Mechanisms of Quality Preservation in Golden Pomfret Fish Balls Treated with Ultra-High Pressure During Freeze–Thaw Cycles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Freeze–Thaw Cycle of Sample
2.2. Water Holding Capacity (WHC)
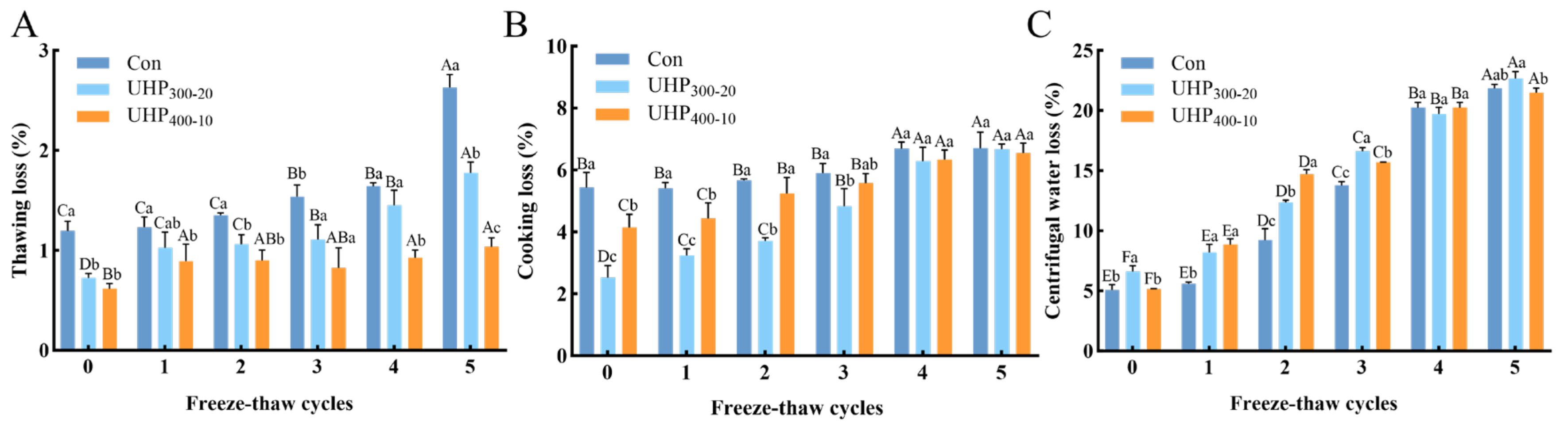
2.2.1. Thawing Loss
2.2.2. Centrifugal Water Loss
2.2.3. Cooking Loss
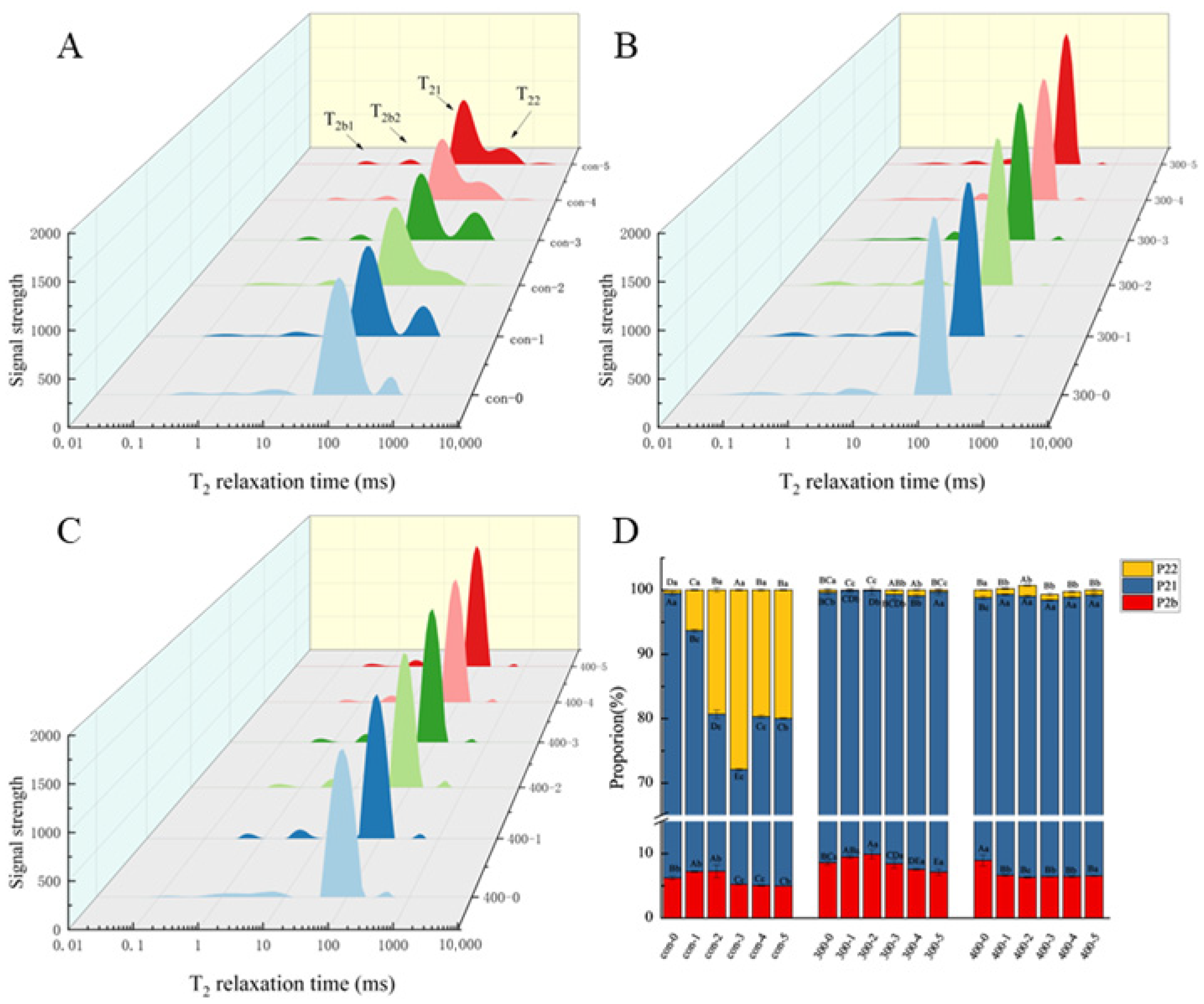
2.3. Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.4. Determination of Texture
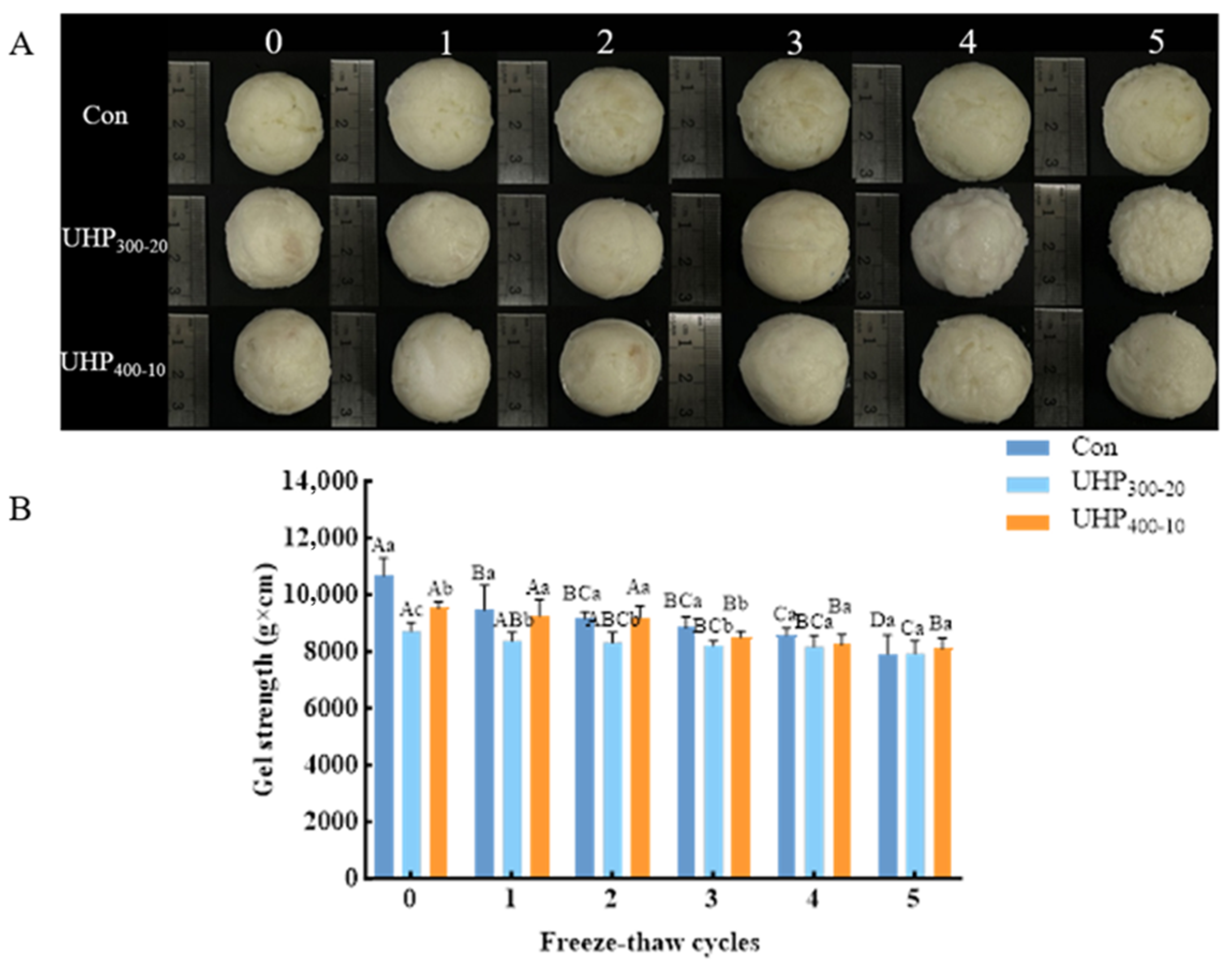
2.5. Determination of Gel Strength
2.6. Color Evaluation
2.7. Chemical Interaction
2.8. Morphology of Ice Crystals
2.9. Scanning Electron Microscopy (SEM)
2.10. Myofibrillar Protein (MP) Extraction
2.11. Protein Oxidation
2.12. Peroxide Value (POV)
2.13. Thiobarbituric Acid Reactive Substances (TBARSs)
2.14. Statistical Analysis
3. Results and Discussion
3.1. Changes in WHC of Fish Balls
3.2. Changes in Water State and Water Distribution in Fish Ball
3.3. Changes in Physicochemical Properties and Color in Fish Balls
| Freeze–Thaw Cycles | Group | Hardness(kg) | Springiness | Chewiness | Adhesive Properties |
|---|---|---|---|---|---|
| 0 | Con | 345.194 ± 18.390 Aa | 0.734 ± 0.115 Ab | 64.086 ± 3.062 Ab | 0.504 ± 0.050 Ac |
| UHP300-20 | 172.501 ± 10.709 Ac | 0.963 ± 0.018 Aa | 39.988 ± 4.927 Ac | 0.575 ± 0.042 Ab | |
| UHP400-10 | 262.143 ± 3.592 Ab | 0.991 ± 0.012 Aa | 73.826 ± 3.564 Aa | 0.684 ± 0.022 Aa | |
| 1 | Con | 319.825 ± 12.939 Ba | 0.707 ± 0.036 Ab | 63.775 ± 2.585 Aa | 0.486 ± 0.114 Aa |
| UHP300-20 | 167.544 ± 3.228 Ac | 0.927 ± 0.037 Ba | 35.094 ± 2.362 Bb | 0.575 ± 0.060 Aa | |
| UHP400-10 | 199.236 ± 7.002 Bb | 0.954 ± 0.025 Ba | 60.905 ± 3.172 Ba | 0.596 ± 0.074 Ba | |
| 2 | Con | 289.863 ± 14.156 Ca | 0.772 ± 0.093 Ab | 63.449 ± 2.125 Aa | 0.540 ± 0.050 Aa |
| UHP300-20 | 151.126 ± 9.578 Bb | 0.842 ± 0.025 Cb | 30.852 ± 2.979 Bc | 0.538 ± 0.023 ABa | |
| UHP400-10 | 78.392 ± 23.434 Dc | 0.927 ± 0.033 BCa | 50.635 ± 1.937 Cb | 0.541 ± 0.027 BCDa | |
