Chitosan-Based Coating Incorporated with Lemon Essential Oil/Rutin Composite Nanoemulsion for Pork Preservation
Abstract
1. Introduction
2. Materials
2.1. Materials and Methods
2.2. Preparation and Characterization of Lemon Essential Oil/Rutin Composite Nanoemulsion (LEO-NE-R)
2.2.1. Preparation of LEO-NE-R
2.2.2. Particle Size, Zeta Potential (ZP) and Polydispersity Index (PDI) Analysis
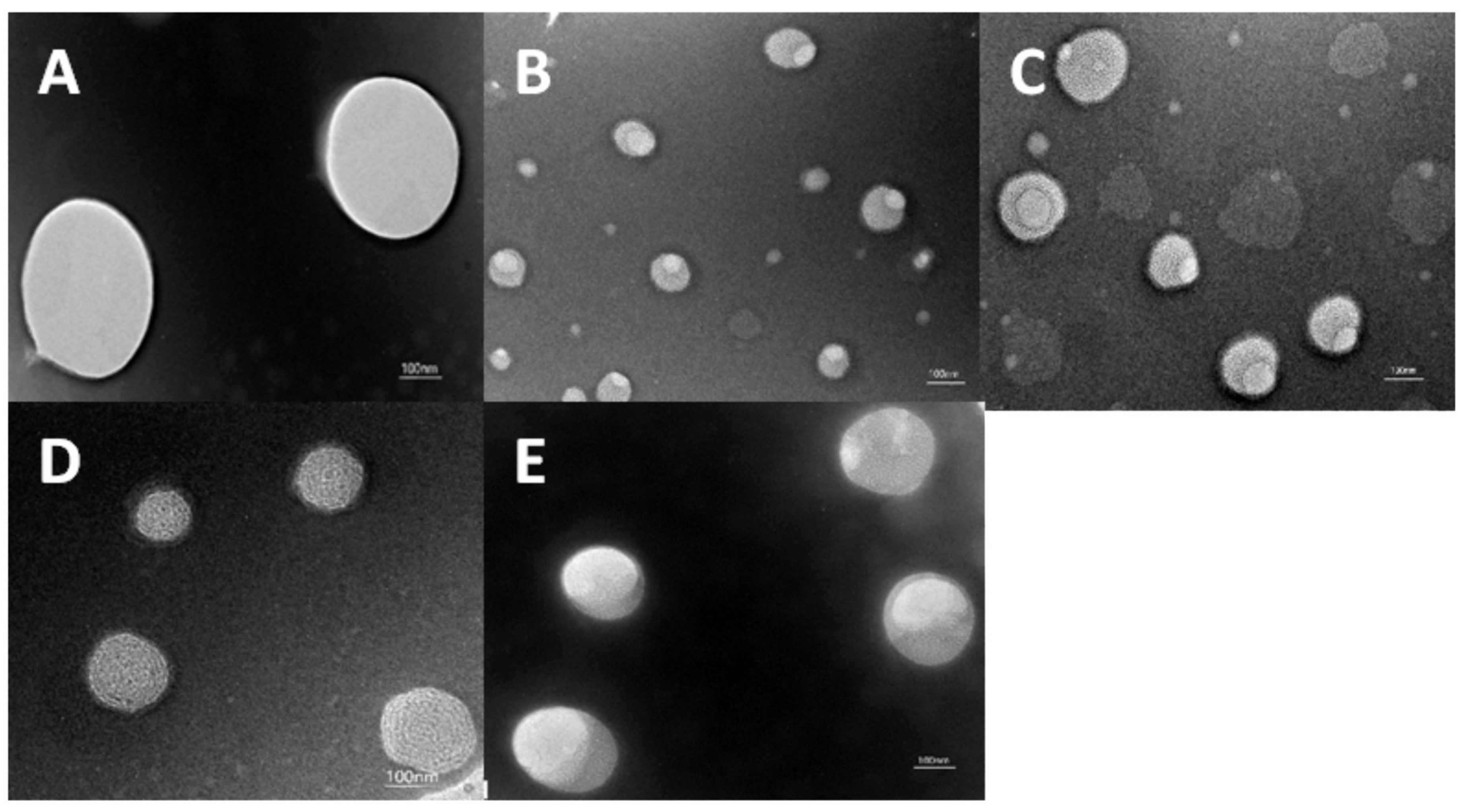
2.2.3. Transmission Electron Microscopy (TEM) Observation
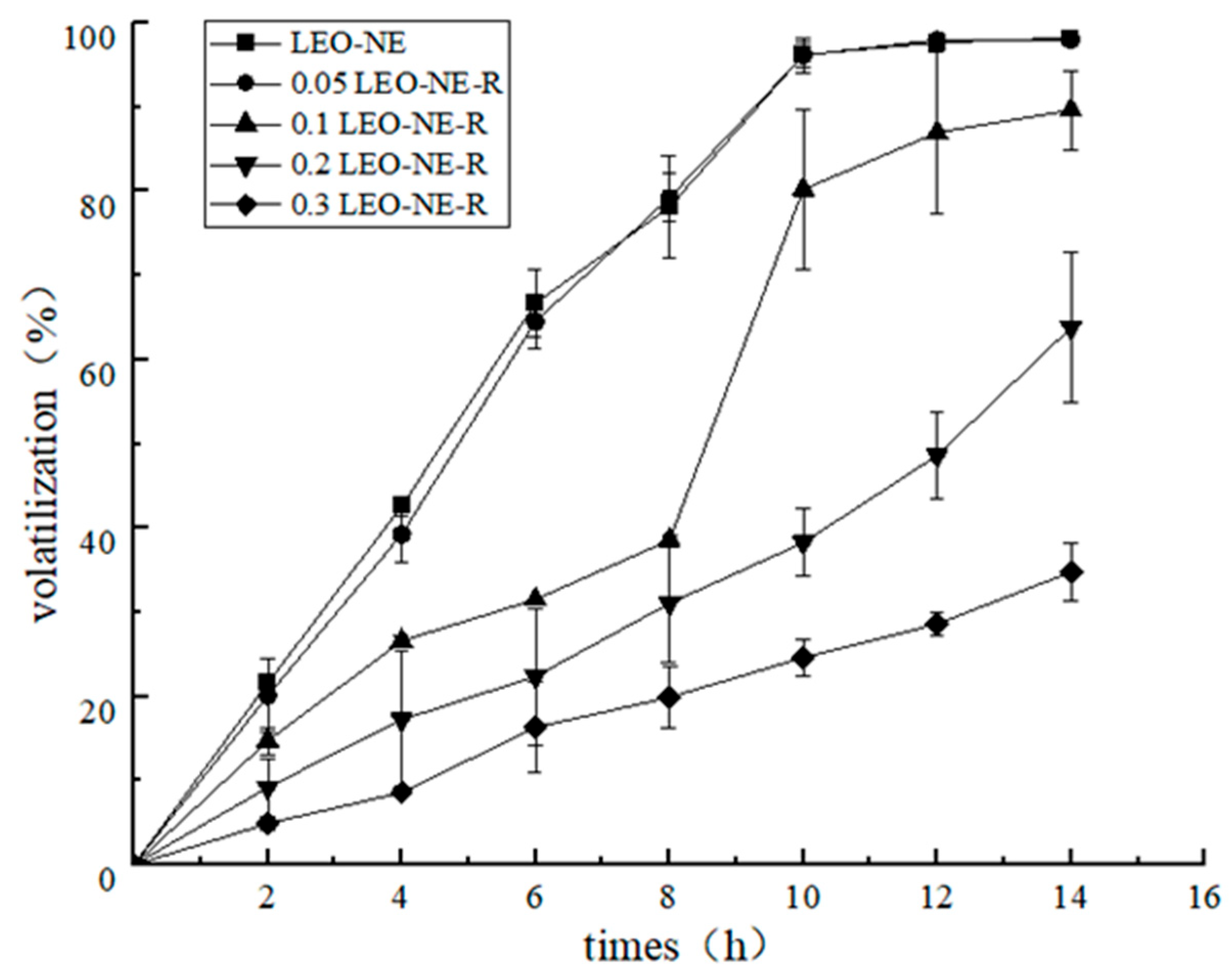
2.2.4. Slow-Release Analysis
2.3. Preparation and Characterization of CS-Based Coating Incorporated with LEO-NE-R
2.3.1. Preparation of Coating
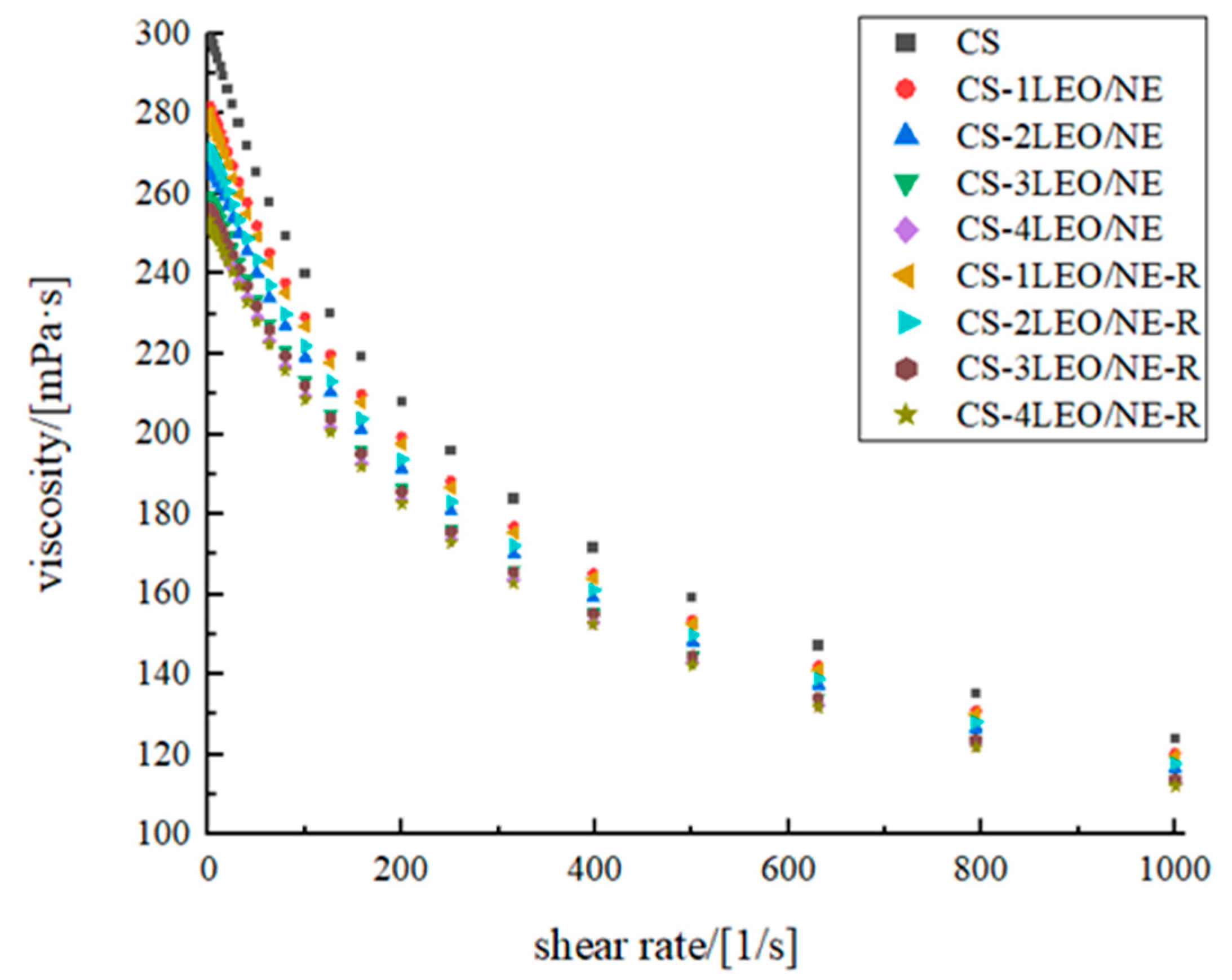
2.3.2. Rheological Behavior Analysis
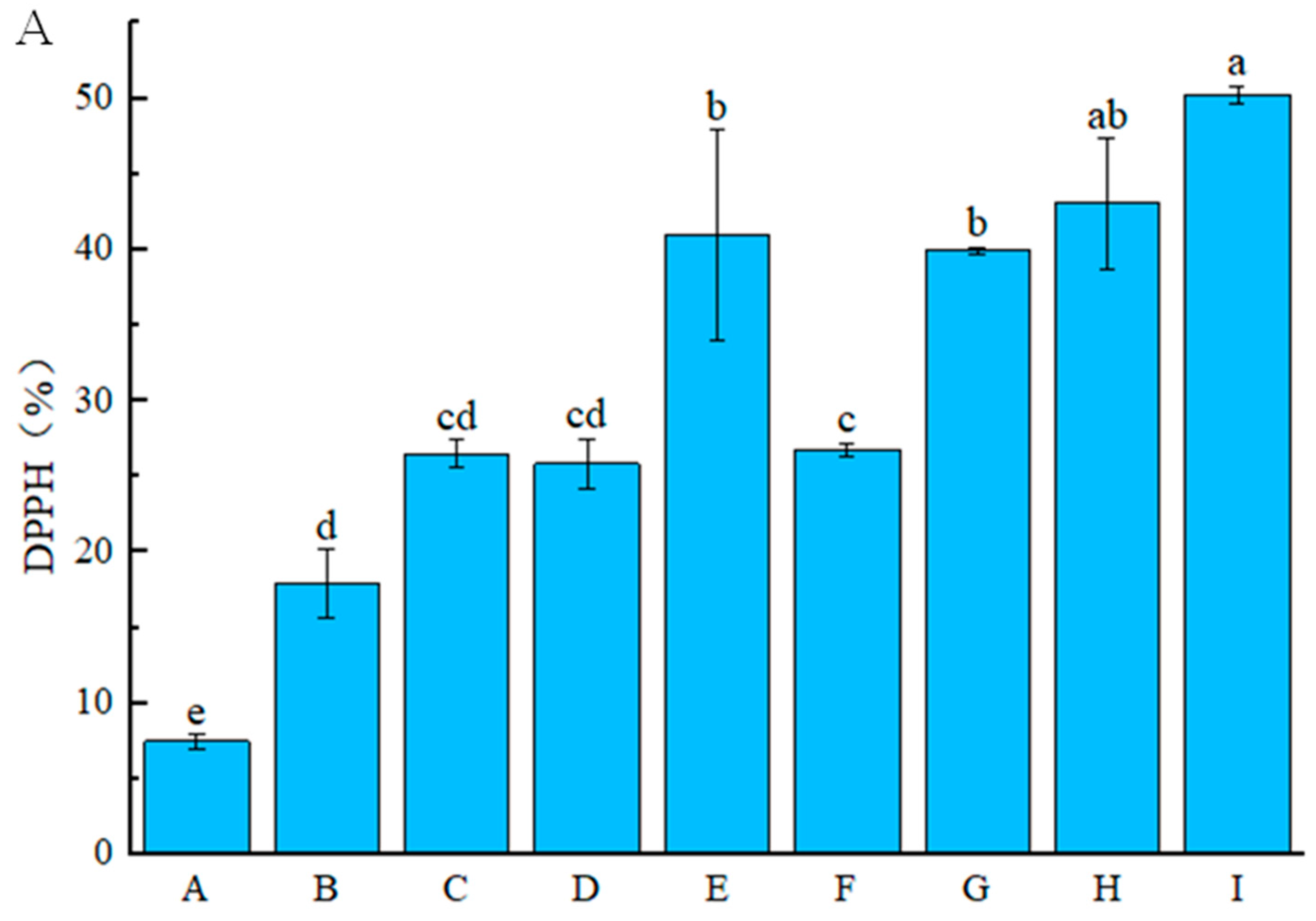
2.3.3. Antioxidant Activity Analysis
2.3.4. Bacteriostatic Activity Analysis
2.4. Pork Preservation
2.4.1. Processing of Meat Samples
2.4.2. Color Analysis
2.4.3. pH Analysis
2.4.4. Drip Loss Analysis
2.4.5. Total Volatile Basic Nitrogen (TVB-N) Analysis
2.4.6. Thiobarbituric Acid-Reactive Substances (TBARS) Analysis
2.4.7. Total Viable Count (TVC) Analysis
2.5. Statistical Analysis
3. Results and Discussions
3.1. Characterization of LEO-NE-R
3.1.1. Particle Size, PDI and ZP
3.1.2. TEM Observation
3.1.3. Slow-Release Performance
3.2. Characterization of CS-Based Coating
3.2.1. Rheological Behavior
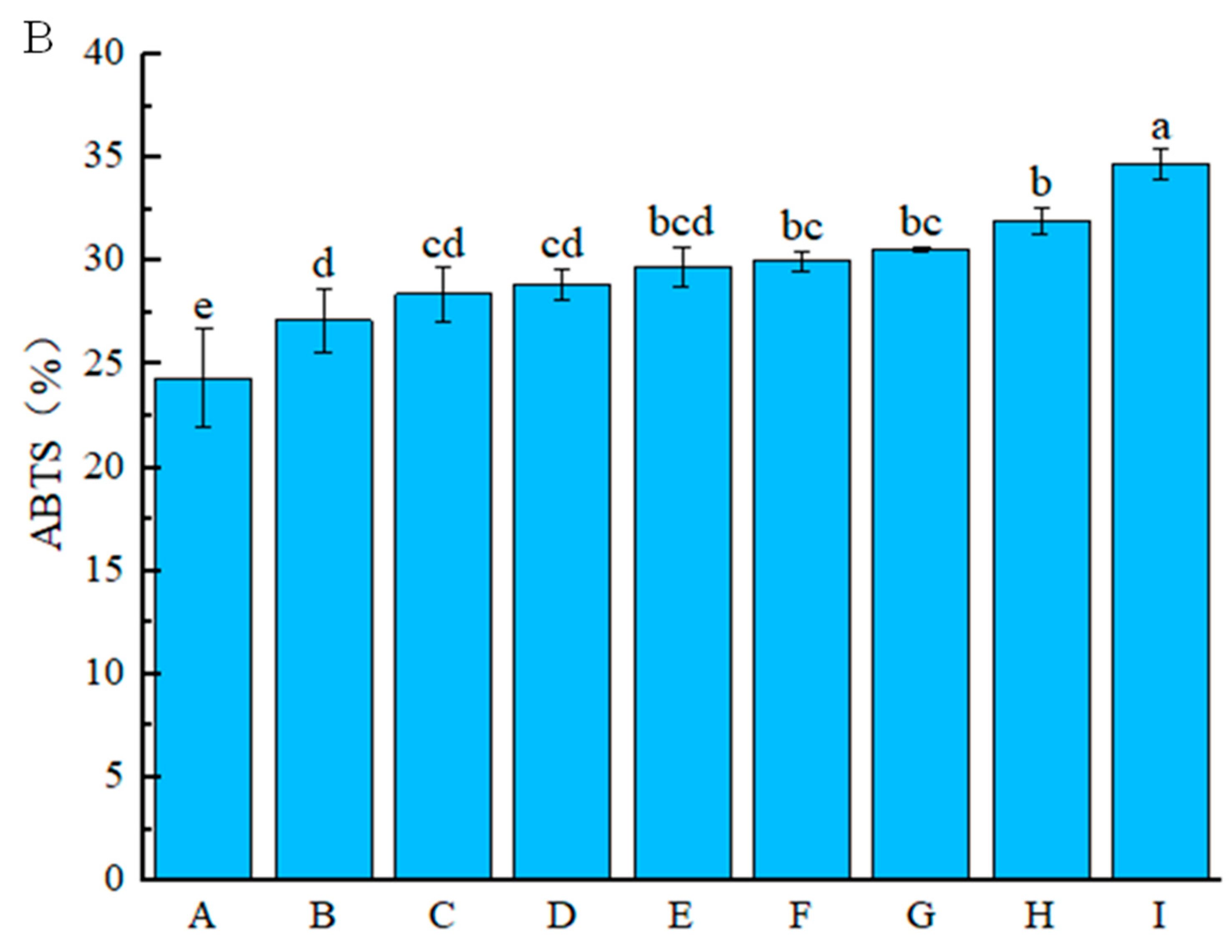
3.2.2. Antioxidant Activity
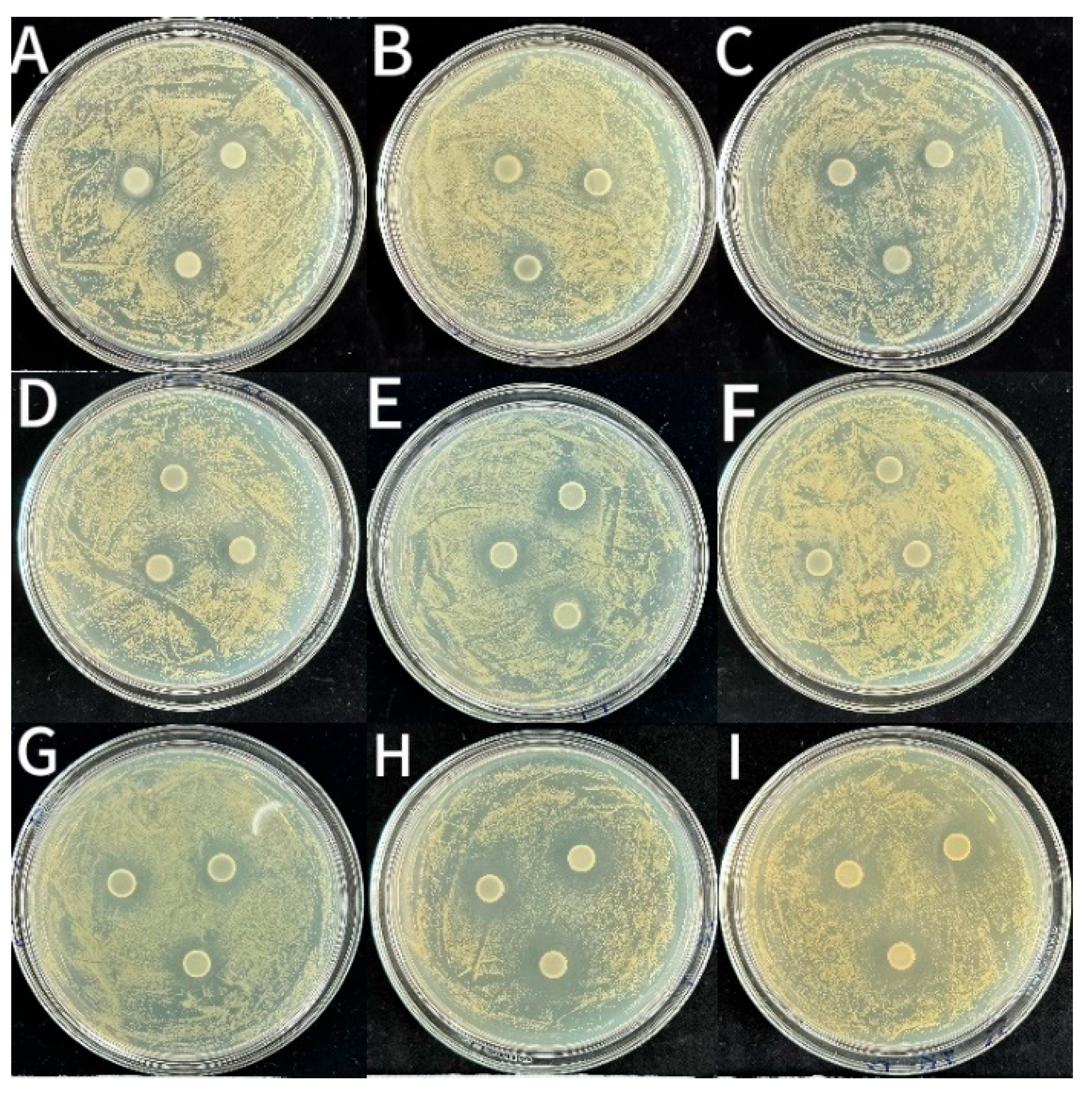
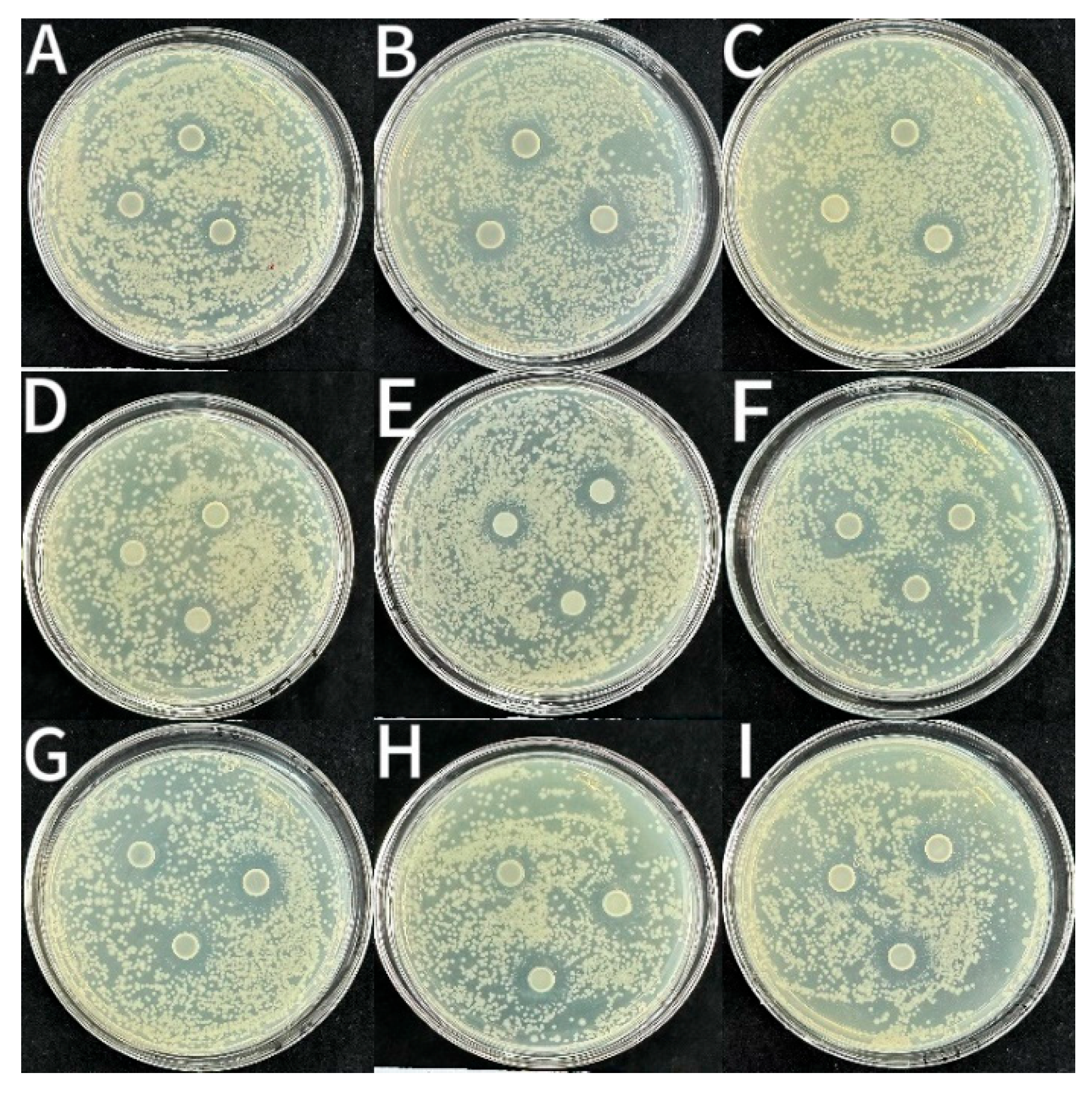
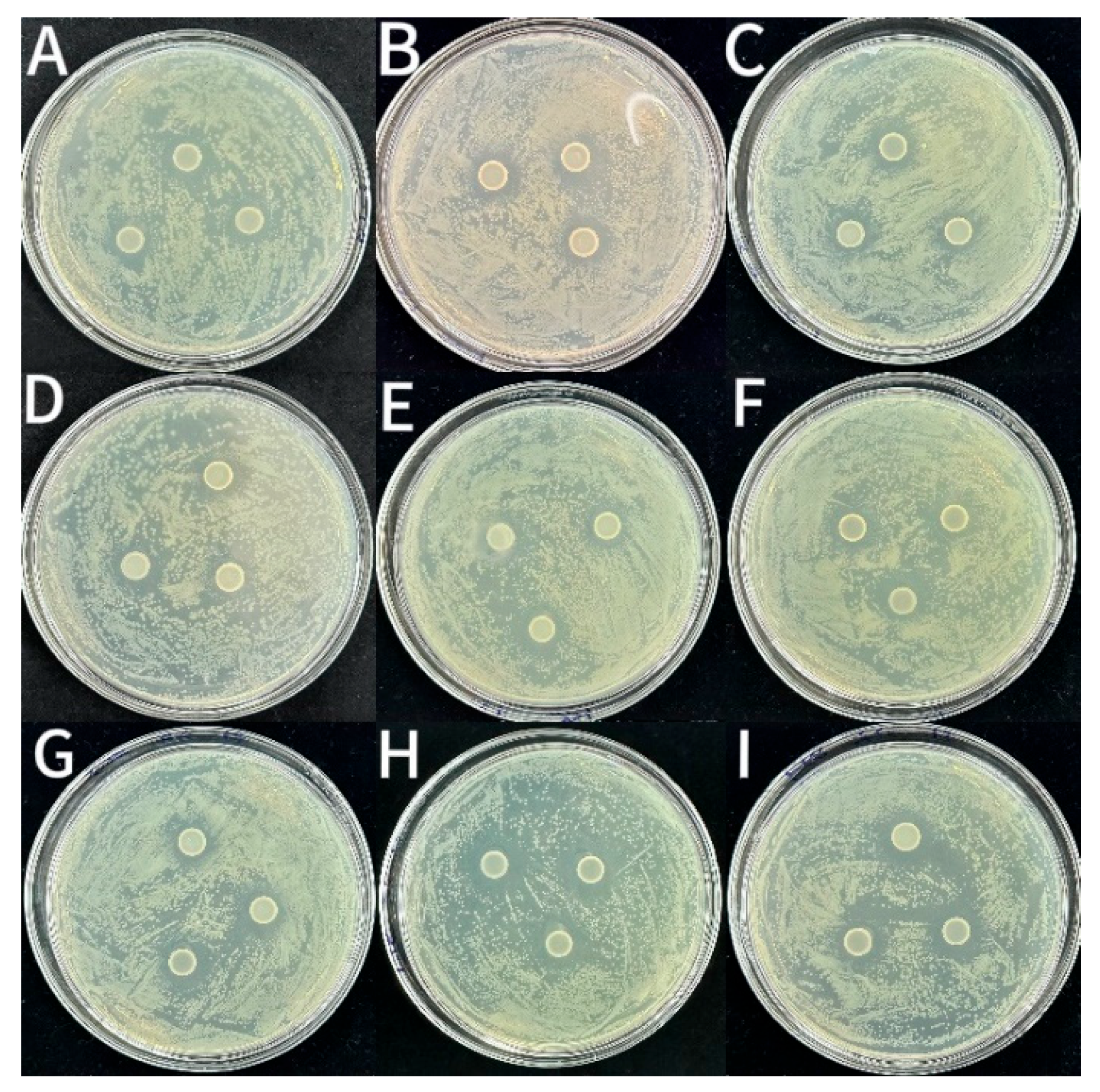
3.2.3. Bacteriostatic Activity
3.3. Pork Preservation
3.3.1. Color
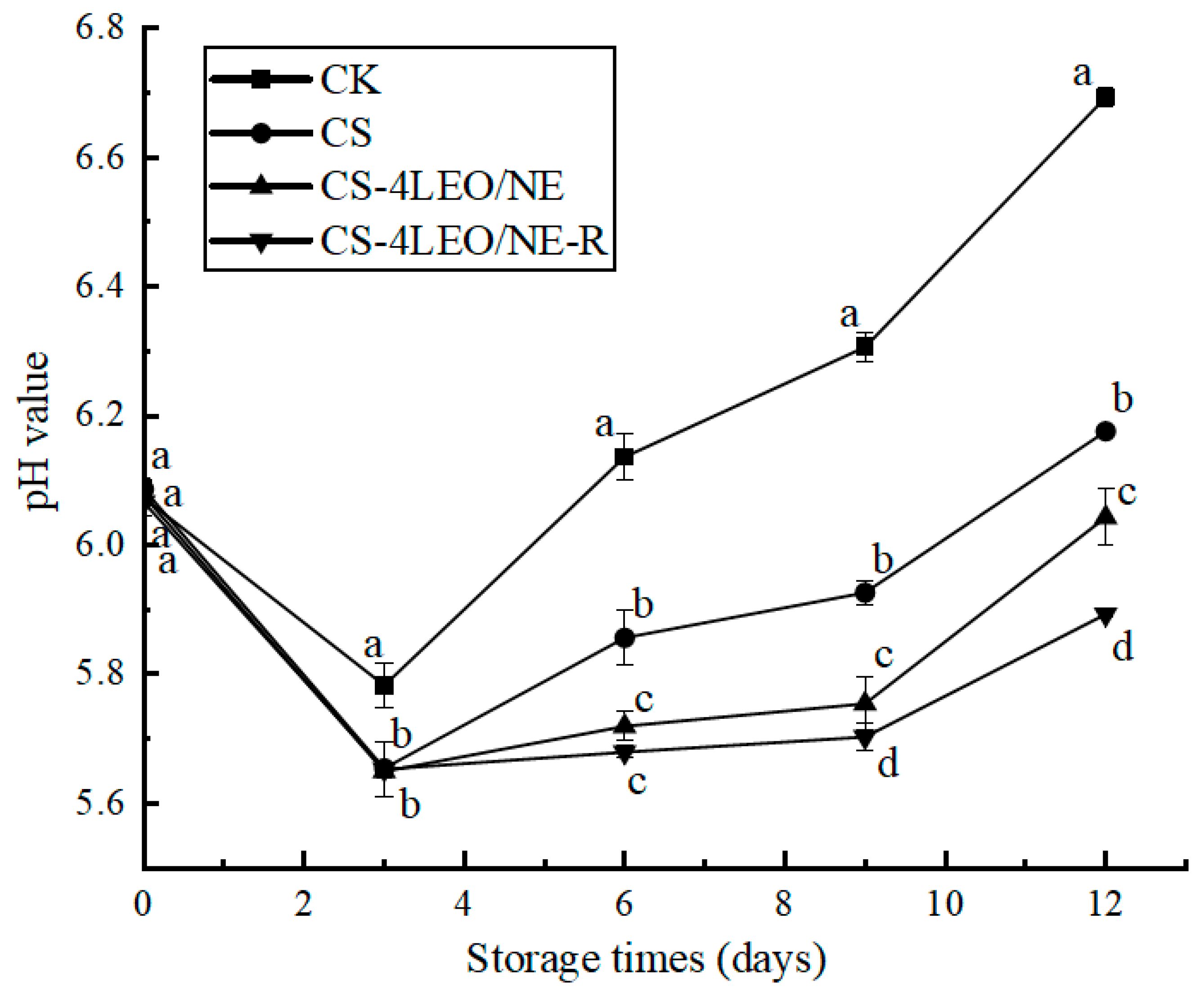
3.3.2. pH
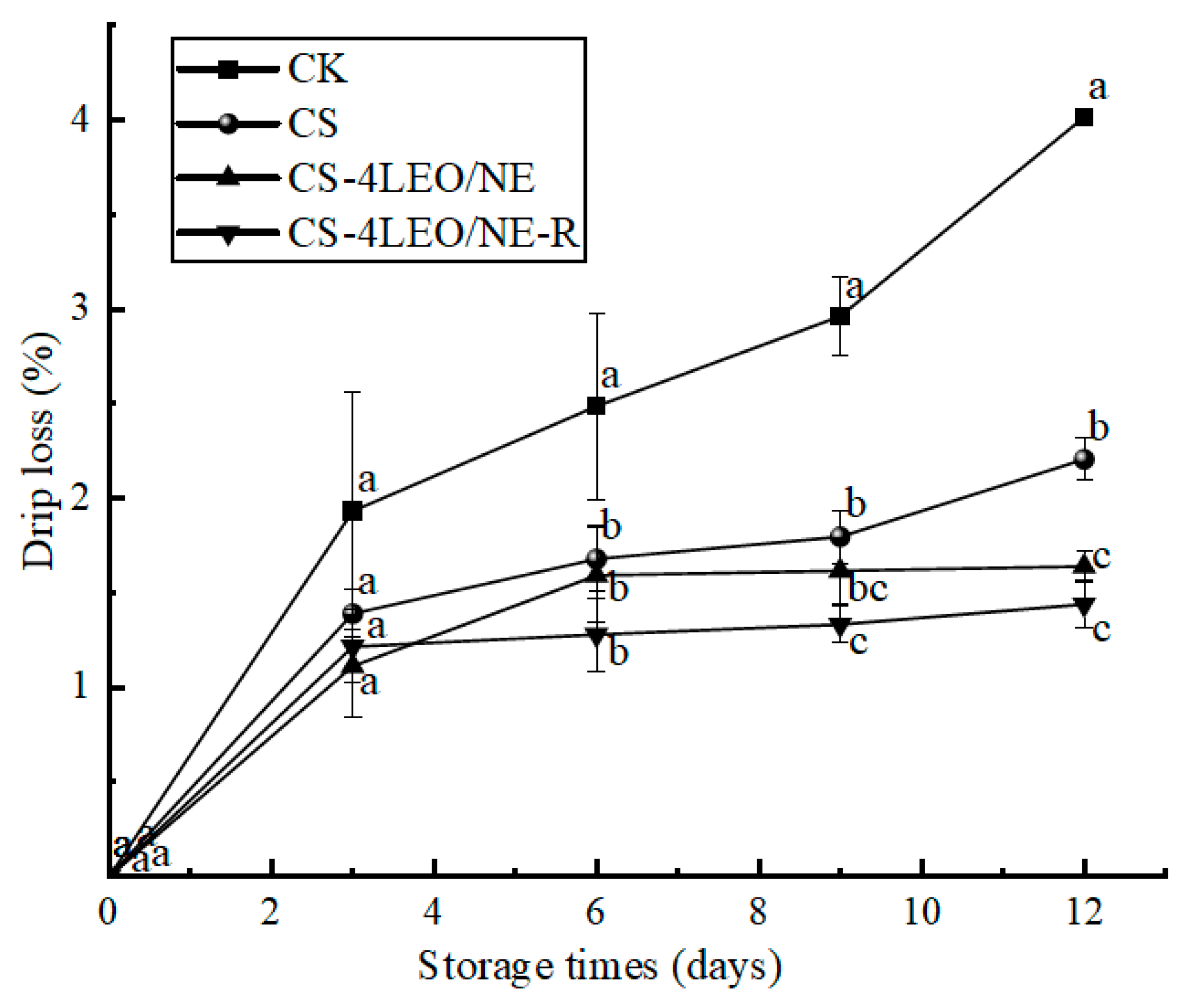
3.3.3. Drip Loss
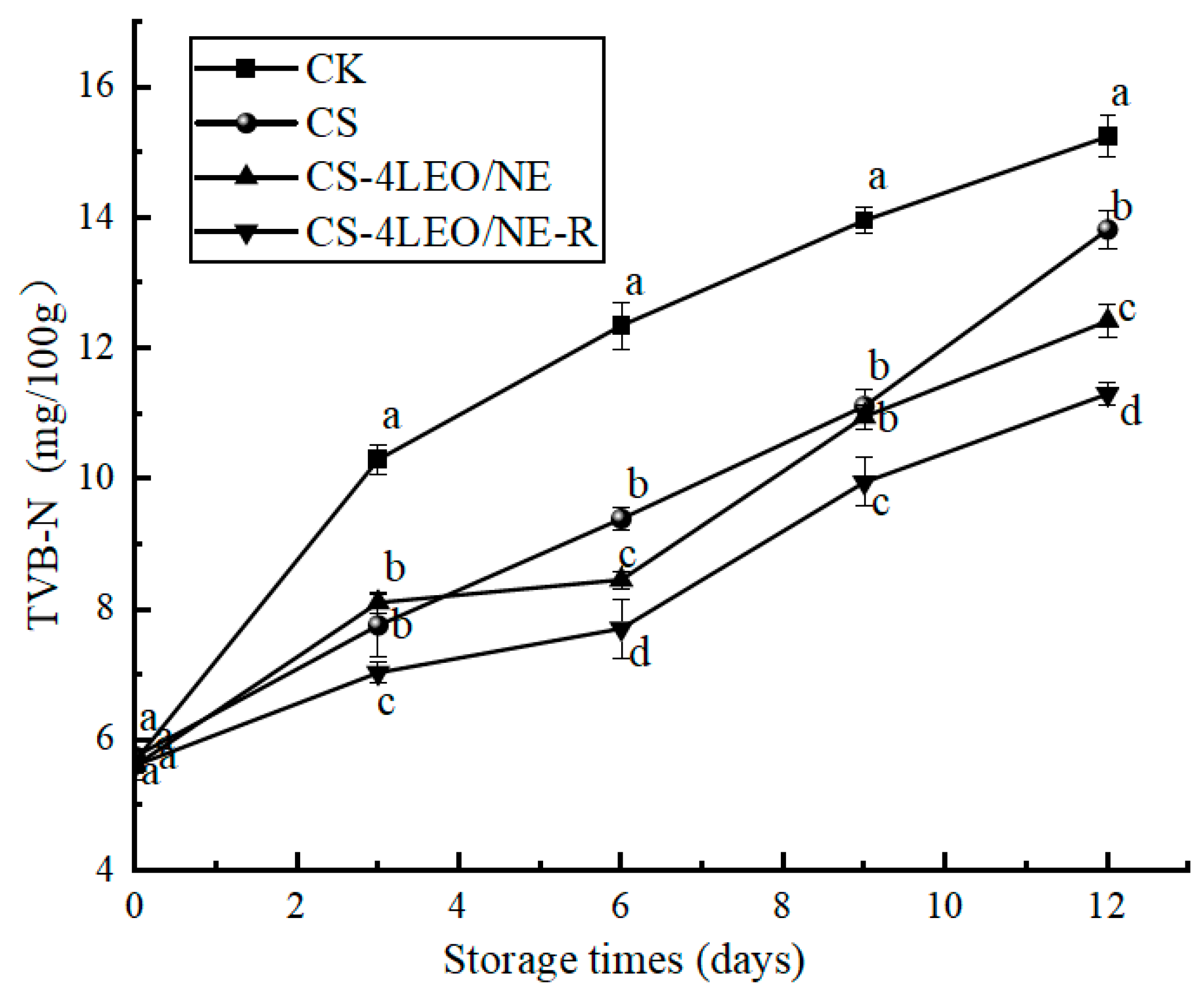
3.3.4. TVB-N
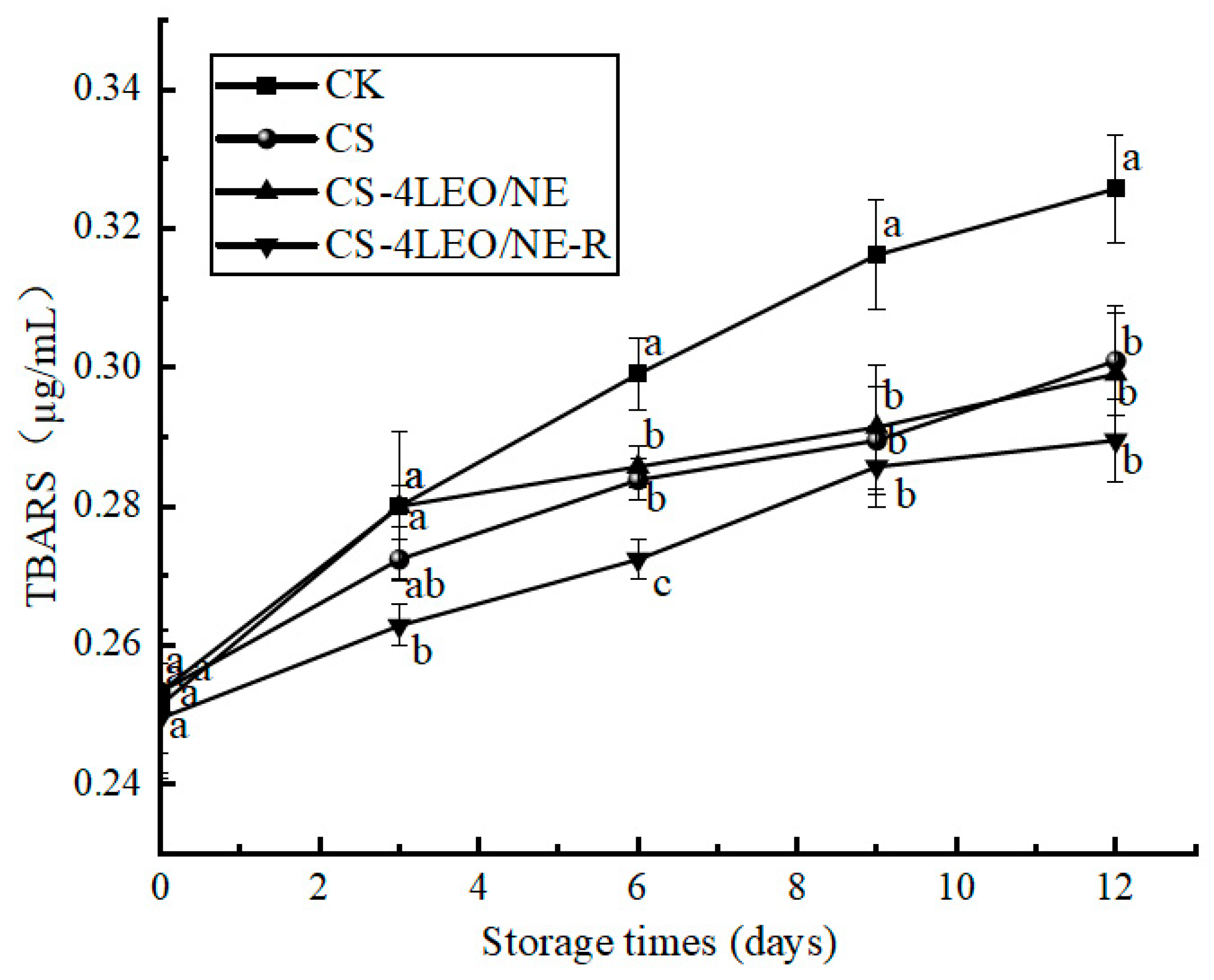
3.3.5. TBARS
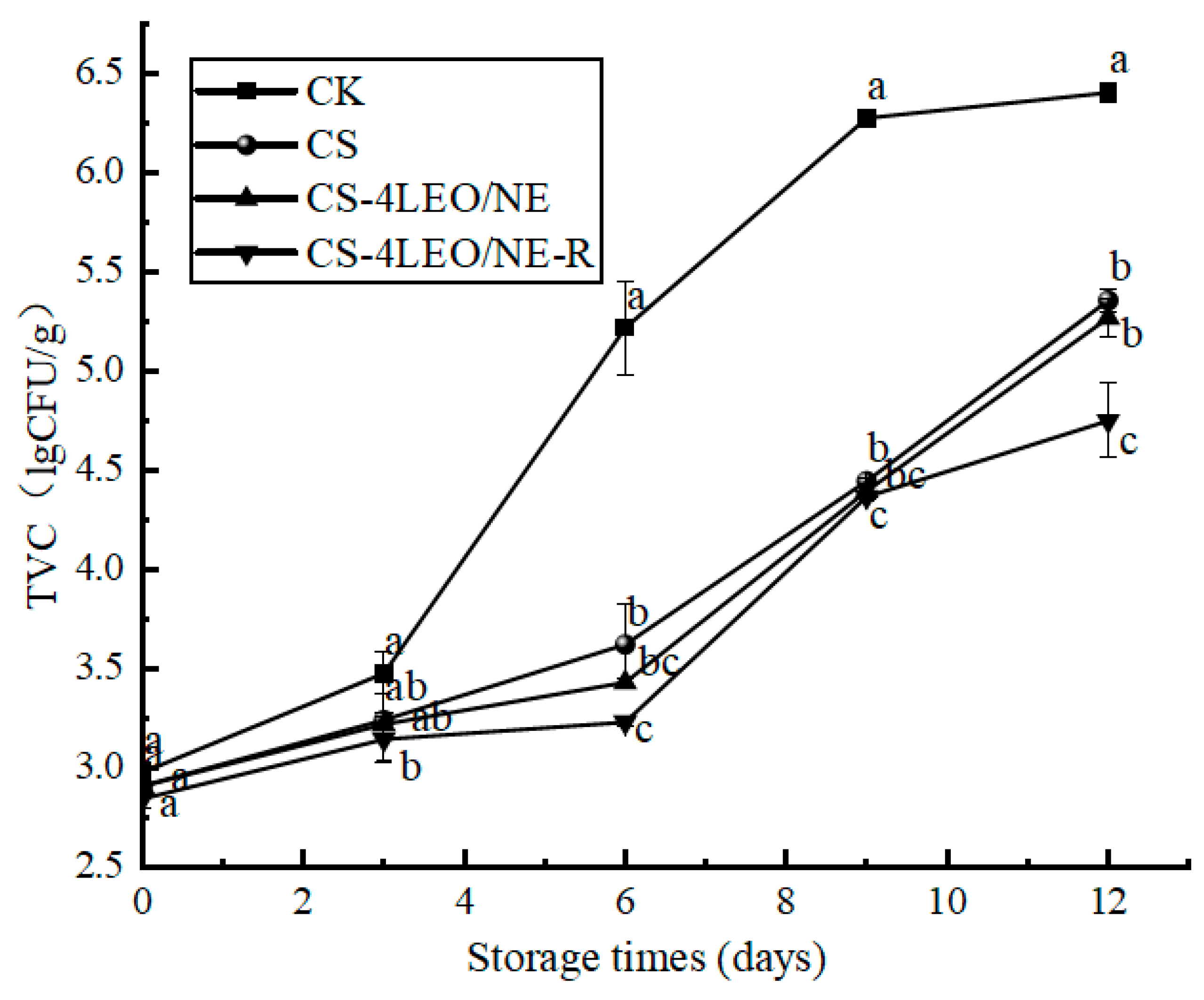
3.3.6. TVC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, G.; Chen, Y.; Luan, W.; Wang, X. Study of the freezing process and ice crystal growth during freezing and storage of pork. LWT 2025, 225, 117921. [Google Scholar] [CrossRef]
