Light-Quality-Dependent Greening and Steroidal Glycoalkaloid Accumulation in Potato Tubers: Regulatory Mechanisms and Postharvest Strategies to Reduce Food Safety Risks
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Light Exposure Treatment
2.3. Calculation of Tuber Greening Rate and Analysis of Color Difference
2.4. Determination of Chlorophyll, Carotenoid, and Anthocyanin Content
2.5. Determination of α-Solanine and α-Chaconine Content
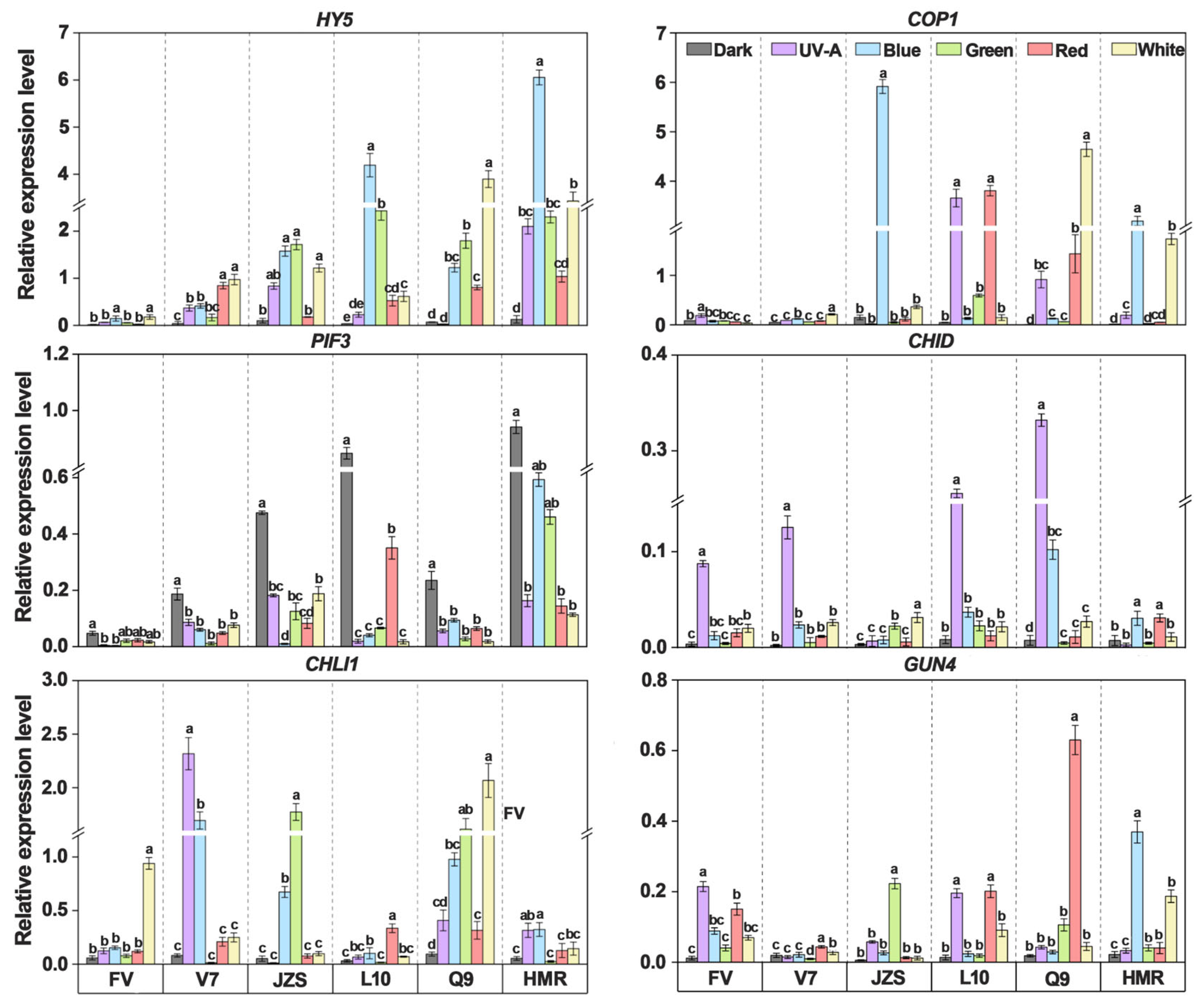
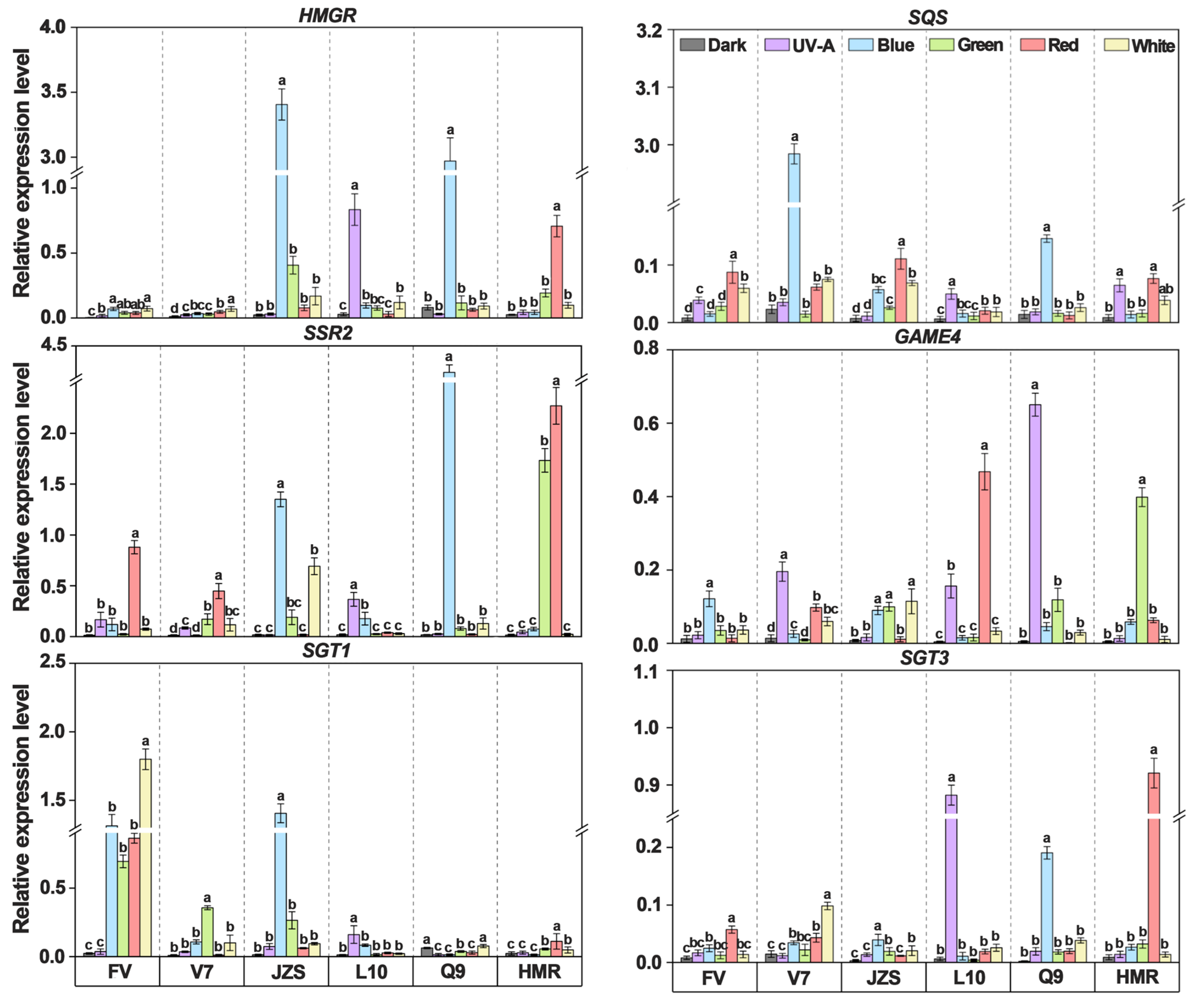
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
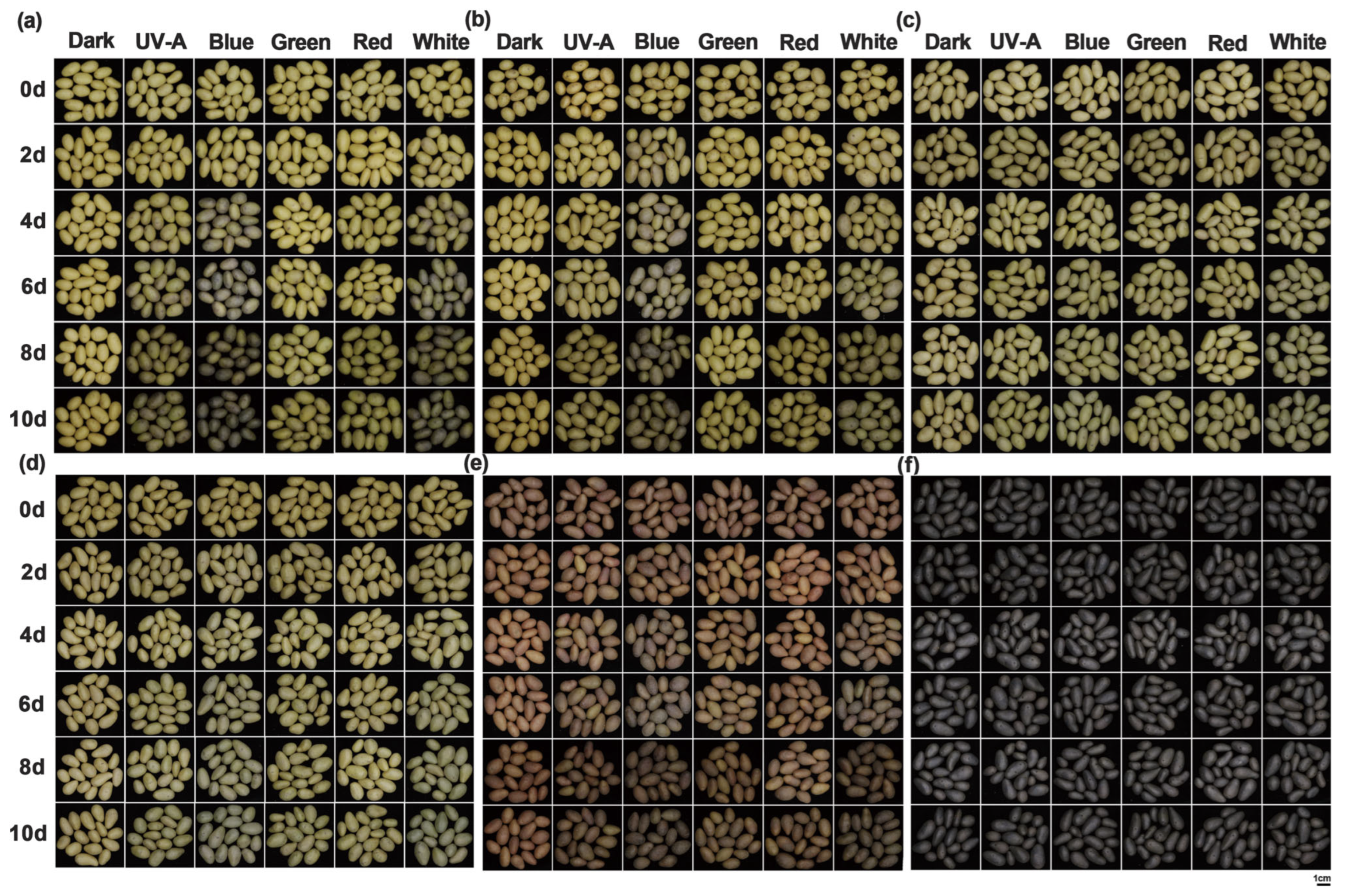
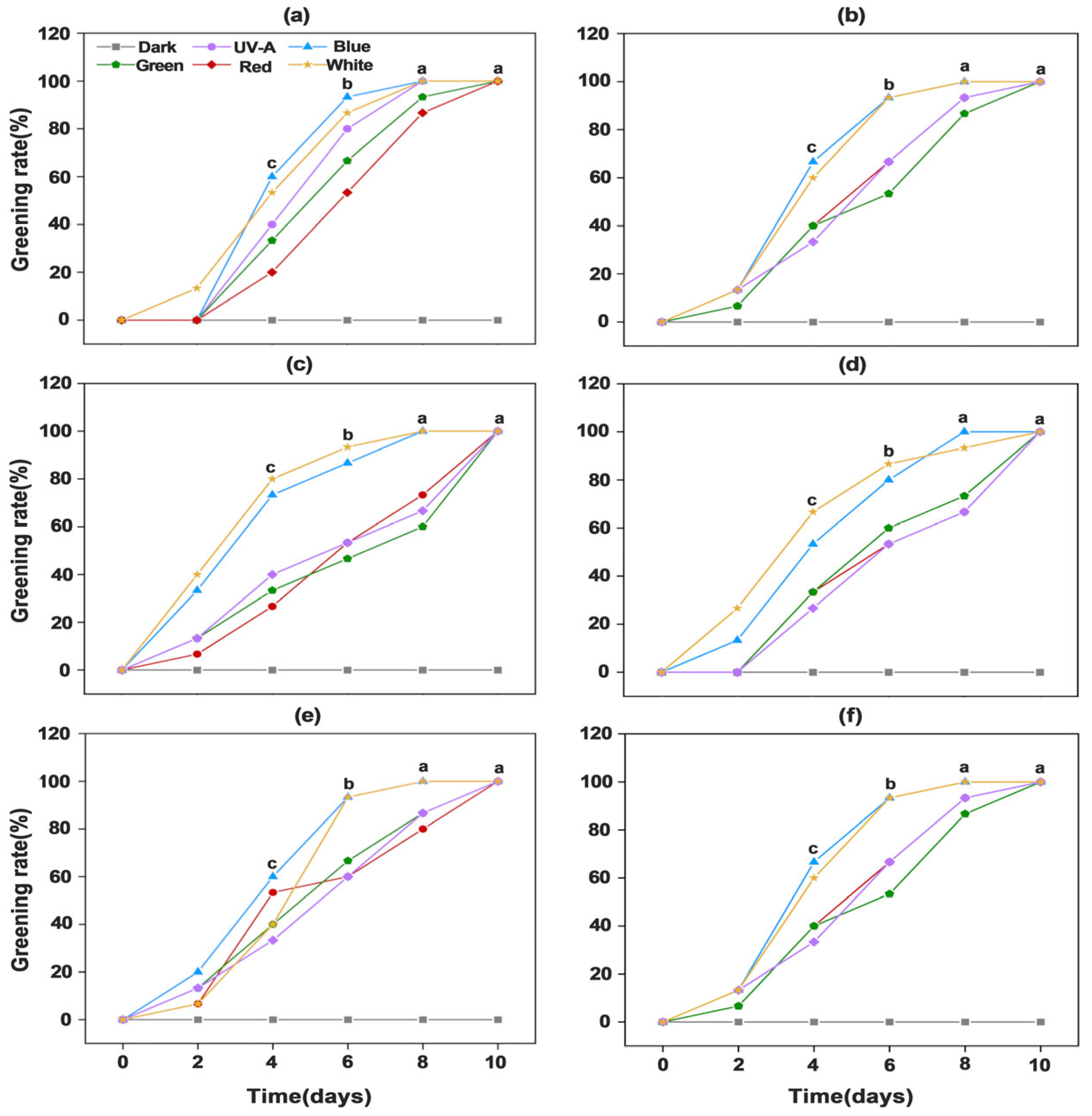
3.1. Analysis of Greening Phenotypes and Greening Rates Statistics of Potato Tuber Peels Induced by Different Light Spectra
3.2. Color Difference Analysis of Potato Tuber Peels Induced by Different Light Qualities
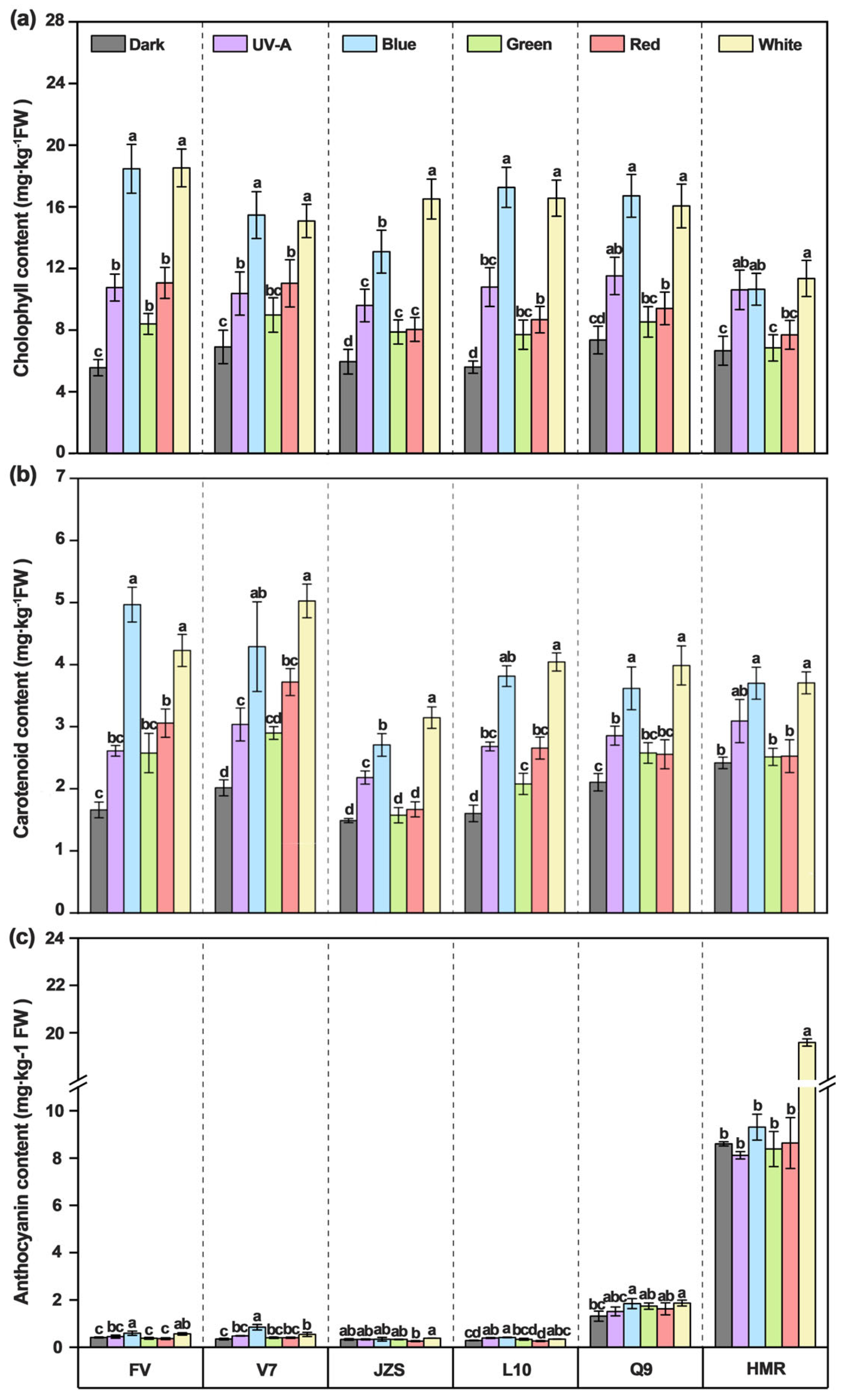
3.3. Effects of Different Light Quality Treatments on Chlorophyll, Carotenoid, and Anthocyanin Contents in Potato Tuber Peels
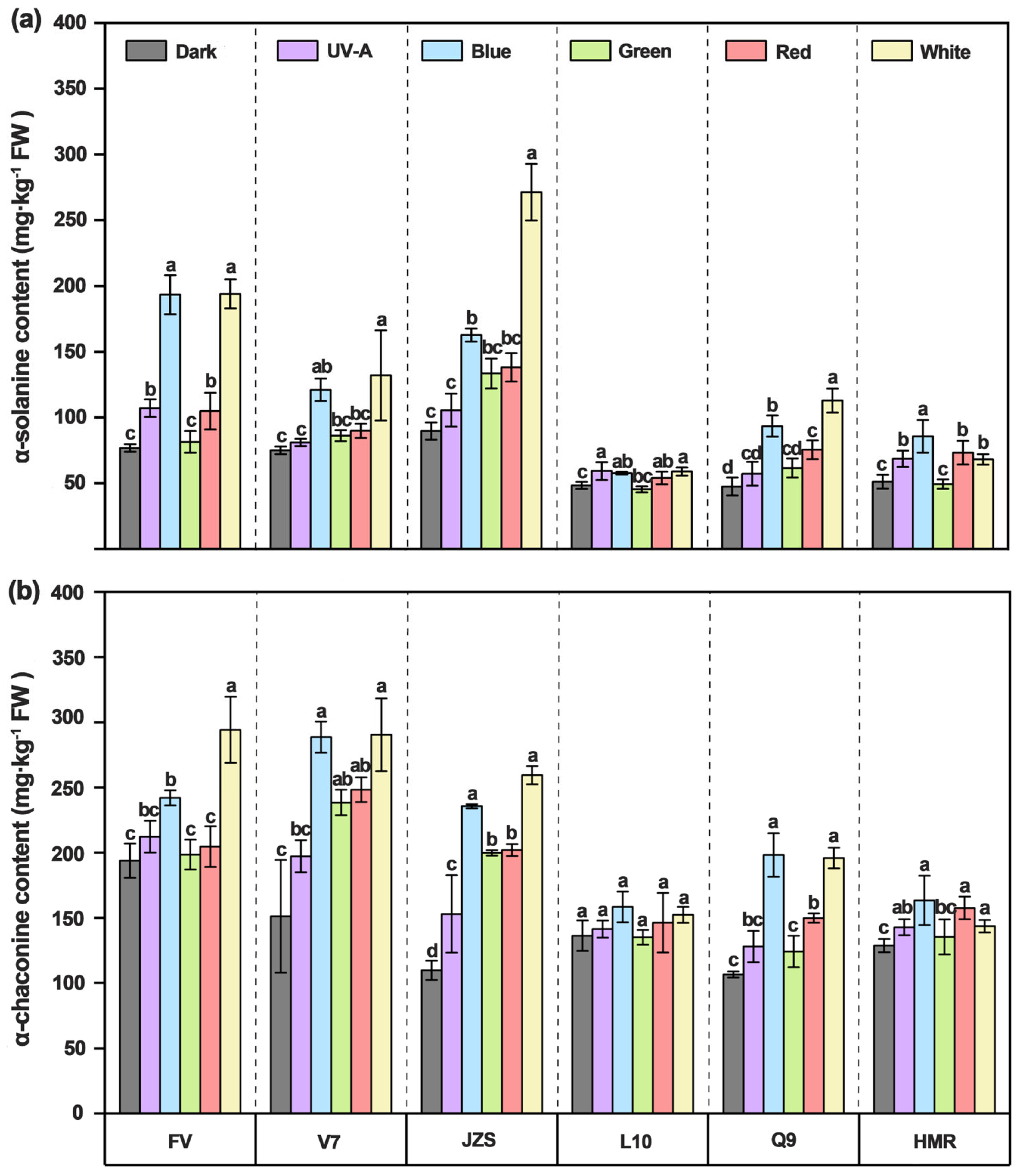