| 3 | Con | 249.506 ± 6.427 DEa | 0.750 ± 0.135 Ab | 54.499 ± 2.777 Ba | 0.451 ± 0.057 ABb |
| UHP300-20 | 146.877 ± 2.540 Bb | 0.740 ± 0.012 Db | 32.291 ± 3.035 Bc | 0.533 ± 0.026 ABa | |
| UHP400-10 | 96.603 ± 6.965 Cc | 0.910 ± 0.023 Ca | 45.633 ± 1.838 Db | 0.580 ± 0.058 BCa | |
| 4 | Con | 265.788 ± 19.928 Da | 0.752 ± 0.140 Ab | 47.231 ± 2.420 Ca | 0.454 ± 0.037 ABc |
| UHP300-20 | 148.689 ± 3.587 Bb | 0.740 ± 0.003 Db | 33.507 ± 4.121 Bb | 0.545 ± 0.021 ABa | |
| UHP400-10 | 84.858 ± 4.682 CDc | 0.906 ± 0.039 Ca | 46.431 ± 2.869 Da | 0.495 ± 0.038 Db | |
| 5 | Con | 232.834 ± 17.367 Ea | 0.479 ± 0.092 Bb | 43.678 ± 1.998 Da | 0.377 ± 0.112 Bb |
| UHP300-20 | 74.509 ± 7.645 Cc | 0.931 ± 0.023 Ba | 31.188 ± 2.757 Bc | 0.501 ± 0.039 Ba | |
| UHP400-10 | 86.056 ± 16.466 CDb | 0.943 ± 0.012 Ba | 35.637 ± 1.873 Eb | 0.535 ± 0.031 CDa |
3.4. Changes in Gel Strength in Fish Balls
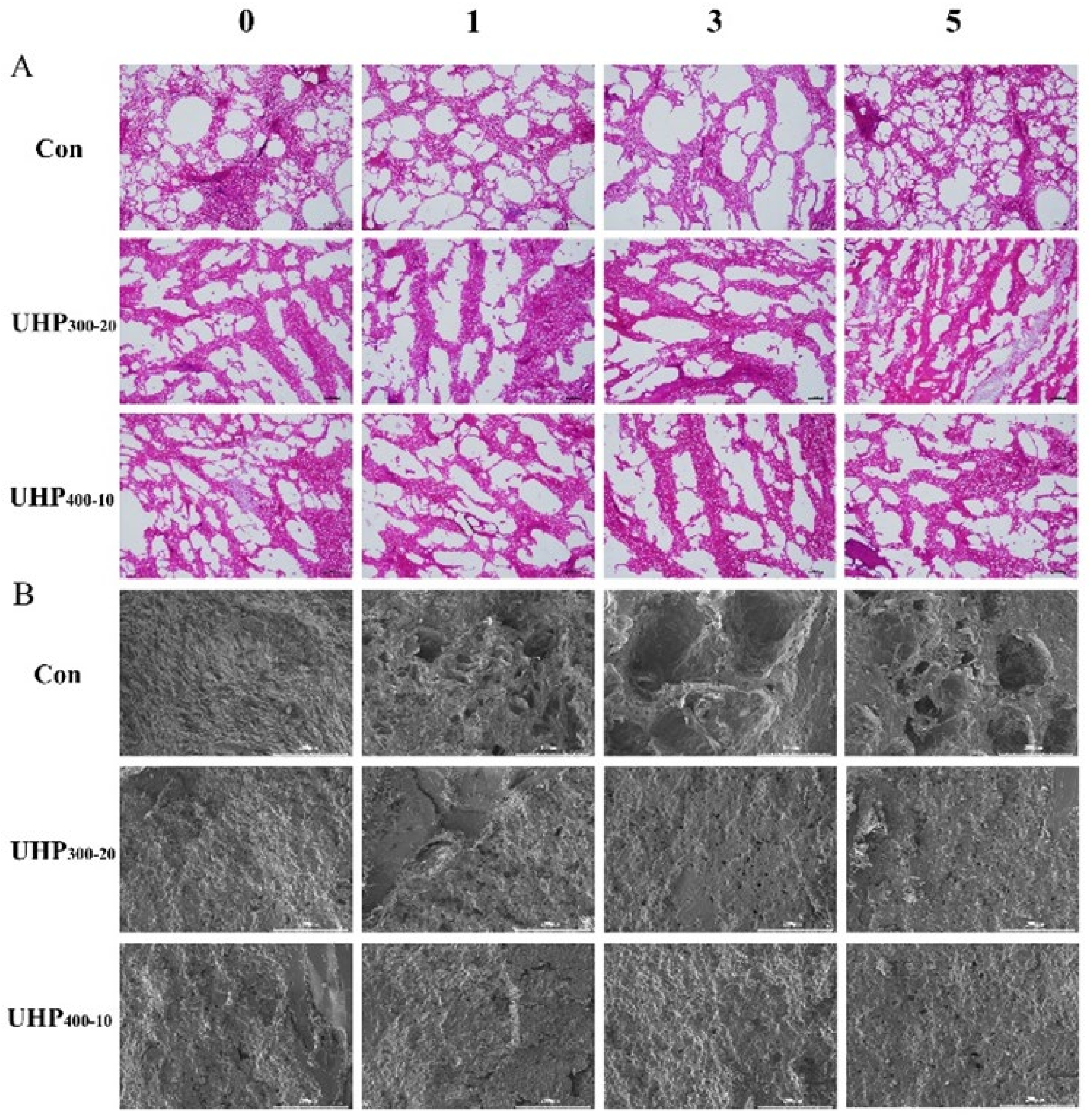
3.5. Changes in Ice Crystal Morphology and Microstructure in Fish Balls
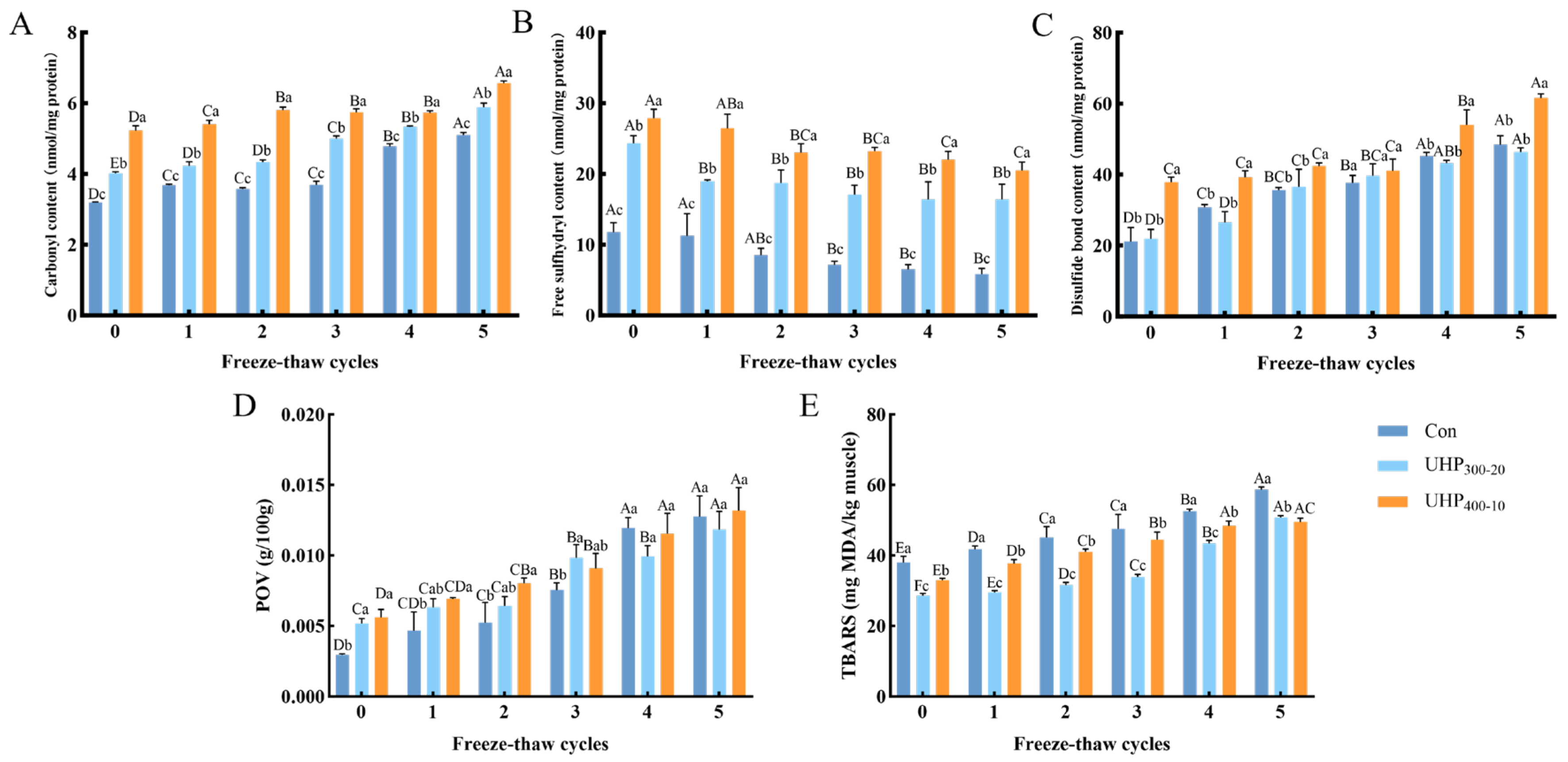
3.6. Changes in Protein and Lipid Oxidation in Fish Balls
3.6.1. Protein Oxidation
3.6.2. Lipid Oxidation
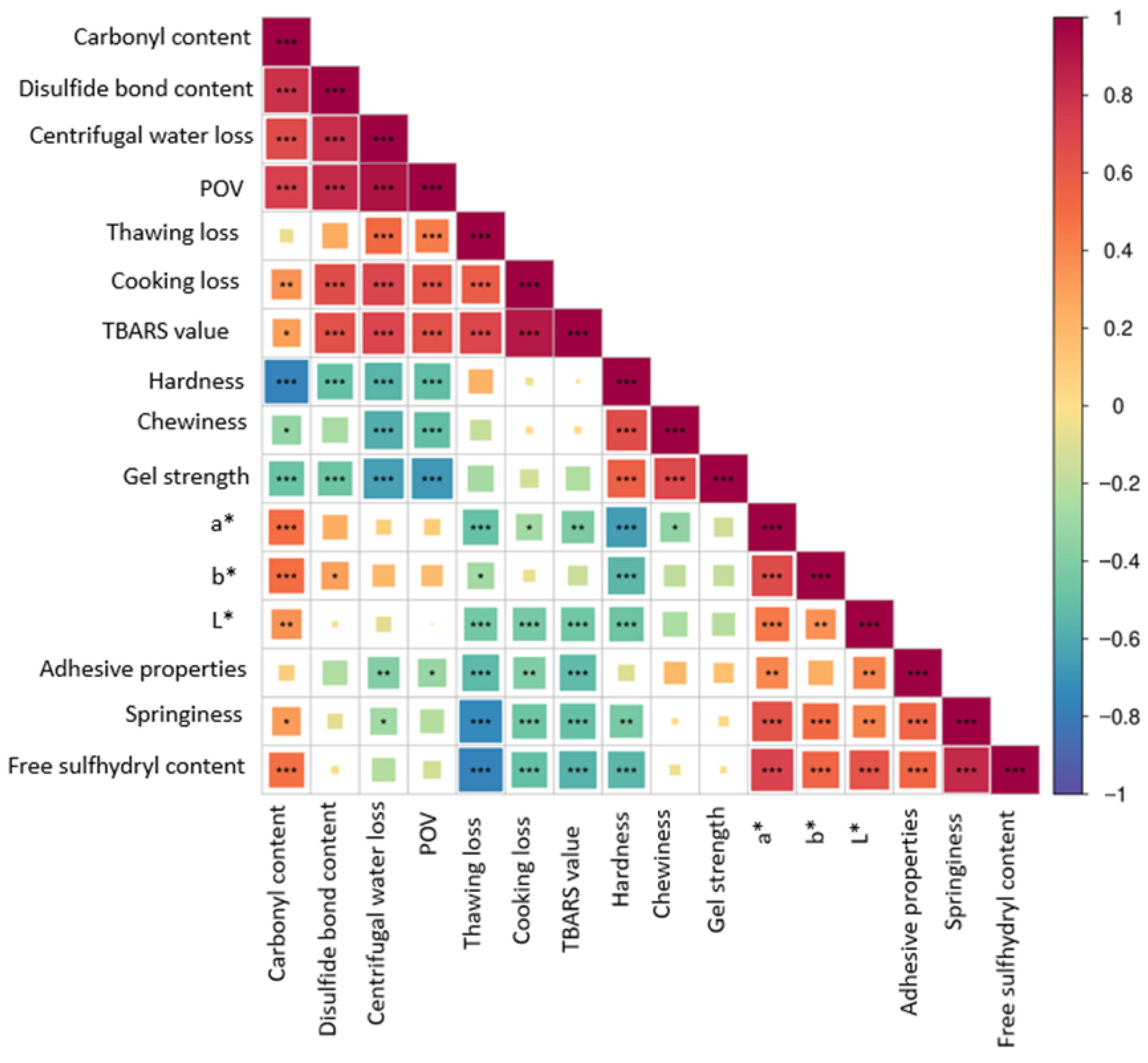
3.7. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UHP | Ultra-high pressure |
| F-T | Freeze–thaw |
| LF-NMR | Low-field nuclear magnetic resonance |
| WHC | Water Holding Capacity |
| SEM | Scanning electron microscopy |
| MP | Myofibrillar protein |
| POV | Peroxide value |
| TBARS | Thiobarbituric acid-reactive substances |
References
- Zhu, Z.; Zhou, Q.; Sun, D.-W. Measuring and controlling ice crystallization in frozen foods: A review of recent developments. Trends Food Sci. Technol. 2019, 90, 13–25. [Google Scholar] [CrossRef]
- Li, N.; Tan, Z.; Ma, R.; Song, Y.; Liu, R.; Zhao, J.; Qin, N.; Li, Y.; Liu, X.; Zhou, D. Using multi-modal spectroscopy technology and microscopic analysis to explore the regulation of ultra-high pressure heat-assisted treatment on the texture of ready-to-eat shrimp during storage. Food Chem. 2025, 464, 141604. [Google Scholar]
