiang, B.; Chen, J.; Zhou, L.; Zhong, F. Volatile compounds analysis and biodegradation strategy of beany flavor in pea protein. Food Chem. 2023, 402, 134275. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Tan, W.; Ren, J.; Hou, M.; Wang, Z.; Guo, C.; Gao, Z. Lactic acid bacteria fermentation of prebiotic-supplemented apple juice: Viable counts and flavor evolution revealed by HS-SPME-GC–MS coupled with electronic sensory and chemometrics. Food Chem. 2025, 486, 144634. [Google Scholar] [CrossRef]
- Ge, Y.; Wu, Y.; Aihaiti, A.; Wang, L.; Wang, Y.; Xing, J.; Zhu, M.; Hong, J. The Metabolic Pathways of Yeast and Acetic Acid Bacteria During Fruit Vinegar Fermentation and Their Influence on Flavor Development. Microorganisms 2025, 13, 477. [Google Scholar] [CrossRef]
- Kompoura, V.; Karapantzou, I.; Mitropoulou, G.; Parisis, N.A.; Gkalpinos, V.K.; Anagnostou, V.A.; Tsiailanis, A.D.; Vasdekis, E.P.; Koutsaliaris, I.K.; Tsouka, A.N.; et al. Exploiting the beneficial effects of Salvia officinalis L. extracts in human health and assessing their activity as potent functional regulators of food microbiota. Food Chem. 2024, 441, 138175. [Google Scholar] [CrossRef]
- Magdum, A.B.; Waghmode, R.S.; Shinde, K.V.; Mane, M.P.; Kamble, M.V.; Kamble, R.S.; Jangam, A.P.; Pawar, K.D.; Sonawane, K.D.; Patil, P.S.; et al. Biogenic synthesis of silver nanoparticles from leaves extract of Decaschistia trilobata an endemic shrub and its application as antioxidant, antibacterial, anti-inflammatory and dye reduction. Catal. Commun. 2024, 187, 106865. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, X.; Liu, Z.; Wu, X.; Bao, C.; Sun, Y.; Su, N.; Cui, J. Transcriptome Analysis Reveals the Molecular Mechanism of GABA Accumulation during Quinoa (Chenopodium quinoa Willd.) Germination. J. Agric. Food Chem. 2021, 69, 12171–12186. [Google Scholar] [CrossRef] [PubMed]
- Pannerchelvan, S.; Rios-Solis, L.; Faizal Wong, F.W.; Zaidan, U.H.; Wasoh, H.; Mohamed, M.S.; Tan, J.S.; Mohamad, R.; Halim, M. Strategies for improvement of gamma-aminobutyric acid (GABA) biosynthesis via lactic acid bacteria (LAB) fermentation. Food Funct. 2023, 14, 3929–3948. [Google Scholar] [CrossRef]
- Kadyan, P.; Singh, L. Unraveling the mechanistic interplay of mediators orchestrating the neuroprotective potential of harmine. Pharmacol. Rep. 2024, 76, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liu, W.; Dang, R.; Hu, X.; Cai, F.; Xiang, Z.; Zhao, X.; Cheng, X.; Wang, C. Effects and mechanisms of harmine on ameliorating ethanol-induced memory impairment. J. Ethnopharmacol. 2025, 337, 118789. [Google Scholar] [CrossRef]
- Niu, X.; Yao, Q.; Li, W.; Zang, L.; Li, W.; Zhao, J.; Liu, F.; Zhi, W. Harmine mitigates LPS-induced acute kidney injury through inhibition of the TLR4-NF-kappaB/NLRP3 inflammasome signalling pathway in mice. Eur. J. Pharmacol. 2019, 849, 160–169. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Lv, Y.X.; Guo, C.Y.; Xiao, Z.M.; Jiang, Q.G.; Kuang, H.; Zhang, W.H.; Hu, P. Harmine inhibits the proliferation and migration of glioblastoma cells via the FAK/AKT pathway. Life Sci. 2021, 270, 119112. [Google Scholar] [CrossRef]
- Pervaiz, I.; Mehta, Y.; Sherill, K.; Patel, D.; Al-Ahmad, A.J. Ketone bodies supplementation restores the barrier function, induces a metabolic switch, and elicits beta-hydroxybutyrate diffusion across a monolayer of iPSC-derived brain microvascular endothelial cells. Microvasc. Res. 2023, 150, 104585. [Google Scholar] [CrossRef]
- Zhen, A.X.; Piao, M.J.; Hyun, Y.J.; Kang, K.A.; Ryu, Y.S.; Cho, S.J.; Kang, H.K.; Koh, Y.S.; Ahn, M.J.; Kim, T.H.; et al. Purpurogallin Protects Keratinocytes from Damage and Apoptosis Induced by Ultraviolet B Radiation and Particulate Matter 2.5. Biomol. Ther. 2019, 27, 395–403. [Google Scholar] [CrossRef]
