Effect of Selenium Fortification on Growth Performance and Nutritional Compounds of Kale (Brassica oleracea L. Var. acephala DC.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Experimental Site, Growing Conditions and Cultivation
2.3. Determination of Biometric Measurements of Kale
2.4. Determination of Chlorophyll Contents in Kale Leaf
2.5. Determination of Total Phenolic Contents in Kale Leaf
2.6. Determination of Total Se Contents in Kale Leaf
2.7. Determination of Speciation of Se Compounds in Kale Leaf
2.8. Quasi-Targeted Metabolomics Analysis of Kale Leaf
2.8.1. Sample Preparation
2.8.2. HPLC-MS/MS Analysis
2.8.3. Metabolite Identification and Quantification
2.8.4. Data Analysis
2.9. Correlation Analyses Between Metabolic Biomarkers, Se Contents and Se Speciation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of Se Fortification on the Growth Performance of Kale
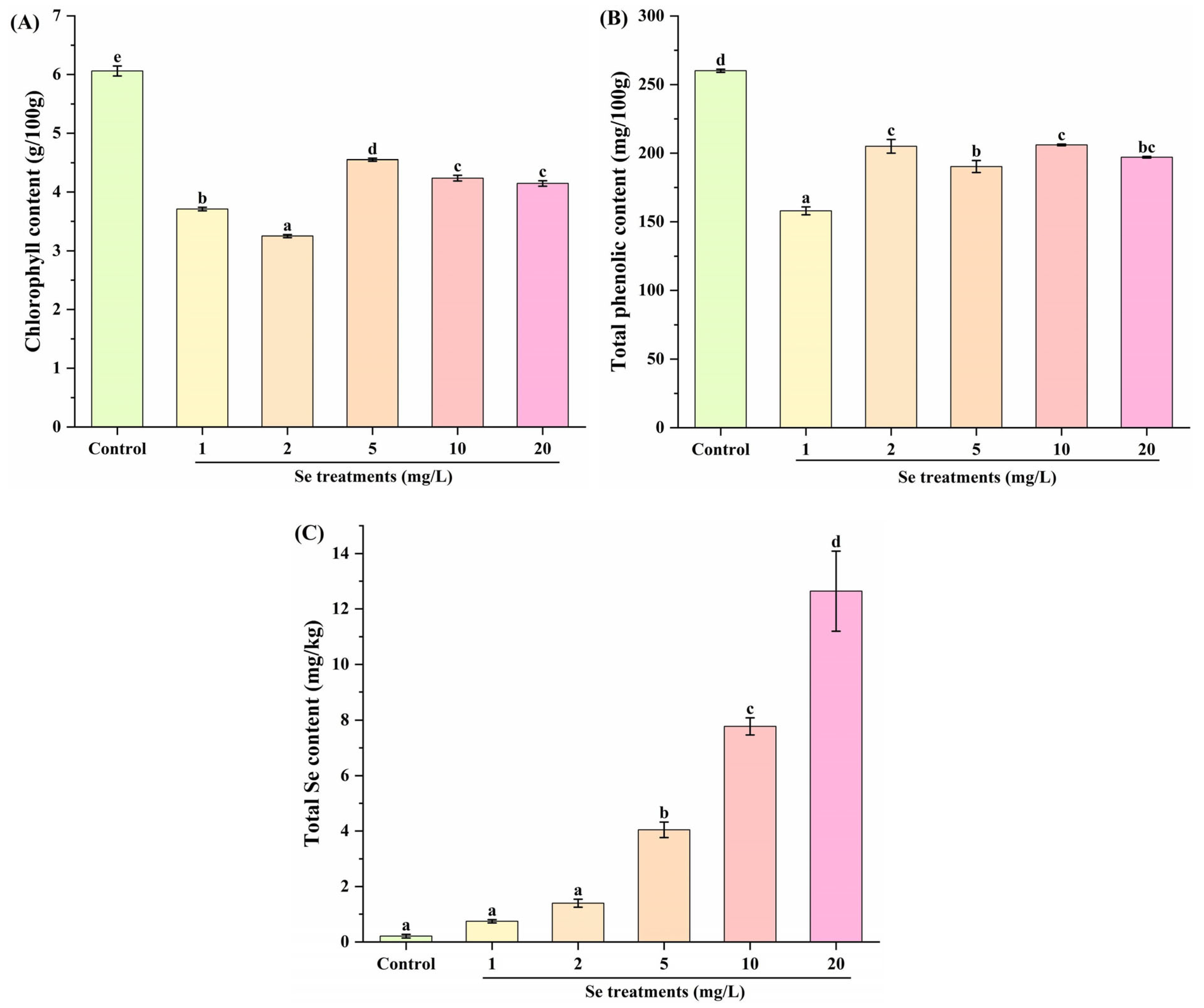
3.2. Effect of Se Fortification on Chlorophyll, Total Phenolic and Total Se Contents in Kale Leaf
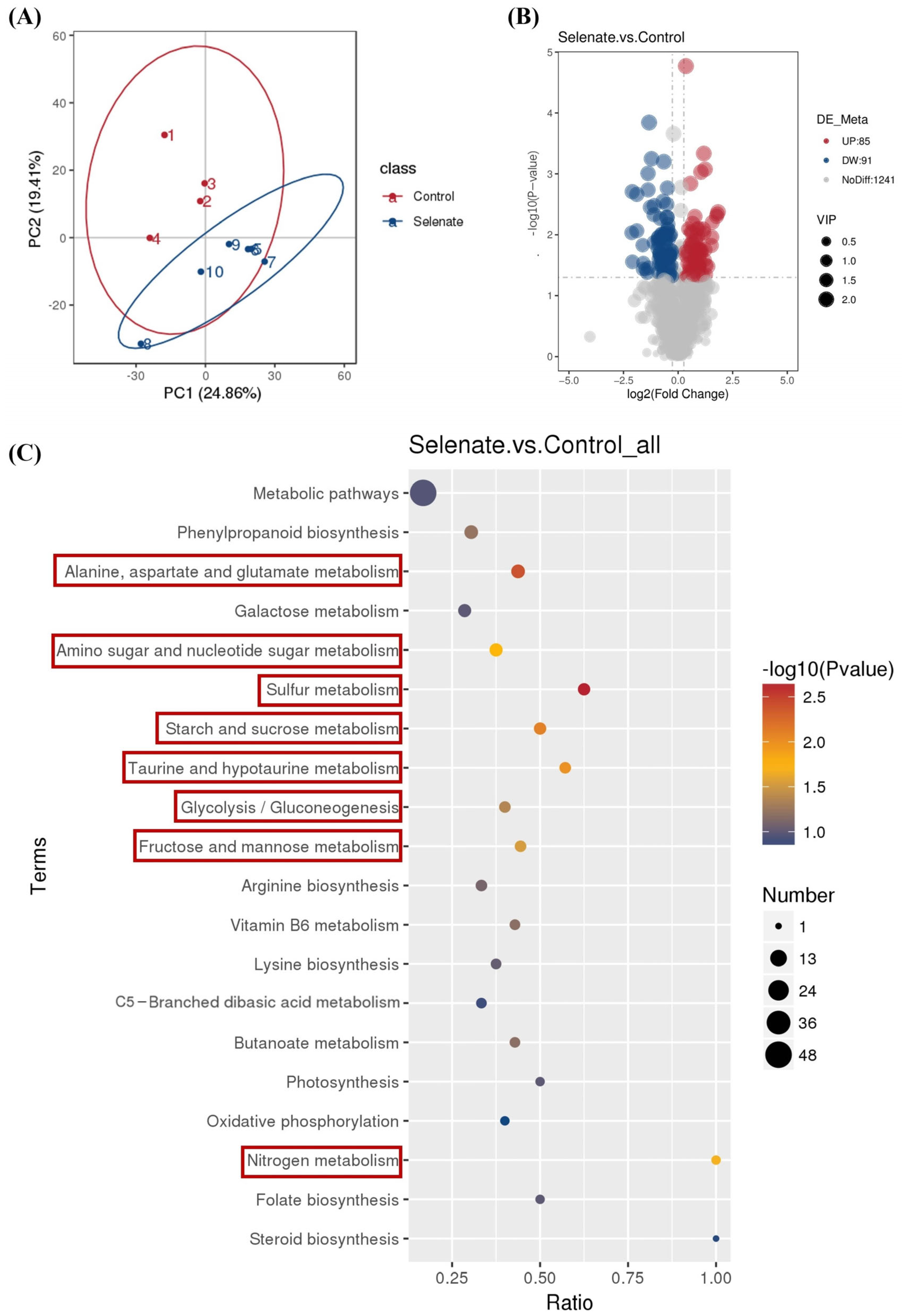
3.3. Effect of Se Fortification on Speciation of Se Compounds in Kale Leaf
3.4. Effect of Se Fortification on Bioactive Compounds in Kale Leaf
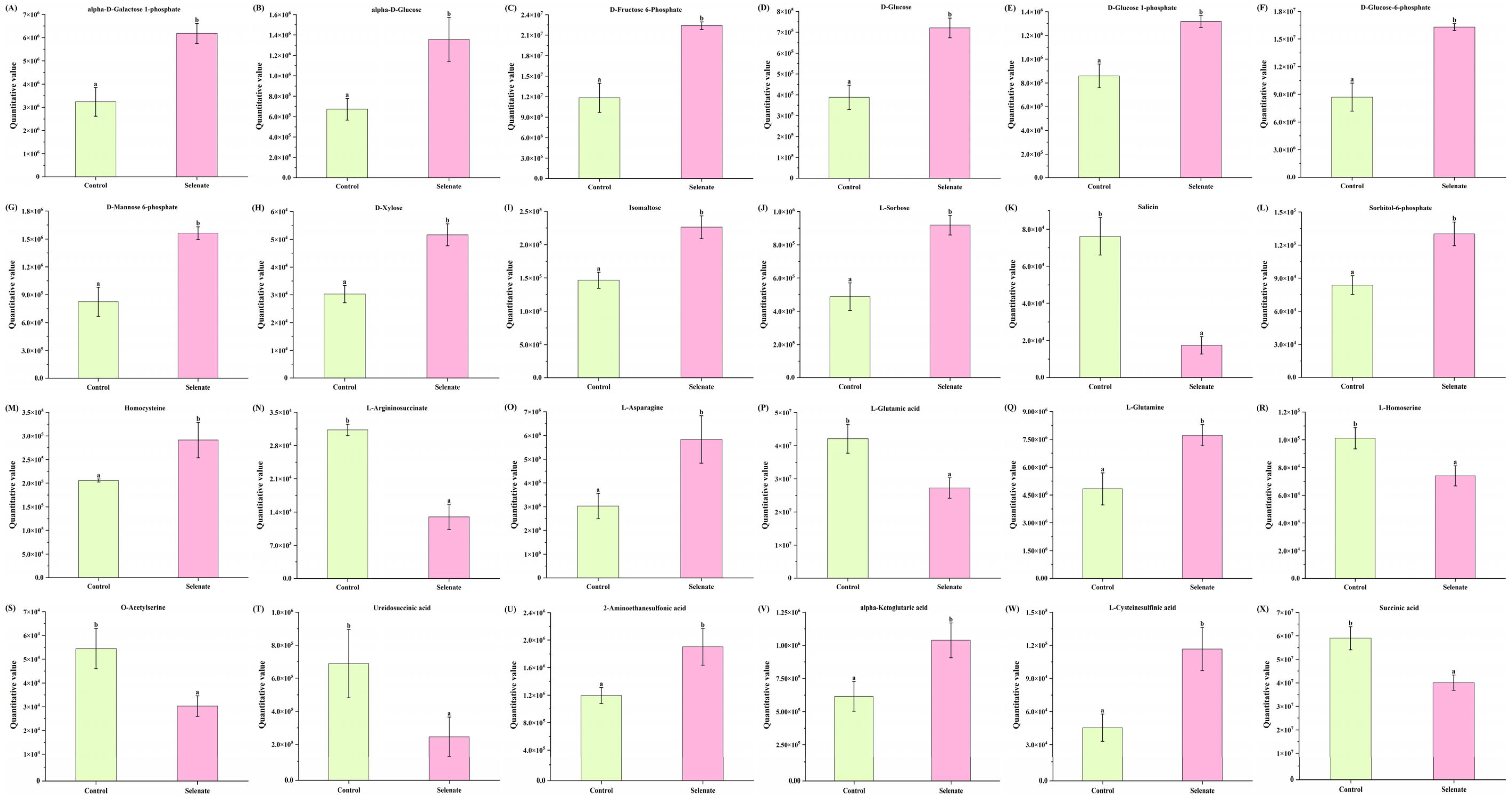
3.4.1. Effect of Se Fortification on Amino Acid and Its Derivatives in Kale Leaf
3.4.2. Effect of Se Fortification on Organic Acid and Its Derivatives in Kale Leaf
3.4.3. Effect of Se Fortification on Carbohydrates and Their Derivatives in Kale Leaf
3.4.4. Effect of Se Fortification on Lipids in Kale Leaf
3.4.5. Effect of Se Fortification on Flavonoids in Kale Leaf
3.4.6. Effect of Se Fortification on Organoheterocyclic Compounds in Kale Leaf
3.4.7. Effect of Se Fortification on Other Differential Metabolites in Kale Leaf
3.5. Metabolic Biomarkers in Kale Leaf Affected by Se Fortification
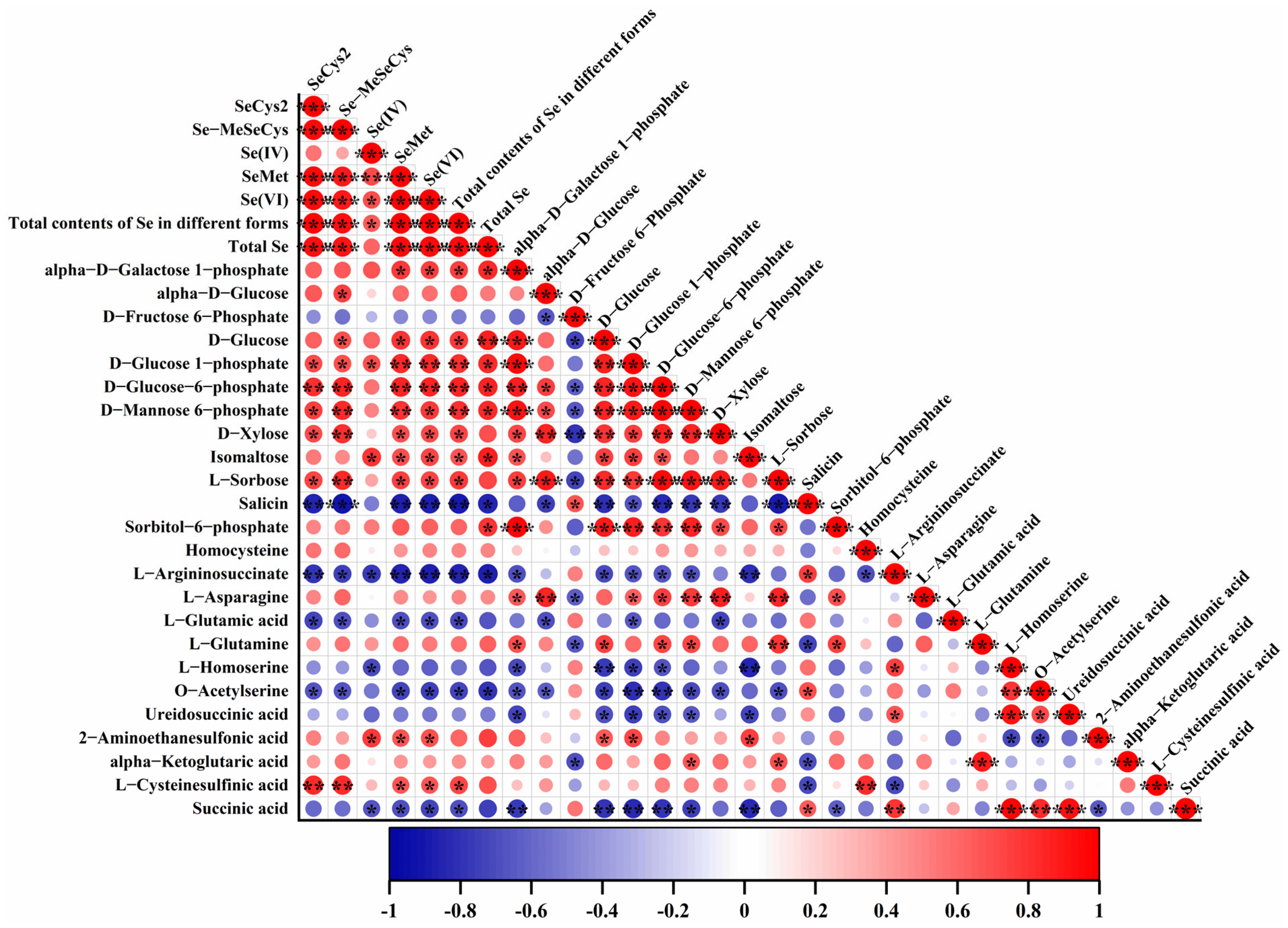
3.6. Correlations Between Metabolic Biomarkers and Se Contents as Well as Speciation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. 2019, 59, 2411–2422. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, H.M.; Shin, M.J.; Arasu, M.V.; Chung, D.Y.; Al-Dhabi, N.A.; Kim, S.J. Effect of different proportion of sulphur treatments on the contents of glucosinolate in kale (Brassica oleracea var. acephala) commonly consumed in Republic of Korea. Saudi J. Biol. Sci. 2018, 25, 349–353. [Google Scholar] [CrossRef]
- Khalid, W.; Fareed, A.; Abdul, R.M.; Asma, A.R.; Hadiqa, F.u.R.; Sajid, A.M.; Saadia, A.; Muhammad, Z.; Saira, S.; Ammar, A.F.; et al. Industrial applications of kale (Brassica oleracea var. sabellica) as a functional ingredient: A review. Int. J. Food Prop. 2023, 26, 489–501. [Google Scholar] [CrossRef]
- Satheesh, N.; Workneh Fanta, S. Kale: Review on nutritional composition, bio-active compounds, anti-nutritional factors, health beneficial properties and value-added products. Cogent Food Agric. 2020, 6, 1811048. [Google Scholar] [CrossRef]