- Aubourg, S.P. Impact of high-pressure processing on chemical constituents and nutritional properties in aquatic foods: A review. Int. J. Food Sci. Technol. 2018, 53, 873–891. [Google Scholar]
- Chen, L.; Jiao, D.; Yu, X.; Zhu, C.; Sun, Y.; Liu, M.; Liu, H. Effect of high pressure processing on the physicochemical and sensorial properties of scallop (Mizuhopecten yessoensis) during iced storage. Int. J. Food Sci. Technol. 2022, 57, 1226–1236. [Google Scholar] [CrossRef]
- Zeng, X.; Jiao, D.; Yu, X.; Chen, L.; Sun, Y.; Guo, A.; Zhu, C.; Wu, J.; Liu, J.; Liu, H. Effect of ultra-high pressure on the relationship between endogenous proteases and protein degradation of Yesso scallop (Mizuhopecten yessoensis) adductor muscle during iced storage. Food Chem. X 2022, 15, 100438. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiao, D.; Liu, H.; Zhu, C.; Sun, Y.; Wu, J.; Zheng, M.; Zhang, D. Effects of water distribution and protein degradation on the texture of high pressure-treated shrimp (Penaeus monodon) during chilled storage. Food Control. 2022, 132, 108555. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, A.; Yang, R.; Jia, R.; Zhang, J.; Xu, D.; Yang, W. Myofibrillar protein structure and gel properties of trichiurus haumela surimi subjected to high pressure or high pressure synergistic heat. Food Bioprocess Technol. 2020, 13, 589–598. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, A.; Benjakul, S.; Zou, Y.; Liu, X.; Xiao, S. The mechanism of low-level pressure coupled with heat treatment on water migration and gel properties of Nemipterus virgatus surimi. LWT 2021, 150, 112086. [Google Scholar] [CrossRef]
- Jiang, Y. Frozen surimi and surimi products production process technology. Meat Ind. 2011, 10, 12–14. [Google Scholar]
- Zhang, K.; Liang, Y.; Tan, Z.; Zhou, D.; Li , D. Characterization of a novel chickpea protein-pullulan hydrogels that efficiently load and release sodium salts: Microstructures, molecular dynamics, and rheological properties. Food Res. Int. 2025, 204, 115951. [Google Scholar]
