Impact of Different Microbial Biostimulants and Salt Stress on the Endophytome of the Edible Part of Lettuce and Tomato Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Biological Material
2.2. Inoculation Treatments
2.3. Experimental Design and Growth Conditions
2.3.1. Lettuce Experiment
2.3.2. Tomato Experiment
2.4. Bacterial Colonization of the Rhizosphere and Symbiotic Development
2.5. Microbial Metagenomic DNA Extraction and Quantification
2.6. High Throughput Sequencing of 16S rRNA and ITS2 Genes and Bioinformatics Analysis
3. Results
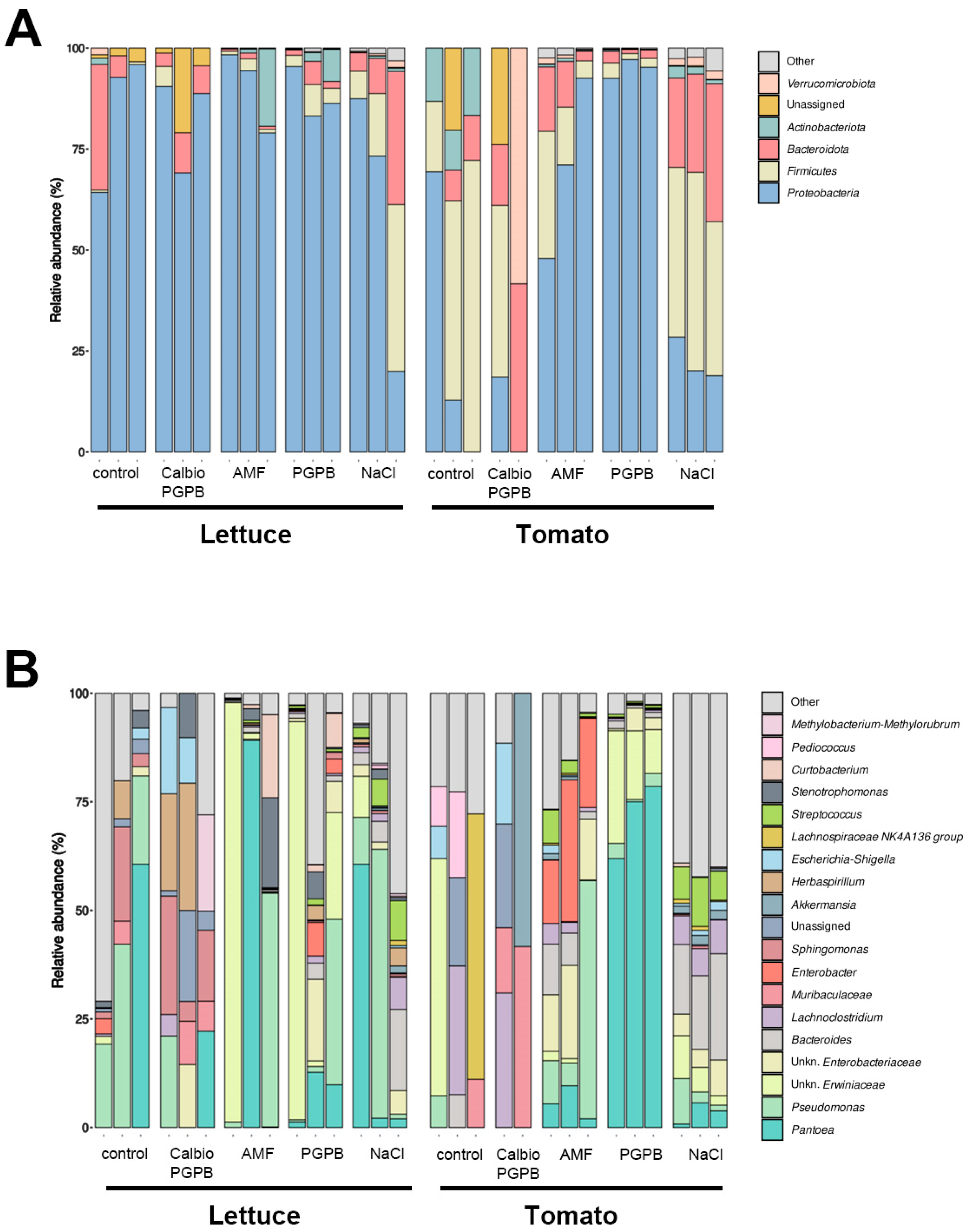
3.1. Bacterial Communities
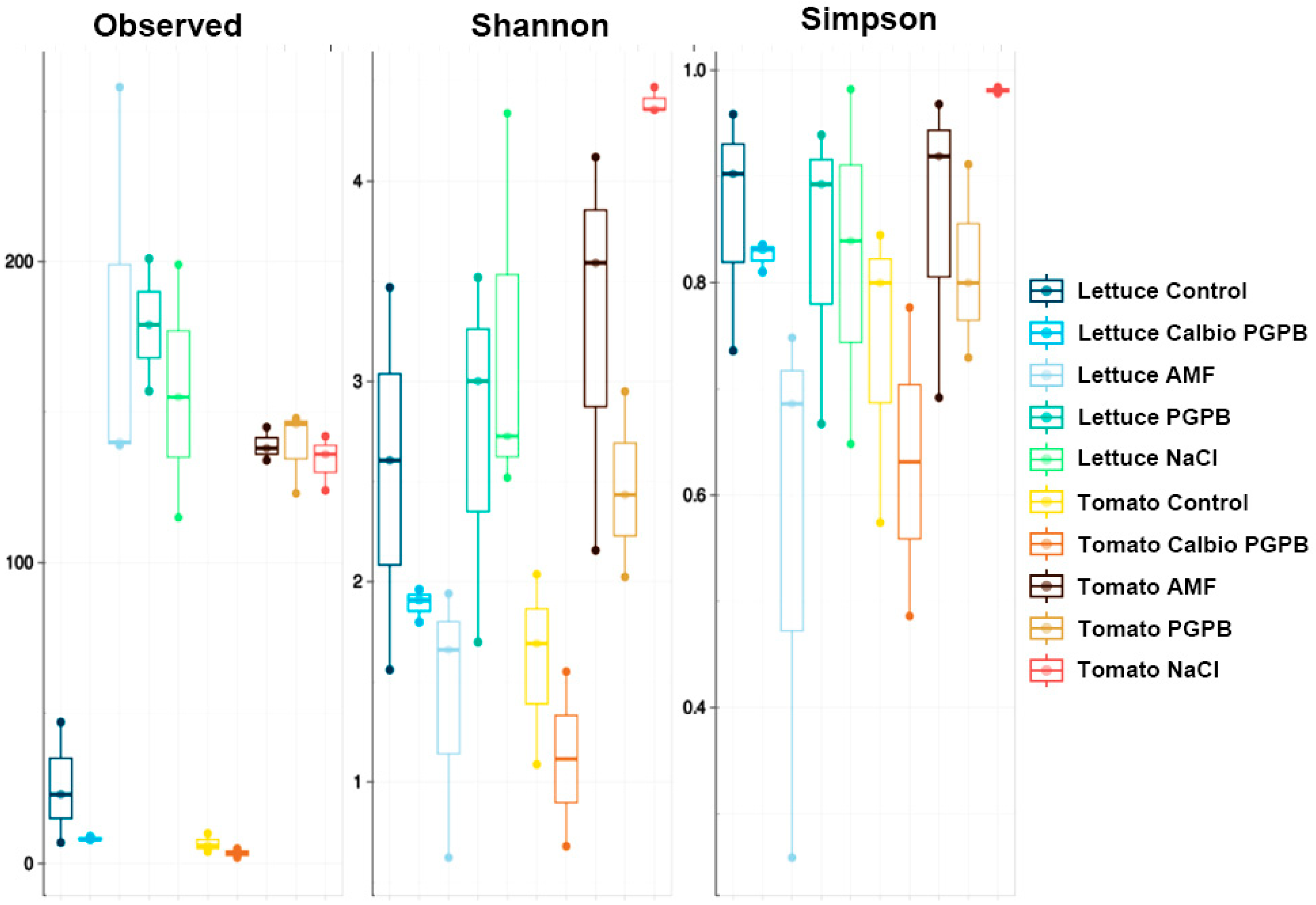
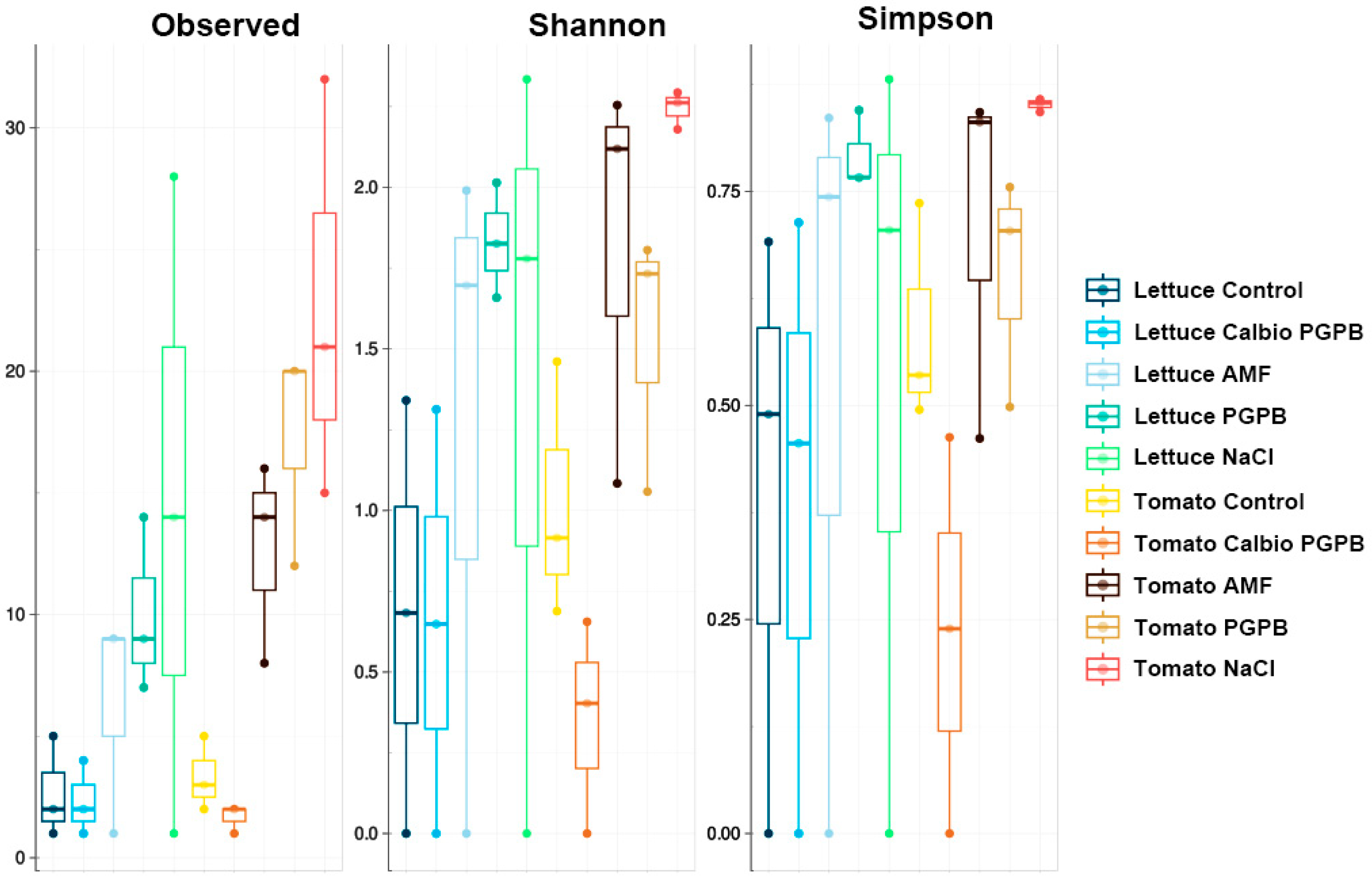
3.2. Alpha-Diversity
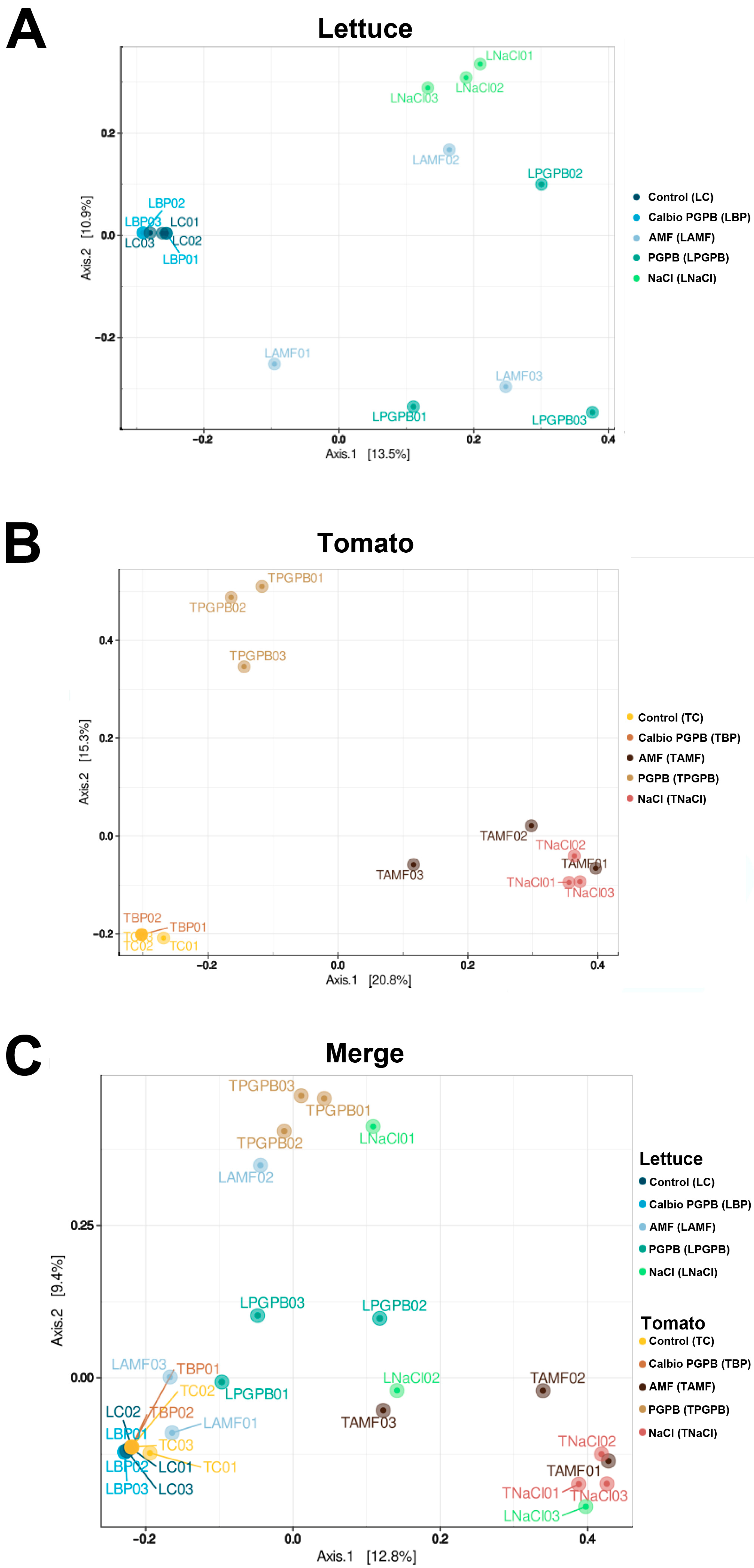
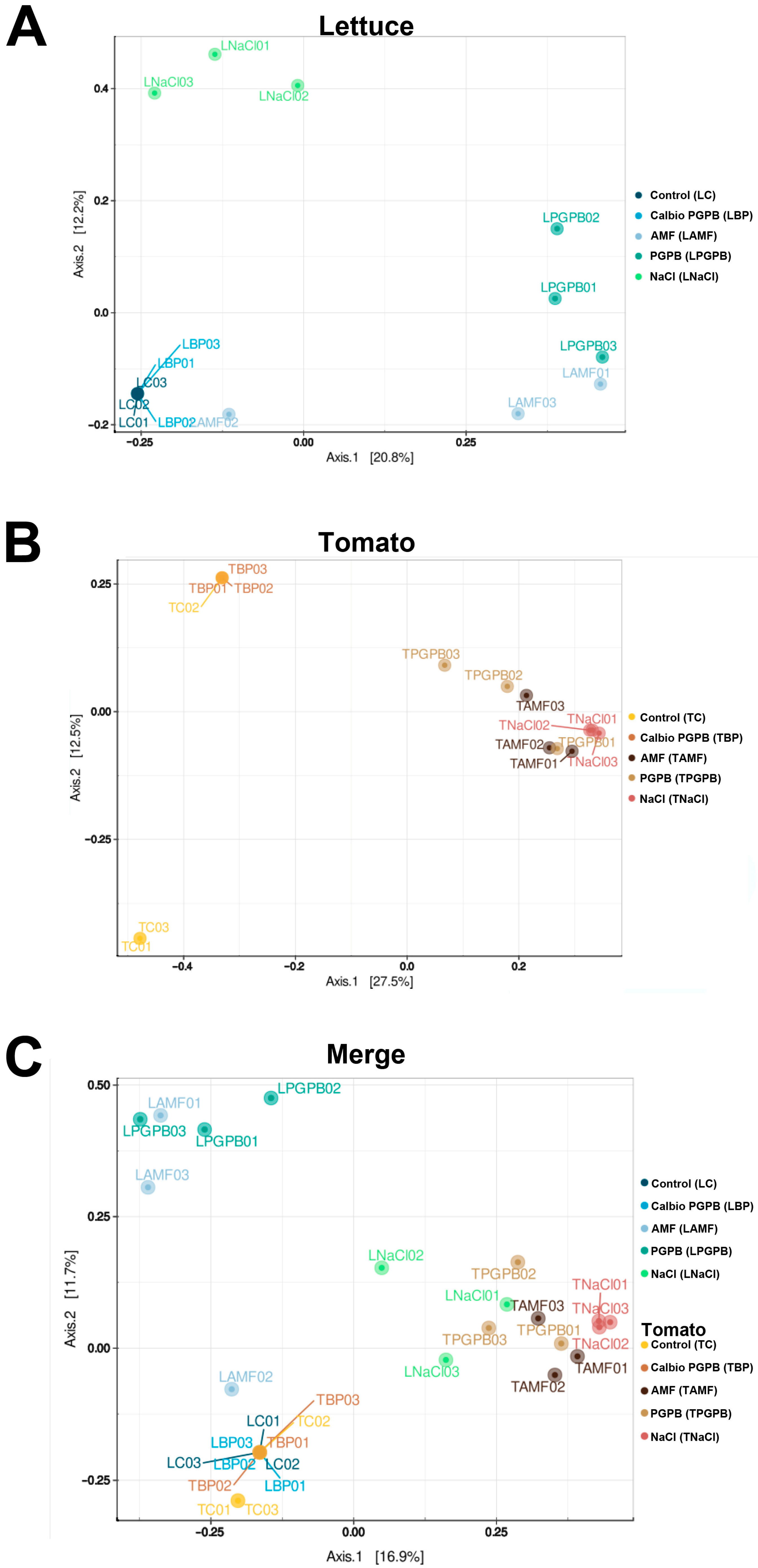
3.3. Beta-Diversity
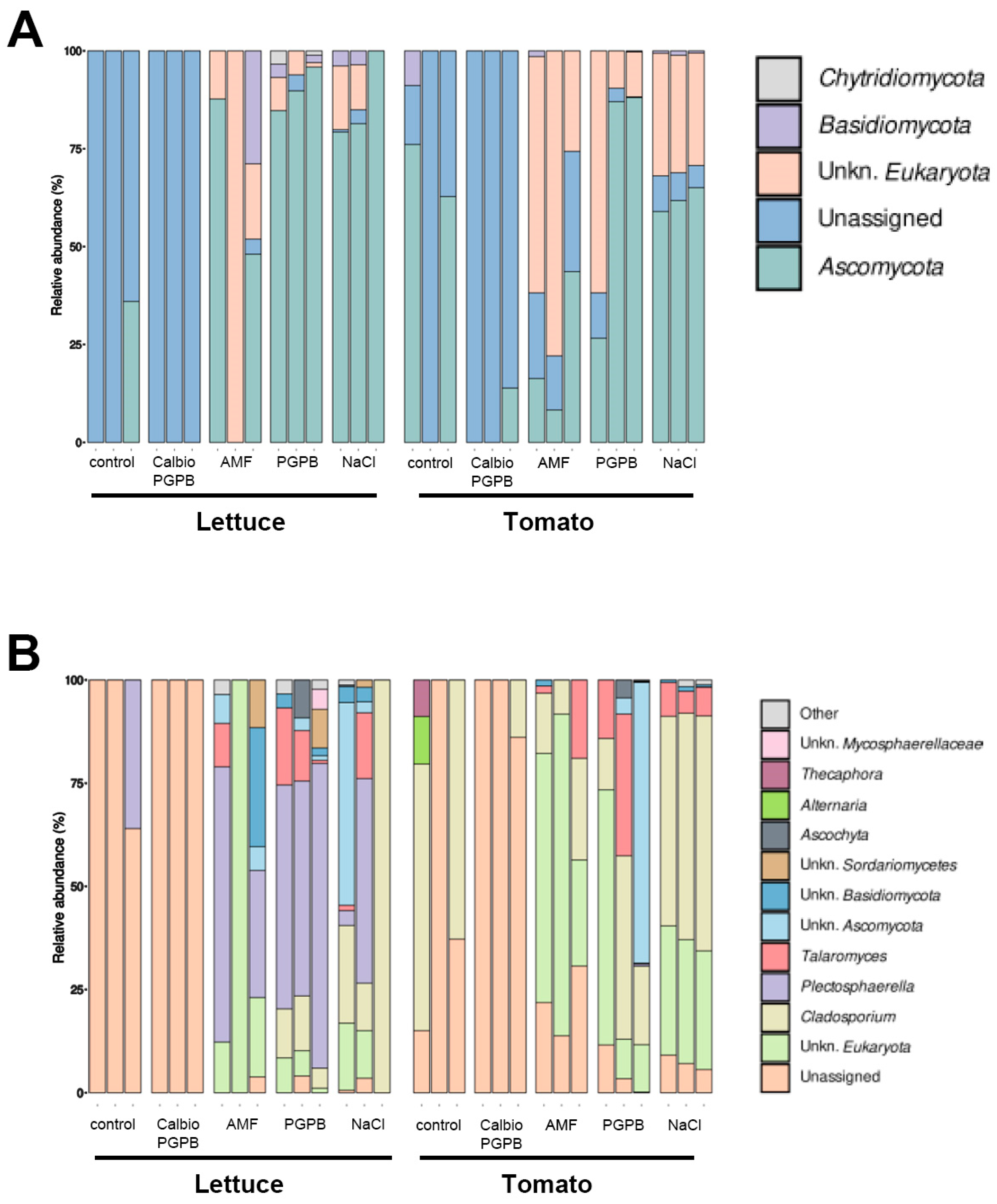
