Exploiting Marker Genes for Reliable Botanical Authentication of Bacopa monnieri Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Species and Commercial Samples
2.2. DNA Extraction
2.3. Screening of Molecular Markers
2.4. Qualitative PCR
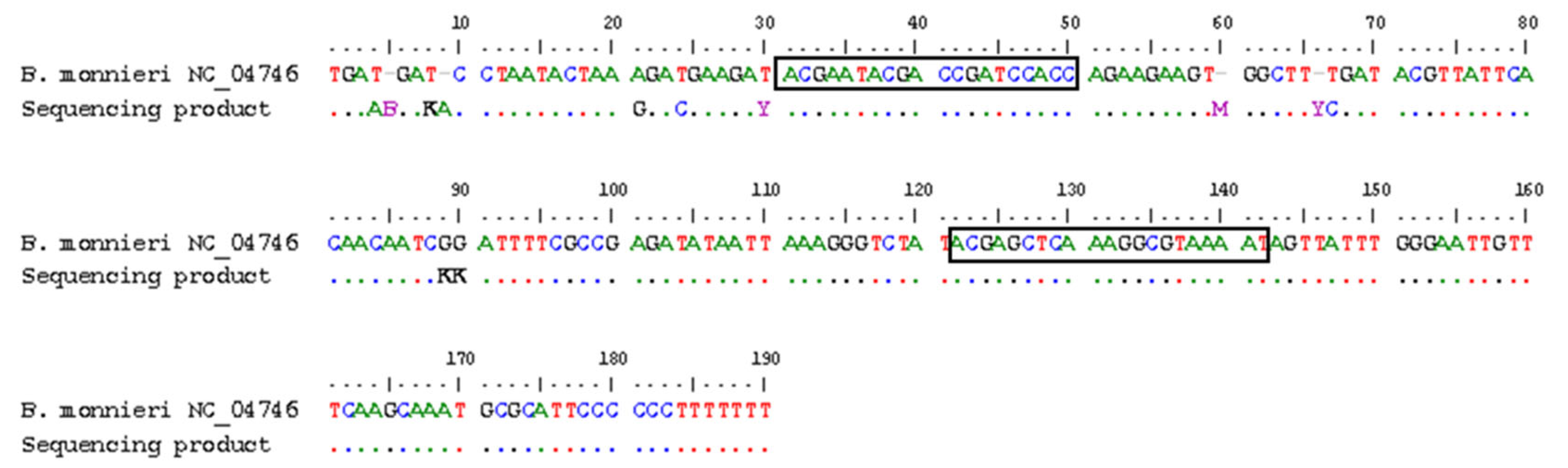
2.5. Sequencing
2.6. Real-Time PCR
3. Results
3.1. DNA Quality, Selection of Target Markers and Specificity
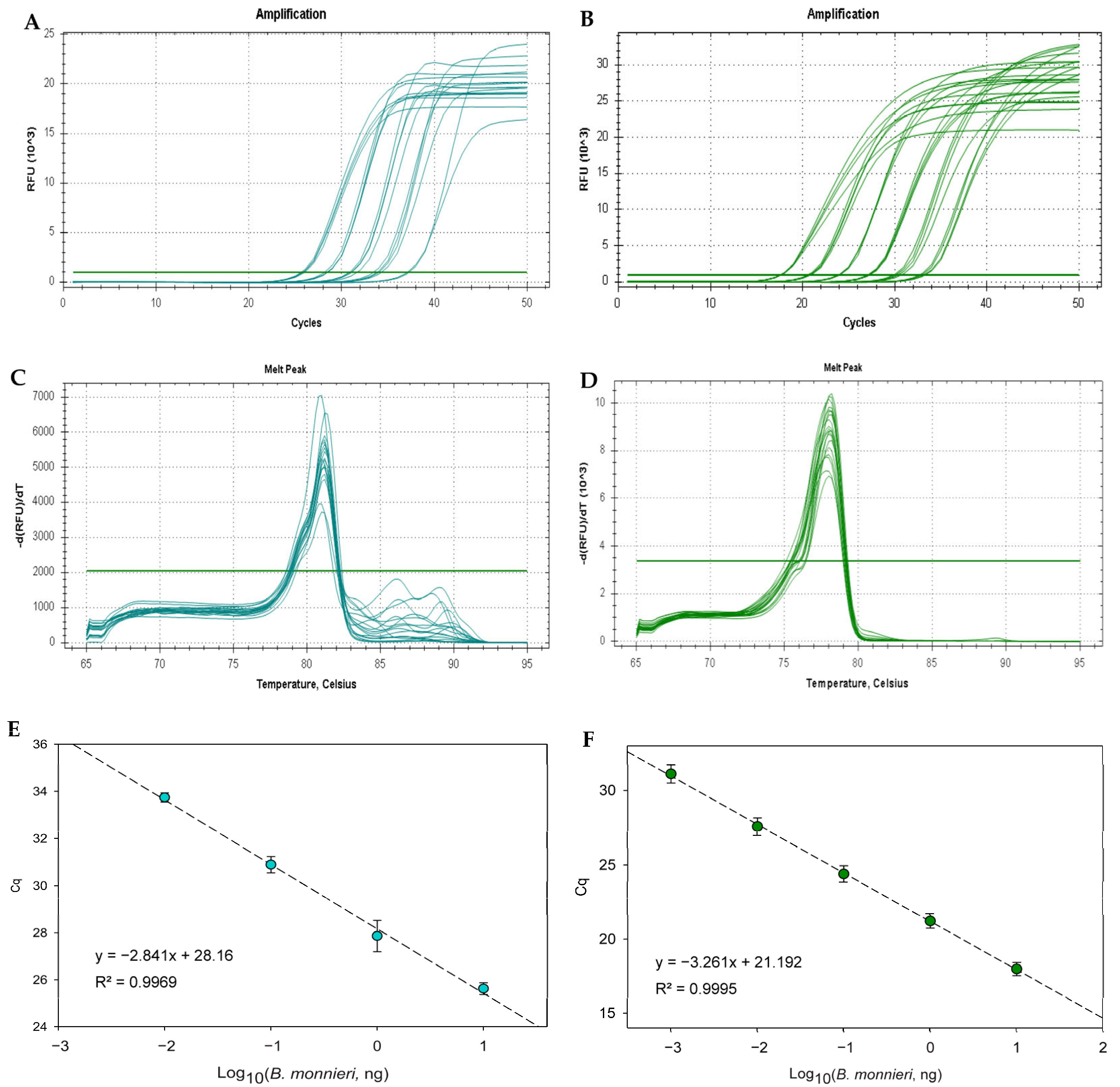
3.2. Real-Time PCR Method Development
3.3. Validation of Method
3.4. Authentication of Commercial Herbal Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAGR | Compound annual growth rate |
| Cq | Cycle of quantification |
| CV | Coefficient of variation |
| EU | European Union |
| FDA | Food and Drug Administration |
| Flag | Flavonoid glucosyltransferase gene |
| GMO | Genetically modified organisms |
| HRM | High resolution melting |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MIQE | Minimum information for publication of quantitative real-time PCR experiments |
| PCR | Polymerase chain reaction |
| RAPD | Randomly amplified polymorphic DNA |
| SCAR | Sequence-characterised amplified region |
| SD | Standard deviation |
| Ycf1 | Ycf1 photosystem I assembly protein gene |
References
- Pandey, A.K. Bacopa monnieri (Linn.) Pennell—A possible plant for impossible diseases. Adv. Pharmacol. Pharm. 2022, 10, 35–53. [Google Scholar] [CrossRef]
- Kuruvalli, G.; Wankhade, I.; Wankhede, S.; Ramesh, S.B.; Narayanaswamy, C.K.; Munikrishnappa, G.N.; Reddy, V.D. A comprehensive review on the ethno-medicinal and pharmacological properties of Bacopa monnieri. Pharmacogn. Rev. 2023, 17, 418–425. [Google Scholar] [CrossRef]