3.4. Effects of Different Light Quality Treatments on α-Solanine and α-Chaconine Contents in Potato Tuber Peels
3.5. Effects of Different Light Quality Treatments on Tuber Peel Pigments and Key Gene Expression in SGAs Synthesis
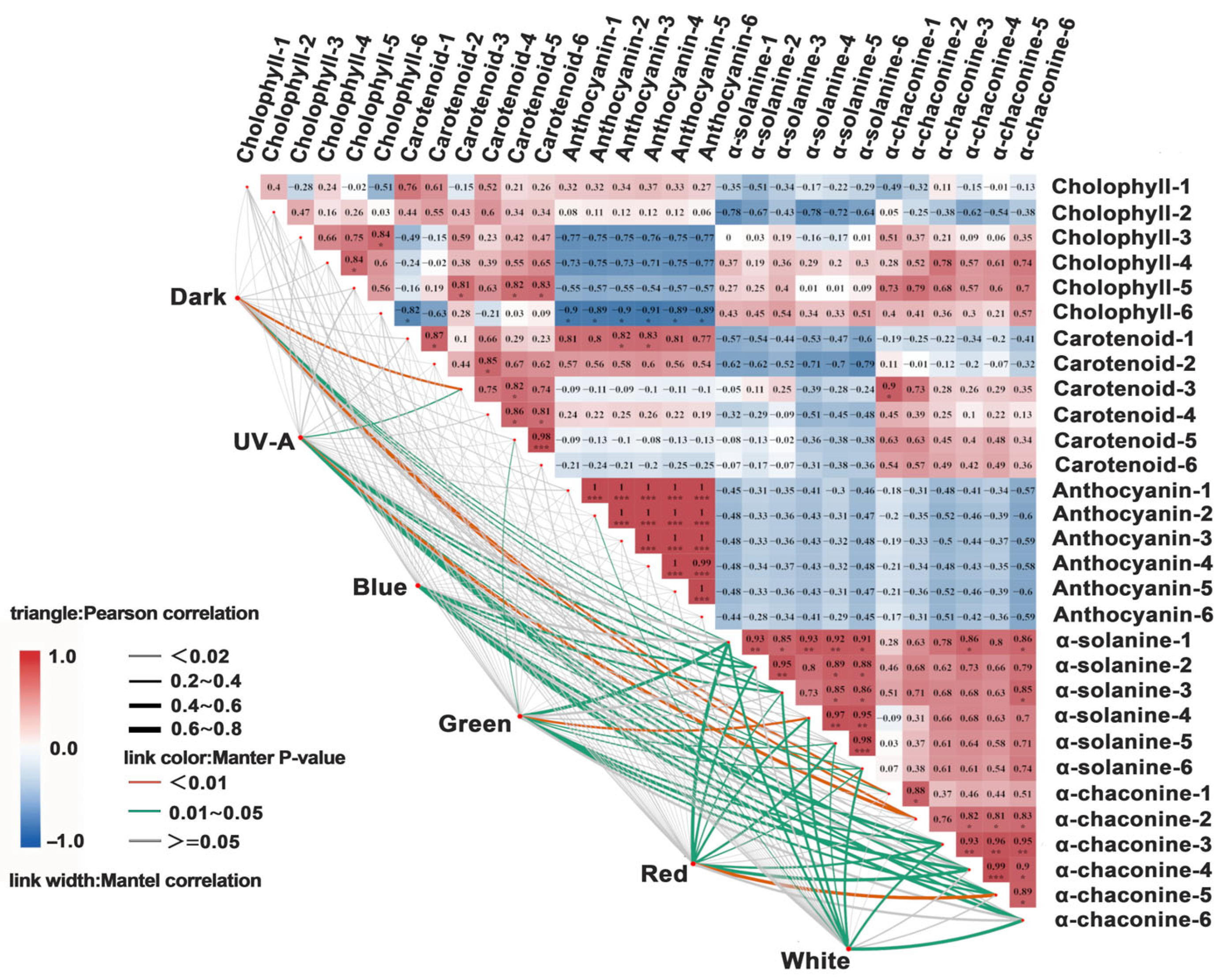
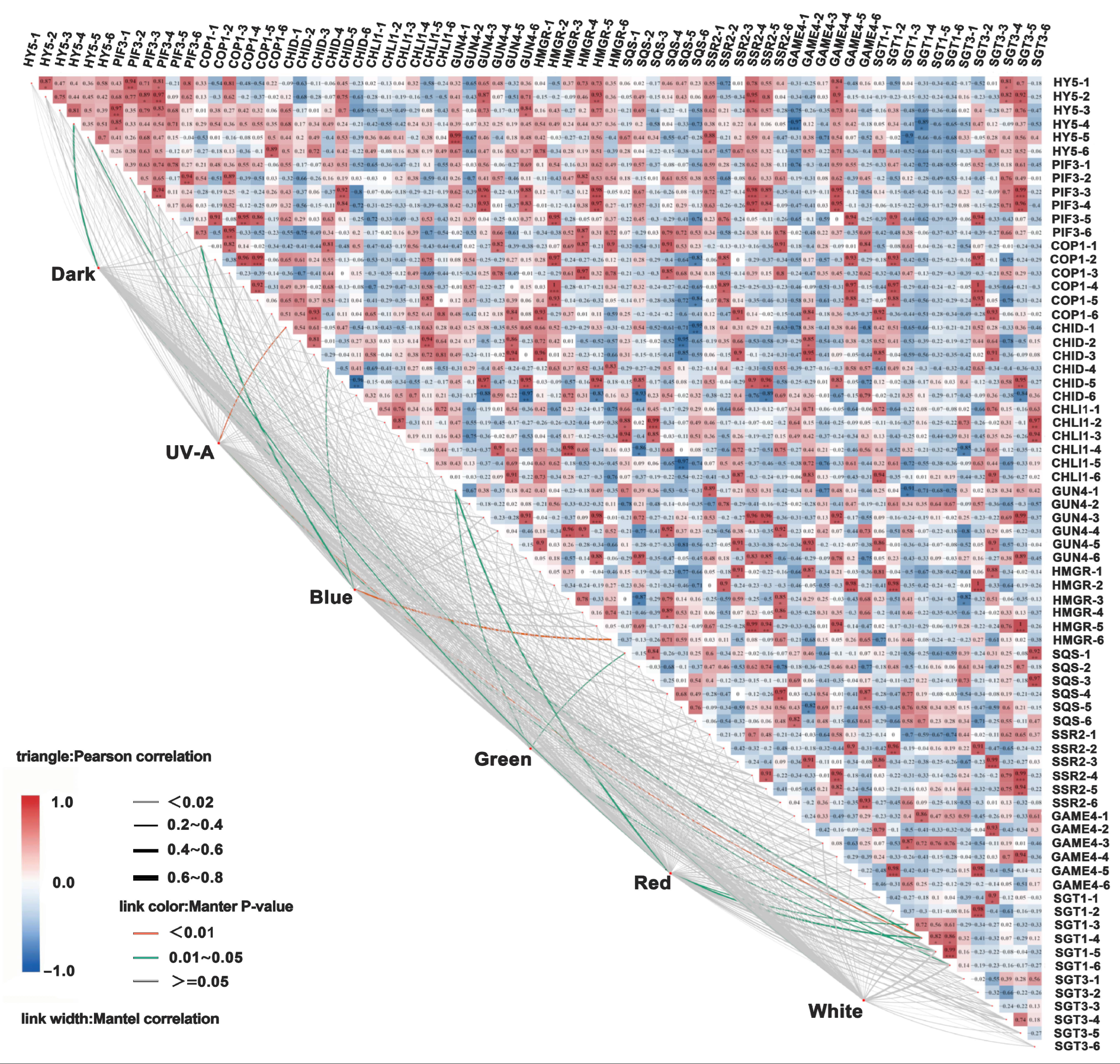
3.6. Correlation Analysis of Different Light Quality Treatments on Tuber Peel Color, Glycoalkaloid Content, and Expression Levels of Key Genes
4. Discussion
4.1. Light Quality Specifically Regulates Pigment Accumulation and the Synthesis of SGAs in Potato Tuber Peels
4.2. Genetic Determinants of Greening Resistance in Potato Cultivars
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmadu, T.; Abdullahi, A.; Ahmad, K. The Role of Crop Protection in Sustainable Potato (Solanum tuberosum L.) Production to Alleviate Global Starvation Problem: An Overview; IntechOpen: London, UK, 2021. [Google Scholar]
- Chauhan, V.B.S.; Mallick, S.N.; Pati, K.; Arutselvan, R.; Nedunchezhiyan, M. Status and Importance of Underexploited Tuber Crops in Relation to Nutritional Security and Economic Prosperity. In Compendium for Winter School on “Unexpected Vegetables: Unexplored Treasure Trove for Food”; ICAR-Indian Institute of Vegetable Research: Varanasi, India, 2022; pp. 246–264. [Google Scholar]
