Optimization of Nano-SiO2/Tea Polyphenol/Pullulan Edible Composite Films for Postharvest Preservation of Cherry Tomatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Composite Film Preparation and Optimization
2.2.1. Preparation of Nano-SiO2/TP/PUL Composite Film
2.2.2. Single-Factor Experimental Design
2.2.3. Response Surface Experimental Design
2.3. Mechanical Properties of Edible Composite Films
2.4. Physical Properties of Edible Composite Films
2.4.1. The Light Transmittance of Edible Composite Films
2.4.2. Water Vapor Permeability of Edible Composite Films
2.5. Functional Properties of Composite Films
2.5.1. Determination of Antioxidant Properties
2.5.2. Determination of the Antibacterial Property
2.6. Structural Characterization of Films
2.6.1. Scanning Electron Microscopy
2.6.2. ATR-FTIR Spectra
2.7. Evaluation of the K Value for the Composite Films
2.8. Coating Application for the Storage of Cherry Tomatoes
2.8.1. Coating Treatment for Cherry Tomato
2.8.2. Determination of Weight Loss
2.8.3. Determination of Decay Rate
2.8.4. Determination of Firmness
2.8.5. Determination of Total Soluble Content
2.8.6. Determination of Titratable Acid
2.8.7. Determination of VC
2.8.8. Determination of Respiration Rate
2.8.9. Sensory Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Single-Factor Test Results
3.1.1. Influence of Nano-SiO2 for Composite Film
3.1.2. Influence of PUL for Composite Film
3.1.3. Influence of TP for Composite Film
3.1.4. Influence of Glycerol for Composite Film
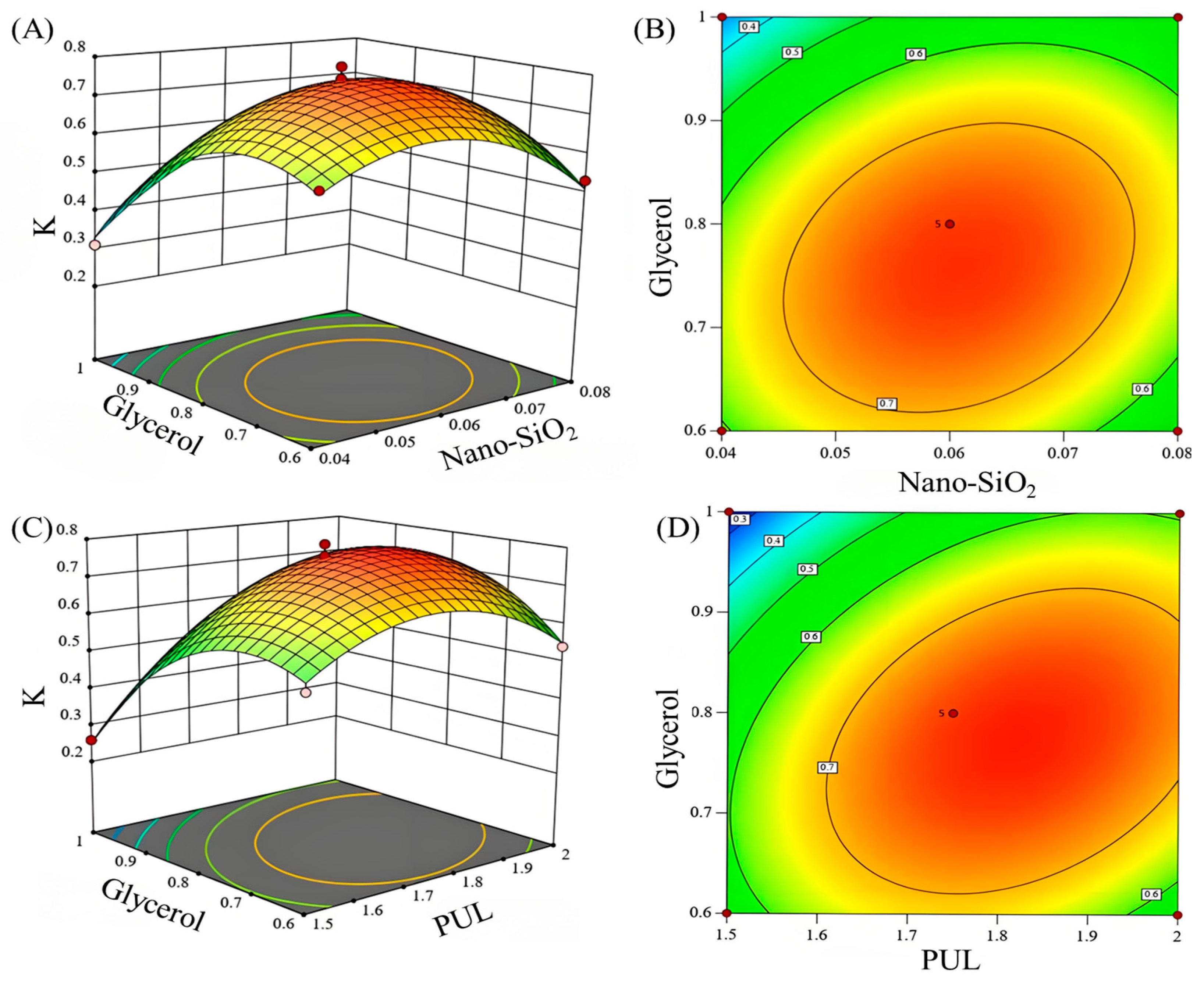
3.2. Optimization by Response Surface Methodology
3.2.1. Establishment of the Regression Model
3.2.2. Validation Results
3.3. Analysis of Functional Characteristics of Composite Films
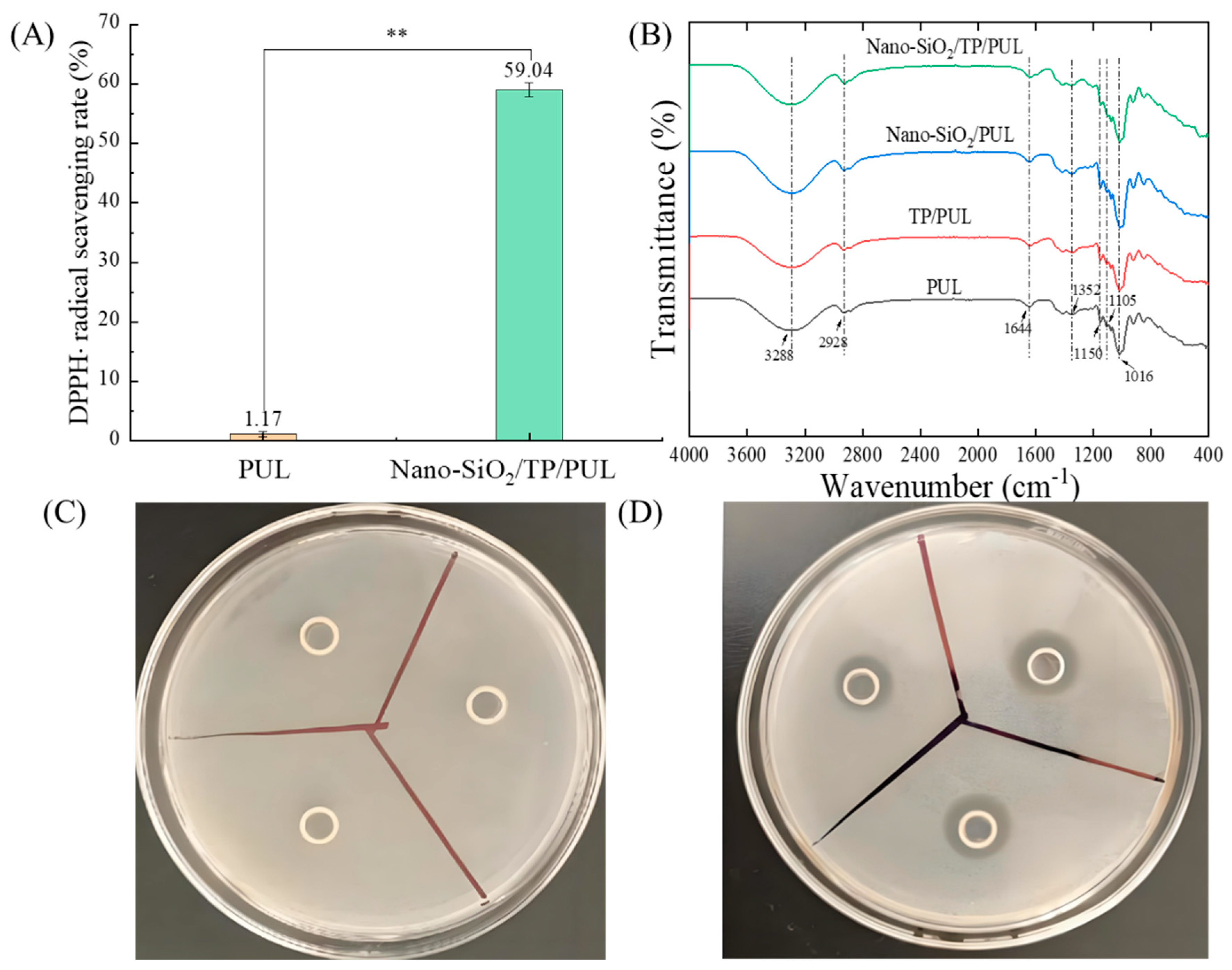
3.3.1. Analysis of Antioxidant Properties
3.3.2. Antibacterial Assessment
3.4. Analysis of Construction Characteristics of Composite Films
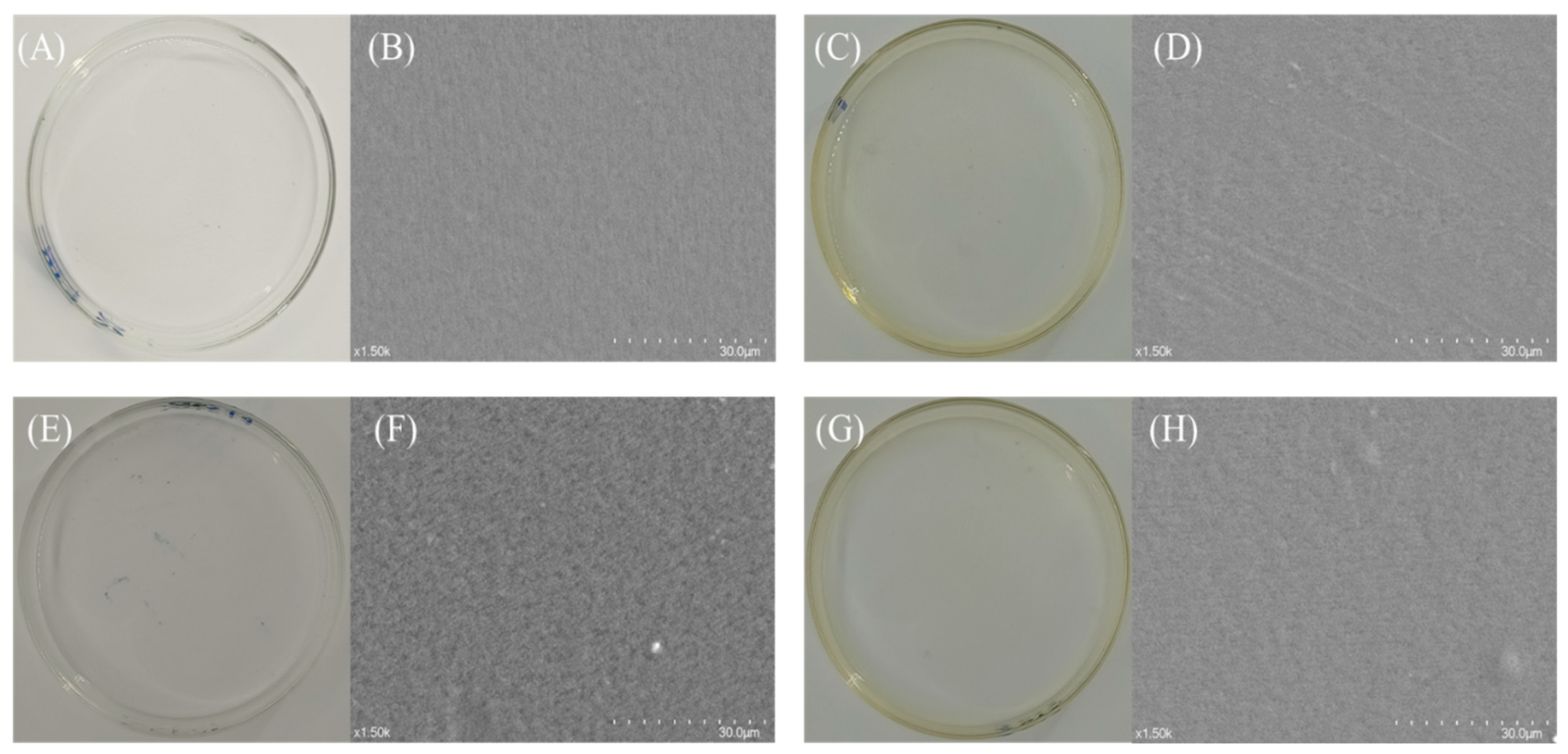
3.4.1. SEM
3.4.2. FTIR
3.5. Application of Composite Coating for Cherry Tomatoes
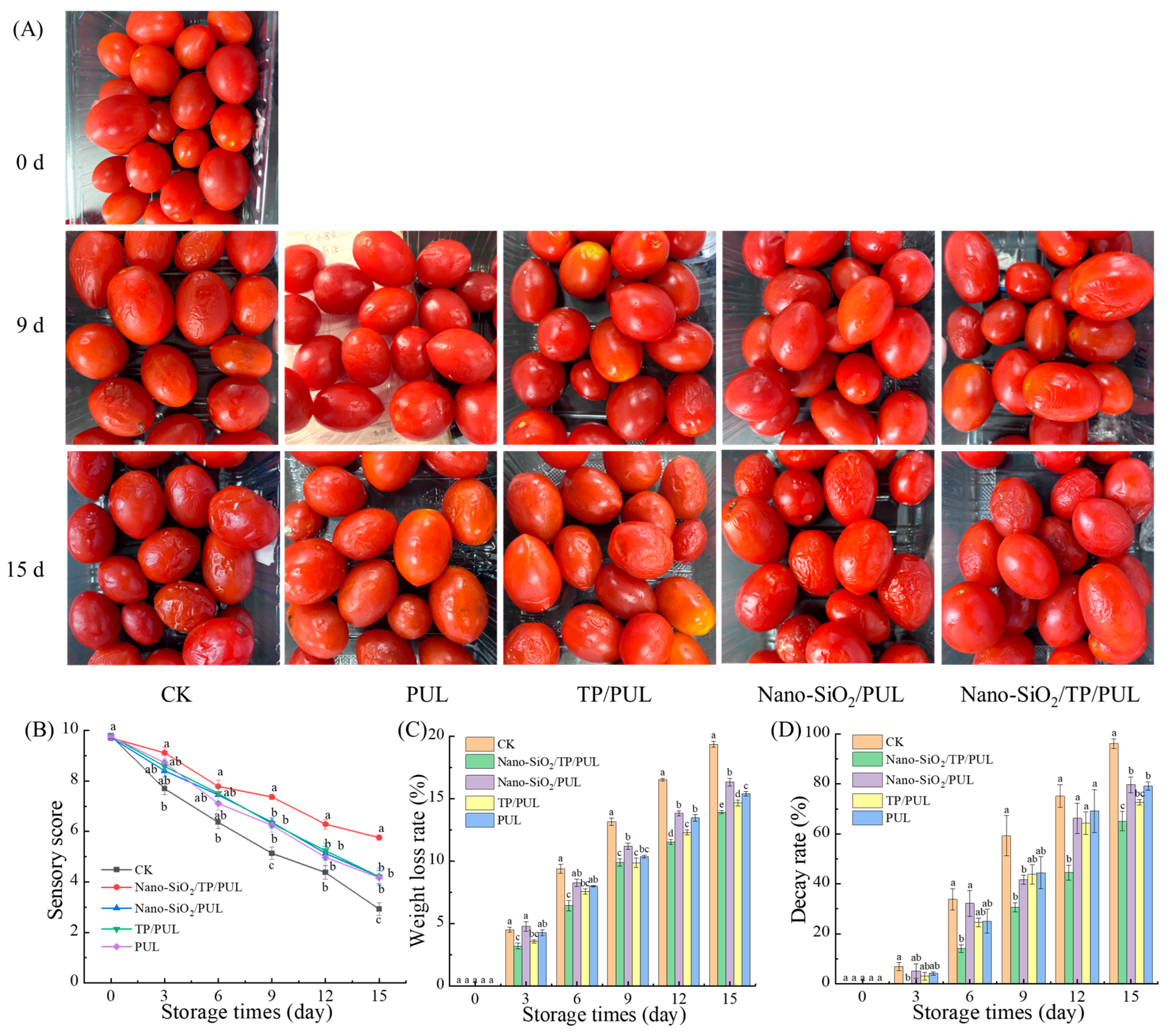
3.5.1. Effect of Coating on Appearance and Sensory Evaluation
3.5.2. Effect of Coating on Weight Loss Rate and Decay Rate
3.5.3. Effect of Coating on Respiration Rate
3.5.4. Effect of Coating on the Firmness and VC
3.5.5. Effect of Coating on the TSS and TA
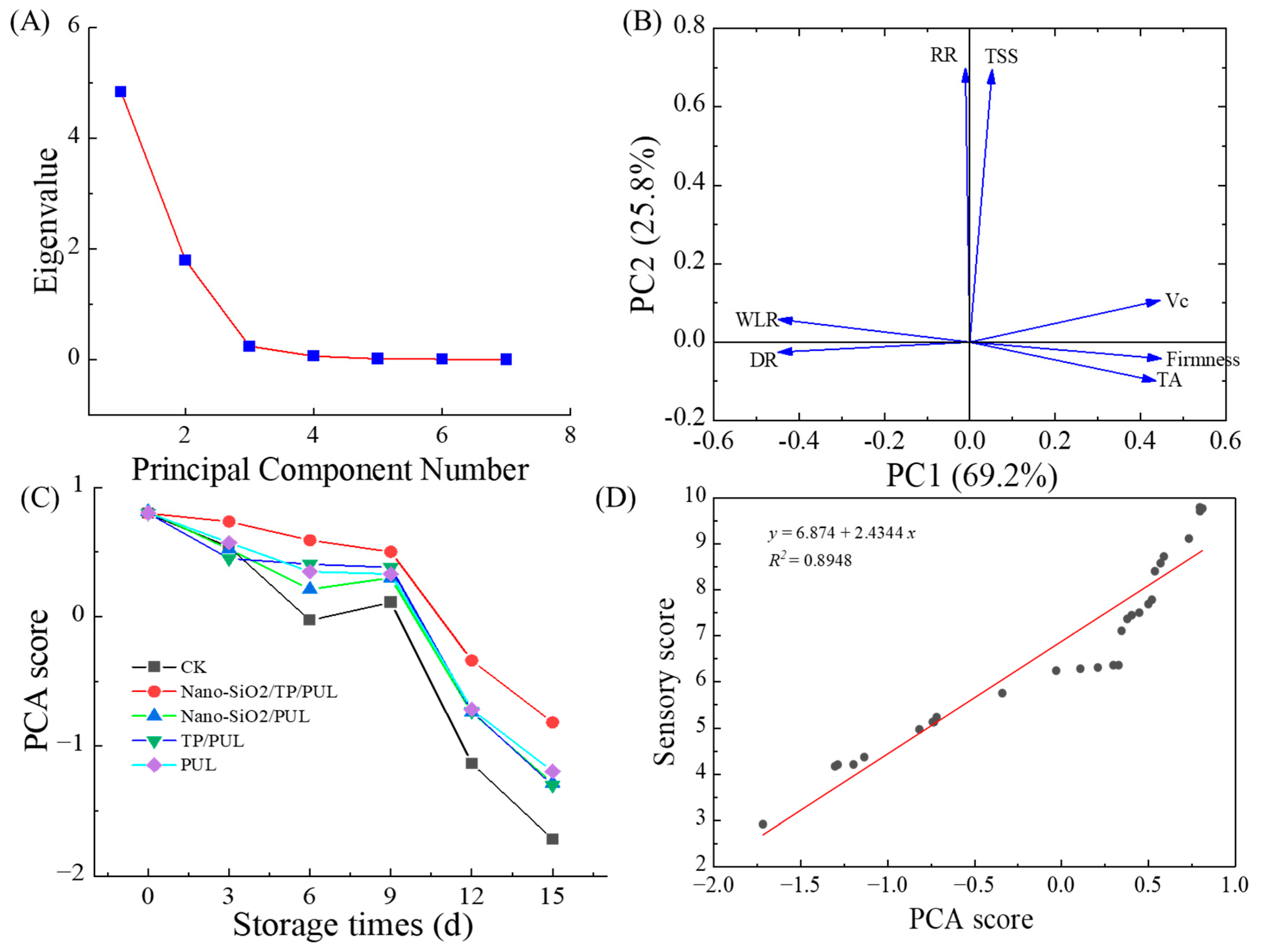
3.6. PCA of Coating Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR-FTIR | Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy |
| CK | Control check |
| DCPIP | 2,6-Dichlorophenol Indophenol |
| DPPH | 1,1-Diphenylsulfonylbenzhydrazide |
| DR | Decay Rate |
| EB | Elongation at Break |
| LT | Light Transmittance |
| Nanno-SiO2 | Nanoscale Silicon Dioxide |
| PCA | Principal Component Analysis |
| PUL | Pullulan |
| PVA | Polyvinyl Alcohol |
| RR | Respiration Rate |
| SEM | Scanning Electron Microscopy |
| TA | Titratable Acid |
| TP | Tea Polyphenols |
| TS | Tensile Strength |
| TSS | Total Soluble Solids |
| Vitamin C | VC |
| WLR | Water Loss Rate |
| WVP | Water Vapor Permeability |
| ZnO-Nps | Nanoscale of Zinc Oxide |
Appendix A
| Groups | Treatment Composition (w/v) |
|---|---|
| Nano-SiO2/TP/PUL | 0.06% Nano-SiO2 + 0.1% TP + 1.8% PUL coating solution |
| Nano-SiO2/PUL | 0.06% Nano-SiO2 + 1.8% PUL coating solution |
| TP/PUL | 0.1% TP + 1.8% PUL coating solution |
| PUL | 1.8% PUL coating solution |
| CK | Distilled water (uncoated group) |
| Score Range | Appearance | Odor | Texture | Decay Degree |
|---|---|---|---|---|
| 8.1–10.0 | Vibrant color with gloss | Intense fruity fragrance | Firm | No decay |
| 6.1–8.0 | Slight discoloration, partial dehydration | Mild fruity notes | Moderately firm | No visible decay |
| 4.1–6.0 | Darkened color, significant dehydration | Slight sour/rotten odor | Softened, non-elastic | Minor decay |
| 2.1–4.0 | Dull color, severe wrinkles | Alcoholic off-odor | Spoiled | Severe decay |
| 0–2.0 | Blackened appearance | Strong alcoholic odor | Severely mushy | Fully decayed |
References
