Foodborne Titanium Dioxide Nanoparticles Aggravated Secondary Liver Injury in DSS-Induced Colitis: Role of the NLRP3 Inflammasome
Abstract
1. Introduction
2. Materials and Methods
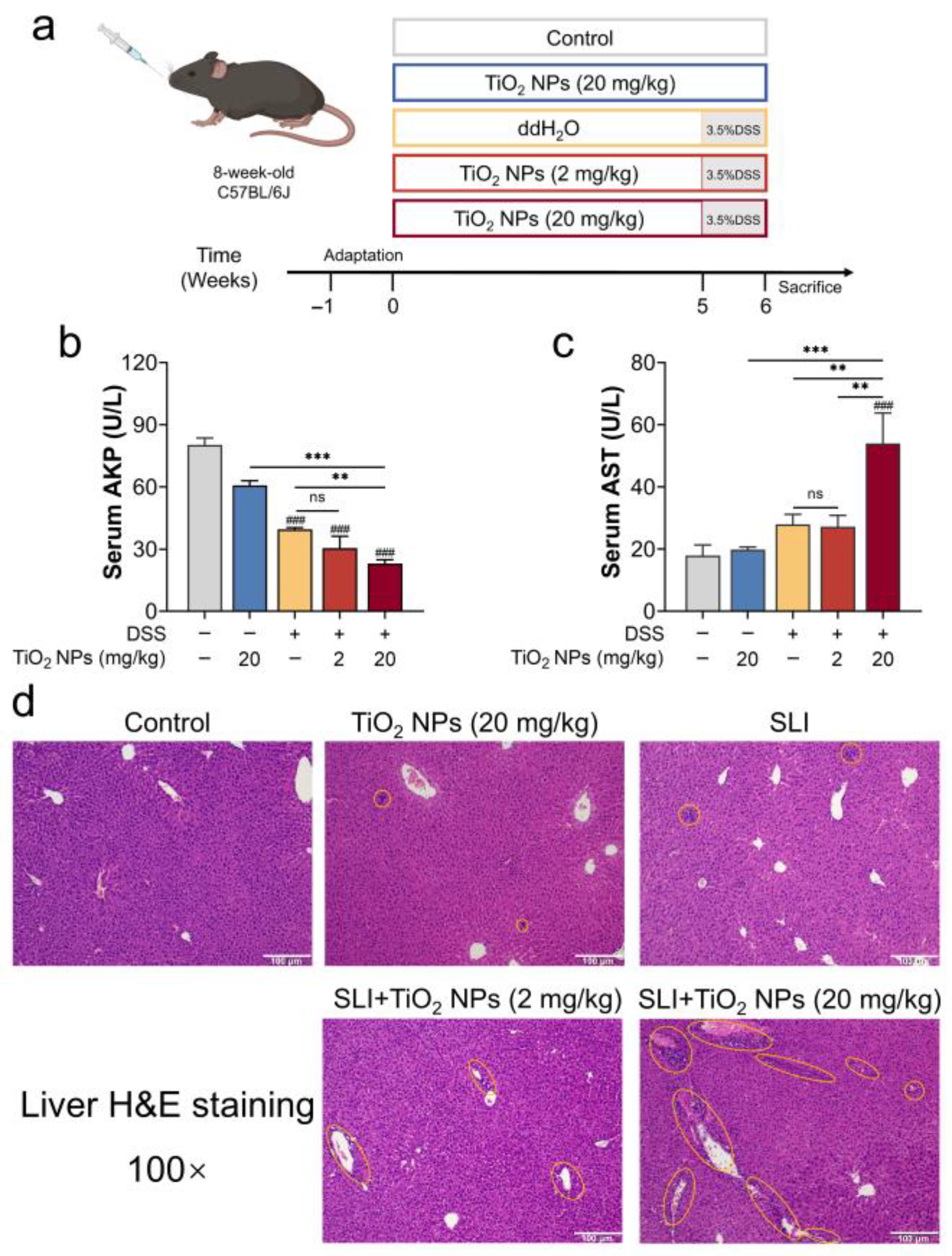
2.1. Animal Experiment and Design
2.2. Histopathological Analysis
2.3. Liver Function Assessment
2.4. Liver Inflammation and Oxidative Injury
2.5. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.6. Statistical Analysis
3. Results
3.1. TiO2 NPs Aggravated the Symptoms of DSS-Induced SLI
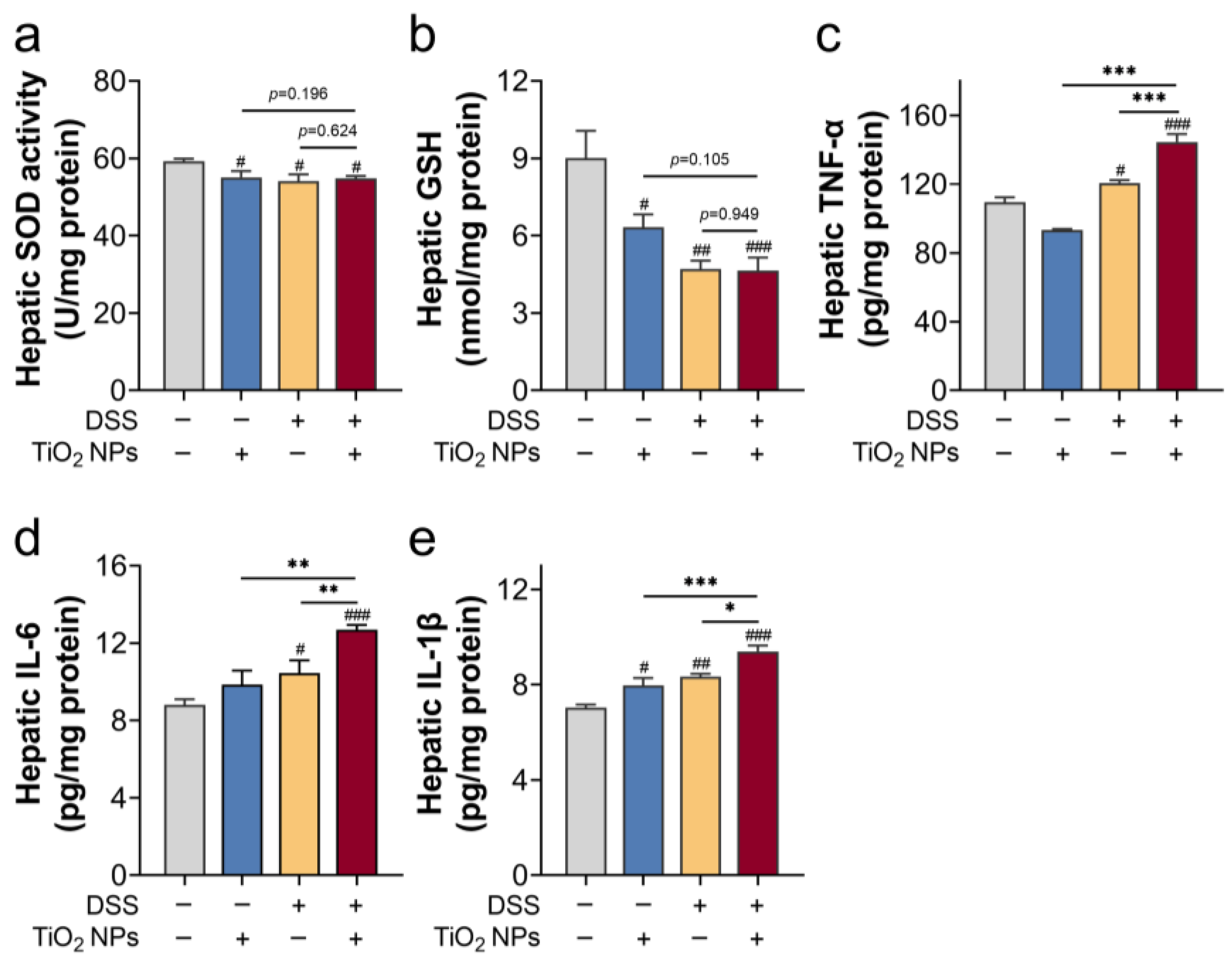
3.2. TiO2 NPs Induced Liver Inflammation and Oxidative Injury in DSS-Induced SLI Mice
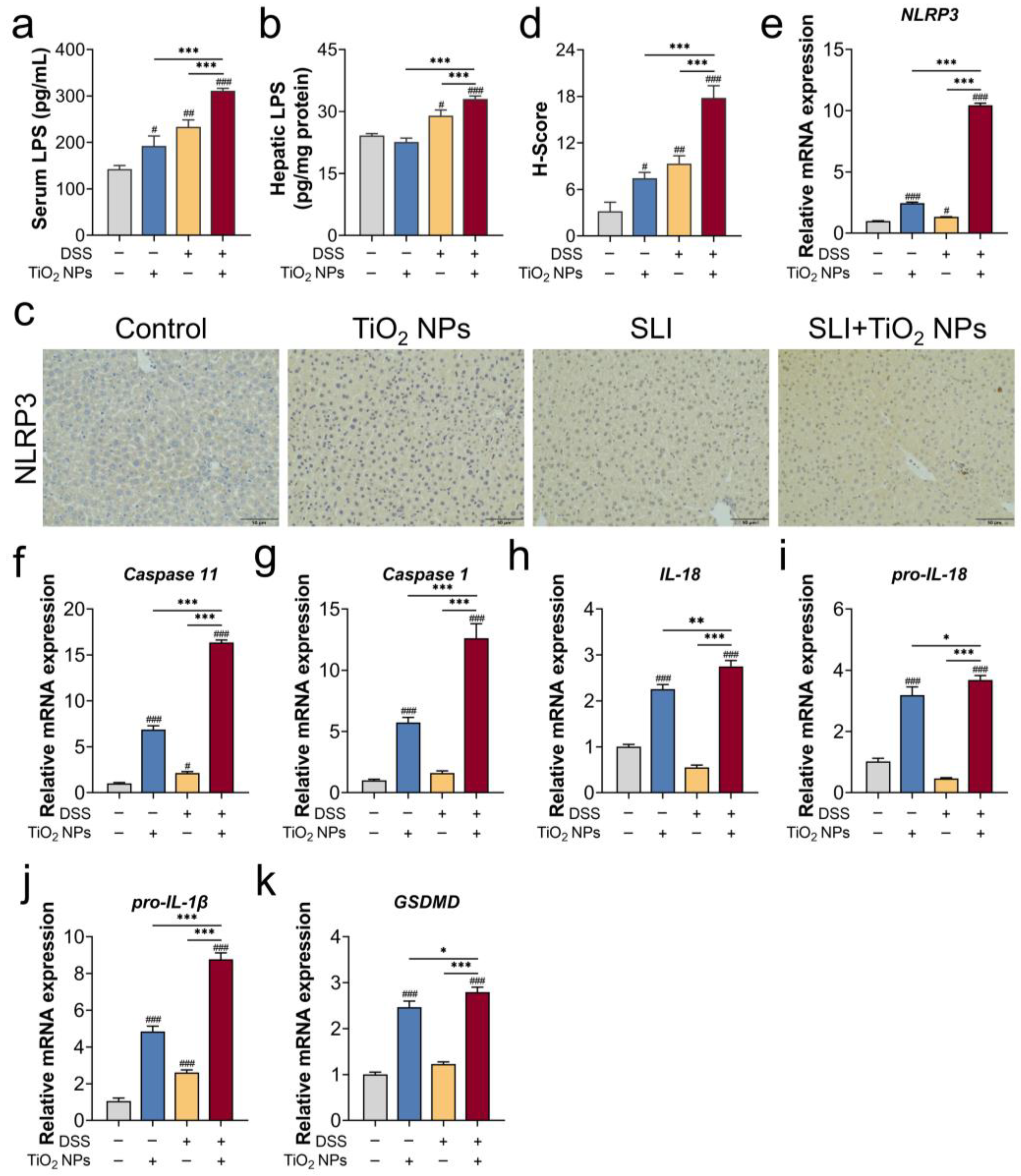
3.3. The NLRP3 Inflammasome Was Probably Involved in TiO2 NP-Aggravated SLI
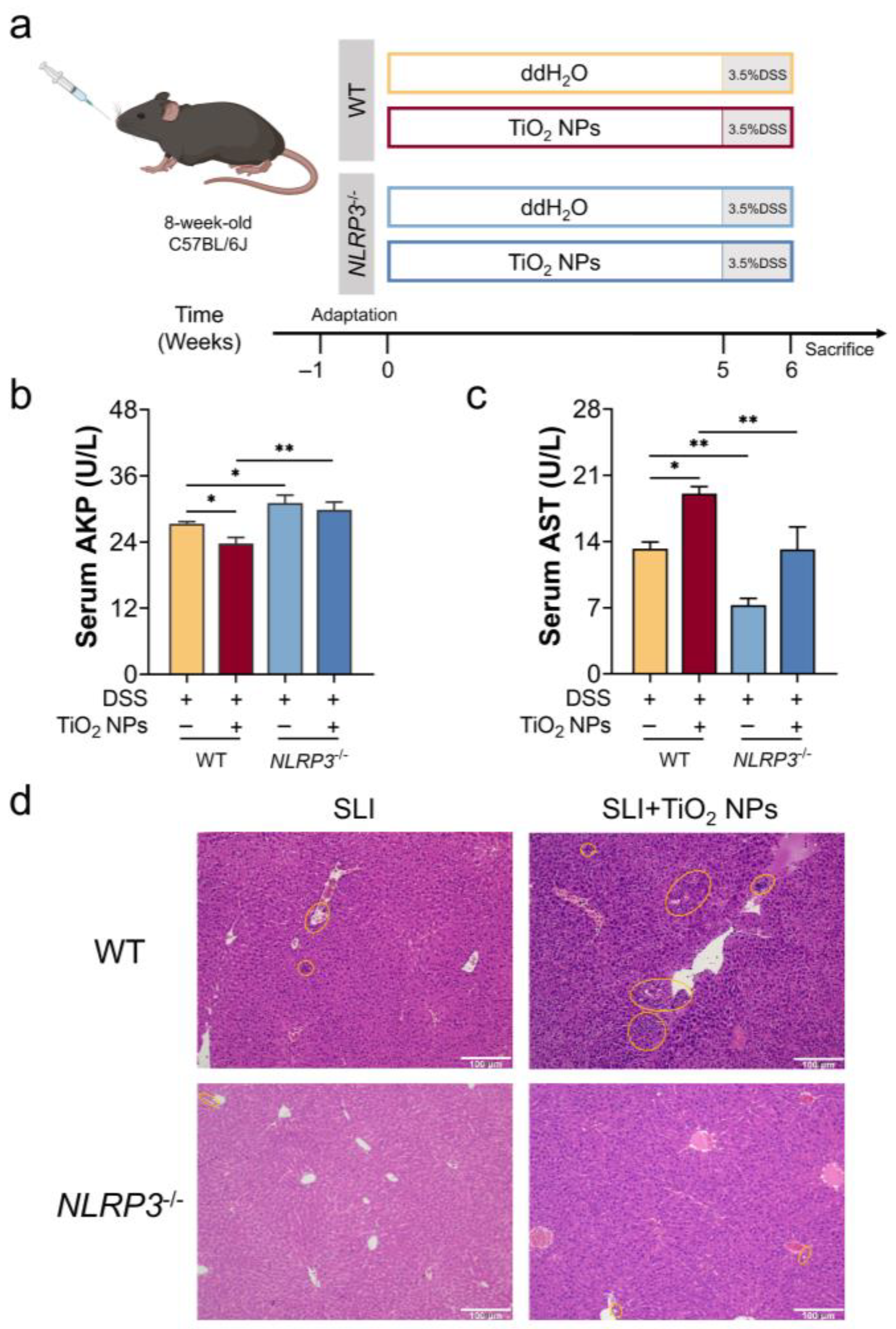
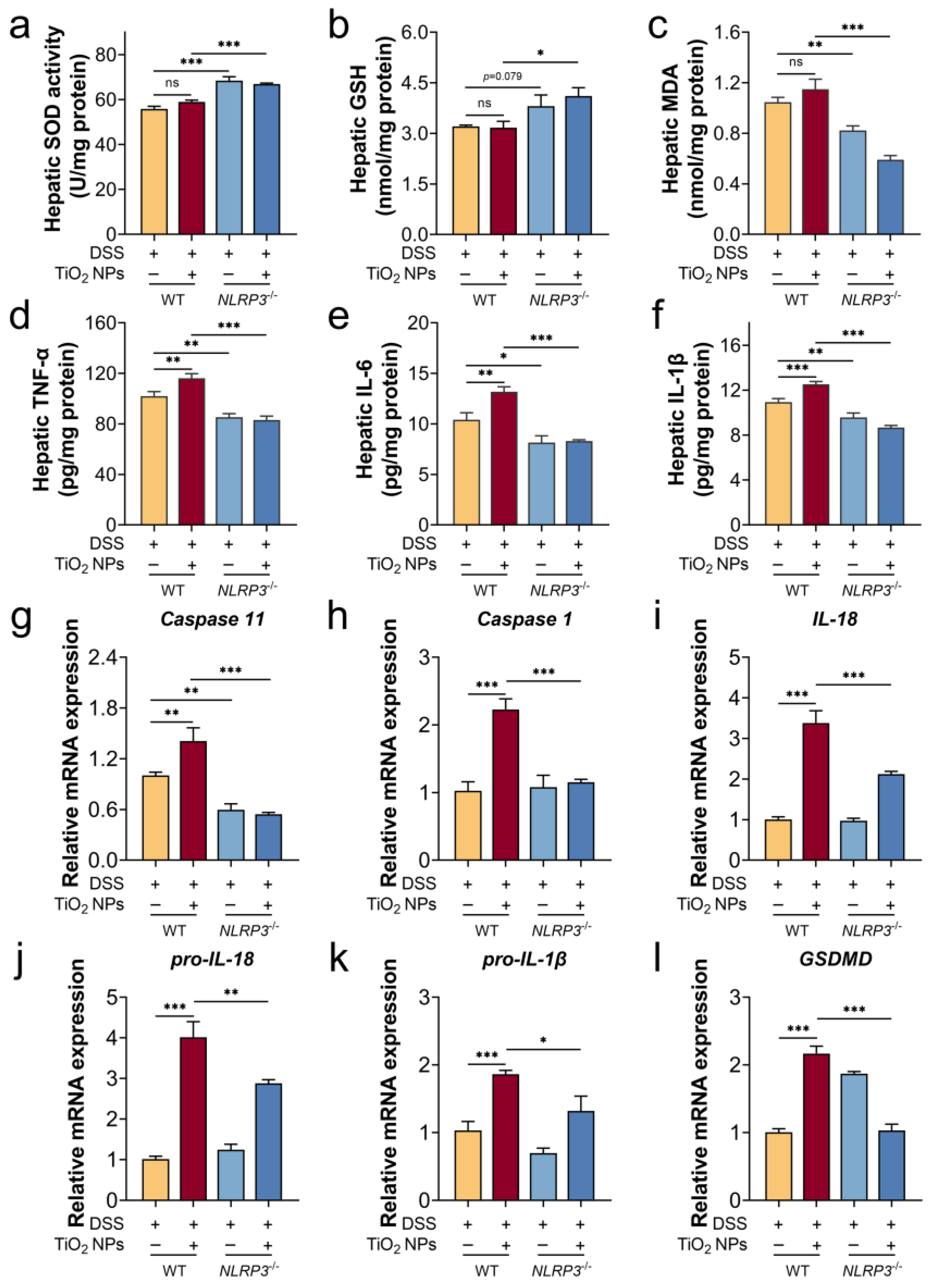
3.4. The Absence of NLRP3 Alleviated the Symptoms of DSS-Induced SLI with TiO2 NPs Exposure
3.5. The Absence of NLRP3 Mitigated Liver Injury in SLI Mice with TiO2 NPs Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boccatonda, A.; Balletta, M.; Vicari, S.; Hoxha, A.; Simioni, P.; Campello, E. The journey through the pathogenesis and treatment of venous thromboembolism in inflammatory bowel diseases: A narrative review. Semin. Thromb. Hemost. 2022, 49, 744–755. [Google Scholar] [CrossRef]
- Liu, C.; Qi, X.; Liu, X.; Sun, Y.; Mao, K.; Shen, G.; Ma, Y.; Li, Q. Anti-inflammatory probiotics HF05 and HF06 synergistically alleviate ulcerative colitis and secondary liver injury. Food Funct. 2024, 15, 3765–3777. [Google Scholar] [CrossRef]