- Dhalsamant, K.; Singh, C.B.; Lankapalli, R. A Review on Greening and Glycoalkaloids in Potato Tubers: Potential Solutions. J. Agric. Food Chem. 2022, 70, 13819–13831. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Y.; Zhao, X.; Wang, E.; Liu, T.; Zhang, H.; Botao, S. The Transcription Factor StMYB113 Regulates Light-Induced Greening by Modulating Steroidal Glycoalkaloid Biosynthesis in Potatoes (Solanum tuberosum L.). Hortic. Adv. 2024, 2, 7. [Google Scholar] [CrossRef]
- Pavlista, A.D. Green Potatoes: The Problem and the Solution; Cooperative Extension, Institute of Agriculture and Natural Resources, University of Nebraska-Lincoln: Lincoln, NE, USA, 2001. [Google Scholar]
- Li, Y.; Cao, T.; Guo, Y.; Grimm, B.; Li, X.; Duanmu, D.; Lin, R. Regulatory and Retrograde Signaling Networks in the Chlorophyll Biosynthetic Pathway. J. Integr. Plant Biol. 2025, 67, 887–911. [Google Scholar] [CrossRef] [PubMed]
- Muraja-Fras, J.; Krsnik-Rasol, M.; Wrischer, M. Plastid Transformation in Greening Potato Tuber Tissue. J. Plant Physiol. 1994, 144, 58–63. [Google Scholar] [CrossRef]
- Tanios, S.; Thangavel, T.; Eyles, A.; Tegg, R.S.; Nichols, D.S.; Corkrey, R.; Wilson, C.R. Suberin Deposition in Potato Periderm: A Novel Resistance Mechanism Against Tuber Greening. New Phytol. 2020, 225, 1273–1284. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of Photosynthetic Processes and the Accumulation of Secondary Metabolites in Plants in Response to Monochromatic Light Environments: A Review. Biochim. Biophys. Acta 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Hoffmann, A.M.; Noga, G.; Hunsche, M. Alternating High and Low Intensity of Blue Light Affects PSII Photochemistry and Raises the Contents of Carotenoids and Anthocyanins in Pepper Leaves. Plant Growth Regul. 2016, 79, 275–285. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current Status and Recent Achievements in the Field of Horticulture with the Use of Light-Emitting Diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Rodríguez-Villalón, A.; Gas, E.; Rodríguez-Concepción, M. Phytoene