- Huang, X.W.; Zhao, W.Y.; Zhang, K.; Shi, J.Y.; Zhai, X.D.; Zhang, J.J.; Shen, T.T.; Liu, H.C.; Lin, T.; Zou, X.B.; et al. Cinnamon essential oil pickering emulsion-gellan gum composite films: A sustainable active packaging strategy for pork preservation. Int. J. Biol. Macromol. 2025, 318, 145227. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Shi, H.; Zhang, X.; Dong, P.; Luo, X.; Qin, H.; Zhang, Y.; Mao, Y.; Holman, B.W.B. Influence of low-energy electron beam irradiation on the quality and shelf-life of vacuum-packaged pork stored under chilled and superchilled conditions. Meat Sci. 2023, 195, 109019. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Wang, S.; Wang, F.; Li, C.; Jiang, F.; Xiao, M. Fast water absorption and antibacterial konjac glucomannan/xanthan gum/carboxymethyl cellulose film incorporated with rosmarinic acid for extending shelf life of chilled pork. LWT 2025, 229, 118102. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Xavier, K.A.M.; Porayil, L.; Balange, A.K.; Nayak, B.B. Effects of chitosan molecular weight on proteins and lipids interactions in fish mince emulsion sausages. Int. J. Biol. Macromol. 2025, 319, 145562. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Q.; Manickam, S.; Li, Y.; Zhang, M.; Luo, X.; Xing, J.; Wan, Y.; Ouyang, D.; Shen, J.; et al. Fabrication of Plantaricin FB-2 loaded chitosan/agar coatings: Enhancing antimicrobial activity and prolonging fresh pork shelf life. Int. J. Food Microbiol. 2025, 441, 111329. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.Y.; Dai, R.T. Study on the Preservation Effect of Lemon Essential Oil Nanoemulsion on Seasoned Pork Patties. J. Food Saf. Qual. 2022, 13, 2550–2557. [Google Scholar]
- Shan, M.Y.; Song, L.L.; Hu, Q.J.; Xv, D.L. Effect of Fish Scale Gelatin-based Films on the Preservation of Fresh Tuna Meat. J. Nucl. Agric. Sci. 2019, 33, 1137–1145. [Google Scholar]