- Haile, A.; Ayalew, T. Comparative study on the effect of bio-slurry and inorganic N-fertilizer on growth and yield of kale (Brassica oleracea L.). Afr. J. Plant Sci. 2018, 12, 81–87. [Google Scholar]
- Rodríguez, V.M.; Soengas, P.; Alonso-Villaverde, V.; Sotelo, T.; Cartea, M.E.; Velasco, P. Effect of temperature stress on the early vegetative development of Brassica oleracea L. BMC Plant Biol. 2015, 15, 145. [Google Scholar] [CrossRef]
- Šamec, D.; Ljubej, V.; Redovniković, I.R.; Fistanić, S.; Salopek-Sondi, B. Low temperatures affect the physiological status and phytochemical content of flat leaf kale (Brassica oleracea var. acephala) Sprouts. Foods 2022, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.X.; Chai, X.R.; Zhong, M.; Zhang, L.; Zhao, P.Y.; Kang, Y.Y.; Guo, J.X.; Yang, X. Effects of nitrogen and magnesium nutrient on the plant growth, quality, photosynthetic characteristics, antioxidant metabolism, and endogenous hormone of Chinese kale (Brassica albograbra Bailey). Sci. Hortic. 2022, 303, 111243. [Google Scholar] [CrossRef]
- Krawczyk, K.K.; Smoleń, S.; Wisła-Świder, A.; Kowalska, I.; Kiełbasa, D.; Pitala, J.; Krzemińska, J.; Waśniowska, J.; Koronowicz, A. Kale (Brassica oleracea L. var. sabellica) biofortified with iodoquinolines: Effectiveness of enriching with iodine and influence on chemical composition. Sci. Hortic. 2024, 323, 112519. [Google Scholar] [CrossRef]
- Leamsamrong, K.; Tongjaroenbuangam, W.; Maneetong, S.; Chantiratikul, A.; Chinrasri, O.; Chantiratikul, P. Physicochemical contents, antioxidant activities, and acute toxicity assessment of selenium-enriched Chinese Kale (Brassica oleracea var. alboglabra L.) seedlings. J. Chem. 2019, 2019, 7983038. [Google Scholar] [CrossRef]
- Zagrodzki, P.; Paśko, P.; Galanty, A.; Tyszka-Czochara, M.; Wietecha-Posłuszny, R.; Rubió, P.S.; Bartoń, H.; Prochownik, E.; Muszyńska, B.; Sułkowska-Ziaja, K.; et al. Does selenium fortification of kale and kohlrabi sprouts change significantly their biochemical and cytotoxic properties? J. Trace Elem. Med. Biol. 2020, 59, 126466. [Google Scholar] [CrossRef] [PubMed]
- Maneetong, S.; Chookhampaeng, S.; Chantiratikul, A.; Chinrasri, O.; Thosaikham, W.; Sittipout, R.; Chantiratikul, P. Hydroponic cultivation of selenium-enriched kale (Brassica oleracea var. alboglabra L.) seedling and speciation of selenium with HPLC-ICP-MS. Microchem. J. 2013, 108, 87–91. [Google Scholar] [CrossRef]
- Li, J.; Liang, D.L.; Qin, S.Y.; Feng, P.Y.; Wu, X.P. Effects of selenite and selenate application on growth and shoot selenium accumulation of pak choi (Brassica chinensis L) during successive planting conditions. Environ. Sci. Pollut. R 2015, 22, 11076–11086. [Google Scholar] [CrossRef]