- Tang, B.; Wu, X.; Liu, L.; Xu, J.; Ma, J.; Zhang, H. Preparation of multi-functional active packaging film of Galla chinensis waste CDs/pullulan. Int. J. Biol. Macromol. 2024, 275, 133221. [Google Scholar] [CrossRef]
- Ali, M.; Ali, A.; Ali, S.; Chen, H.; Wu, W.; Liu, R.; Chen, H.; Ahmed, Z.F.R.; Gao, H. Global insights and advances in edible coatings or films toward quality maintenance and reduced postharvest losses of fruit and vegetables: An updated review. Compr. Rev. Food. Sci. Food Saf. 2025, 24, e70103. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, N.; Upadhyay, A.; Sethi, S.; Singh, A. Edible coating as postharvest management strategy for shelf-life extension of fresh tomato (Solanum lycopersicum L.): An overview. J. Food Sci. 2022, 87, 2256–2290. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Lin, L.; Zhang, B.; Yang, Q.; Niu, J.; Zhang, S.; Miao, J. Preparation and characterization of ginger essential oil nanoemulsion composite films and its effect on preservation of cherry tomatoes. Food Control. 2025, 177, 111455. [Google Scholar] [CrossRef]
- Álvarez, A.; Manjarres, J.J.; Ramírez, C.; Bolívar, G. Use of an exopolysaccharide-based edible coating and lactic acid bacteria with antifungal activity to preserve the postharvest quality of cherry tomato. LWT 2021, 151, 112225. [Google Scholar] [CrossRef]
- Giacondino, C.; De Bruno, A.; Puntorieri, D.; Pizzimenti, M.; Piscopo, A. Impact of Antioxidant-Enriched Edible Gel Coatings and Bio-Based Packaging on Cherry Tomato Preservation. Gels 2024, 10, 549. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.; Wang, L.; Cheng, S.; Cheng, H. Chitosan derived nano-selenium based coatings for postharvest safety of cherry tomato. LWT 2025, 217, 117441. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, J.; Tian, Y.; Guo, Y.; He, T.; Xie, Y.; Wu, K.; Zhu, F.; Jiang, F. Improved konjac glucomannan/curdlan-based emulsion coating by mung bean protein addition for cherry tomato preservation. Int. J. Biol. Macromol. 2025, 291, 139080. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Hernández-Varela, J.D.; Burelo, M.; Elizondo-Luevano, J.H.; Araújo, R.G.; Treviño-Quintanilla, C.D.; Medina, D.I. Progress in chitosan-based materials: Enhancing edible coatings and films through modifications and functionalization for food preservation. Process Biochem. 2025, 156, 175–190. [Google Scholar] [CrossRef]
- Chen, C.; Ding, Y.; Sun, Y.; Li, X.; Sun, C.; Guo, F.; Zeng, X.; Gong, H.; Fan, X. Chitosan/pullulan edible coatings containing thyme essential oil nanoemulsion: Preparation, characterization and application in strawberry preservation. Int. J. Biol. Macromol. 2025, 309, 143043. [Google Scholar] [CrossRef]
- Guan, Y.; Lu, X.; Cheng, J.; Lu, S.; Yin, L.; Cheng, J.; Yang, M.; Chen, Y.; Sun, J.; Lu, G.; et al. Montmorillonite-based edible coating enhances the postharvest quality of sweetpotato by regulating ROS and membrane lipid metabolism. Food Control 2024, 158, 110259. [Google Scholar] [CrossRef]
- Wang, M.; Miao, X.; Guo, F.; Deng, Z.; Bian, F.; Xiao, T.; Chen, C. Optimized hybrid edible surface coating prepared with gelatin and cellulose nanofiber for cherry tomato preservation. Int. J. Biol. Macromol. 2024, 279, 134822. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wu, W.; Liu, R.; Niu, B.; Chen, H.; Fang, X.; Chen, H.; Shen, C.; Gao, H. Preparation and application of thyme essential oil@halloysite nanotubes-loaded multifunctional pullulan/gelatin/PVA aerogels. Int. J. Biol. Macromol. 2025, 309, 142917. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Bai, J.; Pilon, L.; Torres, R.; Casals, C.; Solsona, C.; Teixidó, N. Fundamentals of Edible Coatings and Combination with Biocontrol Agents: A Strategy to Improve Postharvest Fruit Preservation. Foods 2024, 13, 2980. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, K.; Adhikary, T.; Gill, P.P.S.; Kumar, A. Edible coatings enriched with plant-based extracts preserve postharvest quality of fruits: A review. Prog. Org. Coat. 2023, 182, 107669. [Google Scholar] [CrossRef]
- Huang, P.; Ding, J.; Liu, C.; Li, H.; Wang, C.; Lin, Y.; Sameen, D.E.; Hossen, M.A.; Chen, M.; Yan, J.; et al. Konjac glucomannan/low-acyl gellan gum edible coating containing thymol microcapsule regulates cell wall polysaccharides disassembly and delays postharvest softening of blueberries. Postharvest Biol. Technol. 2023, 204, 112449. [Google Scholar] [CrossRef]