- Yazgan, H.; Ozogul, Y.; Kuley, E. Antimicrobial influence of nanoemulsified lemon essential oil and pure lemon essential oil on food-borne pathogens and fish spoilage bacteria. Int. J. Food Microbiol. 2019, 306, 108266. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, S.; Zhang, H.; Kai, Y.; Yang, H. Preservative effect of gelatin/chitosan-based films incorporated with lemon essential oil on grass carp (Ctenopharyngodon idellus) fillets during storage. Int. J. Food Microbiol. 2023, 407, 110437. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ge, S.; Liu, H.; Wang, S.; Chen, S.; Wang, J.; Li, J.; Zhang, Q. Properties and antimicrobial activity of silver carp (Hypophthalmichthys molitrix) skin gelatin-chitosan films incorporated with oregano essential oil for fish preservation. Food Packag. Shelf Life 2014, 2, 7–16. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Dima, C.; Can Karaca, A.; Jafari, S.M. A review on rutin-loaded nanocarriers: Fundamentals, bioavailability, application in functional foods, and challenges. Eur. Polym. J. 2024, 219, 113385. [Google Scholar] [CrossRef]
- Bin, Y.; Wu, X.; Shi, J.; Zhao, Y.; Yue, X.; Xu, X.; Zuo, J.; Yuan, S.; Wang, Q. Rutin treatment delays postharvest chilling injury in green pepper fruit by modulating antioxidant defense capacity. Postharvest Biol. Technol. 2025, 230, 113753. [Google Scholar] [CrossRef]
- Luo, Z.J.; Murray, B.S.; Yusoff, A.; Morgan, M.R.A.; Povey, M.J.W.; Day, A.J. Particle-stabilizing effects of flavonoids at the oil-water interface. J. Agric. Food Chem. 2011, 59, 2636–2645. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, Y.; Wu, W.; Zhu, H.; Zhang, Y.; Li, S.; Sun, J.; Wang, W.; Zhu, Y.; Ma, Q. Chitosan-based active films loaded with lemon essential oil nanoemulsions for improving the shelf life and quality of chilled fresh pork. J. Food Meas. Charact. 2025, 1–18. [Google Scholar] [CrossRef]
- Peng, X.; Ren, W.; Jia, M.; Zhou, Q.; Li, B.; Li, G.; Xie, Y.; Dai, X.; Cao, H.; Shi, X. Multi-scale study on the volatility of essential oil and its modulation methods. J. Drug Deliv. Sci. Technol. 2025, 112, 107227. [Google Scholar] [CrossRef]
- Yang, Z.K.; Zou, X.B.; Li, Z.H.; Huang, X.W.; Zhai, X.D.; Zhang, W.; Shi, J.Y.; Tahir, H.E. Improved Postharvest Quality of Cold Stored Blueberry by Edible Coating Based on Composite Gum Arabic/Roselle Extract. Food Bioprocess Technol. 2019, 12, 1537–1547. [Google Scholar] [CrossRef]