3.4. Fungal Communities
3.5. Alpha-Diversity
3.6. Beta-Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Johnson, A.J.; Vangay, P.; Al-Ghalith, G.A.; Hillmann, B.M.; Ward, T.L.; Shields-Cutler, R.R.; Kim, A.D.; Shmagel, A.K.; Syed, A.N.; Walter, J.; et al. Daily Sampling Reveals Personalized Diet-Microbiome Associations in Humans. Cell Host Microbe 2019, 25, 789–802.e5. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fibre–microbiota interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef] [PubMed]
- Soto-Giron, M.J.; Kim, J.N.; Schott, E.; Tahmin, C.; Ishoey, T.; Mincer, T.J.; DeWalt, J.; Toledo, G. The Edible Plant Microbiome represents a diverse genetic reservoir with functional potential in the human host. Sci. Rep. 2021, 11, 24017. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros Azevedo, T.; Aburjaile, F.F.; Ferreira-Neto, J.R.C.; Pandolfi, V.; Benko-Iseppon, A.M. The endophytome (plant-associated microbiome): Methodological approaches, biological aspects, and biotech applications. World J. Microbiol. Biotechnol. 2021, 37, 206. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Organic food and the impact on human health. Crit. Rev. Food Sci. Nutr. 2019, 59, 704–714. [Google Scholar] [CrossRef]
- Taïbi, K.; del Campo, A.D.; Aguado, A.; Mulet, J.M. The effect of genotype by environment interaction, phenotypic plasticity and adaptation on Pinus halepensis reforestation establishment under expected climate drifts. Ecol. Eng. 2015, 84, 218–228. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Yuan, Z. Plant Responses and Adaptations to Salt Stress: A Review. Horticulturae 2024, 10, 1221. [Google Scholar] [CrossRef]