- Meng, Z.; Fu, B.; Yang, Z.; Xu, Y.; Huang, H.; Bai, Y.; Fang, X.; Shen, S.; Yang, J.; Yong, J.; et al. Polydopamine-coated thalidomide nanocrystals promote DSS-induced murine colitis recovery through Macrophage M2 polarization together with the synergistic anti-inflammatory and anti-angiogenic effects. Int. J. Pharm. 2023, 630, 122376. [Google Scholar] [CrossRef]
- Ng, S.; Shi, H.; Hamidi, N.; Underwood, F.; Tang, W.; Benchimol, E.; Panaccione, R.; Ghosh, S.; Wu, J.; Chan, F.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Zhu, L.; Zong, X.; Xiao, X.; Cheng, Y.; Fu, J.; Lu, Z.; Jin, M.; Wang, F.; Wang, Y. Multi-omics analysis of the gut-liver axis reveals the mechanism of liver injury in colitis mice. Front. Immunol. 2022, 12, 773070. [Google Scholar] [CrossRef]
- Silva, J.; Brito, B.; Silva, I.; Nóbrega, V.; Silva, M.; Gomes, H.; Fortes, F.; Pimentel, A.; Mota, J.; Almeida, N.; et al. Frequency of hepatobiliary manifestations and concomitant liver disease in inflammatory bowel disease patients. BioMed. Res. Int. 2019, 2019, 7604939. [Google Scholar] [CrossRef]
- Trivedi, P.; Jena, G. Ulcerative colitis-induced hepatic damage in mice: Studies on inflammation, fibrosis, oxidative DNA damage and GST-P expression. Chem-Biol. Interact. 2013, 201, 19–30. [Google Scholar] [CrossRef]
- Lama, S.; Pagano, E.; Borrelli, F.; Maisto, M.; Tenore, G.; Nanì, M.; Chacon-Millan, P.; Novellino, E.; Stiuso, P. Polyphenol-rich extract from ‘Limoncella’ apple variety ameliorates dinitrobenzene sulfonic acid-induced colitis and linked liver damage. Int. J. Mol. Sci. 2024, 25, 3210. [Google Scholar] [CrossRef]