- Xu, T.; Gao, C.C.; Feng, X.; Yang, Y.L.; Shen, X.C.; Tang, X.Z. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Someya, T.; Sano, K.; Sagane, Y.; Watanabe, T.; Wijesekara, R. Antioxidant activities of traditional plants in Sri Lanka by DPPH free radical-scavenging assay. Data Brief 2018, 17, 870–875. [Google Scholar] [CrossRef]
- Huang, K.; Liu, R.N.; Zhang, Y.; Guan, X. Characteristics of two cedarwood essential oil emulsions and their antioxidant and antibacterial activities. Food Chem. 2021, 346, 128970. [Google Scholar] [CrossRef]
- Das, A.K.; Mallik, M.; Kalita, P.; Tag, H. Optimization of biogenic calcium carbonate production using bamboo dust as a nutrient source by Bacillus subtilis and Bacillus cereus for enhanced self-healing concrete. J. Indian Chem. Soc. 2025, 102, 101715. [Google Scholar] [CrossRef]
- Zhang, D.J.; Lillevang, S.K.; Shah, N.P. Influence of pre-acidification, and addition of KGM and whey protein-based fat replacers CH-4560, and YO-8075 on texture characteristics and pizza bake properties of low-fat Mozzarella cheese. Lwt-Food Sci. Technol. 2021, 137, 110384. [Google Scholar] [CrossRef]
- Janisch, S.; Krischek, C.; Wicke, M. Color values and other meat quality characteristics of breast muscles collected from 3 broiler genetic lines slaughtered at 2 ages. Poult. Sci. 2011, 90, 1774–1781. [Google Scholar] [CrossRef]
- GB 5009.228-2016; Determination of Volatile Base Nitrogen in Food Safety National Standards. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- Peng, C.; Qin, J.; Li, Y.; Chen, K.; Jiang, F.; Xiao, M. Enhanced water and oxygen barrier properties of deacetylated konjac glucomannan/high acyl gellan gum water gradient film for improved frozen fish fillet preservation. Int. J. Biol. Macromol. 2024, 279, 135203. [Google Scholar] [CrossRef]
- GB 4789.2-2016; National food safety standard Food microbiological examination: Aerobic plate count. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- Stoica, M.; Bichescu, C.I.; Crețu, C.-M.; Dragomir, M.; Ivan, A.S.; Podaru, G.M.; Stoica, D.; Stuparu-Crețu, M. Review of Bio-Based Biodegradable Polymers: Smart Solutions for Sustainable Food Packaging. Foods 2024, 13, 3027. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, C.; Xiang, Q.; Wu, J.; Li, J.; Cao, Z.; Xiao, F. Probing the stability of emulsified asphalts: A dual analysis of zeta potential and particle size. Fuel 2025, 396, 135266. [Google Scholar] [CrossRef]