- Ding, J.; Zhao, X.; Li, X.; Huang, Q. Effects of different recovered sarcoplasmic proteins on the gel performance, water distribution and network structure of silver carp surimi. Food Hydrocoll. 2022, 131, 107835. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Pan, Y.; Wei, S.; Xia, Q.; Liu, S.; Ji, H.; Deng, C.; Hao, J. Investigation on the correlation between changes in water and texture properties during the processing of surimi from golden pompano (Trachinotus ovatus). J. Food Sci. 2021, 86, 376–384. [Google Scholar] [CrossRef]
- Luo, H.; Sheng, Z.; Guo, C.; Jia, R.; Yang, W. Quality attributes enhancement of ready-to-eat hairtail fish balls by high-pressure processing. LWT 2021, 147, 111658. [Google Scholar] [CrossRef]
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Oliver, C.N.; Ahn, B.-W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Chen, X.-W.; Li, J.; Bi, Y.-L. Approach to evaluate the sensory quality deterioration of chicken seasoning using characteristic oxidation indicators. Food Chem. X 2023, 17, 100564. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, D.-Y.; Liu, Z.-Y.; Yin, F.-W.; Liu, Z.-Q.; Li, D.-Y.; Shahidi, F. Hydrolysis and oxidation of lipids in mussel Mytilus edulis during cold storage. Food Chem. 2019, 272, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Yang, X.; Zhao, W.; Ma, J.; Fan, X.; Liu, Z.; Dong, X. Modifying yellowfin tuna myofibrillar proteins under ultra-high pressure auxiliary heat treatment: Impact on the conformation, gel properties and digestive characteristics. Food Chem. 2025, 475, 143365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhai, Z.; Yu, X.; Li, D. The Effects of Ultra-High Pressure Combined with Egg White Protein on the Gel Physical Properties of Reduced-Salt Shrimp Surimi. Foods 2025, 14, 2144. [Google Scholar] [CrossRef]