- Petersen, M.; Hans, J.; Matern, U. Biosynthesis of Phenylpropanoids and Related Compounds. In Annual Plant Reviews Volume 40: Biochemistry of Plant Secondary Metabolism, 2nd ed; Wiley: Hoboken, NJ, USA, 2010; pp. 182–257. [Google Scholar] [CrossRef]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef]
- Margret, A.A.; Mareeswari, R.; Kumar, K.A.; Jerley, A.A. Relative profiling of L-tryptophan derivatives from selected edible mushrooms as psychoactive nutraceuticals to inhibit P-glycoprotein: A paradigm to contest blood-brain barrier. BioTechnologia 2021, 102, 55–64. [Google Scholar] [CrossRef]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22, 181. [Google Scholar] [CrossRef]
- Lee, J.-y.; Lee, J.-h. Recent Research Progress in the Microbial Production of Aromatic Compounds Derived from L-Tryptophan. J. Life Sci. 2020, 30, 919–929. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, Y.; Lin, L.; Tunyaluk, B.; Li, P. Dynamic changes in volatile components during dark tea wine processing. LWT 2024, 194, 115783. [Google Scholar] [CrossRef]
- Tieman, D.; Taylor, M.; Schauer, N.; Fernie, A.R.; Hanson, A.D.; Klee, H.J. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc. Natl. Acad. Sci. USA 2006, 103, 8287–8292. [Google Scholar] [CrossRef] [PubMed]
- Nain, S.; Mathur, G.; Anthwal, T.; Sharma, S.; Paliwal, S. Synthesis, Characterization, and Antibacterial Activity of New Isatin Derivatives. Pharm. Chem. J. 2023, 57, 196–203. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Li, X.; Zhang, Y.; Dong, B.; Hu, S.; Liu, C.; Fu, D.; Shen, L.; Liu, G. Unveiling the mechanism of fermentation induced antioxidant activity enhancement in asparagus juice: Metabolomic and transcriptomic insights into the functional role of Lactiplantibacillus plantarum JGS 49. Food Biosci. 2025, 69, 106907. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. eBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Sha, M.; Feng, Z.; Liu, Y. Natural bioactive flavonoids as promising agents in alleviating exercise-induced fatigue. Food Biosci. 2023, 51, 102360. [Google Scholar] [CrossRef]
- Pires, A.S.; Sundaram, G.; Heng, B.; Krishnamurthy, S.; Brew, B.J.; Guillemin, G.J. Recent advances in clinical trials targeting the kynurenine pathway. Pharmacol. Ther. 2022, 236, 108055. [Google Scholar] [CrossRef]
- Bertollo, A.G.; Mingoti, M.E.D.; Ignacio, Z.M. Neurobiological mechanisms in the kynurenine pathway and major depressive disorder. Rev. Neurosci. 2025, 36, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Fan, M.; Qi, J.; Yao, L. Design, synthesis, and antitumor activity evaluation of pretubulysin analogs. Chem. Biol. Drug Des. 2021, 98, 341–351. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, Q.; Wang, J.; Huang, X.; Wang, Y.; Qiao, M.; Ma, Y.; Hai, D. Metabolic Remodeling and Flavor Enhancement of Mulberry Juice Through Lactic Acid Bacteria Fermentation: A GC-IMS and Untargeted Metabolomics Approach. Foods 2025, 14, 3398. https://doi.org/10.3390/foods14193398
Liu Y, Liu Q, Wang J, Huang X, Wang Y, Qiao M, Ma Y, Hai D. Metabolic Remodeling and Flavor Enhancement of Mulberry Juice Through Lactic Acid Bacteria Fermentation: A GC-IMS and Untargeted Metabolomics Approach. Foods. 2025; 14(19):3398. https://doi.org/10.3390/foods14193398
Chicago/Turabian StyleLiu, Yufei, Quanjun Liu, Jinglong Wang, Xianqing Huang, Yanrui Wang, Mingwu Qiao, Yan Ma, and Dan Hai. 2025. "Metabolic Remodeling and Flavor Enhancement of Mulberry Juice Through Lactic Acid Bacteria Fermentation: A GC-IMS and Untargeted Metabolomics Approach" Foods 14, no. 19: 3398. https://doi.org/10.3390/foods14193398
APA StyleLiu, Y., Liu, Q., Wang, J., Huang, X., Wang, Y., Qiao, M., Ma, Y., & Hai, D. (2025). Metabolic Remodeling and Flavor Enhancement of Mulberry Juice Through Lactic Acid Bacteria Fermentation: A GC-IMS and Untargeted Metabolomics Approach. Foods, 14(19), 3398. https://doi.org/10.3390/foods14193398





