Probiotics and Postbiotics for Green Control of Foodborne Pathogens: Intelligent Detection and Biopreservation Strategies for Safer Foods
Abstract
1. Introduction
2. Chemical Preservatives in Food Safety: Risks and Limitations
2.1. Common Chemical Preservatives and Their Uses
2.2. Documented Health Risks
2.3. Consumer-Driven Demand for Alternatives
3. Probiotics and Postbiotics in the Context of Food Safety
3.1. Definitions and Differences
3.2. Key Microbial Strains and Metabolites
3.3. Mechanistic Pathways in Inhibiting Pathogens
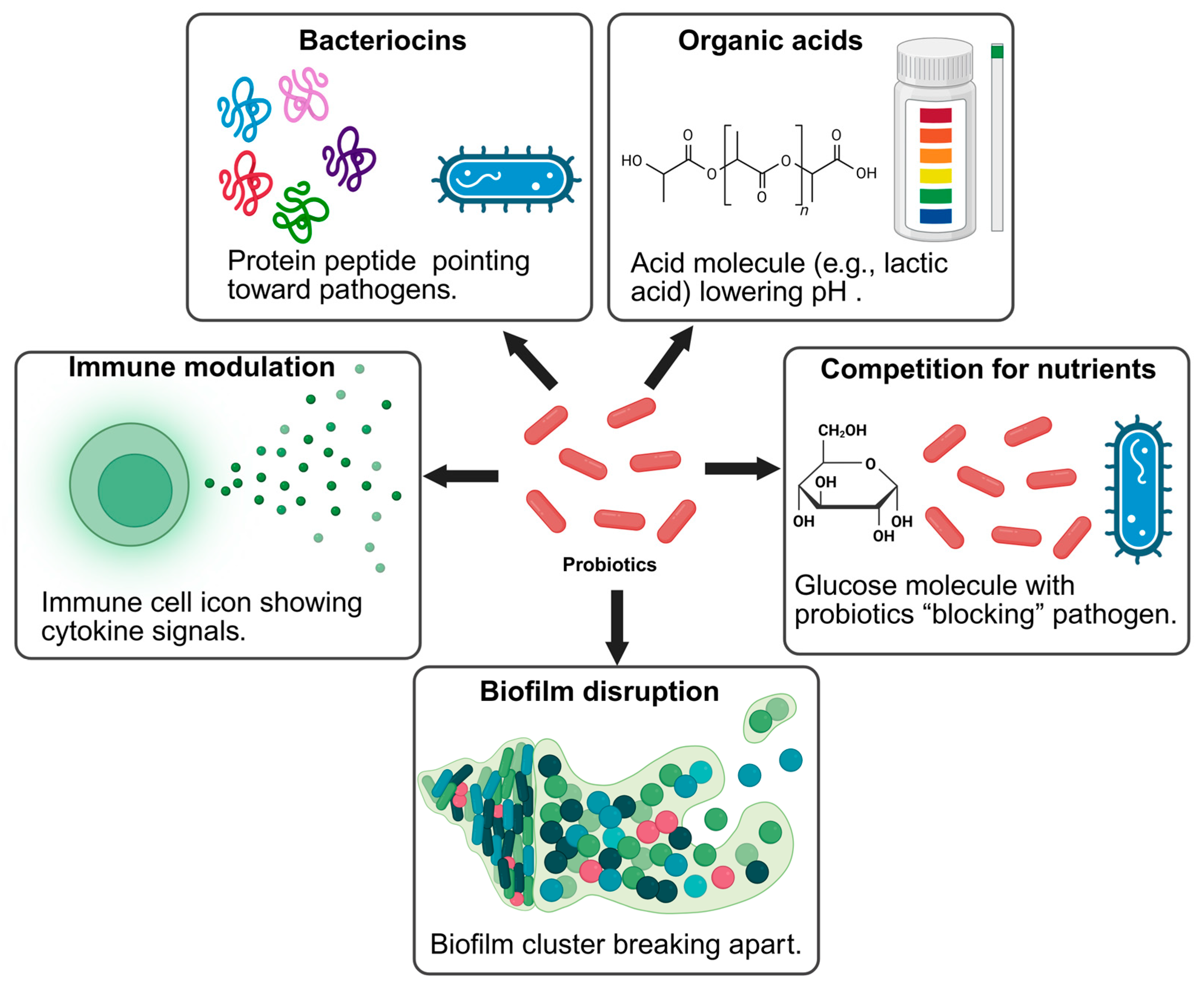
4. Mechanisms of Biopreservation Against Foodborne Pathogens
4.1. Antibacterial Effects: Bacteriocins, Organic Acids, and Hydrogen Peroxide
4.2. Antifungal Activity
4.3. Biofilm Disruption and Quorum Sensing Inhibition
4.4. Antioxidant Effects
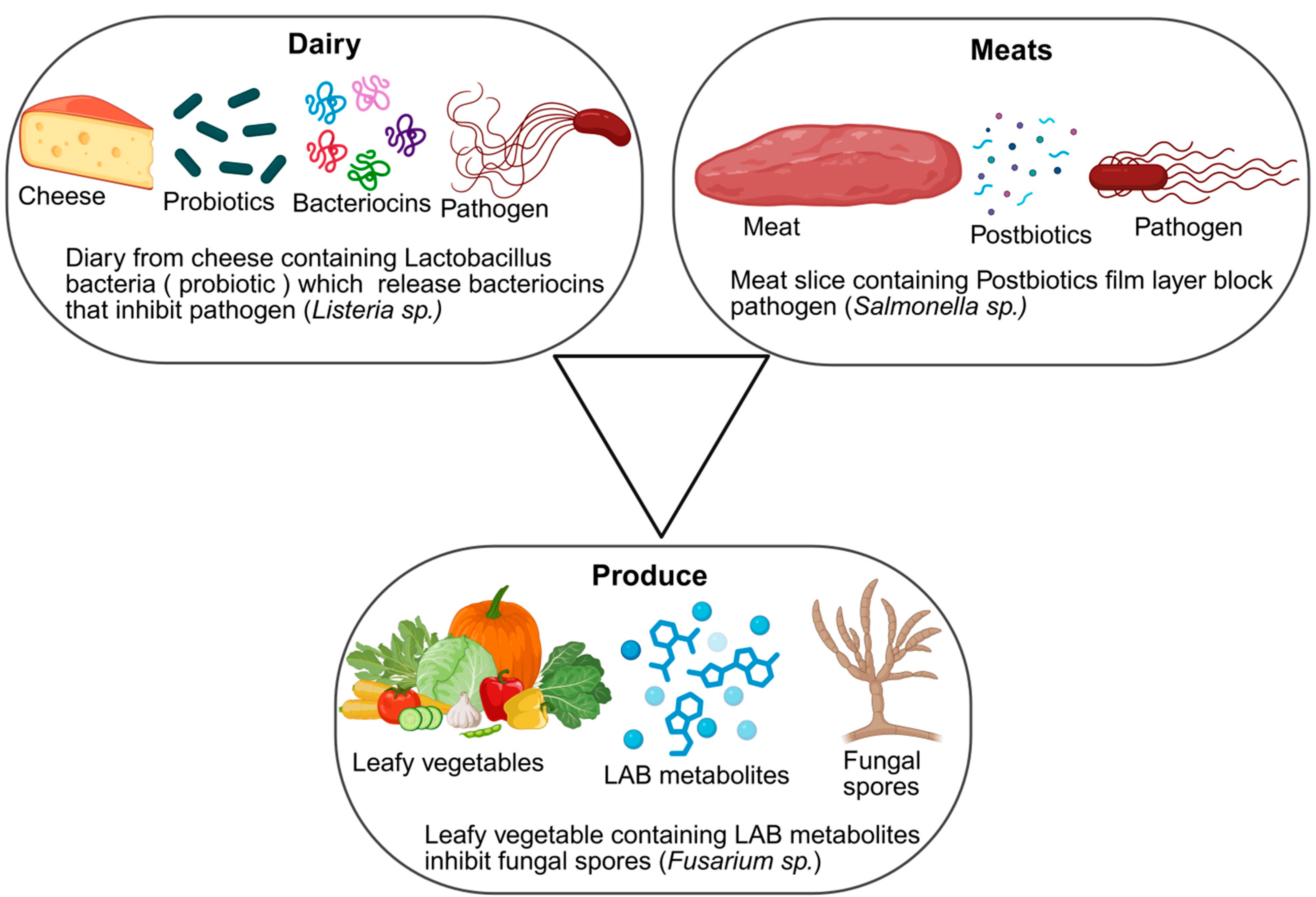
4.5. Case Examples in Dairy, Meat, and Fresh Produce
5. Intelligent Detection and Monitoring Tools
5.1. Biosensors (Electrochemical, Aptamer, CRISPR)
5.2. Omics-Based Approaches (Metabolomics, Proteomics, Microbiome Shifts)
5.3. AI/Machine Learning for Pathogen Risk Prediction
5.4. Integration into Food Production Pipelines
6. Review of Research Findings & Applications
6.1. Evidence from Bench to Industry
6.2. Case Studies: Dairy, Meats, Beverages, Plant-Based Foods
6.3. Regional Evidence (Africa, Asia, EU, USA)
6.4. Comparison with Chemical Preservatives
7. Innovations & Technological Advances
7.1. Encapsulation for Stability
7.2. Edible Coatings & Films Enriched with Postbiotics
7.3. Synergistic Preservation with Essential Oils and Nanomaterials
7.4. Smart Packaging Linking Probiotic/Postbiotic Release and Detection
8. Challenges & Limitations
8.1. Stability and Viability in Food Matrices
8.2. Regulatory Ambiguity Around “Postbiotics”
8.3. Cost and Scalability
8.4. Risk of Antimicrobial Resistance
9. Future Directions
9.1. Precision Fermentation and Synthetic Biology
9.2. Multi-Strain, Matrix-Specific Formulations
9.3. Deployment in LMICs Using Local LAB Strains
9.4. Global Regulatory Harmonization
9.5. Consumer Perception and Acceptance Studies
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mesías, F.J.; Martín, A.; Hernández, A. Consumers’ growing appetite for natural foods: Perceptions towards the use of natural preservatives in fresh fruit. Food Res. Int. 2021, 150, 110749. [Google Scholar] [CrossRef]
- Chauhan, K.; Rao, A. Clean-label alternatives for food preservation: An emerging trend. Heliyon 2024, 10, e35815. [Google Scholar] [CrossRef]
- Kim, M.; Bae, S.M.; Yoo, Y.; Park, J.; Jeong, J.Y. Clean-Label Strategies for the Replacement of Nitrite, Ascorbate, and Phosphate in Meat Products: A Review. Foods 2025, 14, 2442. [Google Scholar] [CrossRef] [PubMed]
- Rafique, N.; Jan, S.Y.; Dar, A.H.; Dash, K.K.; Sarkar, A.; Shams, R.; Pandey, V.K.; Khan, S.A.; Amin, Q.A.; Hussain, S.Z. Promising bioactivities of postbiotics: A comprehensive review. J. Agric. Food Res. 2023, 14, 100708. [Google Scholar] [CrossRef]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of Probiotics in Human World: A Nonstop Source of Benefactions till the End of Time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef] [PubMed]
- Jahedi, S.; Pashangeh, S. Bioactivities of postbiotics in food applications: A review. Iran. J. Microbiol. 2025, 17, 348–357. [Google Scholar] [CrossRef]
- Ali, S.; Hamayun, M.; Siraj, M.; Khan, S.A.; Kim, H.-Y.; Lee, B. Recent advances in prebiotics: Classification, mechanisms, and health applications. Future Foods 2025, 12, 100680. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Mir, T.U.G.; Chopra, C.; Singh, R.; Hong, J.C.; Kadam, U.S. CRISPR/Cas12a-based biosensors for environmental monitoring and diagnostics. Environ. Technol. Innov. 2024, 34, 103625. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef]
- Imran, A.; Ahmed, F.; Ali, Y.A.; Naseer, M.S.; Sharma, K.; Bisht, Y.S.; Alawadi, A.H.; Shehzadi, U.; Islam, F.; Shah, M.A. A comprehensive review on carbon dot synthesis and food applications. J. Agric. Food Res. 2025, 21, 101847. [Google Scholar] [CrossRef]
- Said, M.I.; Rageh, A.H.; Mohammed, A.A.K.; Nevoigt, I.; Schulz, F.; Parak, W.J.; Chakraborty, I. Towards development of luminescent silver-based metal organic frameworks for selective detection of trifluralin. Inorg. Chem. Commun. 2025, 178, 114429. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Dabash, H.; Ponnamma, D.; Abbas, M.K.G. Carbon dots as versatile nanomaterials in sensing and imaging: Efficiency and beyond. Heliyon 2024, 10, e31634. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Q.; Xiao, X.; Wang, T.; Yan, H.; Wu, W.; Wang, Q. Lychee-like plasmonic nanocomplex with programmable hierarchical structure: A high-performance SERS platform for monitoring diazepam in aquatic products. Microchem. J. 2025, 215, 114177. [Google Scholar] [CrossRef]
- Nnaji, N.D.; Onyeaka, H.; Ughamba, K.T.; Ononugbo, C.M.; Olovo, C.V.; Mazi, I.M. Chemical Toxicants Used for Food Preservation in Africa. Is it a Case of Ignorance or Food Fraud? A Review. Health Sci. Rep. 2025, 8, e70333. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Nagababu, B.H. Applications of food color and bio-preservatives in the food and its effect on the human health. Food Chem. Adv. 2022, 1, 100019. [Google Scholar] [CrossRef]