- Jiang, X.; Liang, Q.; Shi, W. Influence of starch on freeze-thaw stability of Hypophthalmichthys molitrix surimi gel observed via ice crystal distribution and gel properties. Food Chem. X 2024, 24, 101995. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Ding, Z.; Yang, D.; Xie, J. The quality properties of frozen large yellow croaker fillets during temperature fluctuation cycles: Improvement by cellobiose and carboxylated cellulose nanofibers. Int. J. Biol. Macromol. 2022, 194, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, C.; Tan, Y.; McClements, D.J.; Wang, L. Insight of rheology, water distribution and in vitro digestive behavior of starch based-emulsion gel: Impact of potato starch concentration. Food Hydrocoll. 2022, 132, 107859. [Google Scholar] [CrossRef]
- Zhu, C.; Zeng, X.; Chen, L.; Liu, M.; Zheng, M.; Liu, J.; Liu, H. Changes in quality characteristics based on protein oxidation and microbial action of ultra-high pressure-treated grass carp (Ctenopharyngodon idella) fillets during magnetic field storage. Food Chem. 2024, 434, 137464. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Li, K.; Ren, D.; Wan, H.; Shi, L.; Cheng, F.; Zhao, W.; Cao, J.; Cheng, Y. Ultra-high pressure treatment improves the gel properties of Lentinan edodes polysaccharides-chicken myofibrillar protein composite. LWT 2025, 223, 117751. [Google Scholar] [CrossRef]
- Bai, X.; Shi, S.; Kong, B.; Chen, Q.; Liu, Q.; Li, Z.; Wu, K.; Xia, X. Analysis of the influencing mechanism of the freeze–thawing cycles on in vitro chicken meat digestion based on protein structural changes. Food Chem. 2023, 399, 134020. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Zhou, A.; Liu, X.; Benjakul, S. Elucidating the molecular mechanism of water migration in myosin gels of Nemipterus virgatus during low pressure coupled with heat treatment. Int. J. Biol. Macromol. 2023, 253, 126815. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, K.; Tan, Z.; Xu, W.; Liu, X.; Zhou, D.; Li, D. Effects of ultrahigh pressure heat-assisted technology on the physicochemical and gelling properties of myofibrillar protein from Penaeus vannamei. Food Chem. 2025, 464, 141697. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kelly, A.; Kerry, J. Effects of high-pressure and heat treatments on physical and biochemical characteristics of oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 2007, 8, 30–38. [Google Scholar] [CrossRef]