- Romanazzi, G.; Moumni, M. Chitosan and other edible coatings to extend shelf life, manage postharvest decay, and reduce loss and waste of fresh fruits and vegetables. Curr. Opin. Biotechnol. 2022, 78, 102834. [Google Scholar] [CrossRef]
- Utami, M.R.; Kusumaningsih, T.; Firdaus, M.; Istiqomah, A. Optimization of edible film based on chitosan-L-lysine incorporating cinnamon essential oil using response surface methodology. J. Phys. Conf. Ser. 2022, 2190, 12026. [Google Scholar] [CrossRef]
- Ali, M.M.; Zhang, Z.; Liang, X.; Ali, S.; Ejaz, S.; Maryam, A.; Ibrahim, M.; Ercisli, S.; Chen, F. Pullulan coating enhances postharvest quality retention and extends shelf life of Chinese plums by regulating enzymatic activity and gene expression related to ROS scavenging and cell wall metabolism. Int. J. Biol. Macromol. 2025, 309, 143020. [Google Scholar] [CrossRef]
- Lee, C.R.; Lee, S.J.; Kim, T.I.; Chathuranga, K.; Lee, J.S.; Kim, S.; Kim, M.H.; Park, W.H. Chitosan-gallic acid conjugate edible coating film for perishable fruits. Food Chem. 2025, 463, 141322. [Google Scholar] [CrossRef]
- Benlloch-Tinoco, M.; Gentile, P.; Taylor, L.; Girón-Hernández, J. Alginate edible films as delivery systems for green tea polyphenols. Food Hydrocoll. 2025, 158, 110518. [Google Scholar] [CrossRef]
- Wani, S.M.; Rizwan, D.; Khanday, F.A.; Mir, S.A.; Masoodi, F.A. Effect of pullulan and pullulan-chitosan composite coating on the antioxidant activity, texture, color and shelf-life of a local cultivar of sweet cherry. Food Humanit. 2024, 3, 100432. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, B.; Yuan, Z.; Sheng, Q.; Jin, C.; Qi, J.; Yu, M.; Liu, Y.; Xiong, G. Preparation and performance testing of corn starch/pullulan/gallic acid multicomponent composite films for active food packaging. Food Chem. X 2023, 19, 100782. [Google Scholar] [CrossRef] [PubMed]
- Gniewosz, M.; Pobiega, K.; Kraśniewska, K.; Synowiec, A.; Chaberek, M.; Galus, S. Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging. Foods 2022, 11, 2319. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Wang, W.; Li, L.; Xia, Q. Preparation and characterization of tamarind seed polysaccharide-pullulan sublingual films loaded with muco-inert nanoparticles for mucosal co-delivery of BSA and resveratrol. Carbohydr. Polym. 2025, 356, 123420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, J.; Chen, Z.; Yu, Y.; Zhang, X.; Zhang, W. Widely targeted metabolomics to analyze the effect of polyvinyl alcohol/pullulan/ZnO-Nps composite film on postharvest storage of Allium mongolicum Regel. Int. J. Biol. Macromol. 2025, 306, 141667. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, H.; Song, S.; Li, Y.; Zhang, X.; Zhang, W. Preparation and characterization of polyvinyl alcohol/pullulan/ZnO-Nps composite film and its effect on the postharvest quality of Allium mongolicum Regel. Int. J. Biol. Macromol. 2024, 279, 135380. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Liang, Q.; Kang, L.; Raza, H.; Chi, Z.; Chi, R.; Ren, X.; Ma, H. Preparation and characterization of ultrasound-assisted essential oil-loaded nanoemulsions stimulated pullulan-based bioactive film for strawberry fruit preservation. Food Chem. 2023, 422, 136254. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, W.; Wang, X.; Aslam, M.M.; Li, W.; Shao, Y. Tea polyphenols coating improves physiological properties, microstructure and chemical composition of cuticle to suppress quality deterioration of passion fruit during cold storage. Food Chem. 2025, 463, 141524. [Google Scholar] [CrossRef]
- Wang, Y.; Muzammal Aslam, M.; Wang, X.; Gu, H.; Jia, W.; Li, W.; Shao, Y. Aloe vera and tea polyphenols composite coating delays passion fruit senescence by promoting phenolic and flavonoid accumulation. Food Res. Int. 2024, 190, 114594. [Google Scholar] [CrossRef]