- Tavan, M.; Wee, B.; Fuentes, S.; Pang, A.; Brodie, G.; Viejo, C.G.; Gupta, D. Biofortification of kale microgreens with selenate-selenium using two delivery methods: Selenium-rich soilless medium and foliar application. Sci. Hortic. 2024, 323, 112522. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Żmudzki, P.; Bieniek, U.; Prochownik, E.; Domínguez-Álvarez, E.; Bierła, K.; Łobiński, R.; Szpunar, J.; et al. Varied effect of fortification of kale sprouts with novel organic selenium compounds on the synthesis of sulphur and phenolic compounds in relation to cytotoxic, antioxidant and anti-inflammatory activity. Microchem. J. 2022, 179, 107509. [Google Scholar] [CrossRef]
- Niu, Y.Q.; Zheng, M.X.; Tian, D.G.; Zhu, Y.M.; Chen, H.; Zhu, Y.J.; Su, H.L. Quasi-targeted metabonomics reveals metabolites associated with antioxidant activity of Mesona chinensis Benth Cultivar Xiaoye. Plants 2025, 14, 1585. [Google Scholar] [CrossRef]
- Fang, X.; Xue, R.; Xiao, J.Y.; Pu, Q.; Wang, Y.F.; Yuan, Y.; Liu, B.; Sui, M.Y.; Jiang, G.X.; Niaz, R.; et al. Effects of different fermentation modes on tea leaves: Revealing the metabolites modification by quasi-targeted metabolomics. Food Biosci. 2024, 62, 105223. [Google Scholar] [CrossRef]
- Ma, X.Y.; Jin, W.; Wang, L.F.; Chen, W.W.; Wang, Y.F.; Wen, H.B.; Cao, X.J. Metabolic response of Sinosolenaia oleivora to heat and drought stress using a quasi-targeted metabolomics approach. Comp. Biochem. Phys. D 2025, 55, 101499. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, M.C.; Zhen, L.; Wang, Y.D.; Wang, Y.F.; Qin, Y.Y.; Zhang, Z.H.; Zhao, T.R.; Cao, J.X.; Liu, Y.P.; et al. Ultra-high hydrostatic pressure pretreatment on White Que Zui Tea: Chemical constituents, antioxidant, cytoprotective, and anti-Inflammatory activities. Foods 2023, 12, 628. [Google Scholar] [CrossRef]
- Ren, J.; Yang, L.; Cao, R.F.; Wang, Y.D.; Zhang, C.; Yu, X.J.; Meng, W.D.; Ye, X.L. Integrated metabolome and transcriptome analysis provides new insights into the glossy graft cucumber fruit (Cucumis sativus L.). Int. J. Mol. Sci. 2023, 24, 12147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Hu, L.P.; Liu, G.M.; Zhang, D.S.; He, H.J. Evaluation of the nutritional quality of Chinese Kale (Brassica alboglabra Bailey) using UHPLC-Quadrupole-Orbitrap MS/MS-based metabolomics. Molecules 2017, 22, 1262. [Google Scholar] [CrossRef]
- NY/T 3082-2017; Determination of Chlorophyll Content in Fruits, Vegetables and Derived Products-Spectrophotometry Method. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2017.
- Liao, Q.; Liang, P.X.; Xing, Y.; Yao, Z.F.; Chen, J.P.; Pan, L.P.; Deng, Y.Q.; Liu, Y.X.; Huang, D. Optimizing selenium application for enhanced quality and nutritional value of spring tea (Camellia sinensis). Horticulturae 2025, 11, 423. [Google Scholar] [CrossRef]
- GB 5009.93-2017; National Food Safety Standard Determination of Selenium in Food. China Standard Publishing House: Beijing, China, 2017.
- NY/T 3556-2020; Determination of Selenocysteine and Selenomethionine in Cereals– Liquid Chromatography–Inductively Coupled Plasma Mass Spectrometry. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2020.