- Munir, M.T.; Mtimet, N.; Guillier, L.; Meurens, F.; Fravalo, P.; Federighi, M.; Kooh, P. Physical Treatments to Control Clostridium botulinum Hazards in Food. Foods 2023, 12, 1580. [Google Scholar] [CrossRef] [PubMed]
- Lien, K.-W.; Hsieh, D.P.H.; Huang, H.-Y.; Wu, C.-H.; Ni, S.-P.; Ling, M.-P. Food safety risk assessment for estimating dietary intake of sulfites in the Taiwanese population. Toxicol. Rep. 2016, 3, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Geng, Y.; Yao, J.; Ji, J.; Chen, F.; Xiao, J.; Hu, X.; Ma, L. N-nitrosamines in processed meats: Exposure, formation and mitigation strategies. J. Agric. Food Res. 2023, 13, 100645. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Sodium Benzoate—Harmfulness and Potential Use in Therapies for Disorders Related to the Nervous System: A Review. Nutrients 2022, 14, 1497. [Google Scholar] [CrossRef]
- Awaad, S.S.; Sherief, M.A.; Mousa, S.M.; Orabi, A.; Abdel-Salam, A.B. A comparative study on the antifungal effect of potassium sorbate, chitosan, and nano-chitosan against Rhodotorula mucilaginosa and Candida albicans in skim milk acid-coagulated (Karish) cheese. Vet. World 2023, 16, 1991–2001. [Google Scholar] [CrossRef]
- Irwin, S.V.; Fisher, P.; Graham, E.; Malek, A.; Robidoux, A. Sulfites inhibit the growth of four species of beneficial gut bacteria at concentrations regarded as safe for food. PLoS ONE 2017, 12, e0186629. [Google Scholar] [CrossRef]
- Zavišić, G.; Ristić, S.; Petričević, S.; Janković, D.; Petković, B. Microbial Contamination of Food: Probiotics and Postbiotics as Potential Biopreservatives. Foods 2024, 13, 2487. [Google Scholar] [CrossRef]
- Atli, E. The effects of ethylparaben and propylparaben on the development and fecundity of Drosophila melanogaster. Environ. Toxicol. Pharmacol. 2022, 92, 103856. [Google Scholar] [CrossRef]
- Dassarma, B.; Nandi, D.K.; Gangopadhyay, S.; Samanta, S. Hepatoprotective effect of food preservatives (butylated hydroxyanisole, butylated hydroxytoluene) on carbon tetrachloride-induced hepatotoxicity in rat. Toxicol. Rep. 2018, 5, 31–37. [Google Scholar] [CrossRef]
- Sun, X.; Yang, C.; Zhang, W.; Zheng, J.; Ou, J.; Ou, S. Toxicity of formaldehyde, and its role in the formation of harmful and aromatic compounds during food processing. Food Chem. X 2025, 25, 102225. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, R.; Hasan, S.; Zzaman, W.; Rana, M.R.; Ahmed, S.; Roy, M.; Sayem, A.; Matin, A.; Raposo, A.; et al. A Comprehensive Review on Bio-Preservation of Bread: An Approach to Adopt Wholesome Strategies. Foods 2022, 11, 319. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef]
- Aldabayan, Y.S. Effect of Artificial Food Additives on Lung Health—An Overview. Medicina 2025, 61, 684. [Google Scholar] [CrossRef]
- Nowak, K.; Ratajczak–Wrona, W.; Górska, M.; Jabłońska, E. Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Z.; Kuang, J. Artificial Intelligence-Driven Recommendations and Functional Food Purchases: Understanding Consumer Decision-Making. Foods 2025, 14, 976. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Kołożyn-Krajewska, D. The Role of Microorganisms and Their Antibacterial Compounds in Food Biopreservation. Appl. Sci. 2024, 14, 5557. [Google Scholar] [CrossRef]
- Mafe, A.N.; Smart, O.O.; Edo, G.I.; Akpoghelie, P.O.; Gaaz, T.S.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; Ismael, S.A.; Essaghah, A.E.A.; et al. Genesis, Health Benefits, and Future Perspectives of Probiotics: Exploring Endogenous and Exogenous Classes, Innovations, and Research Gaps. Probiot. Antimicrob. Proteins 2025, 1. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Makia, R.S.; Joshua, O.A.; Akpoghelie, P.O.; Gaaz, T.S.; Jikah, A.N.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; et al. A review on food spoilage mechanisms, food borne diseases and commercial aspects of food preservation and processing. Food Chem. Adv. 2024, 5, 100852. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Shi, C.; Maktabdar, M. Lactic Acid Bacteria as Biopreservation Against Spoilage Molds in Dairy Products—A Review. Front. Microbiol. 2022, 12, 819684. [Google Scholar] [CrossRef]
- Anumudu, C.K.; Miri, T.; Onyeaka, H. Multifunctional Applications of Lactic Acid Bacteria: Enhancing Safety, Quality, and Nutritional Value in Foods and Fermented Beverages. Foods 2024, 13, 3714. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Divyashree, S.; Shruthi, B.; Vanitha, P.R.; Sreenivasa, M.Y. Probiotics and their postbiotics for the control of opportunistic fungal pathogens: A review. Biotechnol. Rep. 2023, 38, e00800. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Nanda, P.K.; Pateiro, M.; Lorenzo, J.M.; Dhar, P.; Das, A.K. Lactic Acid Bacteria and Bacteriocins: Novel Biotechnological Approach for Biopreservation of Meat and Meat Products. Microorganisms 2022, 10, 2058. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; da Silva, R.C.; Ibrahim, S.A. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Wu, M.; Ma, Y.; Dou, X.; Zohaib Aslam, M.; Liu, Y.; Xia, X.; Yang, S.; Wang, X.; Qin, X.; Hirata, T.; et al. A review of potential antibacterial activities of nisin against Listeria monocytogenes: The combined use of nisin shows more advantages than single use. Food Res. Int. 2023, 164, 112363. [Google Scholar] [CrossRef]