- Locascio, A.; Andrés-Colás, N.; Mulet, J.M.; Yenush, L. Saccharomyces cerevisiae as a tool to investigate plant potassium and sodium transporters. Int. J. Mol. Sci. 2019, 20, 2133. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Azcon, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef]
- Saporta, R.; Bou, C.; Frías, V.; Mulet, J.M. A method for a fast evaluation of the biostimulant potential of different natural extracts for promoting growth or tolerance against abiotic stress. Agronomy 2019, 9, 143. [Google Scholar] [CrossRef]
- Benito, P.; Ligorio, D.; Bellón, J.; Yenush, L.; Mulet, J.M. A fast method to evaluate in a combinatorial manner the synergistic effect of different biostimulants for promoting growth or tolerance against abiotic stress. Plant Methods 2022, 18, 111. [Google Scholar] [CrossRef]
- Benito, P.; Celdrán, M.; Bellón, J.; Arbona, V.; González-Guzmán, M.; Porcel, R.; Yenush, L.; Mulet, J.M. The combination of a microbial and a non-microbial biostimulant increases yield in lettuce (Lactuca sativa) under salt stress conditions by up-regulating cytokinin biosynthesis. J. Integr. Plant Biol. 2024, 66, 2140–2157. [Google Scholar] [CrossRef]
- Benito, P.; Trigueros, S.; Celdrán, M.; Sánchez, V.; Coronado, A.; Bellón, J.; Arbona, V.; González-Guzmán, M.; Porcel, R.; Yenush, L.; et al. The combined effect of a newly designed biostimulant and a plant growth-promoting bacterium increases tomato yield under salt stress by increasing the cytokinin isopentenyladenine riboside content. Chem. Biol. Technol. Agric. 2025, 12, 117. [Google Scholar] [CrossRef]
- Mulet, J.M.; Alejandro, S.; Romero, C.; Serrano, R. The trehalose pathway and intracellular glucose phosphates as modulators of potassium transport and general cation homeostasis in yeast. Yeast 2004, 21, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA Sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Gagne-Bourgue, F.; Aliferis, K.A.; Seguin, P.; Rani, M.; Samson, R.; Jabaji, S. Isolation and characterization of indigenous endophytic bacteria associated with leaves of switchgrass (Panicum virgatum L.) cultivars. J. Appl. Microbiol. 2013, 114, 836–853. [Google Scholar] [CrossRef]
- Safari, R.; Jacques, J.R.; Brostaux, Y.; Willems, L. Ablation of non-coding RNAs affects bovine leukemia virus B lymphocyte proliferation and abrogates oncogénesis. PLoS Pathog. 2020, 16, e1008502. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial. 2015. Available online: https://john-quensen.com/wp-content/uploads/2018/10/Oksanen-Jari-vegantutor.pdf (accessed on 24 September 2025).