- Wu, Y.; Xiao, H.W.; Zhang, H.; Pan, A.L.; Shen, J.; Sun, J.; Liang, Z.H.; Pi, J.S. Quasi-targeted metabolomics approach reveal the metabolite differences of three poultry eggs. Foods 2023, 12, 2765. [Google Scholar] [CrossRef] [PubMed]
- Lefsrud, M.; Kopsell, D.; Wenzel, A.; Sheehan, J. Changes in kale (Brassica oleracea L. var. acephala) carotenoid and chlorophyll pigment concentrations during leaf ontogeny. Sci. Hortic. 2007, 112, 136–141. [Google Scholar] [CrossRef]
- Viltres-Portales, M.; Sánchez-Martín, M.J.; Llugany, M.; Boada, R.; Valiente, M. Selenium biofortification of microgreens: Influence on phytochemicals, pigments and nutrients. Plant Physiol. Biochem. 2024, 206, 108283. [Google Scholar] [CrossRef]
- Saeedi, M.; Soltani, F.; Babalar, M.; Izadpanah, F.; Wiesner-Reinhold, M.; Baldermann, S. Selenium fortification alters the growth, antioxidant characteristics and secondary metabolite profiles of cauliflower (Brassica oleracea var. botrytis) cultivars in hydroponic culture. Plants 2021, 10, 1537. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Picchi, V.; Tava, A.; Doria, F.; Walley, P.G.; Dever, L.; di Bella, M.C.; Arena, D.; Ben Ammar, H.; Lo Scalzo, R.; et al. Insights into the phytochemical composition of selected genotypes of organic kale (Brassica oleracea L. var. acephala). J. Food Compos. Anal. 2024, 125, 105721. [Google Scholar] [CrossRef]
- Ortega-Hernández, E.; Antunes-Ricardo, M.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Selenium, sulfur, and methyl jasmonate treatments improve the accumulation of lutein and glucosinolates in kale Sprouts. Plants 2022, 11, 1271. [Google Scholar] [CrossRef]
- Tangjaidee, P.; Swedlund, P.; Xiang, J.Q.; Yin, H.Q.; Quek, S.Y. Selenium-enriched plant foods: Selenium accumulation, speciation, and health functionality. Front. Nutr. 2023, 9, 962312. [Google Scholar] [CrossRef]
- Silva, M.A.; de Sousa, G.F.; Bañuelos, G.; Amaral, D.; Brown, P.H.; Guilherme, L.R.G. Selenium speciation in Se-enriched soybean grains from biofortified plants grown under different methods of selenium application. Foods 2023, 12, 1214. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Freeman, J.L.; Arroyo, I.S. Selenium content and speciation differences in selenium enriched soups made from selenium biofortified plants. J. Food Compos. Anal. 2022, 105, 104255. [Google Scholar] [CrossRef]
- Ortega-Hernández, E.; Antunes-Ricardo, M.; Jacobo-Velázquez, D.A. Improving the health-benefits of kales (Brassica oleracea L. var. acephala DC) through the application of controlled abiotic stresses: A review. Plants 2021, 10, 2629. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Liu, C.; Shen, C.X.; Ran, B.H.; Yang, Z.P.; Zhou, L.; Xu, H.H.; Tang, Y. The impact of high voltage electrostatic field on the storage quality and metabolism of Chinese kale. J. Food Meas. Charact. 2024, 18, 3405–3424. [Google Scholar] [CrossRef]
- Ren, Y.R.; Zhang, Q.; Li, X.; Zhang, T.Y.; Tian, D.C.; Liu, L.; Dong, X.Y.; Wang, Z.Y.; Chai, M.F. Effects of selenium content on growth, antioxidant activity, and key selenium-enriched gene expression in alfalfa sprouts. Foods 2024, 13, 2261. [Google Scholar] [CrossRef]
- Chao, W.; Rao, S.; Chen, Q.W.; Zhang, W.W.; Liao, Y.L.; Ye, J.B.; Cheng, S.Y.; Yang, X.Y.; Xu, F. Advances in research on the involvement of selenium in regulating plant ecosystems. Plants 2022, 11, 2712. [Google Scholar] [CrossRef]
- Skrypnik, L.; Feduraev, P.; Golovin, A.; Maslennikov, P.; Styran, T.; Antipina, M.; Riabova, A.; Katserov, D. The integral boosting effect of selenium on the secondary metabolism of higher plants. Plants 2022, 11, 3432. [Google Scholar] [CrossRef]
- Zagrodzki, P.; Wiesner, A.; Marcinkowska, M.; Jamrozik, M.; Domínguez-Álvarez, E.; Bierła, K.; Łobiński, R.; Szpunar, J.; Handzlik, J.; Galanty, A.; et al. Relationships between molecular characteristics of novel organic selenium compounds and the formation of sulfur compounds in selenium biofortified kale sprouts. Molecules 2023, 28, 2062. [Google Scholar] [CrossRef]
- Hu, H.F.; Hu, J.K.; Wang, Q.D.; Xiang, M.L.; Zhang, Y.R. Transcriptome analysis revealed accumulation-assimilation of selenium and physio-biochemical changes in alfalfa (Medicago sativa L.) leaves. J. Sci. Food Agric. 2022, 102, 4577–4588. [Google Scholar] [CrossRef]
- Xiong, Y.Z.; Xiang, X.M.; Xiao, C.M.; Zhang, N.; Cheng, H.; Rao, S.; Cheng, S.Y.; Li, L. Illumina RNA and SMRT sequencing reveals the mechanism of uptake and transformation of selenium nanoparticles in soybean seedlings. Plants 2023, 12, 789. [Google Scholar] [CrossRef]
- Liu, N.X.; Yu, Q.T.; Chen, B.C.; Li, C.X.; Bu, F.S.; Li, J.R.; Peng, X.L.; Lu, Y.C. Selenium alleviates low-temperature stress in rice by regulating metabolic networks and functional genes. Agriculture 2025, 15, 1489. [Google Scholar] [CrossRef]
- Maas, M.N.; Hintzen, J.C.J.; Porzberg, M.R.B.; Mecinović, J. Trimethyllysine: From carnitine biosynthesis to epigenetics. Int. J. Mol. Sci. 2020, 21, 9451. [Google Scholar] [CrossRef]
- Tian, J.; He, G.; Mai, K.S.; Liu, C.D.; Zhou, H.H.; Wen, H. Dietary ala-Gln ameliorated growth suppression and intestinal injury induced by soya saponin in zebrafish. Aquaculture 2020, 529, 735748. [Google Scholar] [CrossRef]
- Liu, J.; Zong, C.G.; Yu, X.; Ding, Y.; Chang, B.; Wang, R.Y.; Sang, L.X. Alanyl-glutamine (Ala-Gln) ameliorates dextran sulfate sodium (DSS)-induced acute colitis by regulating the gut microbiota, PI3K-Akt/NF-κB/STAT3 signaling, and associated pulmonary injury. ACS Infect. Dis. 2023, 9, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wu, Y.J.; Zeng, C.X.; Wu, H.R.; Jia, G.F.; Ye, J.H.; Qin, S.; Liu, Z.H.; Shi, M. Phytochemicals from fractioned dark tea water extract enhance the digestive enzyme inhibition, antioxidant capacities and glucose-lipid balance. Food Res. Int. 2025, 204, 115957. [Google Scholar] [CrossRef]
- Sumalan, R.L.; Croitor, L.; Petric, M.; Radulov, I.; Bourosh, P.; Sumalan, R.M.; Crisan, M. p-Aminobenzoate organic salts as potential plant growth regulators for tomatoes. Molecules 2020, 25, 1635. [Google Scholar] [CrossRef] [PubMed]