- Chernyshev, V.S.; Rachamadugu, R.; Tseng, Y.H.; Belnap, D.M.; Jia, Y.; Branch, K.J.; Butterfield, A.E.; Pease III, L.F.; Bernard, P.S.; Skliar, M. Size and shape characterization of hydrated and desiccated exosomes. Anal. Bioanal. Chem. 2015, 407, 3285–3301. [Google Scholar] [CrossRef] [PubMed]
- Zembyla, M.; Aris, L.; Murray, B.S.; Sarkar, A. Stability of water-in-oil emulsions co-stabilized by polyphenol crystal-protein complexes as a function of shear rate and temperature. J. Food Eng. 2020, 281, 109991. [Google Scholar] [CrossRef]
- Ashaq, B.; Rasool, K.; Habib, S.; Bashir, I.; Nisar, N.; Mustafa, S.; Ayaz, Q.; Nayik, G.A.; Uddin, J.; Ramniwas, S.; et al. Insights into chemistry, extraction and industrial application of lemon grass essential oil-A review of recent advances. Food Chem. 2024, 22, 101521. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar]
- Du, H.J.; Hu, Q.H.; Yang, W.J.; Pei, F.; Kimatu, B.M.; Ma, N.; Fang, Y.; Cao, C.J.; Zhao, L.Y. Development, physiochemical characterization and forming mechanism of Flammulina velutipes polysaccharide-based edible films. Carbohydr. Polym. 2016, 152, 214–221. [Google Scholar] [CrossRef]
- Pandey, V.K.; Islam, R.U.; Shams, R.; Dar, A.H. A comprehensive review on the application of essential oils as bioactive compounds in Nanoemulsion based edible coatings of fruits and vegetables. Appl. Food Res. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Brito, J.; Hlushko, H.; Abbott, A.; Aliakseyeu, A.; Hlushko, R.; Sukhishvili, S.A. Integrating antioxidant functionality into polymer materials: Fundamentals, strategies, and applications. ACS Appl. Mater. Interfaces 2021, 13, 41372–41395. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, W.; Edziri, H.; Elmsehli, S.; Horchani, M.; Bechi, S.; Chaieb, I.; Vilhena, K.D.S.d.S.; de Oliveira, M.S. Chemical composition and ecological bioactivity of Citrus sinensis essential oil. Biochem. Syst. Ecol. 2025, 123, 105079. [Google Scholar] [CrossRef]