- Saturio, S.; Nogacka, A.M.; Alvarado-Jasso, G.M.; Salazar, N.; de los Reyes-Gavilán, C.G.; Gueimonde, M.; Arboleya, S. Role of Bifidobacteria on Infant Health. Microorganisms 2021, 9, 2415. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Zhang, P. Properties of Listeria monocytogenes on Acquisition of Pediocin Resistance. Ann. Microbiol. 2019, 69, 123–130. [Google Scholar] [CrossRef]
- de Paula, B.P.; Chávez, D.W.H.; Lemos Junior, W.J.F.; Guerra, A.F.; Corrêa, M.F.D.; Pereira, K.S.; Coelho, M.A.Z. Growth Parameters and Survivability of Saccharomyces boulardii for Probiotic Alcoholic Beverages Development. Front. Microbiol. 2019, 10, 2092. [Google Scholar] [CrossRef]
- Ahmed, S.; Singh, S.; Singh, V.; Roberts, K.D.; Zaidi, A.; Rodriguez-Palacios, A. The Weissella Genus: Clinically Treatable Bacteria with Antimicrobial/Probiotic Effects on Inflammation and Cancer. Microorganisms 2022, 10, 2427. [Google Scholar] [CrossRef]
- Kyrylenko, A.; Eijlander, R.T.; Alliney, G.; de Bos, E.L.; Wells-Bennik, M.H.J. Levels and types of microbial contaminants in different plant-based ingredients used in dairy alternatives. Int. J. Food Microbiol. 2023, 407, 110392. [Google Scholar] [CrossRef]
- Chae, J.K.; Han, S.; Kim, D.H.; Park, S.H.; Ha, S.-D. Growth characterization of Propionibacterium and propionic acid production capabilities at different temperatures and pH levels. Food Sci. Biotechnol. 2022, 31, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Vandera, E.; Lianou, A.; Kakouri, A.; Feng, J.; Koukkou, A.-I.; Samelis, J. Enhanced Control of Listeria monocytogenes by Enterococcus faecium KE82, a Multiple Enterocin–Producing Strain, in Different Milk Environments. J. Food Prot. 2017, 80, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, Z.; Šarić, L.; Tomičić, R. Potential Future Applications of Postbiotics in the Context of Ensuring Food Safety and Human Health Improvement. Antibiotics 2025, 14, 674. [Google Scholar] [CrossRef]
- Bucataru, C.; Ciobanasu, C. Antimicrobial peptides: Opportunities and challenges in overcoming resistance. Microbiol. Res. 2024, 286, 127822. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Salman, M.K.; Abuqwider, J.; Mauriello, G. Anti-Quorum Sensing Activity of Probiotics: The Mechanism and Role in Food and Gut Health. Microorganisms 2023, 11, 793. [Google Scholar] [CrossRef]
- Udayakumar, S.; Rasika, D.M.D.; Priyashantha, H.; Vidanarachchi, J.K.; Ranadheera, C.S. Probiotics and Beneficial Microorganisms in Biopreservation of Plant-Based Foods and Beverages. Appl. Sci. 2022, 12, 11737. [Google Scholar] [CrossRef]
- Rahman, M.S.; Soltani, S.; LaPointe, G.; Karboune, S.; Fliss, I. Lactic acid bacteria: Beyond fermentation to bio-protection against fungal spoilage and mycotoxins in food systems. Front. Microbiol. 2025, 16, 1580670. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Q.; Ren, K.; Xu, T.; Zhang, Z.; Xu, M.; Rao, Z.; Zhang, X. A Review of Antimicrobial Peptides: Structure, Mechanism of Action, and Molecular Optimization Strategies. Fermentation 2024, 10, 540. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Neetoo, H.; Al-Asmari, F. Antimicrobial Activity of Hydrogen Peroxide for Application in Food Safety and COVID-19 Mitigation: An Updated Review. J. Food Prot. 2024, 87, 100306. [Google Scholar] [CrossRef]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, Potent Antimicrobial Peptides and the Fight against Multi Drug Resistant Species: Resistance Is Futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, J.; Wang, Q.; Suo, H.; Hamdy, A.M.; Song, J. Antifungal Effect of Metabolites from a New Strain Lactiplantibacillus Plantarum LPP703 Isolated from Naturally Fermented Yak Yogurt. Foods 2023, 12, 181. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, B. Role of cell membrane homeostasis in the pathogenicity of pathogenic filamentous fungi. Virulence 2024, 15, 2299183. [Google Scholar] [CrossRef]
- de Carvalho, T.B.; Silva, B.N.; Tomé, E.; Teixeira, P. Preventing Fungal Spoilage from Raw Materials to Final Product: Innovative Preservation Techniques for Fruit Fillings. Foods 2024, 13, 2669. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Ali, A.B.M.; Akpoghelie, P.O.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; Ajiduku, L.A.; Owheruo, J.O.; et al. Next-Generation Biopolymers for Sustainable Food Packaging: Innovations in Material Science, Circular Economy, and Smart Technologies. Food Bioprocess Technol. 2025, 10. [Google Scholar] [CrossRef]
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, A.; Kheyrolahzadeh, K.; Hosseiniyan Khatibi, S.M.; Sharifi, S.; Memar, M.Y.; Zununi Vahed, S. The Battle of Probiotics and Their Derivatives Against Biofilms. Infect. Drug Resist. 2020, 13, 659–672. [Google Scholar] [CrossRef]