- Li, W.; Bai, X.; Xia, X.; Chen, H. Effect of sodium alginate ice glazing on the quality of the freeze-thawed fish balls. Int. J. Biol. Macromol. 2024, 254, 128097. [Google Scholar] [CrossRef]
- Hector, D.; Brew-Graves, C.; Hassen, N.; Ledward, D. Relationship between myosin denaturation and the colour of low-voltage-electrically-stimulated beef. Meat Sci. 1992, 31, 299–307. [Google Scholar] [CrossRef]
- Zhang, S.; Meenu, M.; Xiao, T.; Hu, L.; Ren, J.; Ramaswamy, H.S.; Yu, Y. Insight into the mechanism of pressure shift freezing on water mobility, microstructure, and rheological properties of grass carp surimi gel. Innov. Food Sci. Emerg. Technol. 2024, 91, 103528. [Google Scholar] [CrossRef]
- Wu, G.; Yang, C.; Lin, H.; Hu, F.; Li, X.; Xia, S.; Bruce, H.L.; Roy, B.C.; Huang, F.; Zhang, C. To What Extent Do Low-Voltage Electrostatic Fields Play a Role in the Physicochemical Properties of Pork during Freezing and Storage? J. Agric. Food Chem. 2024, 72, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, X.; Tan, M.; Chen, Z.; Lin, H.; Gao, J.; Zheng, H.; Cao, W. Molecular insights into the antifreeze mechanism of collagen peptides based on their interaction with ice crystals. Food Chem. X 2025, 26, 102334. [Google Scholar] [CrossRef]
- Ganjeh, A.M.; Pinto, C.A.; Casal, S.; Saraiva, J.A. The effects of pressure-based processing technologies on protein oxidation. Food Biosci. 2024, 59, 103963. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Quan, Z.; Li, J.; Liu, Z.; Guo, X.; Dong, X.; Zhou, D.; Zhu, B. Effects of freeze-thaw cycles on texture and protein digestive properties of scallop adductor muscles: Role of protein oxidative changes. Food Chem. 2025, 475, 143351. [Google Scholar] [CrossRef]