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Sun, Y.; Snow, D.; Walia, H.; Li, X. Transmission Routes of the Microbiome and Resistome from Manure to Soil and Lettuce. Environ. Sci. Technol. 2021, 55, 11102–11112. [Google Scholar] [CrossRef]
- Dong, C.J.; Wang, L.L.; Li, Q.; Shang, Q.M. Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE 2019, 14, e0223847. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Shamim, A.; Sanka Loganathachetti, D.; Chandran, S.; Masmoudi, K.; Mundra, S. Salinity of irrigation water selects distinct bacterial communities associated with date palm (Phoenix dactylifera L.) root. Sci. Rep. 2022, 12, 12733. [Google Scholar] [CrossRef]
- Enya, J.; Koitabashi, M.; Shinohara, H.; Yoshida, S.; Tsukiboshi, T.; Negishi, H.; Suyama, K.; Tsushima, S. Phylogenetic diversities of dominant culturable Bacillus, Pseudomonas and Pantoea species on tomato leaves and their possibility as biological control agents. J. Phytopathol. 2007, 155, 446–453. [Google Scholar] [CrossRef]
- Cassol, I.; Ibañez, M.; Bustamante, J.P. Key features and guidelines for the application of microbial alpha diversity metrics. Sci. Rep. 2025, 15, 622. [Google Scholar] [CrossRef]
- Huang, J.; Mi, J.; Yan, Q.; Wen, X.; Zhou, S.; Wang, Y.; Ma, B.; Zou, Y.; Liao, X.; Wu, Y. Animal manures application increases the abundances of antibiotic resistance genes in soil-lettuce system associated with shared bacterial distributions. Sci. Total Environ. 2021, 787, 147667. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.T.; Wang, W.H.; Tsui, C.K.; Cai, L. Changes in bacterial and fungal microbiomes associated with tomatoes of healthy and infected by Fusarium oxysporum f. sp. lycopersici. Microb. Ecol. 2021, 81, 1004–1017. [Google Scholar] [CrossRef]
- Vlasselaer, L.; Crauwels, S.; Lievens, B.; De Coninck, B. Unveiling the microbiome of hydroponically cultivated lettuce: Impact of Phytophthora cryptogea infection on plant-associated microorganisms. FEMS Microbiol. Ecol. 2024, 100, 10. [Google Scholar] [CrossRef]
- Sherwin, W.B. Bray-Curtis (AFD) differentiation in molecular ecology: Forecasting, an adjustment (AA), and comparative performance in selection detection. Ecol. Evol. 2022, 12, e9176. [Google Scholar] [CrossRef]
- Yang, X.Q.; Ma, S.Y.; Peng, Z.X.; Wang, Z.Q.; Qiao, M.; Yu, Z. Diversity of Plectosphaerella within aquatic plants from southwest China, with P. endophytica and P. sichuanensis spp. nov. MycoKeys 2021, 80, 57–75. [Google Scholar] [CrossRef]
- Kharkwal, A.C.; Joshi, H.; Shandilya, C.; Dabral, S.; Kumar, N.; Varma, A. Isolation and characterization of a newly discovered plant growth-promoting endophytic fungal strain from the genus Talaromyces. Sci. Rep. 2024, 14, 6022. [Google Scholar] [CrossRef]
- Zajc, J.; Zalar, P.; Plemenitaš, A.; Gunde-Cimerman, N. The mycobiota of the salterns. Prog. Mol. Subcell. Biol. 2012, 53, 133–158. [Google Scholar] [CrossRef] [PubMed]
- Roca-Couso, R.; Flores-Félix, J.D.; Deb, S.; Giagnoni, L.; Tondello, A.; Stevanato, P.; Squartini, A.; García-Fraile, P.; Rivas, R. Metataxonomic analysis of endophytic bacteria of blackberry (Rubus ulmifolius Schott) across tissues and environmental conditions. Sci. Rep. 2024, 14, 13388. [Google Scholar] [CrossRef] [PubMed]
- Lengrand, S.; Dubois, B.; Pesenti, L.; Debode, F.; Legrève, A. Humic substances increase tomato tolerance to osmotic stress while modulating vertically transmitted endophytic bacterial communities. Front. Plant Sci. 2024, 15, 1488671. [Google Scholar] [CrossRef] [PubMed]
- López, S.M.Y.; Pastorino, G.N.; Fernández-González, A.J.; Franco, M.E.E.; Fernández-López, M.; Balatti, P.A. The endosphere bacteriome of diseased and healthy tomato plants. Arch. Microbiol. 2020, 202, 2629–2642. [Google Scholar] [CrossRef]