- Mendes, F.; Levy, C.; Enders, F.; Loftus, E., Jr.; Angulo, P.; Lindor, K. Abnormal hepatic biochemistries in patients with inflammatory bowel disease. Off. J. Am. Coll. Gastroenterol. 2007, 102, 344–350. [Google Scholar]
- Li, E.; Wang, T.; Zhou, R.; Zhou, Z.; Zhang, C.; Wu, W.; He, K. Myricetin and myricetrin alleviate liver and colon damage in a chronic colitis mice model: Effects on tight junction and intestinal microbiota. J. Funct. Foods. 2021, 87, 104790. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, S.; Chen, Z.; Liu, Y.; Pei, C.; Huang, H.; Hou, S.; Ning, W.; Liang, J. Gingerenone A alleviates ferroptosis in secondary liver injury in colitis mice via activating Nrf2–Gpx4 signaling pathway. J. Agric. Food Chem. 2022, 70, 12525–12534. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Pei, C.; Liang, J.; Ding, P.; Chen, S.; Hou, S. Monotropein alleviates secondary liver injury in chronic colitis by regulating TLR4/NF-κB signaling and NLRP3 inflammasome. Eur. J. Pharmacol. 2020, 883, 173358. [Google Scholar] [CrossRef]
- Sandys, O.; te Velde, A. Raising the alarm: Environmental factors in the onset and maintenance of chronic (low-grade) inflammation in the gastrointestinal tract. Digest. Dis. Sci. 2022, 67, 4355–4368. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; Wei, X.; Chang, L.; Liu, S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total Environ. 2021, 750, 143085. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Z.; Jin, Y. F-53B induces hepatotoxic effects and slows self-healing in ulcerative colitis in mice. Environ. Pollut. 2023, 317, 120819. [Google Scholar] [CrossRef]