Synthase Activity Controls the Biosynthesis of Carotenoids and the Supply of Their Metabolic Precursors in Dark-Grown Arabidopsis Seedlings. Plant J. 2009, 60, 424–435. [Google Scholar] [CrossRef]
- Gaion, L.A.; Galati, V.C.; Cardoso, A.C.R.; Rossatto, D.R.; Carvalho, R.F. Shifting the Anthocyanins from Light to Shade: What Roles Do These Pigments Play? Theor. Exp. Plant Physiol. 2025, 37, 16. [Google Scholar] [CrossRef]
- Li, Y.; Luo, J. From Steroidal Glycoalkaloids to Steroidal Saponins: Biosynthesis and Ecological Role in the Solanum Genus. Mol. Plant. 2025, 18, 22–24. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Li, Y.; Pei, Y.; Jaleel, A.; Ren, M. Potato Steroidal Glycoalkaloids: Properties, Biosynthesis, Regulation and Genetic Manipulation. Mol. Hortic. 2024, 4, 43. [Google Scholar] [CrossRef]
- McMillan, M.; Thompson, J.C. An Outbreak of Suspected Solanine Poisoning in Schoolboys: Examination of Criteria of Solanine Poisoning. QJM Int. J. Med. 1979, 190, 227–231. [Google Scholar]