- Huang, M.; Wang, H.; Xu, X.; Lu, X.; Song, X.; Zhou, G. Effects of nanoemulsion-based edible coatings with composite mixture of rosemary extract and ε-poly-L-lysine on the shelf life of ready-to-eat carbonado chicken. Food Hydrocoll. 2020, 102, 105576. [Google Scholar]
- Xu, C.; Chen, G.; Chen, X.; Chen, C.; Xia, Q.; Sun, Q.; Wei, S.; Han, Z.; Wang, Z.; Liu, S. Oxidized myoglobin: Revealing new perspectives and insights on factors affecting the water retention of myofibrillar proteins. Food Chem. 2024, 441, 138332. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, L.; Huang, F.; Wei, W.; Barbut, S.; Erasmus, S.; Zhang, C. Lipid-derived odour-active volatile compound formation pathways in Tibetan pork across different cooking methods: Insights from iron properties, lipid oxidation, and lipidomics analysis. Food Chem. 2025, 491, 145256. [Google Scholar]
- Zhou, Y.; Liu, J.J.H.; Kang, Y.; Cui, H.; Yang, H. Effects of acid and alkaline treatments on physicochemical and rheological properties of tilapia surimi prepared by pH shift method during cold storage. Food Res. Int. 2021, 145, 110424. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Lipová, P.; Ladero, L.; Fernández-García, J.L.; Cava, R. Glycogen and lactate contents, pH and meat quality and gene expression in muscle Longissimus dorsi from iberian pigs under different rearing conditions. Livest. Sci. 2020, 240, 104167. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, F.; Wang, Y.; Han, J.; Gao, F.; Tian, J.; Zhang, K.; Jin, Y. The changes occurring in proteins during processing and storage of fermented meat products and their regulation by lactic acid bacteria. Foods 2022, 11, 2427. [Google Scholar] [CrossRef]
- Sujiwo, J.; Kim, D.; Jang, A. Relation among quality traits of chicken breast meat during cold storage: Correlations between freshness traits and torrymeter values. Poult. Sci. 2018, 97, 2887–2894. [Google Scholar] [CrossRef]
- Lebret, B.; Čandek-Potokar, M. Pork quality attributes from farm to fork. Part I. Carcass and fresh meat. Animal 2022, 16, 100402. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.D. The eating quality of meat: IV-Water holding capacity and juiciness. In Lawrie’s Meat Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 457–508. [Google Scholar]