- Li, W.; Qin, Y.; Zhang, B.; Zhang, W.; Yao, D.; Zeng, C.; Ning, D.; Zhuang, Y.; Li, L.; Huang, R. Effect of different zein/tea polyphenol/apple pectin coating on nutritional, physicochemical properties of fresh walnut kernel preservation. Food Chem. 2025, 475, 143230. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, Y.; Qin, Y.; Chen, H.; Zhou, L. Release of clove essential oil loaded by mesoporous nano-silica in polylactic acid-based food packaging on postharvest preservation of white button mushroom. Int. J. Food Sci. Technol. 2022, 57, 457–465. [Google Scholar] [CrossRef]
- Sodano, V. Nanotechnology and Food System: Assessing the European Union Regulatory System. Eur. J. Dev. Stud. 2023, 3, 25–31. [Google Scholar] [CrossRef]
- Zhang, W.; Ahari, H.; Zhang, Z.; Jafari, S.M. Role of silica (SiO2) nano/micro-particles in the functionality of degradable packaging films/coatings and their application in food preservation. Trends Food Sci. Technol. 2023, 133, 75–86. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Li, L.; Cheng, M.; Zhang, L. Optimization of konjac glucomannan/carrageenan/nano-SiO2 coatings for extending the shelf-life of Agaricus bisporus. Int. J. Biol. Macromol. 2019, 122, 857–865. [Google Scholar] [CrossRef]
- Wang, L.; Shao, S.; Madebo, M.P.; Hou, Y.; Zheng, Y.; Jin, P. Effect of nano-SiO2 packing on postharvest quality and antioxidant capacity of loquat fruit under ambient temperature storage. Food Chem. 2020, 315, 126295. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Xu, R.; Liu, J. Preparation of a Novel Perilla Essential Oil/Grape Seed Extract–Chitosan/Gelatin Composite Edible Gel Film and Its Application in the Preservation of Grass Carp. Gels 2025, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Tsague Donjio, R.; Aghofack Nguemezi, J.; Anoumaa, M.; Tafre Phounzong, E.; Kenfack, J.O.; Fonkou, T. Using Response Surface Methodology to Optimize Edible Coating Formulations to Delay Ripening and Preserve Postharvest Quality of Tomatoes. J. Food Qual. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Ma, Q.; Mu, J.; Li, X.; Liu, H. Preparation and Characterization of Yellow Peach Peel/Sodium Alginate/Glycerol Antioxidant Film Applicable for Oil Package. Polymers 2022, 14, 1693. [Google Scholar] [CrossRef]
- Gond, D.K.; Vandna; Vishwakarma, S.K.; Dixit, S.; Mishra, P.K.; Yadav, V.L. Optimization of PVA/alkali treated ramie fiber-based composite film for green packaging application using response surface methodology approach. Surf. Interfaces 2025, 56, 105562. [Google Scholar] [CrossRef]
- Nkede, F.N.; Wardak, M.H.; Fanze, M.; Kondo, N.; Wardana, A.A.; Jothi, J.S.; Tanaka, F.; Tanaka, F. The potential of Helichrysum italicum essential oil-infused alginate coatings and film for prolonging the shelf-life of cherry tomatoes. Food Packag. Shelf Life 2024, 46, 101381. [Google Scholar] [CrossRef]
- Hashemi Tabatabaei, R.; Jafari, S.M.; Mirzaei, H.; Mohammadi Nafchi, A.; Dehnad, D. Preparation and characterization of nano-SiO2 reinforced gelatin-k-carrageenan biocomposites. Int. J. Biol. Macromol. 2018, 111, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Abdelkader, M.F.M.; Lo’ay, A.A.; El-Ansary, D.O.; Shaaban, F.K.M.; Osman, S.O.; Shenawy, I.E.; Osman, H.-E.H.; Limam, S.A.; Abdein, M.A.; et al. The Combined Effect of Hot Water Treatment and Chitosan Coating on Mango (Mangifera indica L. cv. Kent) Fruits to Control Postharvest Deterioration and Increase Fruit Quality. Coatings 2022, 12, 83. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.; Ribeiro, C.; Vilela, A.; Meyer, A.S.; Gonçalves, B. Innovative edible coatings for postharvest storage of sweet cherries. Sci. Hortic. 2023, 310, 111738. [Google Scholar] [CrossRef]
- Chikhala, T.; Seke, F.; Slabbert, R.M.; Sultanbawa, Y.; Sivakumar, D. Utilizing Xanthan Gum Coatings as Probiotic Bacteria Carriers to Enhance Postharvest Quality and Antioxidants in Fresh-Cut Cantaloupe and Honeydew (Cucumis melo L.) Melons. Foods 2024, 13, 940. [Google Scholar] [CrossRef]