- Luo, T.; Wang, D.; Zhao, Y.; Li, X.; Yang, G.; Jin, Y. Polystyrene microplastics exacerbate experimental colitis in mice tightly associated with the occurrence of hepatic inflammation. Sci. Total. Environ. 2022, 844, 156884. [Google Scholar] [CrossRef]
- Yang, H.; Sanidad, K.; Wang, W.; Xie, M.; Gu, M.; Cao, X.; Xiao, H.; Zhang, G. Triclocarban exposure exaggerates colitis and colon tumorigenesis: Roles of gut microbiota involved. Gut Microbes 2019, 12, 1690364. [Google Scholar] [CrossRef]
- Candás-Zapico, S.; Kutscher, D.; Montes-Bayón, M.; Bettmer, J. Single particle analysis of TiO2 in candy products using triple quadrupole ICP-MS. Talanta 2018, 180, 309–315. [Google Scholar] [CrossRef]
- Peters, R.; van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.; Marvin, H.; Weigel, S.; Tromp, P.; Oomen, A.; Rietveld, A.; Bouwmeester, H. Characterization of titanium dioxide nanoparticles in food products: Analytical methods to define nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef]
- Cui, Y.; Gong, X.; Duan, Y.; Li, N.; Hu, R.; Liu, H.; Hong, M.; Zhou, M.; Wang, L.; Wang, H.; et al. Hepatocyte apoptosis and its molecular mechanisms in mice caused by titanium dioxide nanoparticles. J. Hazard. Mater. 2010, 183, 874–880. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Winiarska-Mieczan, A. A review of research on the impact of E171/TiO2 NPs on the digestive tract. J. Trace Elem. Med. Biol. 2022, 72, 126988. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Tang, Y.; You, T.; Xu, H. Lactobacillus rhamnosus GG ameliorated long-term exposure to TiO2 nanoparticles induced microbiota-mediated liver and colon inflammation and fructose-caused metabolic abnormality in metabolism syndrome mice. J. Agric. Food Chem. 2021, 69, 9788–9799. [Google Scholar] [CrossRef]
- Lomer, M.; Hutchinson, C.; Volkert, S.; Greenfield, S.; Catterall, A.; Thompson, R.; Powell, J. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn’s disease. Br. J. Nutr. 2004, 92, 947–955. [Google Scholar] [CrossRef]
- Ruiz, P.; Moron, B.; Becker, H.; Lang, S.; Atrott, K.; Spalinger, M.; Scharl, M.; Wojtal, K.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef]
- Duan, S.; Wang, H.; Gao, Y.; Wang, X.; Lyu, L.; Wang, Y. Oral intake of titanium dioxide nanoparticles affect the course and prognosis of ulcerative colitis in mice: Involvement of the ROS-TXNIP-NLRP3 inflammasome pathway. Part. Fibre Toxicol. 2023, 20, 24. [Google Scholar] [CrossRef]
- Urrutia-Ortega, I.; Garduño-Balderas, L.; Delgado-Buenrostro, N.; Freyre-Fonseca, V.; Flores-Flores, J.; González-Robles, A.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Rodríguez-Sosa, M.; León-Cabrera, S.; et al. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem. Toxicol. 2016, 93, 20–31. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, D.; Han, S.; Zhou, S.; Jia, G. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles. Part. Fibre Toxicol. 2019, 16, 48. [Google Scholar] [CrossRef]