- Levent, G.; Božić, A.; Petrujkić, B.T.; Callaway, T.R.; Poole, T.L.; Crippen, T.L.; Harvey, R.B.; Ochoa-García, P.; Corral-Luna, A.; Yeater, K.M.; et al. Assessment of potential anti-methanogenic and antimicrobial activity of ethyl nitroacetate, α-lipoic acid, taurine and L-cysteinesulfinic acid in vitro. Microorganisms 2024, 12, 34. [Google Scholar] [CrossRef]
- Li, B.; Förster, C.; Robert, C.A.M.; Züst, T.; Hu, L.; Machado, R.A.R.; Berset, J.D.; Handrick, V.; Knauer, T.; Hensel, G.; et al. Convergent evolution of a metabolic switch between aphid and caterpillar resistance in cereals. Sci. Adv. 2018, 4, eaat6797. [Google Scholar] [CrossRef]
- Megías-Pérez, R.; Hahn, C.; Ruiz-Matute, A.I.; Behrends, B.; Albach, D.C.; Kuhnert, N. Changes in low molecular weight carbohydrates in kale during development and acclimation to cold temperatures determined by chromatographic techniques coupled to mass spectrometry. Food Res. Int. 2020, 127, 108727. [Google Scholar] [CrossRef]
- Ganesh, M.; Mohankumar, M. Extraction and identification of bioactive components in Sida cordata (Burm. f.) using gas chromatography-mass spectrometry. J. Food Sci. Tech. 2017, 54, 3082–3091. [Google Scholar] [CrossRef]
- Cui, W.W.; Liu, D.; Gu, W.; Chu, B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 2021, 28, 2536–2551. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Zhang, X.J.; Zeng, F.K. Biological functions and health benefits of flavonoids in fruits and vegetables: A contemporary review. Foods 2025, 14, 155. [Google Scholar] [CrossRef]
- Peng, P.; Zou, J.R.; Zhong, B.; Zhang, G.X.; Zou, X.F.; Xie, T.P. Protective effects and mechanisms of flavonoids in renal ischemia-reperfusion injury. Pharmacology 2023, 108, 27–36. [Google Scholar] [CrossRef]
- Naguib, A.E.M.M.; El-Baz, F.K.; Salama, Z.A.; Hanaa, H.A.E.B.; Ali, H.F.; Gaafar, A.A. Enhancement of phenolics, flavonoids and glucosinolates of Broccoli (Brassica olaracea, var. Italica) as antioxidants in response to organic and bio-organic fertilizers. J. Saudi Soc. Agric. Sci. 2012, 11, 135–142. [Google Scholar] [CrossRef]
- Wei, F.; Shi, Z.G.; Wan, R.; Li, Y.X.; Wang, Y.J.; An, W.; Qin, K.; Cao, Y.L.; Chen, X.Y.; Wang, X.Y.; et al. Impact of phosphorus fertilizer level on the yield and metabolome of goji fruit. Sci. Rep. 2020, 10, 14656. [Google Scholar] [CrossRef]
- Wang, T.; Tang, C.Y.; Chen, J.B.; Liang, J.; Li, Y.L.; Li, X.Z. Accumulation characteristics of natural Ophiocordyceps sinensis metabolites driven by environmental factors. Metabolites 2024, 14, 414. [Google Scholar] [CrossRef]
- Díaz-Urbano, M.; Velasco, P.; Cartea, M.E.; Rodríguez, V.M. Metabolism reorganization in kale (Brassica oleracea L. var acephala) populations with divergent glucosinolate content under thermal stresses. Agronomy 2022, 12, 2652. [Google Scholar] [CrossRef]
- Leverson, J.D.; Zhang, H.; Chen, J.; Tahir, S.K.; Phillips, D.C.; Xue, J.; Nimmer, P.; Jin, S.; Smith, M.; Xiao, Y.; et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 2015, 6, e1590. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, K.; Mulugeta, K.; Hailu, E.; Tamene, W.; Alagar Yadav, S. Insights for integrative medicinal potentials of Ethiopian Kale (Brassica carinata): Investigation of antibacterial, antioxidant potential and phytocompounds composition of its leaves. Chin. Herb. Med. 2021, 13, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Moldovan, A.; Kekina, H.; Kharchenko, V.; Sekara, A.; Vasileva, V.; Skrypnik, L.; Tallarita, A.; Caruso, G. Joint biofortification of plants with selenium and iodine: New field of discoveries. Plants 2021, 10, 1352. [Google Scholar] [CrossRef]
- Kiełkowska, A.; Adamus, A. Exogenously applied polyamines reduce reactive oxygen species, enhancing cell division and the shoot regeneration from Brassica oleracea L. var. capitata protoplasts. Agronomy 2021, 11, 735. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Hayırlıoglu-Ayaz, S.; Alpay-Karaoglu, S.; Grúz, J.; Valentová, K.; Ulrichová, J.; Strnad, M. Phenolic acid contents of kale (Brassica oleraceae L. var. acephala DC.) extracts and their antioxidant and antibacterial activities. Food Chem. 2008, 107, 19–25. [Google Scholar] [CrossRef]
- Zhou, C.R.; Zhang, J.B.; Wu, Y.L.; Cheng, H.Y.; Pang, Q.L.; Xiao, Y.H.; Li, D.; Pan, C.P. Metabolomic analysis on the mechanism of nanoselenium biofortification improving the Siraitia grosvenorii nutritional and health value. Foods 2022, 11, 3019. [Google Scholar] [CrossRef]
- Sun, P.B.; Ge, G.T.; Sun, L.; Bao, J.; Zhao, M.Q.E.; Hao, J.F.; Zhang, Y.H.; Liu, G.S.; Wang, Z.J.; Jia, Y.S. Metabolomics combined with physiology and transcriptomics reveal the regulation of key nitrogen metabolic pathways in alfalfa by foliar spraying with nano-selenium. J. Nanobiotechnol. 2025, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Miao, P.J.; Li, D.; Wu, Y.L.; Zhou, C.R.; Pan, C.P. Improving red pitaya fruit quality by nano-selenium biofortification to enhance phenylpropanoid and betalain biosynthesis. Ecotoxicol. Environ. Saf. 2023, 267, 115653. [Google Scholar] [CrossRef]
- Schaffer, S.; Kim, H.W. Effects and mechanisms of taurine as a therapeutic agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Q.; Liu, H.Q.; Peng, L.X.; He, W.G.; Li, S.Y. Potential clinical applications of alpha-ketoglutaric acid in diseases (Review). Mol. Med. Rep. 2022, 25, 151. [Google Scholar] [CrossRef] [PubMed]
- Maione, S.; Leyva, J.; Palazzo, E.; Stella, L.; Rossi, F. L-Cysteinesulfinic acid modulates cardiovascular function in the periaqueductal gray area of rat. J. Cardiovasc. Pharmacol. 1998, 32, 650–653. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Wu, H.; Peng, Y.H.; Du, M.Y.; Luo, H.H. Metabolomics revealed the enrichment of nutritional components in purple rice grains exposed to different selenium concentrations. J. Cereal Sci. 2025, 125, 104249. [Google Scholar] [CrossRef]


| Number | RT (min) | Name | Formula | Molecular Weight (Da) | Fold Change | p-Value | Change |
|---|---|---|---|---|---|---|---|
| ESI+ mode | |||||||