- Wan, Z.; Wei, R.; Yang, M.; Xu, X.; Tian, X. Mechanism of brittleness deterioration of pork meatballs induced by freeze–thaw cycles based on ice crystals and molecular conformation. Food Res. Int. 2025, 202, 115711. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Guo, A. Animal and plant protein oxidation: Chemical and functional property significance. Foods 2020, 10, 40. [Google Scholar] [CrossRef]
- Peng, X.; Li, Y.; Yu, J.; Gao, Y.; Zhao, X.; Jia, N. Assessment of the impact of whey protein hydrolysate on myofibrillar proteins in surimi during repeated freeze–thaw cycles: Quality enhancement and antifreeze potential. Food Chem. 2024, 460, 140552. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Wu, J. Marine proteins and peptides: Production, biological activities, and potential applications. Food Innov. Adv. 2023, 2, 69–84. [Google Scholar] [CrossRef]
- Cartagena, L.; Puértolas, E.; de Marañón, I.M. Evolution of quality parameters of high pressure processing (HPP) pretreated albacore (Thunnus alalunga) during long-term frozen storage. Innov. Food Sci. Emerg. Technol. 2020, 62, 102334. [Google Scholar] [CrossRef]
| Freeze–Thaw Cycles | Group | L* | a* | b* | W |
|---|---|---|---|---|---|
| 0 | Con | 69.690 ± 0.909 Ec | −3.132 ± 0.19 BCc | 1.568 ± 0.456 Bc | 69.485 ± 0.906 Ec |
| UHP300-20 | 78.153 ± 0.582 Db | −2.478 ± 0.197 Bb | 2.687 ± 0.438 Bb | 77.846 ± 0.593 Db | |
| UHP400-10 | 81.227 ± 0.902 Ca | −2.033 ± 0.136 Aa | 3.598 ± 0.251 Aa | 80.775 ± 0.884 BCa | |
| 1 | Con | 66.310 ± 0.762 Fb | −2.823 ± 0.185 Ab | 1.970 ± 0.853 ABb | 66.126 ± 0.800 Fb |
| UHP300-20 | 83.417 ± 0.244 Aa | −2.078 ± 0.035 Aa | 3.215 ± 0.188 ABa | 82.980 ± 0.263 Aa | |
| UHP400-10 | 82.823 ± 0.594 Ba | −2.203 ± 0.287 ABa | 3.045 ± 0.699 ABa | 82.403 ± 0.566 Ba | |
| 2 | Con | 74.082 ± 0.359 Bb | −3.322 ± 0.153 Cb | 2.605 ± 0.473 Ab | 73.736 ± 0.359 Bb |
| UHP300-20 | 82.837 ± 0.997 ABa | −2.032 ± 0.06 Aa | 3.043 ± 0.784 ABab | 82.435 ± 0.960 Aa | |