- Bekhit, A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pirouzi, S.; Yaghoubi, M.; Karimi-Dehkordi, M.; Jafarzadeh, S.; Khaneghah, A.M. Packaging of beef fillet with active chitosan film incorporated with ɛ-polylysine: An assessment of quality indices and shelf life. Meat Sci. 2021, 176, 108475. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Zhang, Y.; Yang, Z.; Ma, Z.; Chen, J.; Chen, X.; Qiu, Z.; Tian, J.; Pu, A. Formation and regulation strategies for volatile off-flavor compounds in livestock meat, poultry meat, and their products: A comprehensive review. Trends Food Sci. Technol. 2024, 152, 104689. [Google Scholar] [CrossRef]
- GB2707-2016; National Food Safety Standard—Fresh (frozen) livestock and poultry products. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
| Samples | MD (nm) | PDI | ZP (mV) |
|---|---|---|---|
| LEO-NE | 239.67 ± 2.83 a | 0.28 ± 0.04 a | −21.07 ± 0.06 b |
| 0.05 LEO-NE-R | 175.50 ± 0.98 d | 0.21 ± 0.01 b | −25.57 ± 0.85 a |
| 0.1 LEO-NE-R | 178.93 ± 0.67 d | 0.22 ± 0.01 b | −25.83 ± 0.31 a |
| 0.2 LEO-NE-R | 199.37 ± 2.06 c | 0.23 ± 0.01 b | −20.80 ± 0.26 b |
| 0.3 LEO-NE-R | 233.63 ± 3.00 b | 0.23 ± 0.01 b | −21.17 ± 0.40 b |
| Samples | Inhibition Zone Diameter (mm) | ||
|---|---|---|---|
| S. aureus | L. monocytogenes | E. coli | |
| CS | 14.29 ± 0.20 g | 12.88 ± 0.13 g | 12.51 ± 0.07 f |
| CS-1LEO/NE | 14.89 ± 0.53 f | 13.09 ± 0.14 g | 13.09 ± 0.14 e |
| CS-2LEO/NE | 14.90 ± 0.23 f | 13.64 ± 0.08 ef | 13.47 ± 0.10 cd |
| CS-3LEO/NE | 15.88 ± 0.05 cd | 13.77 ± 0.07 de | 13.44 ± 0.21 d |
| CS-4LEO/NE | 16.57 ± 0.08 ab | 14.10 ± 0.03 c | 13.81 ± 0.03 b |
| CS-1LEO/NE-R | 15.27 ± 0.12 ef | 13.45 ± 0.03 f | 13.29 ± 0.20 de |
| CS-2LEO/NE-R | 15.61 ± 0.16 e | 14.04 ± 0.22 cd | 13.76 ± 0.14 bc |
| CS-3LEO/NE-R | 16.19 ± 0.13 bc | 14.41 ± 0.14 b | 14.01 ± 0.10 b |
| CS-4LEO/NE-R | 16.74 ± 0.09 a | 15.27 ± 0.18 a | 14.40 ± 0.14 a |
| Parameter | Sample | Storage Times/d | ||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | ||