| 1 | 0.760 | Trimethyllysine | C9H21N2O2 | 189.275 | 2.96414 | 0.006252 | Up |
| 2 | 0.840 | 2-Amino-2-deoxy-D-gluconate | C6H13NO6 | 195.171 | 1.49011 | 0.016303 | Up |
| 3 | 0.840 | Homocysteine | C4H9NO2S | 135.185 | 1.41606 | 0.033335 | Up |
| 4 | 0.860 | D-Threonine | C4H9NO3 | 119.119 | 0.69360 | 0.048622 | Down |
| 5 | 0.880 | N-Hydroxyl-tryptamine | C10H12N2O | 176.215 | 1.64435 | 0.045438 | Up |
| 6 | 0.886 | L-Glutamic acid | C5H9NO4 | 147.130 | 0.64700 | 0.025971 | Down |
| 7 | 0.960 | N-Acetyl-L-valine | C7H13NO3 | 159.183 | 1.58498 | 0.006555 | Up |
| 8 | 1.000 | D-Proline betaine | C7H13NO2 | 143.180 | 1.30347 | 0.022577 | Up |
| 9 | 1.008 | Stachydrine | C7H13NO2 | 143.180 | 1.82899 | 0.008002 | Up |
| 10 | 1.040 | S-Methyl-L-cysteine | C4H9NO2S | 135.185 | 1.28090 | 1.70E-05 | Up |
| 11 | 1.040 | N alpha-Acetyl-L-arginine | C8H16N4O3 | 216.238 | 0.69416 | 0.024535 | Down |
| 12 | 1.830 | Homocysteic acid | C4H9NO5S | 183.180 | 0.79828 | 0.035047 | Down |
| 13 | 1.840 | L-Methionine sulfone | C5H11NO4S | 181.210 | 0.77200 | 0.010351 | Down |
| 14 | 1.840 | N-Acetyl-Dl-glutamic acid | C7H11NO5 | 189.166 | 1.39598 | 0.019346 | Up |
| 15 | 1.860 | D-Homocysteine | C4H9NO2S | 135.185 | 0.73808 | 0.011723 | Down |
| 16 | 2.270 | DL-m-Tyrosine | C9H11NO3 | 181.190 | 0.69750 | 0.005192 | Down |
| 17 | 3.780 | Kynurenine | C10H12N2O3 | 208.214 | 0.23397 | 0.027744 | Down |
| 18 | 8.680 | Santacruzamate A | C15H22N2O3 | 278.350 | 0.26958 | 0.002170 | Down |
| 19 | 18.290 | N-Acetyltryptamine | C12H14N2O | 202.252 | 1.24839 | 0.049374 | Up |
| ESI− mode | |||||||
| 1 | 0.843 | L-Asparagine | C4H8N2O3 | 132.120 | 1.92649 | 0.030460 | Up |
| 2 | 0.850 | Aspartate | C4H7NO4 | 133.103 | 1.46312 | 0.014558 | Up |
| 3 | 0.850 | Ala-Gln | C8H15N3O4 | 217.220 | 2.42031 | 0.044904 | Up |
| 4 | 0.868 | L-Glutamine | C5H10N2O3 | 146.140 | 1.59856 | 0.045253 | Up |
| 5 | 1.031 | Ureidosuccinic acid | C5H8N2O5 | 176.130 | 0.35666 | 0.037044 | Down |
| 6 | 1.056 | O-Acetylserine | C5H9NO4 | 147.130 | 0.55573 | 0.020191 | Down |
| 7 | 1.110 | Allysine | C6H11NO3 | 145.074 | 1.65456 | 0.017013 | Up |
| 8 | 1.900 | L-Homoserine | C4H9NO3 | 119.119 | 0.73223 | 0.035539 | Down |
| 9 | 2.150 | N-Acetylalanine | C5H9NO3 | 131.130 | 0.60878 | 0.029064 | Down |
| 10 | 4.090 | Phenprobamate | C10H13NO2 | 179.216 | 0.74104 | 0.025886 | Down |
| 11 | 5.410 | L-Argininosuccinate | C10H18N4O6 | 290.273 | 0.41486 | 0.003546 | Down |
| 12 | 6.070 | 3-(2-Naphthyl)-L-alanine | C13H13NO2 | 215.095 | 0.26935 | 0.008577 | Down |
| 13 | 6.220 | 3-(2-Naphthyl)-D-alanine | C13H13NO2 | 215.248 | 0.76105 | 0.021510 | Down |
| Number | RT (min) | Name | Formula | Molecular Weight (Da) | Fold Change | p-Value | Change |
|---|---|---|---|---|---|---|---|
| ESI+ mode | |||||||
| 1 | 0.980 | N-Acetyl-L-carnosine | C11H16N4O4 | 268.270 | 1.39197 | 0.008014 | Up |
| 2 | 1.490 | Maleamic acid | C4H5NO3 | 115.090 | 2.00340 | 0.047445 | Up |
| 3 | 4.120 | 4-Hydroxybenzoate | C7H6O3 | 138.121 | 1.27311 | 0.018775 | Up |
| 4 | 4.450 | p-Aminobenzoate | C7H7NO2 | 137.136 | 3.45323 | 0.004585 | Up |
| 5 | 6.070 | 4-Hydroxy-3,5-diisopropylbenzaldehyde | C13H18O2 | 206.200 | 1.69562 | 0.029888 | Up |
| 6 | 6.470 | 2-Aminoethylphosphonate | C2H8NO3P | 125.064 | 0.56516 | 0.021502 | Down |
| 7 | 9.452 | Dodecanedioic acid | C12H22O4 | 230.300 | 0.32932 | 0.036416 | Down |
| 8 | 10.950 | trans-Aconitic acid | C6H6O6 | 174.016 | 0.69121 | 0.009376 | Down |
| 9 | 11.010 | Phosphoric acid | H3O4P | 97.995 | 0.79450 | 0.018272 | Down |
| ESI− mode | |||||||
| 1 | 0.870 | alpha-Ketoglutaric acid | C5H6O5 | 146.098 | 1.68713 | 0.041975 | Up |
| 2 | 0.880 | L-Cysteinesulfinic acid | C3H7NO4S | 153.160 | 2.57192 | 0.033105 | Up |
| 3 | 0.900 | Hydroxycitric acid | C6H8O8 | 208.124 | 1.44818 | 0.037908 | Up |
| 4 | 1.040 | 2-Aminoethanesulfonic acid | C2H7NO3S | 125.015 | 1.59222 | 0.045750 | Up |
| 5 | 1.936 | Succinic acid | C4H6O4 | 118.090 | 0.67941 | 0.011762 | Down |
| 6 | 1.938 | Methylmalonate | C4H6O4 | 118.090 | 0.66665 | 0.013200 | Down |
| 7 | 3.420 | Glutaconic acid | C5H6O4 | 130.099 | 0.64917 | 0.009588 | Down |
| 8 | 4.450 | 3-Hydroxyanthranilic acid | C7H7NO3 | 153.135 | 0.58887 | 0.031454 | Down |
| 9 | 5.901 | Vanillic acid | C8H8O4 | 168.150 | 0.53096 | 0.011337 | Down |
| 10 | 6.100 | Isovanillic acid | C8H8O4 | 168.150 | 0.55737 | 0.012052 | Down |
| Number | RT (min) | Name | Formula | Molecular Weight (Da) | Fold Change | p-Value | Change |
|---|---|---|---|---|---|---|---|
| ESI+ mode | |||||||
| 1 | 0.900 | Isomaltose | C12H22O11 | 342.297 | 1.54350 | 0.008773 | Up |
| 2 | 1.660 | D-Threose | C4H8O4 | 120.042 | 0.66552 | 0.024747 | Down |
| 3 | 4.260 | DIMBOA glucoside | C15H19NO10 | 374.108 | 2.88963 | 0.010864 | Up |
| 4 | 5.730 | Pteroside A | C21H30O8 | 410.464 | 0.74440 | 0.045354 | Down |
| 5 | 6.050 | Icariside B2 | C19H30O8 | 386.194 | 1.95132 | 0.007890 | Up |
| 6 | 7.470 | 7-O-Methylaloeresin A | C29H30O11 | 554.550 | 1.96790 | 0.022436 | Up |
| 7 | 10.150 | Sedoheptulose anhydride | C7H12O6 | 192.167 | 0.79070 | 0.009938 | Down |
| ESI− mode | |||||||
| 1 | 0.860 | Sorbitol-6-phosphate | C6H15O9P | 262.045 | 1.55425 | 0.013097 | Up |
| 2 | 0.860 | D-Glucose 1-phosphate | C6H13O9P | 260.136 | 1.53388 | 0.022855 | Up |
| 3 | 0.860 | D-Fructose 6-phosphate | C6H13O9P | 260.136 | 1.89067 | 0.033529 | Up |
| 4 | 0.860 | D-Glucose-6-phosphate | C6H13O9P | 260.030 | 1.87176 | 0.036772 | Up |
| 5 | 0.870 | D-Glucopyranose | C6H12O6 | 180.156 | 1.73418 | 0.019685 | Up |
| 6 | 0.870 | D-Galactaric acid | C6H10O8 | 210.038 | 1.44618 | 0.021811 | Up |
| 7 | 0.870 | alpha-D-Galactose 1-phosphate | C6H13O9P | 260.136 | 1.91270 | 0.032810 | Up |
| 8 | 0.870 | D-Mannose 6-phosphate | C6H13O9P | 260.136 | 1.89630 | 0.044741 | Up |
| 9 | 0.890 | L-Sorbose | C6H12O6 | 180.160 | 1.88091 | 0.033118 | Up |
| 10 | 0.894 | D-Glucuronic acid | C6H10O7 | 194.140 | 1.88042 | 0.046651 | Up |
| 11 | 0.900 | D-Galactose | C6H12O6 | 180.160 | 1.82545 | 0.033758 | Up |