- Mafe, A.N.; Nkene, I.H.; Ali, A.B.M.; Edo, G.I.; Akpoghelie, P.O.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; Ismael, S.A.; Essaghah, A.E.A.; et al. Smart Probiotic Solutions for Mycotoxin Mitigation: Innovations in Food Safety and Sustainable Agriculture. Probiot. Antimicrob. Proteins 2025, 7. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Li, J.; Qin, G. New Strategies for Biocontrol of Bacterial Toxins and Virulence: Focusing on Quorum-Sensing Interference and Biofilm Inhibition. Toxins 2023, 15, 570. [Google Scholar] [CrossRef]
- Al-Hazmi, N.E.; Naguib, D.M. Antioxidant and Antibacterial Activities of Nano-probiotics Versus Free Probiotics Against Gastrointestinal Pathogenic Bacteria. Indian J. Microbiol. 2024, 64, 141–152. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef]
- Dabija, A.; Afloarei, C.Ș.; Dabija, D.; Chetrariu, A. Conventional and Innovative Methods for Reducing the Incidence of Listeria monocytogenes in Milk and Dairy Products. Appl. Sci. 2025, 15, 6580. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Mafe, A.N.; Büsselberg, D. The Effect of Microbiome-Derived Metabolites in Inflammation-Related Cancer Prevention and Treatment. Biomolecules 2025, 15, 688. [Google Scholar] [CrossRef]
- Reddyvari, R.; Lu, S.; Kosuri, P.; Amalaradjou, M.A. Incorporation of probiotics in post-harvest wash treatments reduces Salmonella contamination and improves egg safety. Poult. Sci. 2025, 104, 105146. [Google Scholar] [CrossRef]
- Inês, A.; Cosme, F. Biosensors for Detecting Food Contaminants—An Overview. Processes 2025, 13, 380. [Google Scholar] [CrossRef]
- Bodkhe, G.A.; Kumar, V.; Li, X.; Pei, S.; Ma, L.; Kim, M. Biosensors in Microbial Ecology: Revolutionizing Food Safety and Quality. Microorganisms 2025, 13, 1706. [Google Scholar] [CrossRef]
- Bruce-Tagoe, T.A.; Bhaskar, S.; Kavle, R.R.; Jeevanandam, J.; Acquah, C.; Ohemeng-Boahen, G.; Agyei, D.; Danquah, M.K. Advances in aptamer-based biosensors for monitoring foodborne pathogens. J. Food Sci. Technol. 2024, 61, 1252–1271. [Google Scholar] [CrossRef]
- He, Y.; Hu, Q.; San, S.; Kasputis, T.; Splinter, M.G.D.; Yin, K.; Chen, J. CRISPR-based biosensors for human health: A novel strategy to detect emerging infectious diseases. TrAC Trends Anal. Chem. 2023, 168, 117342. [Google Scholar] [CrossRef]
- Martínez, A.; Abanto, M.; Días, N.B.; Olate, P.; Pérez Nuñez, I.; Díaz, R.; Sepúlveda, N.; Paz, E.A.; Quiñones, J. Recent Trends in Food Quality and Authentication: The Role of Omics Technologies in Dairy and Meat Production. Int. J. Mol. Sci. 2025, 26, 4405. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.B.; Green, E.; Adebiyi, J.A.; Ogundele, O.M.; Gbashi, S.; Adefisoye, M.A.; Oyeyinka, S.A.; Adebo, O.A. Metabolomic approaches for the determination of metabolites from pathogenic microorganisms: A review. Food Res. Int. 2021, 140, 110042. [Google Scholar] [CrossRef] [PubMed]
- Abril, A.G.; Quintela-Baluja, M.; Villa, T.G.; Calo-Mata, P.; Barros-Velázquez, J.; Carrera, M. Proteomic Characterization of Virulence Factors and Related Proteins in Enterococcus Strains from Dairy and Fermented Food Products. Int. J. Mol. Sci. 2022, 23, 10971. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C.; Dittoe, D.K.; Brown, J.A.; Thompson, D.R. Practical opportunities for microbiome analyses and bioinformatics in poultry processing. Poult. Sci. 2022, 101, 101787. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Zhang, Y.; Feng, Y.; Liu, J.; Zhu, H. Artificial Intelligence in Food Safety: A Decade Review and Bibliometric Analysis. Foods 2023, 12, 1242. [Google Scholar] [CrossRef]
- Sonwani, E.; Bansal, U.; Alroobaea, R.; Baqasah, A.M.; Hedabou, M. An Artificial Intelligence Approach Toward Food Spoilage Detection and Analysis. Front. Public Health 2022, 9, 816226. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Meivelu, M.; Praburaman, L.; Mujahid Alam, M.; Al-Sehemi, A.G. Integrating AI in food contaminant analysis: Enhancing quality and environmental protection. J. Hazard. Mater. Adv. 2024, 16, 100509. [Google Scholar] [CrossRef]
- Akbari Nakhjavani, S.; Mirzajani, H.; Carrara, S.; Onbaşlı, M.C. Advances in biosensor technologies for infectious diseases detection. TrAC Trends Anal. Chem. 2024, 180, 117979. [Google Scholar] [CrossRef]
- Dubey, V.K.; Veeramani, D. A decision-making framework for automating distribution centers in the Retail supply. Heliyon 2024, 10, e30854. [Google Scholar] [CrossRef]
- Borges, F.; Briandet, R.; Callon, C.; Champomier-Vergès, M.-C.; Christieans, S.; Chuzeville, S.; Denis, C.; Desmasures, N.; Desmonts, M.-H.; Feurer, C.; et al. Contribution of omics to biopreservation: Toward food microbiome engineering. Front. Microbiol. 2022, 13, 951182. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Yang, R.-S.; Ci, B.-Q.; Xin, W.-G.; Zhang, Q.-L.; Lin, L.-B.; Wang, F. A novel bacteriocin against multiple foodborne pathogens from Lacticaseibacillus rhamnosus isolated from juice ferments: ATF perfusion-based preparation of viable cells, characterization, antibacterial and antibiofilm activity. Curr. Res. Food Sci. 2023, 6, 100484. [Google Scholar] [CrossRef] [PubMed]