- Duan, S.; Du, X.; Chen, S.; Liang, J.; Huang, S.; Hou, S.; Gao, J.; Ding, P. Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed. Pharmacother. 2020, 121, 109683. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Gao, L.; Zhang, L.; Wang, J. Study on the protective effect and mechanism of umbilicaria esculenta polysaccharide in DSS-induced mice colitis and secondary liver injury. J. Agric. Food Chem. 2024, 72, 10923–10935. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, L.; Liu, Z.; Zhou, Q.; Zhu, Y.; Zhao, Y.; Yang, X. Long-term exposure to titanium dioxide nanoparticles promotes diet-induced obesity through exacerbating intestinal mucus layer damage and microbiota dysbiosis. Nano Res. 2020, 14, 1512–1522. [Google Scholar] [CrossRef]
- Yan, J.; Wang, D.; Li, K.; Chen, Q.; Lai, W.; Tian, L.; Lin, B.; Tan, Y.; Liu, X.; Xi, Z. Toxic effects of the food additives titanium dioxide and silica on the murine intestinal tract: Mechanisms related to intestinal barrier dysfunction involved by gut microbiota. Environ. Toxicol. Phar. 2020, 80, 103485. [Google Scholar] [CrossRef]
- Zhang, C.; He, A.; Liu, S.; He, Q.; Luo, Y.; He, Z.; Chen, Y.; Tao, A.; Yan, J. Inhibition of HtrA2 alleviated dextran sulfate sodium (DSS)-induced colitis by preventing necroptosis of intestinal epithelial cells. Cell Death. Dis. 2019, 10, 344. [Google Scholar] [CrossRef]
- Jiang, N.; Wei, Y.; Cen, Y.; Shan, L.; Zhang, Z.; Yu, P.; Wang, Y.; Xu, L. Andrographolide derivative AL-1 reduces intestinal permeability in dextran sulfate sodium (DSS)-induced mice colitis model. Life Sci. 2020, 241, 117164. [Google Scholar] [CrossRef]