| 12 | 0.910 | 6-Phosphogluconic acid | C6H13O10P | 276.135 | 1.64561 | 0.039733 | Up |
| 13 | 0.912 | alpha-D-Glucose | C6H12O6 | 180.160 | 2.01494 | 0.027591 | Up |
| 14 | 0.920 | D-Ribose | C5H10O5 | 150.130 | 1.48251 | 0.001443 | Up |
| 15 | 0.940 | D-Glucose | C6H12O6 | 180.156 | 1.85706 | 0.031938 | Up |
| 16 | 0.984 | N-Acetylglucosamine | C8H15NO6 | 221.210 | 1.54461 | 0.009389 | Up |
| 17 | 1.010 | D-Xylose | C5H10O5 | 150.131 | 1.70502 | 0.005010 | Up |
| 18 | 4.989 | Salicin | C13H18O7 | 286.280 | 0.22890 | 0.009265 | Down |
| Number | RT (min) | Name | Formula | Molecular Weight (Da) | Fold Change | p-Value | Change |
|---|---|---|---|---|---|---|---|
| ESI+ mode | |||||||
| 1 | 1.060 | Methyl oleate | C19H36O2 | 296.488 | 1.81502 | 0.039804 | Up |
| 2 | 5.250 | 1-Hexadecanol | C16H34O | 242.441 | 3.55889 | 0.004228 | Up |
| 3 | 10.900 | Palmitoleic acid | C16H30O2 | 254.408 | 0.79256 | 0.032195 | Down |
| 4 | 10.990 | LPC (1-acyl 16:2) | C24H46NO7P | 491.600 | 0.55850 | 0.019798 | Down |
| 5 | 11.005 | trans-2-Hexenal | C6H10O | 98.140 | 0.76749 | 0.022241 | Down |
| 6 | 11.330 | LysoPC 18:3 (2n isomer) | C26H48NO7P | 517.200 | 0.69023 | 0.026496 | Down |
| 7 | 11.360 | LysoPC 14:0 (2n isomer) | C22H46NO7P | 467.300 | 0.38479 | 0.000983 | Down |
| 8 | 11.370 | Lysopc 14:0 | C22H46NO7P | 467.577 | 0.38528 | 0.001830 | Down |
| 9 | 11.450 | LysoPC 15:1 | C23H46NO7P | 479.100 | 0.59558 | 0.026791 | Down |
| 10 | 11.540 | LPC(1-acyl 16:1) | C24H48NO7P | 493.610 | 0.62069 | 0.002815 | Down |
| 11 | 11.570 | LysoPC 16:1 | C24H48NO7P | 493.300 | 0.64477 | 0.009996 | Down |
| 12 | 11.940 | LysoPC 15:0 | C23H48NO7P | 481.300 | 0.78497 | 0.044473 | Down |
| ESI− mode | |||||||
| 1 | 1.690 | 9,10-EODE | C18H32O3 | 296.000 | 0.61987 | 0.040417 | Down |
| 2 | 1.860 | (E)-3-(4-Hydroxyphenyl)Propenoic Acid (2S)-3-(beta-D-Glucopyranosyloxy)-2-Hydroxypropyl Ester | C18H24O10 | 400.377 | 2.32277 | 0.025892 | Up |
| 3 | 5.747 | 3-Methyladipic acid | C7H12O4 | 160.170 | 0.74442 | 0.034135 | Down |
| 4 | 11.270 | Lysope 14:0 | C19H40NO7P | 425.500 | 0.42945 | 0.000565 | Down |
| 5 | 11.908 | Hexadecanedioic acid | C16H30O4 | 286.410 | 1.44060 | 0.040142 | Up |
| Number | RT (min) | Name | Formula | Molecular Weight (Da) | Fold Change | p-Value | Change |
|---|---|---|---|---|---|---|---|
| ESI+ mode | |||||||
| 1 | 0.710 | methylLuteolin-C-hexoside | C22H22O11 | 448.400 | 0.39236 | 0.034781 | Down |
| 2 | 0.900 | 8-C-hexosyl-apigenin O-hexosyl-O-hexoside | C33H40O20 | 756.669 | 1.97583 | 0.017199 | Up |
| 3 | 0.970 | methylChrysoeriol-5-O-hexoside | C23H24O11 | 476.430 | 2.06676 | 0.000935 | Up |
| 4 | 0.970 | Irisolidone-7-O-beta-d-glucoside | C23H24O11 | 476.430 | 1.59313 | 0.013537 | Up |
| 5 | 6.180 | Daidzin | C21H20O9 | 416.111 | 0.52885 | 0.016847 | Down |
| 6 | 6.190 | Chrysin O-hexoside | C21H20O9 | 416.200 | 0.46892 | 0.003323 | Down |
| 7 | 6.280 | 4′-O-Glucosylvitexin | C27H30O15 | 594.518 | 0.66480 | 0.042288 | Down |
| 8 | 6.540 | Quercetin-O-glucoside | C21H20O12 | 464.376 | 2.10679 | 0.018089 | Up |
| 9 | 7.520 | C-pentosyl-chrysoeriol 7-O-feruloylhexoside | C37H38O18 | 770.210 | 2.85714 | 0.014988 | Up |
| 10 | 7.590 | Chrysin O-malonylhexoside | C24H22O12 | 502.431 | 2.10725 | 0.019679 | Up |
| ESI− mode | |||||||
| 1 | 1.010 | Sakuranetin | C16H14O5 | 286.279 | 1.72006 | 0.020090 | Up |
| 2 | 6.780 | Genistin | C21H20O10 | 432.380 | 0.53560 | 0.010864 | Down |
| 3 | 7.080 | Heptamethoxyflavone | C22H24O9 | 432.420 | 0.65885 | 0.011248 | Down |
| 4 | 7.300 | Azaleatin | C16H12O7 | 316.265 | 0.37452 | 0.041797 | Down |
| Number | RT (min) | Name | Formula | Molecular Weight (Da) | Fold Change | p-Value | Change |
|---|---|---|---|---|---|---|---|
| ESI+ mode | |||||||
| 1 | 0.730 | Deoxynojirimycin | C6H13NO4 | 163.173 | 1.63225 | 0.044442 | Up |
| 2 | 1.090 | 3-Indoleacetonitrile | C10H8N2 | 156.180 | 1.74573 | 0.028890 | Up |
| 3 | 1.509 | Hypoxanthine | C5H4N4O | 136.110 | 0.58954 | 0.009671 | Down |
| 4 | 1.830 | Zarzissine | C5H5N5 | 135.054 | 0.71998 | 0.008105 | Down |
| 5 | 1.840 | N-Methylnicotinamide | C7H8N2O | 136.151 | 0.72667 | 0.006758 | Down |
| 6 | 2.010 | (S)-2-Phenyloxirane | C8H8O | 120.058 | 0.74614 | 0.032044 | Down |
| 7 | 2.910 | 4-Pyridoxate | C8H9NO4 | 183.161 | 0.23329 | 0.001957 | Down |
| 8 | 3.950 | 4-Methyl-5-thiazoleethanol | C6H9NOS | 143.100 | 0.54310 | 0.030762 | Down |
| 9 | 4.480 | N-gamma-Acetyl-N-2-Formyl-5-Methoxykynurenamine | C13H16N2O4 | 264.277 | 0.54189 | 0.013823 | Down |
| 10 | 5.898 | NNK | C10H13N3O2 | 207.230 | 1.87094 | 0.028485 | Up |
| 11 | 7.142 | Indole-3-carboxylic acid | C9H7NO2 | 161.160 | 0.46336 | 0.004700 | Down |
| 12 | 12.565 | Alantolactone | C15H20O2 | 232.320 | 0.63620 | 0.042875 | Down |
| ESI− mode | |||||||
| 1 | 0.927 | Indole-2-carboxylic acid | C9H7NO2 | 161.160 | 2.19170 | 0.045829 | Up |
| 2 | 5.905 | Brazilin | C16H14O5 | 286.279 | 0.53742 | 0.025127 | Down |
| Number | RT (min) | Name | Formula | Molecular Weight (Da) | Class | Fold Change | p-Value | Change |
|---|---|---|---|---|---|---|---|---|
| ESI+ mode | ||||||||
| 1 | 0.890 | 4-Hydroxycinnamaldehyde | C9H8O2 | 148.159 | Phenylpropanoids and polyketides | 0.68576 | 0.034948 | Down |
| 2 | 1.840 | Caffeic aldehyde | C9H8O3 | 164.100 | Phenylpropanoids and polyketides | 0.70968 | 0.005165 | Down |
| 3 | 5.720 | 4-Methylumbelliferyl phenylphosphonate | C16H13O5P | 316.240 | Phenylpropanoids and polyketides | 1.83270 | 0.048113 | Up |
| 4 | 6.424 | Fangchinoline | C37H40N2O6 | 608.720 | Phenylpropanoids and polyketides | 0.39810 | 0.000144 | Down |
| 5 | 7.158 | Dihydrocoumarin | C9H8O2 | 148.159 | Phenylpropanoids and polyketides | 0.81987 | 0.018214 | Down |
| 6 | 9.238 | 6-Methylcoumarin | C10H8O2 | 160.170 | Phenylpropanoids and polyketides | 1.84664 | 0.010285 | Up |
| 7 | 9.290 | Ethyl coumarin-3-carboxylate | C12H10O4 | 218.205 | Phenylpropanoids and polyketides | 0.58299 | 0.023577 | Down |
| 8 | 10.992 | Methyl eugenol | C11H14O2 | 178.230 | Phenylpropanoids and polyketides | 0.63596 | 0.000633 | Down |