- Muthuvelu, K.S.; Ethiraj, B.; Pramnik, S.; Raj, N.K.; Venkataraman, S.; Rajendran, D.S.; Bharathi, P.; Palanisamy, E.; Narayanan, A.S.; Vaidyanathan, V.K.; et al. Biopreservative technologies of food: An alternative to chemical preservation and recent developments. Food Sci. Biotechnol. 2023, 32, 1337–1350. [Google Scholar] [CrossRef]
- de Niederhäusern, S.; Camellini, S.; Sabia, C.; Iseppi, R.; Bondi, M.; Messi, P. Antilisterial Activity of Bacteriocins Produced by Lactic Bacteria Isolated from Dairy Products. Foods 2020, 9, 1757. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, L.; Qin, Z.; Wang, T. Review of Recent Advances in Intelligent and Antibacterial Packaging for Meat Quality and Safety. Foods 2025, 14, 1157. [Google Scholar] [CrossRef] [PubMed]
- Postigo, V.; García, M.; Crespo, J.; Canonico, L.; Comitini, F.; Ciani, M. Bioactive Properties of Fermented Beverages: Wine and Beer. Fermentation 2025, 11, 234. [Google Scholar] [CrossRef]
- Sar, T.; Kiraz, P.; Braho, V.; Harirchi, S.; Akbas, M.Y. Novel Perspectives on Food-Based Natural Antimicrobials: A Review of Recent Findings Published since 2020. Microorganisms 2023, 11, 2234. [Google Scholar] [CrossRef]
- Wu, M.; Zuo, S.; Maiorano, G.; Kosobucki, P.; Stadnicka, K. How to employ metabolomic analysis to research on functions of prebiotics and probiotics in poultry gut health? Front. Microbiol. 2022, 13, 1040434. [Google Scholar] [CrossRef]
- Kumar, A.; Green, K.M.; Rawat, M. A Comprehensive Overview of Postbiotics with a Special Focus on Discovery Techniques and Clinical Applications. Foods 2024, 13, 2937. [Google Scholar] [CrossRef]
- Singh, V.P. Recent approaches in food bio-preservation—A review. Open Vet. J. 2018, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Wafula, E.N.; Muhonja, C.N.; Kuja, J.O.; Owaga, E.E.; Makonde, H.M.; Mathara, J.M.; Kimani, V.W. Lactic Acid Bacteria from African Fermented Cereal-Based Products: Potential Biological Control Agents for Mycotoxins in Kenya. J. Toxicol. 2022, 2022, 397767. [Google Scholar] [CrossRef]
- Abotsi, E.E.; Panagodage, Y.; English, M. Plant-based seafood alternatives: Current insights on the nutrition, protein-flavour interactions, and the processing of these foods. Curr. Res. Food Sci. 2024, 9, 100860. [Google Scholar] [CrossRef]
- Reuben, R.C.; Torres, C. Bacteriocins: Potentials and prospects in health and agrifood systems. Arch. Microbiol. 2024, 206, 233. [Google Scholar] [CrossRef]
- Thompson, P.B.; Bischof, J.; Powell-Palm, M.J.; Smith, K.; Tiersch, T.R. Biopreservation in Agriculture and Food Systems: A Summary of Ethical Issues. J. Law Med. Ethics 2024, 52, 666–678. [Google Scholar] [CrossRef]
- Ziarno, M.; Zaręba, D.; Ścibisz, I.; Kozłowska, M. Comprehensive studies on the stability of yogurt-type fermented soy beverages during refrigerated storage using dairy starter cultures. Front. Microbiol. 2023, 14, 1230025. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Alagarsamy, K.; Suganthy, N.; Thangaleela, S.; Kesika, P.; Chaiyasut, C. The Role and Significance of Bacillus and Lactobacillus Species in Thai Fermented Foods. Fermentation 2022, 8, 635. [Google Scholar] [CrossRef]
- Bodie, A.R.; O’Bryan, C.A.; Olson, E.G.; Ricke, S.C. Natural Antimicrobials for Listeria monocytogenes in Ready-to-Eat Meats: Current Challenges and Future Prospects. Microorganisms 2023, 11, 1301. [Google Scholar] [CrossRef]
- Simon, B.O.; Nnaji, N.D.; Anumudu, C.K.; Aleke, J.C.; Ekwueme, C.T.; Uhegwu, C.C.; Ihenetu, F.C.; Obioha, P.; Ifedinezi, O.V.; Ezechukwu, P.S.; et al. Microbiome-Based Interventions for Food Safety and Environmental Health. Appl. Sci. 2025, 15, 5219. [Google Scholar] [CrossRef]
- Valentino, V.; Magliulo, R.; Farsi, D.; Cotter, P.D.; O’Sullivan, O.; Ercolini, D.; De Filippis, F. Fermented foods, their microbiome and its potential in boosting human health. Microb. Biotechnol. 2024, 17, e14428. [Google Scholar] [CrossRef]
- Saleem, K.; Ikram, A.; Saeed, F.; Afzaal, M.; Ateeq, H.; Hussain, M.; Raza, A.; Rasheed, A.; Asghar, A.; Asif Shah, M. Nutritional and functional properties of kefir: Review. Int. J. Food Prop. 2023, 26, 3261–3274. [Google Scholar] [CrossRef]
- Ranathunga, N.S.; Wijayasekara, K.N.; Abeyrathne, E.D.N.S. Application of bio-preservation to enhance food safety: A review. Korean J. Food Preserv. 2023, 30, 179–189. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.; Habiba, U.; Pandey, V.K.; Kaur, S.; Rustagi, S. Potential health benefits of postbiotics and its utilization as natural food preservatives. Food Humanit. 2025, 5, 100726. [Google Scholar] [CrossRef]
- Ilieva, G.; Yankova, T.; Ruseva, M.; Dzhabarova, Y.; Klisarova-Belcheva, S.; Dimitrov, A. Consumer Perceptions and Attitudes Towards Ultra-Processed Foods. Appl. Sci. 2025, 15, 3739. [Google Scholar] [CrossRef]