- Mei, C.; Zhou, D.; Chretien, R.L.; Turner, A.; Hou, G.; Evans, M.R.; Lowman, S. A potential application of pseudomonas psychrotolerans ialr632 for lettuce growth promotion in hydroponics. Microorganisms 2023, 11, 376. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, P.; Guo, D.J.; Sharma, A.; Li, D.P.; Li, X.; Verma, K.K.; Malviya, M.K.; Song, X.P.; Lakshmanan, P.; et al. Root-derived endophytic diazotrophic bacteria Pantoea cypripedii af1 and Kosakonia arachidis ef1 promote nitrogen assimilation and growth in sugarcane. Front. Microbiol. 2021, 12, 774707. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; Rocha-Granados, M.d.C.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Mazoyon, C.; Catterou, M.; Alahmad, A.; Mongelard, G.; Guénin, S.; Sarazin, V.; Dubois, F.; Duclercq, J. Sphingomonas sediminicola Dae20 Is a highly promising beneficial bacteria for crop biostimulation due to its positive effects on plant growth and development. Microorganisms 2023, 11, 2061. [Google Scholar] [CrossRef] [PubMed]
- Agnolucci, M.; Avio, L.; Pepe, A.; Turrini, A.; Cristani, C.; Bonini, P.; Cirino, V.; Colosimo, F.; Ruzzi, M.; Giovannetti, M. Bacteria associated with a commercial mycorrhizal inoculum: Community composition and multifunctional activity as assessed by illumina sequencing and culture-dependent tools. Front. Plant Sci. 2019, 9, 427348. [Google Scholar] [CrossRef]
- Lu, L.; Chang, M.; Han, X.; Wang, Q.; Wang, J.; Yang, H.; Guan, Q.; Dai, S. Beneficial effects of endophytic Pantoea ananatis with ability to promote rice growth under saline stress. J. Appl. Microbiol. 2021, 131, 1919–1931. [Google Scholar] [CrossRef]
- Suman, A.; Shukla, L.; Marag, P.S.; Verma, P.; Gond, S.; Sai Prasad, J. Potential use of plant colonizing Pantoea as generic plant growth promoting bacteria for cereal crops. J. Environ. Biol. 2020, 41, 987–994. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Baum, C.; Leinweber, P. Density, metabolic activity, and identity of cultivable rhizosphere bacteria on Salix viminalis in disturbed arable and landfill soils. J. Plant Nutr. Soil Sci. 2010, 173, 747–756. [Google Scholar] [CrossRef]
- Răut, I.; Călin, M.; Capră, L.; Gurban, A.M.; Doni, M.; Radu, N.; Jecu, L. Cladosporium sp. Isolate as fungal plant growth promoting agent. Agronomy 2021, 11, 392. [Google Scholar] [CrossRef]
- Haelewaters, D.; Urbina, H.; Brown, S.; Newerth-Henson, S.; Catherine Aime, M. Isolation and molecular characterization of the romaine lettuce phylloplane mycobiome. J. Fungi 2021, 7, 277. [Google Scholar] [CrossRef]
- García-Gamboa, R.; Díaz-Torres, O.; Senés-Guerrero, C.; Gradilla-Hernández, M.S.; Moya, A.; Pérez-Brocal, V.; Garcia-Gonzalez, A.; González-Avila, M. Associations between bacterial and fungal communities in the human gut microbiota and their implications for nutritional status and body weight. Sci. Rep. 2024, 14, 5703. [Google Scholar] [CrossRef]
- Jin, L.; Jin, N.; Wang, S.; Huang, S.; Yang, X.; Xu, Z.; Jiang, S.; Lyu, J.; Yu, J. Moderate salt stress aids in the enhancement of nutritional and flavor quality in tomato (Solanum lycopersicum L.) fruits. Food Chem. X 2025, 26, 102330. [Google Scholar] [CrossRef] [PubMed]
- Turhan, A.; Kuscu, H.; Ozmen, N.; Serbeci, M.S.; Demir, A.O. Effect of different concentrations of diluted seawater on yield and quality of lettuce. Chil. J. Agric. Res. 2014, 74, 11–116. [Google Scholar] [CrossRef]
- Han, Y.J.; Kim, S.S.; Shin, H.; Kim, H.W.; Park, J.D. Protective effect of gut microbiota restored by fecal microbiota transplantation in a sepsis model in juvenile mice. Front. Immunol. 2024, 15, 1451356. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulet, J.M.; Benito, P.; Celdrán, M.; Yenush, L.; Porcel, R. Impact of Different Microbial Biostimulants and Salt Stress on the Endophytome of the Edible Part of Lettuce and Tomato Plants. Foods 2025, 14, 3366. https://doi.org/10.3390/foods14193366
Mulet JM, Benito P, Celdrán M, Yenush L, Porcel R. Impact of Different Microbial Biostimulants and Salt Stress on the Endophytome of the Edible Part of Lettuce and Tomato Plants. Foods. 2025; 14(19):3366. https://doi.org/10.3390/foods14193366
Chicago/Turabian StyleMulet, José M., Patricia Benito, Marina Celdrán, Lynne Yenush, and Rosa Porcel. 2025. "Impact of Different Microbial Biostimulants and Salt Stress on the Endophytome of the Edible Part of Lettuce and Tomato Plants" Foods 14, no. 19: 3366. https://doi.org/10.3390/foods14193366
APA StyleMulet, J. M., Benito, P., Celdrán, M., Yenush, L., & Porcel, R. (2025). Impact of Different Microbial Biostimulants and Salt Stress on the Endophytome of the Edible Part of Lettuce and Tomato Plants. Foods, 14(19), 3366. https://doi.org/10.3390/foods14193366