- Chen, H.; Wang, B.; Li, J.; Ying, G.; Chen, K. High-strength and super-hydrophobic multilayered paper based on nano-silica coating and micro-fibrillated cellulose. Carbohydr. Polym. 2022, 288, 119371. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Liang, Q.; Kang, L.; Ekumah, J.; Han, X.; Ren, X.; Ma, H. Exploring the potential of pullulan-based films and coatings for effective food preservation: A comprehensive analysis of properties, activation strategies and applications. Int. J. Biol. Macromol. 2024, 260, 129479. [Google Scholar] [CrossRef]
- Hassannia-Kolaee, M.; Khodaiyan, F.; Pourahmad, R.; Shahabi-Ghahfarrokhi, I. Development of ecofriendly bionanocomposite: Whey protein isolate/pullulan films with nano-SiO2. Int. J. Biol. Macromol. 2016, 86, 139–144. [Google Scholar] [CrossRef]
- Zhou, F.; Gu, Z.; Zeng, Z.; Tang, X.; Li, C.; Fang, Z.; Hu, B.; Chen, H.; Wang, C.; Chen, S.; et al. Preparation, characterization and application of Konjac glucomannan/pullulan/microcrystalline cellulose/tea polyphenol active blend film. Food Biosci. 2022, 49, 101898. [Google Scholar] [CrossRef]
- Chen, F.; Chi, C. Development of pullulan/carboxylated cellulose nanocrystal/tea polyphenol bionanocomposite films for active food packaging. Int. J. Biol. Macromol. 2021, 186, 405–413. [Google Scholar] [CrossRef]
- Shinga, M.H.; Kaseke, T.; Pfukwa, T.M.; Fawole, O.A. Optimization of glycerol and cellulose nanofiber concentrations in Opuntia ficus-indica mucilage films functionalized with pomegranate peel extract for postharvest preservation of banana. Food Packag. Shelf Life 2025, 47, 101428. [Google Scholar] [CrossRef]
- Mehraj, S.; Sistla, Y.S. Optimization of process conditions for the development of pectin and glycerol based edible films: Statistical design of experiments. Electron. J. Biotechnol. 2022, 55, 27–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Q.; Wang, Y.; Liu, J.; Zhang, Y. Mechanical and Barrier Properties Optimization of Carboxymethyl Chitosan-Gelatin-Based Edible Film Using Response Surface Methodology. Coatings 2023, 13, 1529. [Google Scholar] [CrossRef]
- Kang, L.; Liang, Q.; Rashid, A.; Qayum, A.; Chi, Z.; Ren, X.; Ma, H. Ultrasound-assisted development and characterization of novel polyphenol-loaded pullulan/trehalose composite films for fruit preservation. Ultrason. Sonochem. 2023, 92, 106242. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.U.; Abidin, M.Z.; Abdullah, M.S.; Razak, A.F.A.; Salleh, M.H.; Basri, S.N.M. Optimisation of Edible Coating to Improve the Postharvest Shelf-life of Guava Using Response Surface Methodology. ASM Sci. J. 2023, 18, 1–10. [Google Scholar] [CrossRef]
- Fathiraja, P.; Gopalrajan, S.; Karunanithi, M.; Obaiah, M.C.; Rajasekaran, B.; Vedhi, C. Process optimization and characterization of composite biopolymer films obtained from fish scale gelatin, agar and chitosan using response surface methodology. Polym. Bull. 2023, 80, 10775–10807. [Google Scholar] [CrossRef]
- Lee, D.; Gwon, J.; Huang, R.; Picha, D.H.; Wu, Q. Bio-based nanomaterial suspensions as sprayable coatings for maintaining blueberry postharvest quality. Food Hydrocoll. 2024, 150, 109743. [Google Scholar] [CrossRef]
- Aquinas, N.; Chithra, C.H.; Bhat, M.R. Progress in bioproduction, characterization and applications of pullulan: A review. Polym. Bull. 2024, 81, 12347–12382. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Bacha, S.A.S.; Liang, Q.; Liu, Y.; Kang, L.; Chi, Z.; Chi, R.; Han, X.; Ekumah, J.; et al. Preparation and functional characterization of pullulan-sodium alginate composite film enhanced with ultrasound-assisted clove essential oil Nanoemulsions for effective preservation of cherries and mushrooms. Food Chem. 2024, 457, 140048. [Google Scholar] [CrossRef]
- Ding, R.; Dai, X.; Zhang, Z.; Bi, Y.; Prusky, D. Composite Coating of Oleaster Gum Containing Cuminal Keeps Postharvest Quality of Cherry Tomatoes by Reducing Respiration and Potentiating Antioxidant System. Foods 2024, 13, 1542. [Google Scholar] [CrossRef]
- Ding, J.; Liu, C.; Huang, P.; Li, H.; Liu, Y.; Sameen, D.E.; Zhang, Y.; Liu, Y.; Qin, W. Effects of konjac glucan-nan/low-acyl gellan edible coatings loaded thymol-β-cyclodextrin microcapsules on postharvest blueberry. Food Chem. 2024, 430, 137080. [Google Scholar] [PubMed]