- Rodríguez-Marca, C.; Domenech-Coca, C.; Nakamura, M.; Ortega-Olivé, N.; Puigbò, P. Use of Live Biopreservatives and Bacteriophages to Enhance the Safety of Meat Products. Life 2025, 15, 197. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, R.A.; Bhutto, N.U.A.H.; Mahar, H.; Khanal, S.; Wang, M.; Iqbal, S.; Fan, Y.; Yi, J. Recent trends in co-encapsulation of probiotics with prebiotics and their applications in the food industry. Trends Food Sci. Technol. 2025, 156, 104829. [Google Scholar] [CrossRef]
- Gao, R.; Zhao, D.; Zhou, X.; Wan, Z.; Wang, X.; Rao, H.; Liu, X.; Gao, X.; Hao, J. Advances in Polysaccharide-Based Biopolymers for Probiotic Encapsulation: From Single Polysaccharides to Composite Systems. Curr. Res. Food Sci. 2025, 11, 101186. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Silva, S.P.M.; Teixeira, J.A.; Silva, C.C.G. Recent advances in the use of edible films and coatings with probiotic and bacteriocin-producing lactic acid bacteria. Food Biosci. 2023, 56, 103196. [Google Scholar] [CrossRef]
- Du, L.; Huang, X.; Li, Z.; Qin, Z.; Zhang, N.; Zhai, X.; Shi, J.; Zhang, J.; Shen, T.; Zhang, R.; et al. Application of Smart Packaging in Fruit and Vegetable Preservation: A Review. Foods 2025, 14, 447. [Google Scholar] [CrossRef]
- Molina, D.; Marinas, I.C.; Angamarca, E.; Hanganu, A.; Stan, M.; Chifiriuc, M.C.; Tenea, G.N. Postbiotic-Based Extracts from Native Probiotic Strains: A Promising Strategy for Food Preservation and Antimicrobial Defense. Antibiotics 2025, 14, 318. [Google Scholar] [CrossRef]
- Guidotti-Takeuchi, M.; de Morais Ribeiro, L.N.; dos Santos, F.A.L.; Rossi, D.A.; Della Lucia, F.; de Melo, R.T. Essential Oil-Based Nanoparticles as Antimicrobial Agents in the Food Industry. Microorganisms 2022, 10, 1504. [Google Scholar] [CrossRef] [PubMed]
- Jasrotia, S.; Gupta, S.; Kudipady, M.L.; Puttaiahgowda, Y.M. Advancing food preservation with quercetin-based Nanocomposites: Antimicrobial, antioxidant, and controlled-release strategies—A review. Curr. Res. Food Sci. 2025, 11, 101159. [Google Scholar] [CrossRef]
- Bhatlawande, A.R.; Ghatge, P.U.; Shinde, G.U.; Anushree, R.K.; Patil, S.D. Unlocking the future of smart food packaging: Biosensors, IoT, and nano materials. Food Sci. Biotechnol. 2024, 33, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-Based Sensors for Smart Food Packaging—Current Applications and Future Trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef]
- Awad, N.M.; Capozzi, V.; Guillard, V.; Goksen, G. An innovative approach for probiotics and postbiotics-based antimicrobial packaging systems: Design, characterization, and benefit for food application. Curr. Opin. Food Sci. 2025, 64, 101315. [Google Scholar] [CrossRef]
- Gidado, M.J.; Gunny, A.A.N.; Gopinath, S.C.B.; Ali, A.; Wongs-Aree, C.; Salleh, N.H.M. Challenges of postharvest water loss in fruits: Mechanisms, influencing factors, and effective control strategies—A comprehensive review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar] [CrossRef]
- Wendel, U. Assessing Viability and Stress Tolerance of Probiotics—A Review. Front. Microbiol. 2022, 12, 818468. [Google Scholar] [CrossRef]
- Popa, E.E.; Miteluț, A.C.; Râpă, M.; Popescu, P.A.; Drăghici, M.C.; Geicu-Cristea, M.; Popa, M.E. Antimicrobial Active Packaging Containing Nisin for Preservation of Products of Animal Origin: An Overview. Foods 2022, 11, 3820. [Google Scholar] [CrossRef]
- Acosta-Piantini, E.; Villarán, M.C.; Martínez, Á.; Lombraña, J.I. Examining the Effect of Freezing Temperatures on the Survival Rate of Micro-Encapsulated Probiotic Lactobacillus acidophilus LA5 Using the Flash Freeze-Drying (FFD) Strategy. Microorganisms 2024, 12, 506. [Google Scholar] [CrossRef]
- Abbasi, A.; Sarabi-Aghdam, V.; Fathi, M.; Abbaszadeh, S. Non-Dairy Fermented Probiotic Beverages: A Critical Review on the Production Techniques, Health Benefits, Safety Considerations and Market Trends. Food Rev. Int. 2025, 28. [Google Scholar] [CrossRef]
- Gangakhedkar, P.S.; Deshpande, H.W.; Törős, G.; El-Ramady, H.; Elsakhawy, T.; Abdalla, N.; Shaikh, A.; Kovács, B.; Mane, R.; Prokisch, J. Fermentation of Fruits and Vegetables: Bridging Traditional Wisdom and Modern Science for Food Preservation and Nutritional Value Improvements. Foods 2025, 14, 2155. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Assadpour, E.; Jafari, S.M. Metabolomics findings associated with the effects of decontamination approaches on foodborne pathogens; a state-of-the-art review. Futur. Foods 2025, 11, 100576. [Google Scholar] [CrossRef]
- Echegaray, N.; Yilmaz, B.; Sharma, H.; Kumar, M.; Pateiro, M.; Ozogul, F.; Lorenzo, J.M. A novel approach to Lactiplantibacillus plantarum: From probiotic properties to the omics insights. Microbiol. Res. 2023, 268, 127289. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, T.; Zolfanelli, C.; Lauciello, V.; Di Ciancia, A.; Vagliasindi, A.; Smaoui, S.; Varzakas, T. Using Postbiotics from Functional Foods for Managing Colorectal Cancer: Mechanisms, Sources, Therapeutic Potential, and Clinical Perspectives. Microorganisms 2025, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Amobonye, A.; Pillay, B.; Hlope, F.; Asong, S.T.; Pillai, S. Postbiotics: An insightful review of the latest category in functional biotics. World J. Microbiol. Biotechnol. 2025, 41, 293. [Google Scholar] [CrossRef] [PubMed]