| UHP400-10 | 83.148 ± 0.523 Ba | −2.162 ± 0.117 ABa | 3.653 ± 0.480 Aa | 80.643 ± 4.027 BCa | |
| 3 | Con | 76.178 ± 0.842 Ac | −2.898 ± 0.349 ABb | 1.877 ± 0.353 Bb | 75.926 ± 0.860 Ac |
| UHP300-20 | 79.932 ± 0.548 Cb | −2.193 ± 0.191 Aa | 2.927 ± 0.768 ABab | 79.588 ± 0.515 Cb | |
| UHP400-10 | 85.635 ± 0.500 Aa | −2.582 ± 0.544 Bab | 3.453 ± 1.224 Aa | 84.951 ± 0.465 Aa | |
| 4 | Con | 72.442 ± 0.179 Cc | −3.302 ± 0.125 Cb | 2.247 ± 0.546 ABb | 72.149 ± 0.184 Cc |
| UHP300-20 | 82.200 ± 0.570 Ba | −2.305 ± 0.183 ABa | 3.305 ± 1.109 ABa | 81.719 ± 0.502 Ba | |
| UHP400-10 | 80.360 ± 0.337 Db | −2.157 ± 0.509 ABa | 3.767 ± 0.605 Aa | 79.873 ± 0.300 Cb | |
| 5 | Con | 71.568 ± 0.217 Dc | −3.377 ± 0.116 Cb | 1.768 ± 0.439 Bb | 71.311 ± 0.227 Dc |
| UHP300-20 | 80.607 ± 0.214 Ca | −2.108 ± 0.430 Aa | 3.822 ± 0.411 Aa | 80.115 ± 0.266 Ca | |
| UHP400-10 | 79.308 ± 0.333 Eb | −2.483 ± 0.559 ABa | 2.375 ± 0.760 Bb | 79.008 ± 0.356 Cb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zeng, X.; Zhao, J.; Chi, Y.; Xiu, L.; Zheng, M.; Liu, H. Mechanisms of Quality Preservation in Golden Pomfret Fish Balls Treated with Ultra-High Pressure During Freeze–Thaw Cycles. Foods 2025, 14, 3342. https://doi.org/10.3390/foods14193342
Liu J, Zeng X, Zhao J, Chi Y, Xiu L, Zheng M, Liu H. Mechanisms of Quality Preservation in Golden Pomfret Fish Balls Treated with Ultra-High Pressure During Freeze–Thaw Cycles. Foods. 2025; 14(19):3342. https://doi.org/10.3390/foods14193342
Chicago/Turabian StyleLiu, Jiawen, Xinyao Zeng, Jiaqi Zhao, Yunfeng Chi, Lin Xiu, Mingzhu Zheng, and Huimin Liu. 2025. "Mechanisms of Quality Preservation in Golden Pomfret Fish Balls Treated with Ultra-High Pressure During Freeze–Thaw Cycles" Foods 14, no. 19: 3342. https://doi.org/10.3390/foods14193342
APA StyleLiu, J., Zeng, X., Zhao, J., Chi, Y., Xiu, L., Zheng, M., & Liu, H. (2025). Mechanisms of Quality Preservation in Golden Pomfret Fish Balls Treated with Ultra-High Pressure During Freeze–Thaw Cycles. Foods, 14(19), 3342. https://doi.org/10.3390/foods14193342