- Hellenas, K.E.; Nyman, A.; Slanina, P.; Loof, L.; Gabrielsson, J. Determination of Potato Glycoalkaloids and Their Aglycones in Blood Serum by High Performance Liquid Chromatography: Application to Pharmacokinetic Studies in Humans. J. Chromatogr. 1992, 573, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Toyoda, M.; Fujiyama, Y.; Saito, Y. Effect of Cooking on the Contents of α-Chaconine and α-Solanine in Potatoes. J. Food Hyg. Soc. Jpn. 1990, 31, 67–73. [Google Scholar] [CrossRef]
- Bushway, R.J.; Bureau, L.J.; McGann, D.F. α-Chaconine and α-Solanine Content of Potato Peels and Potato Peel Products. J. Food Sci. 1983, 48, 84–86. [Google Scholar] [CrossRef]
- Townsend, J.N. Potato Field Greening and Response to Potassium Fertilization in the Columbia Basin. Ph.D. Thesis, Washington State University, Pullman, WA, USA, 2021. [Google Scholar]
- Friedman, M.; McDonald, G.M. Potato Glycoalkaloids: Chemistry, Analysis, Safety and Plant Physiology. Crit. Rev. Plant Sci. 1997, 16, 55–132. [Google Scholar] [CrossRef]
- French-Brooks, J. Reducing Supply Chain and Consumer Potato Waste. In Research Report; WRAP: Banbury, UK, 2012; p. 50. Available online: http://www.wrap.org.uk/ (accessed on 17 September 2025).