- Primi, R.; Grossi, G.; Danieli, P.P.; Vitali, A.; Lacetera, N.; Ronchi, B. State of the art and challenges in the environmental labelling for animal food products. Ital. J. Anim. Sci. 2024, 23, 1104–1123. [Google Scholar] [CrossRef]
- Lenzuni, M.; Converti, A.; Casazza, A.A. From laboratory- to industrial-scale plants: Future of anaerobic digestion of olive mill solid wastes. Bioresour. Technol. 2024, 394, 130317. [Google Scholar] [CrossRef]
- Biswas, R.; Alam, M.; Sarkar, A.; Haque, M.I.; Hasan, M.M.; Hoque, M. Application of nanotechnology in food: Processing, preservation, packaging and safety assessment. Heliyon 2022, 8, e11795. [Google Scholar] [CrossRef]
- Asin-Garcia, E.; Fawcett, J.D.; Batianis, C.; Martins dos Santos, V.A.P. A snapshot of biomanufacturing and the need for enabling research infrastructure. Trends Biotechnol. 2025, 43, 1000–1014. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Ab Rahim, M.S.; Reniers, G.; Yang, M.; Bajpai, S. Risk assessment methods for process safety, process security and resilience in the chemical process industry: A thorough literature review. J. Loss Prev. Process Ind. 2024, 88, 105274. [Google Scholar] [CrossRef]
- Nascimento, A.P.S.; Barros, A.N. Sustainable Innovations in Food Microbiology: Fermentation, Biocontrol, and Functional Foods. Foods 2025, 14, 2320. [Google Scholar] [CrossRef]
- Peña-Castro, J.M.; Muñoz-Páez, K.M.; Robledo-Narvaez, P.N.; Vázquez-Núñez, E. Engineering the Metabolic Landscape of Microorganisms for Lignocellulosic Conversion. Microorganisms 2023, 11, 2197. [Google Scholar] [CrossRef]
- Mannaa, M.; Han, G.; Seo, Y.-S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.; Bermúdez-Luque, A.; Román-Camacho, J.J.; Martín-García, F.J.; Moreno-García, J.; Güngörmüşler, M.; Ruiz-Castilla, F.J. Exploring the future of probiotics with innovations in delivery systems and market insights. Food Biosci. 2025, 71, 107029. [Google Scholar] [CrossRef]
- Loo, J.S.; Oslan, S.N.H.; Mokshin, N.A.S.; Othman, R.; Amin, Z.; Dejtisakdi, W.; Prihanto, A.A.; Tan, J.S. Comprehensive Review of Strategies for Lactic Acid Bacteria Production and Metabolite Enhancement in Probiotic Cultures: Multifunctional Applications in Functional Foods. Fermentation 2025, 11, 241. [Google Scholar] [CrossRef]
- Xie, A.; Dong, Y.; Liu, Z.; Li, Z.; Shao, J.; Li, M.; Yue, X. A Review of Plant-Based Drinks Addressing Nutrients, Flavor, and Processing Technologies. Foods 2023, 12, 3952. [Google Scholar] [CrossRef]
- Raman, J.; Kim, J.-S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.-J.; Kim, S.-J. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef]
- Materia, V.C.; Linnemann, A.R.; Smid, E.J.; Schoustra, S.E. Contribution of traditional fermented foods to food systems transformation: Value addition and inclusive entrepreneurship. Food Secur. 2021, 13, 1163–1177. [Google Scholar] [CrossRef]
- Meena, K.K.; Joshi, M.; Gupta, L.; Meena, S. Comprehensive insights into postbiotics: Bridging the gap to real-world application. Food Nutr. 2025, 1, 100024. [Google Scholar] [CrossRef]
- Fredua-Agyeman, M.; Larbi, E.A. Inaccurate labelling practices in probiotic products: A regulatory shortfall in Accra, Ghana. PLoS ONE 2025, 20, e0322194. [Google Scholar] [CrossRef]
- Divsalar, E.; İncili, G.K.; Shi, C.; Semsari, E.; Hosseini, S.H.; Ebrahimi Tirtashi, F.; Toker, O.S.; Mojgani, N.; Liu, S.-Q.; Moradi, M. Challenges with the use of postbiotics/parabiotics in food industry. Crit. Rev. Food Sci. Nutr. 2025, 13, 2541047. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef] [PubMed]

| Preservative | Food Application | Mode of Action | Documented Risks |
|---|---|---|---|
| Sodium nitrite/nitrate | Processed meats, sausages, cured fish | Inhibits Clostridium botulinum via nitrosylation | Carcinogenic nitrosamine formation; linked to colorectal cancer [18] |
| Sodium benzoate | Beverages, sauces, jams | pH-dependent inhibition of yeasts, molds, some bacteria | Allergic reactions, hyperactivity in children, DNA damage in vitro [19] |
| Potassium sorbate | Cheese, bakery products, soft drinks | Inhibits molds and yeasts via disruption of cell membranes | Potential genotoxic effects; mucosal irritation [20] |
| Sulfites (SO2, sodium metabisulfite) | Dried fruits, wine, juices, seafood | Antioxidant and antimicrobial action | Asthma exacerbation, allergic reactions, gut microbiota disruption [21] |
| Propionic acid and salts | Bakery products, cheeses | Inhibits molds by lowering intracellular pH | Gastrointestinal discomfort; potential microbiome imbalance [22] |
| Parabens (methyl-, propyl-paraben) | Beverages, sauces, cosmetics | Disrupts microbial membranes | Endocrine disruption, estrogenic activity [23] |
| Butylated hydroxyanisole (BHA) & butylated hydroxytoluene (BHT) | Oils, cereals, snacks | Antioxidant to prevent lipid oxidation | Tumorigenic in rodents; oxidative stress induction [24] |
| Hexamethylenetetramine | Fish, caviar, cheese | Converts to formaldehyde in acidic foods, inhibiting bacteria | Formaldehyde toxicity; respiratory irritation [25] |