| L* | CK | 50.33 ± 0.8 a | 50.40 ± 0.49 b | 50.22 ± 0.44 ab | 48.91 ± 1.68 a | 46.45 ± 0.66 c |
| CS | 50.80 ± 1.9 a | 52.64 ± 0.63 a | 48.96 ± 1.16 b | 47.93 ± 1.45 a | 47.41 ± 0.72 bc | |
| CS-LEO/NE | 49.89 ± 0.5 a | 51.23 ± 0.17 b | 49.64 ± 0.35 ab | 49.45 ± 0.71 b | 48.31 ± 0.32 ab | |
| CS-LEO/NE-R | 50.28 ± 0.3 a | 52.97 ± 0.44 a | 50.73 ± 0.48 a | 49.62 ± 0.42 a | 48.95 ± 0.82 a | |
| a* | CK | 9.40 ± 0.13 b | 9.15 ± 0.51 b | 6.77 ± 0.39 b | 5.93 ± 0.33 c | 5.13 ± 1.03 c |
| CS | 8.55 ± 0.16 c | 8.60 ± 0.14 b | 7.40 ± 0.76 b | 6.13 ± 0.17 bc | 5.66 ± 0.17 bc | |
| CS-LEO/NE | 9.06 ± 0.06 b | 9.09 ± 0.34 b | 7.44 ± 0.73 b | 7.09 ± 0.82 b | 6.82 ± 0.28 b | |
| CS-LEO/NE-R | 9.93 ± 0.24 a | 10.42 ± 0.26 a | 9.19 ± 0.31 a | 9.09 ± 0.25 a | 9.24 ± 0.42 a | |
| b* | CK | 5.54 ± 0.09 a | 6.10 ± 0.40 a | 7.53 ± 0.64 a | 7.56 ± 0.54 a | 8.30 ± 0.48 a |
| CS | 5.33 ± 0.13 b | 5.94 ± 0.53 a | 6.75 ± 0.25 ab | 7.07 ± 0.33 ab | 7.65 ± 0.26 ab | |
| CS-LEO/NE | 5.23 ± 0.04 b | 5.33 ± 0.15 a | 6.09 ± 0.26 bc | 6.65 ± 0.71 ab | 7.15 ± 0.29 b | |
| CS-LEO/NE-R | 5.22 ± 0.04 b | 5.32 ± 0.73 a | 5.86 ± 0.11 c | 6.35 ± 0.22 b | 6.88 ± 0.51 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Hou, H.; Zhu, J.; Wang, X.; Meng, F.; Chen, W. Chitosan-Based Coating Incorporated with Lemon Essential Oil/Rutin Composite Nanoemulsion for Pork Preservation. Foods 2025, 14, 3351. https://doi.org/10.3390/foods14193351
Han J, Hou H, Zhu J, Wang X, Meng F, Chen W. Chitosan-Based Coating Incorporated with Lemon Essential Oil/Rutin Composite Nanoemulsion for Pork Preservation. Foods. 2025; 14(19):3351. https://doi.org/10.3390/foods14193351
Chicago/Turabian StyleHan, Jiaxin, Hui Hou, Jiayu Zhu, Xinhui Wang, Fanbing Meng, and Weijun Chen. 2025. "Chitosan-Based Coating Incorporated with Lemon Essential Oil/Rutin Composite Nanoemulsion for Pork Preservation" Foods 14, no. 19: 3351. https://doi.org/10.3390/foods14193351
APA StyleHan, J., Hou, H., Zhu, J., Wang, X., Meng, F., & Chen, W. (2025). Chitosan-Based Coating Incorporated with Lemon Essential Oil/Rutin Composite Nanoemulsion for Pork Preservation. Foods, 14(19), 3351. https://doi.org/10.3390/foods14193351