- Petermann, J.B.; Morris, S.C. The Spectral Responses of Chlorophyll and Glycoalkaloid Synthesis in Potato Tubers (Solanum tuberosum L.). Plant Sci. 1985, 39, 105–110. [Google Scholar] [CrossRef]
- Okamoto, H.; Ducreux, L.J.; Allwood, J.W.; Hedley, P.E.; Wright, A.; Gururajan, V.; Terry, M.J.; Taylor, M.A. Light Regulation of Chlorophyll and Glycoalkaloid Biosynthesis During Tuber Greening of Potato S. tuberosum. Front. Plant Sci. 2020, 11, 753. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, W.; Pan, Q.; Zeng, Q.; Yan, C.; Bai, X.; Liu, Y.; Zhang, L.; Li, B. Effects of Long-Term Blue Light Irradiation on Carotenoid Biosynthesis and Antioxidant Activities in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Food Res. Int. 2023, 174, 113661. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Li, Y.; Chen, Q.; Ye, Y.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H. Effect of Red and Blue Light on Anthocyanin Accumulation and Differential Gene Expression in Strawberry (Fragaria × ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Huang, G.; Li, X.; Xiao, J.; Guo, L. Effect of different LED lights on aliphatic glucosinolates metabolism and biochemical characteristics in broccoli sprouts. Food Res. Int. 2022, 154, 111015. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Eguchi, T.; Deveci, M.; Kubota, C. Tomato Seedling Physiological Responses Under Different Percentages of Blue and Red Photon Flux Ratios Using LEDs and Cool White Fluorescent Lamps. Sci. Hortic. 2016, 213, 270–280. [Google Scholar] [CrossRef]
- Dawson, T.L. Development of Efficient and Durable Sources of White Light. Color. Technol. 2010, 126, 1–10. [Google Scholar] [CrossRef]
- Jayawardena, A.; Duffy, D.; Manahan, J.M. Lighting Matters in Industrial Environments: A Framework Linking Workplace Safety to Lighting Quality Metrics. IEEE Ind. Appl. Mag. 2017, 23, 54–63. [Google Scholar] [CrossRef]
- Li, F.Y.K. White LED for General Illumination Applications. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2007. [Google Scholar]
- Chang, H.Y. Light-Induced Glycoalkaloid and Chlorophyll Synthesis in Potato Tubers: Cultivar Differences and Strategies for Mitigation; University of California: Davis, CA, USA, 2013. [Google Scholar]
- Reeves, A.F. Varietal Differences in Potato Tuber Greening. Am. J. Potato Res. 1988, 65, 651–658. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Mancinelli, A.L.; Rabino, I. Photocontrol of Anthocyanin Synthesis: III. The Action of Streptomycin on the Synthesis of Chlorophyll and Anthocyanin. Plant Physiol. 1975, 55, 251–257. [Google Scholar] [CrossRef]
- Dong, W.; Shi, L.; Li, S.; Xu, F.; Yang, Z.; Cao, S. Hydrogen-Rich Water Delays Fruit Softening and Prolongs Shelf Life of Postharvest Okras. Food Chem. 2023, 399, 133997. [Google Scholar] [CrossRef]
- Zhou, Q.; Bao, Z.; Yu, Y.; Chen, W.; Yang, Z.; Cao, S.; Shi, L. IAA-Regulated Levels of Endogenous Phytohormones in Relation to Chilling Tolerance in Cold-Stored Peaches After Harvest. Postharvest Biol. Technol. 2023, 205, 112490. [Google Scholar] [CrossRef]
- Tanios, S.; Eyles, A.; Corkrey, R.; Tegg, R.S.; Thangavel, T.; Wilson, C.R. Quantifying Risk Factors Associated with Light-Induced Potato Tuber Greening in Retail Stores. PLoS ONE 2020, 15, e0235522. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Su, N.; Shen, W.; Cui, J. Analyzing Photosynthetic Activity and Growth of Solanum lycopersicum Seedlings Exposed to Different Light Qualities. Acta Physiol. Plant. 2014, 36, 1411–1420. [Google Scholar] [CrossRef]
- Liu, C.C.; Ahammed, G.J.; Wang, G.T.; Xu, C.J.; Chen, K.S.; Zhou, Y.H.; Yu, J.Q. Tomato CRY1a Plays a Critical Role in the Regulation of Phytohormone Homeostasis, Plant Development, and Carotenoid Metabolism in Fruits. Plant Cell Environ. 2018, 41, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Tang, J.; Li, Y.; Cheng, W.; Yao, X.; Escalona, V.H.; Qian, G.; Ma, J.; Yu, X.; Li, H. Combination of Light Quality and Melatonin Regulates the Quality in Mustard Sprouts. Food Chem. 2024, 437, 101560. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; An, H.; Zhang, X.; Zhou, B. Integrated Transcriptome and Metabolome Analysis Reveals the Anthocyanin Biosynthesis Mechanisms in Blueberry (Vaccinium corymbosum L.) Leaves Under Different Light Qualities. Front. Plant Sci. 2022, 13, 1073332. [Google Scholar] [CrossRef]
- Mekapogu, M.; Sohn, H.B.; Kim, S.J.; Lee, Y.Y.; Park, H.M.; Jin, Y.I.; Hong, S.Y.; Suh, J.T.; Kweon, K.; Jeong, J.C. Effect of Light Quality on the Expression of Glycoalkaloid Biosynthetic Genes Contributing to Steroidal Glycoalkaloid Accumulation in Potato. Am. J. Potato Res. 2016, 93, 264–277. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Chen, W.; Yang, Z.; Li, X.; Wang, L.; Cao, S.; Shi, L. The Changes in Chlorophyll, Solanine, and Phytohormones During Light-Induced Greening in Postharvest Potatoes. Postharvest Biol. Technol. 2025, 219, 113291. [Google Scholar] [CrossRef]