- Saleem, M.S.; Anjum, M.A.; Naz, S.; Ali, S.; Hussain, S.; Azam, M.; Sardar, H.; Khaliq, G.; Canan, İ.; Ejaz, S. Incorporation of ascorbic acid in chitosan-based edible coating improves postharvest quality and storability of strawberry fruits. Int. J. Biol. Macromol. 2021, 189, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.U.; Kularathnage, N.D.; Du, J.; Liu, R.; Yang, Z.; Zhong, S.; Zhou, J.; Hussain, M.; Shu, X.; Zeng, L. A novel synthesized Vanillin-Based Deep Eutectic Agent (V-DEA) mitigates postharvest fungal decay and improve shelf life and quality of cherry tomatoes. Food Chem. 2024, 453, 139612. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.S.; Ejaz, S.; Anjum, M.A.; Ali, S.; Hussain, S.; Ercisli, S.; Ilhan, G.; Marc, R.A.; Skrovankova, S.; Mlcek, J. Improvement of Postharvest Quality and Bioactive Compounds Content of Persimmon Fruits after Hydrocolloid-Based Edible Coating Application. Horticulturae 2022, 8, 1045. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, P.; Zhu, L. Preparation of modified atmosphere packaging based on the respiratory characteristics of cherry tomato and its freshness preservation application. Sci. Hortic. 2024, 333, 113286. [Google Scholar] [CrossRef]
- Tian, R.; Yuan, S.; Jiang, J.; Kuang, Y.; Wu, K.; Sun, S.; Chen, K.; Jiang, F. Improvement of mechanical, barrier properties, and water resistance of konjac glucomannan/curdlan film by zein addition and the coating for cherry tomato preservation. Int. J. Biol. Macromol. 2024, 276, 134132. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, H.; Liu, Y. Effects of postharvest coating using chitosan combined with natamycin on physicochemical and microbial properties of sweet cherry during cold storage. Int. J. Biol. Macromol. 2022, 214, 1–9. [Google Scholar] [CrossRef]
- Liu, M.; Li, G.; Sun, W.; Li, H.; Fu, J.; Zong, W.; Han, W. Effect of ultrasonic treatment on water-soluble pectin and degrading enzymes in cell wall of persimmon fruit during storage. J. Food Compos. Anal. 2023, 121, 105341. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Chen, C.; Zhang, S.; Zhao, X.; Wu, C.; Kou, X.; Xue, Z. Glycine betaine inhibits postharvest softening and quality decline of winter jujube fruit by regulating energy and antioxidant metabolism. Food Chem. 2023, 410, 135445. [Google Scholar] [CrossRef]
- Wang, D.; Seymour, G.B. Molecular and biochemical basis of softening in tomato. Mol. Hortic. 2022, 2, 5–10. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Lv, Z.; Pan, Z.; Wei, Y.; Shu, C.; Zeng, Q.; Chen, Y.; Zhang, W. Analysis of Nutrients and Volatile Compounds in Cherry Tomatoes Stored at Different Temperatures. Foods 2022, 12, 6. [Google Scholar] [CrossRef]
- Liu, G.; Chen, B.; Liu, H.; Wang, X.; Zhang, Y.; Wang, C.; Liu, C.; Zhong, Y.; Qiao, Y. Effects of Hydroxyethyl Cellulose and Sulfated Rice Bran Polysaccharide Coating on Quality Maintenance of Cherry Tomatoes during Cold Storage. Foods 2023, 12, 3156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, L.; Chen, Y.; Liao, L.; Li, R.; Wang, H.; Mo, Y.; Lin, L.; Liu, K. The combined effect of ascorbic acid and chitosan coating on postharvest quality and cell wall metabolism of papaya fruits. LWT 2022, 171, 114134. [Google Scholar] [CrossRef]
- Gull, S.; Ejaz, S.; Ali, S.; Ali, M.M.; Sardar, H.; Azam, M.; Deng, H.; Yousef, A.F.; Alrefaei, A.F.; Almutairi, M.H. Xanthan gum-based edible coating effectively preserve postharvest quality of ‘Gola’ guava fruits by regulating physiological and biochemical processes. BMC Plant Biol. 2024, 24, 450. [Google Scholar] [CrossRef] [PubMed]
- Tekin, O.; Karatas, M.D.; Cavusoglu, S. Effects of edible coating (guar and tara gam) applications on post-harvest fruit quality and gene expressions in cherry tomatoes. Postharvest Biol. Technol. 2025, 224, 113475. [Google Scholar] [CrossRef]
- Bao, S.; Yin, D.; Zhao, Q.; Zhou, Y.; Hu, Y.; Sun, X.; Liu, X.; Ma, T. Comprehensive evaluation of the effect of five sterilization methods on the quality of black carrot juice based on PCA, TOPSIS and GRA models. Food Chem. X 2023, 17, 100604. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Z.; Hou, X.; Aisa, H.A. A new strategy based on PCA for inter-batches quality consistency evaluation. J. Pharm. Biomed. Anal. 2022, 217, 114838. [Google Scholar] [CrossRef]