| 9 | 0.960 | 2′-deoxy-5-(hydroxymethyl)cytidine | C10H15N3O5 | 257.240 | Nucleotide and its derivates | 1.34418 | 0.048231 | Up |
| 10 | 1.029 | Cytidine | C9H13N3O5 | 243.220 | Nucleotide and its derivates | 1.74257 | 0.034440 | Up |
| 11 | 1.030 | NADP | C21H28N7O17P3 | 743.400 | Nucleotide and its derivates | 0.32475 | 0.014813 | Down |
| 12 | 3.928 | Xanthosine | C10H12N4O6 | 284.225 | Nucleotide and its derivates | 0.46649 | 0.013369 | Down |
| 13 | 4.040 | 1-Methylguanosine | C11H15N5O5 | 297.267 | Nucleotide and its derivates | 0.64768 | 0.023146 | Down |
| 14 | 4.140 | 2′-O-methyladenosine | C11H15N5O4 | 281.268 | Nucleotide and its derivates | 1.96054 | 0.017207 | Up |
| 15 | 4.540 | N6-Succinyl adenosine | C14H17N5O8 | 383.313 | Nucleotide and its derivates | 0.71137 | 0.001703 | Down |
| 16 | 5.130 | 3′-Aenylic acid | C10H14N5O7P | 347.221 | Nucleotide and its derivates | 0.59098 | 0.032129 | Down |
| 17 | 1.060 | Borneol | C10H18O | 154.249 | Terpenoids | 1.55483 | 0.044955 | Up |
| 18 | 6.040 | Citroside A | C19H30O8 | 386.194 | Terpenoids | 2.00210 | 0.018877 | Up |
| 19 | 9.751 | Longifolene | C15H24 | 204.350 | Terpenoids | 0.75707 | 0.048815 | Down |
| 20 | 9.970 | Beta-Ionone | C13H20O | 192.297 | Terpenoids | 0.82862 | 0.048116 | Down |
| 21 | 10.140 | Elemol | C15H26O | 222.198 | Terpenoids | 0.75625 | 0.023813 | Down |
| 22 | 10.795 | Tanshinone II B | C19H18O4 | 310.347 | Terpenoids | 0.76223 | 0.021306 | Down |
| 23 | 0.880 | N1-Acetylspermine | C12H28N4O | 244.377 | Amines | 0.72696 | 0.008203 | Down |
| 24 | 4.430 | N,N-Dimethyl-1,4-phenylenediamine | C8H12N2 | 136.194 | Amines | 2.43345 | 0.031564 | Up |
| 25 | 6.350 | 1-Decanoyl-2-hydroxy-sn-glycero-3-phosphocholine | C18H38NO7P | 411.500 | Amines | 1.39888 | 0.045250 | Up |
| 26 | 9.540 | 4-D-Hydroxysphinganine | C18H39NO3 | 317.507 | Amines | 0.69954 | 0.003165 | Down |
| 27 | 0.800 | Pyridoxamine | C8H12N2O2 | 168.090 | Vitamins | 1.59019 | 0.034441 | Up |
| 28 | 0.970 | Pyridoxine di-O-hexoside | C20H31NO13 | 493.465 | Vitamins | 1.75554 | 0.034493 | Up |
| 29 | 1.050 | Vitamin D2 | C28H44O | 396.648 | Vitamins | 1.35118 | 0.029204 | Up |
| 30 | 1.530 | Pyridoxine | C8H11NO3 | 169.178 | Vitamins | 1.75554 | 0.018088 | Down |
| 31 | 5.100 | N-Feruloyl putrescine | C14H20N2O3 | 264.100 | Phenols and its derivatives | 2.33433 | 0.017474 | Up |
| 32 | 6.490 | Sinapaldehyde | C11H12O4 | 208.211 | Phenols and its derivatives | 0.53038 | 0.012306 | Down |
| 33 | 7.532 | Ferulaldehyde | C10H10O3 | 178.185 | Phenols and its derivatives | 0.82819 | 0.020825 | Down |
| 34 | 9.209 | Bornyl acetate | C12H20O2 | 196.290 | Phenols and its derivatives | 0.56864 | 0.005409 | Down |
| 35 | 4.910 | N-p-Coumaroylputrescine | C13H18N2O2 | 234.290 | Phenolic acids | 2.26059 | 0.000463 | Up |
| 36 | 5.540 | Hydroxycinnamate | C9H8O3 | 164.158 | Phenolic acids | 0.61848 | 0.045476 | Down |
| 37 | 11.630 | 5-O-Caffeoylshikimic acid | C16H16O8 | 336.293 | Phenolic acids | 1.72815 | 0.038816 | Up |
| 38 | 1.000 | iP9G | C16H23N5O5 | 365.384 | Phytohormones | 2.28196 | 0.009327 | Up |
| 39 | 4.620 | trans-zeatin N-glucoside | C16H23N5O6 | 381.200 | Phytohormones | 1.67891 | 0.024310 | Up |
| 40 | 1.540 | Lupinine | C10H19NO | 169.267 | Alkaloids and derivatives | 0.77060 | 0.043363 | Down |
| 41 | 2.834 | Hordenine | C10H15NO | 165.230 | Alkaloids and derivatives | 0.33202 | 0.028973 | Down |
| 42 | 0.680 | Spermidine | C7H19N3 | 145.246 | Polyamine | 0.63059 | 0.035179 | Down |
| 43 | 4.285 | 5-Hydroxymethylfurfural | C6H6O3 | 126.110 | Organooxygen compounds | 0.60215 | 0.020847 | Down |
| 44 | 6.120 | Sattabacin | C13H18O2 | 206.280 | Benzene and substituted derivatives | 1.85913 | 0.011933 | Up |
| ESI− mode | ||||||||
| 1 | 0.690 | Thymidine 5′-diphosphate | C10H16N2O11P2 | 402.190 | Nucleotide and its derivates | 0.59820 | 0.009880 | Down |
| 2 | 0.730 | dATP | C10H16N5O12P3 | 491.001 | Nucleotide and its derivates | 0.71596 | 0.019214 | Down |
| 3 | 0.980 | 2-deoxyglucose-6-phosphate | C6H13O8P | 244.136 | Nucleotide and its derivates | 2.35486 | 0.000842 | Up |
| 4 | 4.289 | Thymidine | C10H14N2O5 | 242.230 | Nucleotide and its derivates | 0.52871 | 0.041315 | Down |
| 5 | 1.038 | Shikimic acid | C7H10O5 | 174.150 | Phenolic acids | 2.22761 | 0.008091 | Up |
| 6 | 5.801 | Caffeic acid | C9H8O4 | 180.160 | Phenolic acids | 3.39126 | 0.004990 | Up |
| 7 | 6.040 | 2,6-Di-tert-butylphenol | C14H22O | 206.320 | Phenolic acids | 1.98445 | 0.033550 | Up |
| 8 | 1.040 | P-Coumaryl alcohol | C9H10O2 | 150.174 | Phenylpropanoids and polyketides | 1.36602 | 0.019139 | Up |
| 9 | 6.970 | Oxyresveratrol | C14H12O4 | 244.240 | Phenylpropanoids and polyketides | 0.65530 | 0.029290 | Down |
| 10 | 0.950 | Bisulfurous acid | C11H8O2 | 172.180 | Vitamins | 1.86849 | 0.017363 | Up |
| 11 | 5.920 | 3-O-p-coumaroyl shikimic acid O-hexoside | C22H26O12 | 482.100 | Alcohols and polyols | 1.74780 | 0.012347 | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.-Y.; Liao, H.; Shen, L.-C.; Zou, Q.; Lv, T.-T.; Wang, M.; Wang, X.-Y. Effect of Selenium Fortification on Growth Performance and Nutritional Compounds of Kale (Brassica oleracea L. Var. acephala DC.). Foods 2025, 14, 3283. https://doi.org/10.3390/foods14183283
Zeng X-Y, Liao H, Shen L-C, Zou Q, Lv T-T, Wang M, Wang X-Y. Effect of Selenium Fortification on Growth Performance and Nutritional Compounds of Kale (Brassica oleracea L. Var. acephala DC.). Foods. 2025; 14(18):3283. https://doi.org/10.3390/foods14183283
Chicago/Turabian StyleZeng, Xiu-Ying, Han Liao, Le-Cheng Shen, Qi Zou, Ting-Ting Lv, Mei Wang, and Xiao-Yin Wang. 2025. "Effect of Selenium Fortification on Growth Performance and Nutritional Compounds of Kale (Brassica oleracea L. Var. acephala DC.)" Foods 14, no. 18: 3283. https://doi.org/10.3390/foods14183283
APA StyleZeng, X.-Y., Liao, H., Shen, L.-C., Zou, Q., Lv, T.-T., Wang, M., & Wang, X.-Y. (2025). Effect of Selenium Fortification on Growth Performance and Nutritional Compounds of Kale (Brassica oleracea L. Var. acephala DC.). Foods, 14(18), 3283. https://doi.org/10.3390/foods14183283