- Xu, Y.; You, C.; Xu, C.; Zhang, C.; Hu, X.; Li, X.; Ma, H.; Gong, J.; Sun, X. Red and Blue Light Promote Tomato Fruit Coloration Through Modulation of Hormone Homeostasis and Pigment Accumulation. Postharvest Biol. Technol. 2024, 207, 112588. [Google Scholar] [CrossRef]
- Wang, C.C.; Meng, L.H.; Gao, Y.; Grierson, D.; Fu, D.Q. Manipulation of light signal transduction factors as a means of modifying steroidal glycoalkaloids accumulation in tomato leaves. Front. Plant Sci. 2018, 9, 437. [Google Scholar] [CrossRef]
- Petersson, E.V.; Arif, U.; Schulzova, V.; Krtkova, V.; Hajslova, J.; Meijer, J.; Andersson, H.C.; Jonsson, L.; Sitbon, F. Glycoalkaloid and Calystegine Levels in Table Potato Cultivars Subjected to Wounding, Light, and Heat Treatments. J. Agric. Food Chem. 2013, 61, 5893–5902. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Merino, I.; Guasca, A.O.; Krmela, A.; Arif, U.; Ali, A.; Westerberg, E.; Jalmi, S.K.; Hajslova, J.; Schulzova, V.; Sitbon, F. Metabolomic and transcriptomic analyses identify external conditions and key genes underlying high levels of toxic glycoalkaloids in tubers of stress-sensitive potato cultivars. Front. Plant Sci. 2023, 14, 1210850. [Google Scholar] [CrossRef]
- Baur, S.; Belle, N.; Hausladen, H.; Wurzer, S.; Brehm, L.; Stark, T.D.; Hücklhoven, R.; Hofmann, T.; Dawid, C. Quantitation of Toxic Steroidal Glycoalkaloids and Newly Identified Saponins in Post-Harvest Light-Stressed Potato (Solanum tuberosum L.) Varieties. J. Agric. Food Chem. 2022, 70, 8300–8308. [Google Scholar] [CrossRef]
- Nahar, N.; Westerberg, E.; Arif, U.; Huchelmann, A.; Guasca, A.O.; Beste, L.; Dalman, K.; Dutta, P.C.; Jonsson, L.; Sitbon, F. Transcript Profiling of Two Potato Cultivars During Glycoalkaloid-Inducing Treatments Shows Differential Expression of Genes in Sterol and Glycoalkaloid Metabolism. Sci. Rep. 2017, 7, 45689. [Google Scholar] [CrossRef]
| Cultivar | Light Quality | Δa* | ΔL* | Δb* | ΔE* ab |
|---|---|---|---|---|---|
| Favorita | Dark | 1.35 a | 2.88 a | 11.50 a | 11.93 a |
| UV-A light | −3.33 c | −2.92 c | 5.73 c | 7.24 e | |
| Blue light | −5.30 d | −9.16 e | 3.88 d | 11.27 b | |
| Green light | −3.52 c | −2.25 c | 7.72 b | 8.78 c | |
| Red light | −2.20 b | −1.12 b | 7.31 b | 7.92 d | |
| White light | −5.14 d | −5.42 d | 1.96 e | 7.72 d | |
| Lucinda | Dark | 1.35 a | 2.88 a | 11.50 a | 11.93 a |
| UV-A light | −3.33 c | −2.92 c | 5.73 c | 8.24 c | |
| Blue light | −5.30 d | −9.16 e | 3.88 d | 9.27 b | |
| Green light | −3.52 c | −2.25 b | 7.72 b | 8.78 b | |
| Red light | −2.20 b | −2.12 b | 7.31 b | 7.92 d | |
| White light | −5.14 d | −5.42 d | 1.96 e | 7.72 d | |
| Jizhang shu 12 | Dark | 1.35 a | 2.88 a | 11.50 a | 11.13 a |
| UV-A light | −3.33 c | −2.92 c | 5.73 c | 7.24 e | |
| Blue light | −5.30 d | −9.16 e | 3.88 d | 8.27 c | |
| Green light | −3.52 c | −2.25 b | 7.72 b | 8.78 b | |
| Red light | −2.20 b | −2.12 b | 7.31 b | 7.92 d | |
| White light | −5.14 d | −6.42 d | 1.96 e | 7.72 d | |
| Long shu 10 | Dark | 1.35 a | 2.28 a | 11.50 a | 10.93 a |
| UV-A light | −3.33 c | −2.92 c | 5.73 d | 7.24 e | |
| Blue light | −5.30 d | −9.16 e | 3.28 e | 9.27 b | |
| Green light | −3.52 c | −2.25 b | 6.72 c | 8.78 c | |
| Red light | −2.20 b | −2.12 b | 7.31 b | 7.92 d | |
| White light | −5.14 d | −6.90 d | 1.96 f | 7.72 d | |
| Qing shu 9 | Dark | 1.35 a | 1.28 a | 11.50 a | 11.93 a |
| UV-A light | −3.33 c | −2.92 c | 5.73 d | 9.04 b | |
| Blue light | −5.30 e | −9.16 e | 3.20 e | 6.17 e | |
| Green light | −3.92 d | −2.25 b | 6.12 c | 8.18 c | |
| Red light | −2.20 b | −2.12 b | 6.81 b | 7.92 d | |
| White light | −5.14 e | −7.72 d | 1.96 f | 5.72 e | |
| Purple Potato | Dark | 1.35 a | 2.08 a | 11.50 a | 11.53 a |
| UV-A light | −3.33 c | −2.92 c | 5.73 d | 7.24 e | |
| Blue light | −5.30 d | −9.16 e | 3.88 e | 8.97 b | |
| Green light | −3.52 c | −2.25 b | 7.02 c | 8.78 c | |
| Red light | −2.20 b | −2.12 b | 9.31 b | 7.92 d | |
| White light | −5.14 d | −7.42 d | 1.96 f | 7.72 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sa, G.; Zao, X.; Yuan, J.; Cheng, L.; Yu, B. Light-Quality-Dependent Greening and Steroidal Glycoalkaloid Accumulation in Potato Tubers: Regulatory Mechanisms and Postharvest Strategies to Reduce Food Safety Risks. Foods 2025, 14, 3394. https://doi.org/10.3390/foods14193394
Sa G, Zao X, Yuan J, Cheng L, Yu B. Light-Quality-Dependent Greening and Steroidal Glycoalkaloid Accumulation in Potato Tubers: Regulatory Mechanisms and Postharvest Strategies to Reduce Food Safety Risks. Foods. 2025; 14(19):3394. https://doi.org/10.3390/foods14193394
Chicago/Turabian StyleSa, Gang, Xiaohua Zao, Jianlong Yuan, Lixiang Cheng, and Bin Yu. 2025. "Light-Quality-Dependent Greening and Steroidal Glycoalkaloid Accumulation in Potato Tubers: Regulatory Mechanisms and Postharvest Strategies to Reduce Food Safety Risks" Foods 14, no. 19: 3394. https://doi.org/10.3390/foods14193394
APA StyleSa, G., Zao, X., Yuan, J., Cheng, L., & Yu, B. (2025). Light-Quality-Dependent Greening and Steroidal Glycoalkaloid Accumulation in Potato Tubers: Regulatory Mechanisms and Postharvest Strategies to Reduce Food Safety Risks. Foods, 14(19), 3394. https://doi.org/10.3390/foods14193394