- Fu, X.Q.; Sun, J.Y.; Lyu, C.M.; Meng, X.J.; Guo, H.; Yang, D. Evaluation on simulative transportation and storage quality of sweet cherry by different varieties based on principal component analysis. Food Sci. Technol. 2022, 42, e30420. [Google Scholar] [CrossRef]
| Factors | LT (%) | WVP (g ∗ mm/(m2 ∗ h ∗ Kpa)) | TS (Mpa) | EB (%) | K |
|---|---|---|---|---|---|
| Nano-SiO2 (g) | |||||
| 0.02 | 93.43 ± 2.35 a | 0.62 ± 0.12 a | 1.25 ± 0.03 c | 29.33 ± 3.12 c | 0.16 ± 0.05 c |
| 0.04 | 90.09 ± 1.78 a | 0.46 ± 0.07 b | 2.13 ± 0.12 b | 31.67 ± 4.01 c | 0.47 ± 0.03 b |
| 0.06 | 87.56 ± 2.36 b | 0.38 ± 0.06 b | 3.03 ± 0.32 a | 44.67 ± 2.35 b | 0.64 ± 0.03 a |
| 0.08 | 81.72 ± 3.52 c | 0.28 ± 0.05 c | 2.73 ± 0.25 a | 47.67 ± 3.81 b | 0.60 ± 0.05 a |
| 0.10 | 83.52 ± 1.26 c | 0.31 ± 0.08 c | 2.07 ± 0.16 b | 59.67 ± 2.36 a | 0.61 ± 0.06 a |
| PUL (g) | |||||
| 1.50 | 82.97 ± 1.44 b | 0.28 ± 0.05 b | 2.64 ± 0.12 b | 70.67 ± 2.36 a | 0.67 ± 0.05 ab |
| 1.75 | 85.70 ± 0.14 ab | 0.17 ± 0.06 c | 3.3 ± 0.25 a | 57 ± 3.52 ab | 0.72 ± 0.04 a |
| 2.00 | 86.07 ± 2.92 ab | 0.22 ± 0.07 c | 2.75 ± 0.08 b | 47.67 ± 4.13 b | 0.61 ± 0.01 b |
| 2.25 | 89.33 ± 1.33 a | 0.40 ± 0.02 c | 2.58 ± 0.05 b | 39.73 ± 3.25 bc | 0.46 ± 0.07 c |
| 2.50 | 87.50 ± 2.56 a | 0.55 ± 0.07 a | 1.98 ± 0.23 c | 24 ± 4.69 c | 0.22 ± 0.03 d |
| TP (g) | |||||
| 0.00 | 90.14 ± 1.56 a | 0.64 ± 0.07 a | 1.76 ± 0.25 b | 22.33 ± 2.13 d | 0.13 ± 0.03 b |
| 0.05 | 87.60 ± 2.36 ab | 0.27 ± 0.01 c | 2.61 ± 0.19 a | 42.01 ± 0.86 b | 0.55 ± 0.06 a |
| 0.10 | 89.03 ± 1.56 ab | 0.22 ± 0.04 c | 2.75 ± 0.08 a | 55.33 ± 3.25 a | 0.63 ± 0.05 a |
| 0.15 | 86.93 ± 0.86 ab | 0.18 ± 0.08 c | 1.33 ± 0.13 c | 33.67 ± 2.32 c | 0.50 ± 0.03 a |
| 0.20 | 86.51 ± 1.23 b | 0.51 ± 0.04 b | 1.16 ± 0.05 d | 26.67 ± 3.26 d | 0.21 ± 0.07 b |
| Glycerol (g) | |||||
| 0.40 | 81.28 ± 2.56 d | 0.41 ± 0.06 b | 7.46 ± 1.23 a | 28.67 ± 2.18 c | 0.58 ± 0.03 b |
| 0.60 | 87.77 ± 1.32 b | 0.29 ± 0.04 bc | 4.42 ± 0.56 b | 35.67 ± 3.25 c | 0.57 ± 0.03 b |
| 0.80 | 92.10 ± 0.69 a | 0.25 ± 0.01 c | 2.72 ± 0.68 c | 52.33 ± 6.23 b | 0.64 ± 0.01 a |
| 1.00 | 90.02 ± 1.96 ab | 0.27 ± 0.06 bc | 1.27 ± 0.32 d | 57.33 ± 7.2 b | 0.57 ± 0.06 b |
| 1.20 | 87.40 ± 1.35 c | 0.75 ± 0.08 a | 0.77 ± 0.67 d | 74.33 ± 4.39 a | 0.30 ± 0.06 c |
| Run | Nano-SiO2 (%, m/v) | PUL (%, m/v) | Glycerol (%, m/v) | K |
|---|---|---|---|---|
| 1 | −1 (0.04) | −1 (1.50) | 0 (0.80) | 0.39 ± 0.02 |
| 2 | 1 (0.08) | −1 (1.50) | 0 (0.80) | 0.48 ± 0.03 |
| 3 | −1 (0.04) | 1 (2.00) | 0 (0.80) | 0.6 ± 0.03 |
| 4 | 1 (0.08) | 1 (2.00) | 0 (0.80) | 0.58 ± 0.05 |
| 5 | −1 (0.04) | 0 (1.75) | −1 (0.60) | 0.6 ± 0.03 |
| 6 | 1 (0.08) | 0 (1.75) | −1 (0.60) | 0.53 ± 0.05 |
| 7 | −1 (0.04) | 0 (1.75) | 1 (1.00) | 0.31 ± 0.01 |
| 8 | 1 (0.08) | 0 (1.75) | 1 (1.00) | 0.49 ± 0.02 |
| 9 | 0 (0.06) | −1 (1.50) | −1 (0.60) | 0.54 ± 0.03 |
| 10 | 0 (0.06) | 1 (2.00) | −1 (0.60) | 0.54 ± 0.04 |
| 11 | 0 (0.06) | −1 (1.50) | 1 (1.00) | 0.19 ± 0.03 |
| 12 | 0 (0.06) | 1 (2.00) | 1 (1.00) | 0.61 ± 0.02 |
| 13 | 0 (0.06) | 0 (1.75) | 0 (0.80) | 0.79 ± 0.03 |
| 14 | 0 (0.06) | 0 (1.75) | 0 (0.80) | 0.75 ± 0.05 |
| 15 | 0 (0.06) | 0 (1.75) | 0 (0.80) | 0.76 ± 0.04 |
| 16 | 0 (0.06) | 0 (1.75) | 0 (0.8) | 0.75 ± 0.03 |
| 17 | 0 (0.06) | 0 (1.75) | 0 (0.8) | 0.74 ± 0.02 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 0.4012 | 9 | 0.0446 | 74.55 | <0.0001 | ** |
| A-Nano SiO2 | 0.0045 | 1 | 0.0045 | 7.49 | 0.0291 | * |
| B-PUL | 0.0551 | 1 | 0.0551 | 92.07 | <0.0001 | ** |
| C-Glycerol | 0.0358 | 1 | 0.0358 | 59.87 | 0.0001 | ** |
| AC | 0.0162 | 1 | 0.0162 | 27.16 | 0.0012 | ** |
| BC | 0.0307 | 1 | 0.0307 | 51.30 | 0.0002 | ** |
| A2 | 0.0668 | 1 | 0.0668 | 111.68 | <0.0001 | ** |
| B2 | 0.0637 | 1 | 0.0637 | 106.60 | <0.0001 | ** |
| C2 | 0.0983 | 1 | 0.0983 | 164.43 | <0.0001 | ** |
| Residual | 0.0042 | 7 | 0.0006 | |||
| Lack of Fit | 0.0024 | 3 | 0.0008 | 1.80 | 0.2865 | |
| Pure Error | 0.0018 | 4 | 0.0004 | |||
| Cor Total | 0.4054 | 16 |
| Indices | Treatments | Storage Times (Days) | |||||
|---|---|---|---|---|---|---|---|