- Faria, S.; Costantini, S.; de Lima, V.; de Andrade, V.; Rialland, M.; Cedric, R.; Budillon, A.; Magalhães, K. NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J. Biomed. Sci. 2021, 28, 26. [Google Scholar] [CrossRef]
- Moretti, J.; Jia, B.; Hutchins, Z.; Roy, S.; Yip, H.; Wu, J.; Shan, M.; Jaffrey, S.; Coers, J.; Blander, J. Caspase-11 interaction with NLRP3 potentiates the noncanonical activation of the NLRP3 inflammasome. Nat. Immunol. 2022, 23, 705–717. [Google Scholar] [CrossRef]
- Matikainen, S.; Nyman, T.; Cypryk, W. Function and regulation of noncanonical caspase-4/5/11 inflammasome. J. Immunol. 2020, 204, 3063–3069. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chng, W. Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer. Mitochondrion 2013, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chi, H.; Zhu, W.; Yang, G.; Song, J.; Mo, L.; Zhang, Y.; Deng, Y.; Xu, F.; Yang, J.; et al. Cadmium induces renal inflammation by activating the NLRP3 inflammasome through ROS/MAPK/NF-κB pathway in vitro and in vivo. Arch. Toxicol. 2021, 95, 3497–3513. [Google Scholar] [CrossRef] [PubMed]
- Lupfer, C.; Anand, P.; Liu, Z.; Stokes, K.; Vogel, P.; Lamkanfi, M.; Kanneganti, T. Reactive oxygen species regulate caspase-11 expression and activation of the non-canonical NLRP3 inflammasome during enteric pathogen infection. PLoS Pathog. 2014, 10, e1004410. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer | Sequence (5′-3′) |
|---|---|---|
| NLRP3 | Forward | ATTACCCGCCCGAGAAAGG |
| Reverse | CATGAGTGTGGCTAGATCCAAG | |
| Caspase 11 | Forward | GTGGTGAAAGAGGAGCTTACAGC |