| Calcium propionate | Bread, baked goods | Inhibits molds and Bacillus spp. | Behavioral effects in sensitive children; GI distress [26] |
| Microbial Strain | Metabolite(s) Produced | Target Pathogen/Spoilage Organism | Food System Application |
|---|---|---|---|
| Lactobacillus plantarum | Lactic acid, bacteriocins (plantaricins), hydrogen peroxide | Listeria monocytogenes, E. coli O157:H7, molds | Fermented meats, dairy, vegetables [40] |
| Lactobacillus rhamnosus | Exopolysaccharides, lactic acid | Salmonella sp., spoilage yeasts | Dairy (yogurt, cheese) [41] |
| Lactococcus lactis | Nisin (bacteriocin) | Gram-positive bacteria (Listeria sp., Staphylococcus sp.) | Cheese, dairy beverages [42] |
| Bifidobacterium bifidum | Short-chain fatty acids, acetate | Enteric pathogens, spoilage bacteria | Infant formula, dairy products [43] |
| Pediococcus acidilactici | Pediocin | Listeria monocytogenes | Meat, fish [44] |
| Saccharomyces boulardii | Organic acids, ethanol, peptides | Spoilage fungi, bacteria | Functional beverages [45] |
| Weissella cibaria | Hydrogen peroxide, antimicrobial peptides | Gram-negative bacteria, molds | Fermented vegetables, kimchi [46] |
| Bacillus subtilis | Subtilin, surfactin | Spore-forming bacteria (Bacillus cereus) | Plant-based foods, soy products [47] |
| Propionibacterium freudenreichii | Propionic acid, acetic acid | Molds, yeasts | Swiss cheese, dairy [48] |
| Enterococcus faecium | Enterocins | Listeria monocytogenes | Meat, dairy [49] |
| Region | Food Products Studied | Probiotic/Postbiotic Applied | Reported Outcomes |
|---|---|---|---|
| Africa | Fermented dairy (nunu, yogurt), fermented cereals | Lactobacillus plantarum, Weissella cibaria | Extended shelf-life, reduced Listeria and fungal spoilage [101] |
| Asia | Kimchi, soy sauce, natto, fermented tea | Lactobacillus sakei, Bacillus subtilis metabolites | Enhanced safety, inhibition of molds and enteric bacteria [102] |
| Europe | Cheese, cured meats, bakery | Nisin, pediocin, L. lactis | Effective Listeria control; consumer acceptance of natural labeling [103] |
| USA | Dairy products, ready-to-eat meats, plant-based beverages | Lactobacillus rhamnosus, nisin, postbiotic blends | Extended shelf-life, reduced recalls due to pathogens [104] |
| Latin America | Fermented maize beverages, cheese | L. plantarum, Bifidobacterium sp. | Improved microbial safety, better consumer acceptance [105] |
| Middle East | Yogurt, kefir, fermented vegetables | Lactobacillus bulgaricus, kefiran exopolysaccharides | Shelf-life extension, antifungal activity [106] |
| Challenge | Description | Example from Literature | Potential Solution/Research Direction |
|---|---|---|---|
| Stability in food matrices | Loss of activity due to pH, heat, oxygen, or storage | Nisin degradation in cheese at high pH | Encapsulation in biopolymers; stabilizers [124] |
| Viability of probiotics | Probiotic cells die before exerting effect | Lactobacillus loss during pasteurization | Freeze-drying, microencapsulation [125] |
| Regulatory ambiguity | No unified definition for “postbiotics” | EFSA lacks harmonized approval pathways | Codex Alimentarius-based global guidelines [126] |
| Cost & scalability | Industrial production costly vs. synthetic preservatives | Nisin > 10× costlier than sodium nitrite | Bioreactor optimization, precision fermentation [127] |
| Consumer skepticism | Concerns about safety, efficacy, and “live microbes” | Low acceptance in some Western markets | Education campaigns, labeling transparency |
| Antimicrobial resistance (AMR) | Risk of transferable resistance genes | Enterococcus strains carrying resistance | Strain screening, use of purified postbiotics [128] |
| Interaction with food matrices | Postbiotics less effective in fatty/complex foods | Nisin in high-fat meats loses potency | Synergistic blends (EOs, nanomaterials) [129] |
| Shelf-life variability | Inconsistent preservation outcomes across foods | L. plantarum effective in vegetables but not meat | Matrix-specific formulations [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafe, A.N.; Büsselberg, D. Probiotics and Postbiotics for Green Control of Foodborne Pathogens: Intelligent Detection and Biopreservation Strategies for Safer Foods. Foods 2025, 14, 3281. https://doi.org/10.3390/foods14183281
Mafe AN, Büsselberg D. Probiotics and Postbiotics for Green Control of Foodborne Pathogens: Intelligent Detection and Biopreservation Strategies for Safer Foods. Foods. 2025; 14(18):3281. https://doi.org/10.3390/foods14183281
Chicago/Turabian StyleMafe, Alice N., and Dietrich Büsselberg. 2025. "Probiotics and Postbiotics for Green Control of Foodborne Pathogens: Intelligent Detection and Biopreservation Strategies for Safer Foods" Foods 14, no. 18: 3281. https://doi.org/10.3390/foods14183281
APA StyleMafe, A. N., & Büsselberg, D. (2025). Probiotics and Postbiotics for Green Control of Foodborne Pathogens: Intelligent Detection and Biopreservation Strategies for Safer Foods. Foods, 14(18), 3281. https://doi.org/10.3390/foods14183281