| 0 d | 3 d | 6 d | 9 d | 12 d | 15 d | ||
| Firmness | CK | 11.85 ± 0.29 a | 9.69 ± 0.36 b | 5.32 ± 0.14 c | 2.73 ± 0.41 c | 1.52 ± 0.07 d | 0.54 ± 0.10 d |
| Nano-SiO2/TP/PUL | 11.85 ± 0.43 a | 10.43 ± 0.25 a | 8.39 ± 0.32 a | 6.20 ± 0.22 a | 4.47 ± 0.43 a | 2.40 ± 0.15 a | |
| Nano-SiO2/PUL | 11.85 ± 0.19 a | 8.85 ± 0.379 c | 6.78 ± 0.34 b | 4.91 ± 0.37 b | 2.34 ± 0.57 c | 1.39 ± 0.22 bc | |
| TP/PUL | 11.85 ± 0.04 a | 9.62 ± 0.077 b | 7.50 ± 0.41 ab | 5.83 ± 0.24 a | 3.20 ± 0.18 b | 1.72 ± 0.21 b | |
| PUL | 11.85 ± 0.42 a | 8.29 ± 0.34 d | 7.32 ± 0.42 ab | 4.89 ± 0.31 b | 3.05 ± 0.15 b | 1.18 ± 0.25 c | |
| TSS | CK | 6.66 ± 0.21 a | 7.17 ± 0.10 d | 8.83 ± 0.07 a | 10.53 ± 0.15 a | 6.34 ± 0.11 e | 5.27 ± 0.06 d |
| Nano-SiO2/TP/PUL | 6.65 ± 0.22 a | 7.73 ± 0.10 a | 8.69 ± 0.12 b | 9.35 ± 0.11 c | 8.32 ± 0.09 a | 7.83 ± 0.04 a | |
| Nano-SiO2/PUL | 6.73 ± 0.16 a | 7.50 ± 0.06 b | 8.21 ± 0.03 d | 9.62 ± 0.16 b | 7.38 ± 0.05 b | 6.27 ± 0.06 b | |
| TP/PUL | 6.65 ± 0.05 a | 7.35 ± 0.02 c | 8.22 ± 0.05 d | 9.02 ± 0.06 d | 6.91 ± 0.05 d | 5.75 ± 0.08 c | |
| PUL | 6.66 ± 0.10 a | 7.29 ± 0.07 c | 8.48 ± 0.05 c | 9.61 ± 0.07 b | 7.14 ± 0.11 c | 5.65 ± 0.10 c | |
| TA | CK | 0.38 ± 0.01 a | 0.37 ± 0.01 a | 0.33 ± 0.06 c | 0.32 ± 0.00 d | 0.31 ± 0.01 c | 0.31 ± 0.00 c |
| Nano-SiO2/TP/PUL | 0.38 ± 0.01 a | 0.37 ± 0.01 a | 0.36 ± 0.00 a | 0.36 ± 0.01 a | 0.35 ± 0.01 a | 0.34 ± 0.00 a | |
| Nano-SiO2/PUL | 0.38 ± 0.01 a | 0.37 ± 0.01 a | 0.34 ± 0.05 bc | 0.33 ± 0.01 c | 0.33 ± 0.01 b | 0.32 ± 0.00 b | |
| TP/PUL | 0.38 ± 0.01 a | 0.37 ± 0.01 a | 0.35 ± 0.05 ab | 0.35 ± 0.01 ab | 0.33 ± 0.01 b | 0.33 ± 0.01 b | |
| PUL | 0.38 ± 0.01 a | 0.37 ± 0.00 a | 0.36 ± 0.05 a | 0.35 ± 0.01 ab | 0.33 ± 0.00 b | 0.33 ± 0.00 b | |
| VC | CK | 39.57 ± 0.09 b | 34.66 ± 0.11 e | 31.34 ± 0.20 d | 28.54 ± 0.11 d | 20.64 ± 0.20 e | 16.57 ± 0.22 e |
| Nano-SiO2/TP/PUL | 39.63 ± 0.17 ab | 38.46 ± 0.11 a | 35.77 ± 0.18 a | 33.68 ± 0.21 a | 27.71 ± 0.17 a | 24.79 ± 0.10 a | |
| Nano-SiO2/PUL | 39.85 ± 0.19 a | 37.52 ± 0.10 c | 34.73 ± 0.21 b | 31.64 ± 0.12 c | 25.45 ± 0.22 c | 21.53 ± 0.16 d | |
| TP/PUL | 39.76 ± 0.11 ab | 37.82 ± 0.14 b | 34.25 ± 0.23 c | 32.23 ± 0.09 b | 26.34 ± 0.17 b | 23.74 ± 0.16 b | |
| PUL | 39.62 ± 0.06 b | 35.50 ± 0.14 d | 34.11 ± 0.05 c | 31.65 ± 0.21 c | 24.77 ± 0.12 d | 22.60 ± 0.14 c | |
| RR | CK | 22.00 ± 0.33 a | 26.82 ± 0.45 a | 33.89 ± 0.34 c | 45.66 ± 0.34 a | 30.03 ± 0.58 a | 21.96 ± 0.66 a |
| Nano-SiO2/TP/PUL | 22.06 ± 0.34 a | 23.13 ± 0.36 b | 28.18 ± 0.37 b | 35.88 ± 0.11 e | 16.33 ± 0.42 e | 13.45 ± 0.46 d | |
| Nano-SiO2/PUL | 22.00 ± 0.23 a | 20.89 ± 0.23 cd | 28.86 ± 0.65 ab | 42.77 ± 00.20 b | 23.75 ± 0.13 c | 18.38 ± 0.15 b | |
| TP/PUL | 22.06 ± 0.44 a | 20.47 ± 0.49 d | 29.46 ± 0.41 a | 38.23 ± 0.31 d | 22.51 ± 0.49 d | 15.76 ± 0.20 c | |
| PUL | 22.07 ± 0.37 a | 21.36 ± 0.26 c | 29.11 ± 0.29 a | 40.73 ± 0.17 c | 25.76 ± 0.36 b | 16.06 ± 0.16 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.; Ding, J.; Han, Y.; Gong, L.; Wu, F.; Liu, Y.; Zhao, P.; Yang, Z.; Ye, L.; Zhou, S.; et al. Optimization of Nano-SiO2/Tea Polyphenol/Pullulan Edible Composite Films for Postharvest Preservation of Cherry Tomatoes. Foods 2025, 14, 3386. https://doi.org/10.3390/foods14193386
Huang P, Ding J, Han Y, Gong L, Wu F, Liu Y, Zhao P, Yang Z, Ye L, Zhou S, et al. Optimization of Nano-SiO2/Tea Polyphenol/Pullulan Edible Composite Films for Postharvest Preservation of Cherry Tomatoes. Foods. 2025; 14(19):3386. https://doi.org/10.3390/foods14193386
Chicago/Turabian StyleHuang, Peng, Jie Ding, Yu Han, Ling Gong, Fang Wu, Yaowen Liu, Pinyao Zhao, Zuying Yang, Lin Ye, Shanshan Zhou, and et al. 2025. "Optimization of Nano-SiO2/Tea Polyphenol/Pullulan Edible Composite Films for Postharvest Preservation of Cherry Tomatoes" Foods 14, no. 19: 3386. https://doi.org/10.3390/foods14193386
APA StyleHuang, P., Ding, J., Han, Y., Gong, L., Wu, F., Liu, Y., Zhao, P., Yang, Z., Ye, L., Zhou, S., & Qin, W. (2025). Optimization of Nano-SiO2/Tea Polyphenol/Pullulan Edible Composite Films for Postharvest Preservation of Cherry Tomatoes. Foods, 14(19), 3386. https://doi.org/10.3390/foods14193386