| Reverse | GCACCAGGAATGTGCTGTCTGA | |
| Caspase 1 | Forward | GGCACATTTCCAGGACTGACTG |
| Reverse | GCAAGACGTGTACGAGTGGTTG | |
| IL-18 | Forward | GACAGCCTGTGTTCGAGGATATG |
| Reverse | TGTTCTTACAGGAGAGGGTAGAC | |
| pro-IL-18 | Forward | GACTCTTGCGTCAACTTCAAGG |
| Reverse | CAGGCTGTCTTTTGTCAACGA | |
| GSDMD | Forward | TGTCAACCTGTCAATCAAGGA |
| Reverse | AGCCAAAACACTCCGGTTC | |
| pro-IL-1β | Forward | GCAACTGTTCCTGAACTCAACT |
| Reverse | ATCTTTTGGGGTCCGTCAACT | |
| β-actin | Forward | AAGGCCAACCGTGAAAAGAT |
| Reverse | GTGGTACGACCAGAGGCATAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Yuan, H.; You, T.; Xu, H. Foodborne Titanium Dioxide Nanoparticles Aggravated Secondary Liver Injury in DSS-Induced Colitis: Role of the NLRP3 Inflammasome. Foods 2025, 14, 3279. https://doi.org/10.3390/foods14183279
Feng X, Yuan H, You T, Xu H. Foodborne Titanium Dioxide Nanoparticles Aggravated Secondary Liver Injury in DSS-Induced Colitis: Role of the NLRP3 Inflammasome. Foods. 2025; 14(18):3279. https://doi.org/10.3390/foods14183279
Chicago/Turabian StyleFeng, Xiaoyan, Hongbin Yuan, Tao You, and Hengyi Xu. 2025. "Foodborne Titanium Dioxide Nanoparticles Aggravated Secondary Liver Injury in DSS-Induced Colitis: Role of the NLRP3 Inflammasome" Foods 14, no. 18: 3279. https://doi.org/10.3390/foods14183279
APA StyleFeng, X., Yuan, H., You, T., & Xu, H. (2025). Foodborne Titanium Dioxide Nanoparticles Aggravated Secondary Liver Injury in DSS-Induced Colitis: Role of the NLRP3 Inflammasome. Foods, 14(18), 3279. https://doi.org/10.3390/foods14183279