- Nemetchek, M.D.; Stierle, A.A.; Stierle, D.B.; Lurie, D.I. The Ayurvedic plant Bacopa monnieri inhibits inflammatory pathways in the brain. J. Ethnopharmacol. 2017, 197, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Mathur, D.; Goyal, K.; Koul, V.; Anand, A. The molecular links of re-emerging therapy: A review of evidence of Brahmi (Bacopa monnieri). Front. Pharmacol. 2016, 7, 44. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Suba, V.; Ramesh, M.; Chen, J.-T. Bacopa monnieri and their bioactive compounds inferred multi-target treatment strategy for neurological diseases: A cheminformatics and system pharmacology approach. Biomolecules 2020, 10, 536. [Google Scholar] [CrossRef]
- Ritter, S.; Urmann, C.; Herzog, R.; Glaser, J.; Bieringer, S.; Geisberger, T.; Eisenreich, W.; Riepl, H. Where is bacosine in commercially available Bacopa monnieri? Planta Medica 2020, 86, 565–570. [Google Scholar] [CrossRef]
- Shalini, V.T.; Neelakanta, S.J.; Sriranjini, J.S. Neuroprotection with Bacopa monnieri—A review of experimental evidence. Mol. Biol. Rep. 2021, 48, 2653–2668. [Google Scholar] [CrossRef]
- Shankar, P.S.; Preeti, B.; Santanu, B.; Gajanan, D.; Rupesh, D. Brahmi (Bacopa monnieri) as functional food ingredient in food processing industry. J. Pharmacogn. Phytochem. 2018, 7, 189–194. [Google Scholar]
- Gouthami, N.; Jain, S.; Jain, N.; Wadhawana, N.; Agarwal, C.; Panwar, N. Innovative approaches for incorporating brahmi (Bacopa monnieri) in postharvest food processing. J. Postharvest Technol. 2023, 11, 122–131. [Google Scholar]
- Saloni, S.M.; Rai, D.C.; Panda, P.; Kumar, S. A comprehensive review on Bacopa monnieri (L.) Pennell (Brahmi): Utilization as a functional food ingredient and health-promoting attributes. Ann. Phytomed. 2022, 11, 142–150. [Google Scholar] [CrossRef]
- Goraya, G.; Ved, D. Medicinal Plants in India: An Assessment of Their Demand and Supply; National Medicinal Plants Board: New Delhi, India; Ministry of AYUSH: New Delhi, India; Government of India: New Delhi, India; New Delhi and Indian Council of Forestry Research & Education: New Delhi, India, 2017.
- IndustryARC. Brahmi Market Forecast (2021–2026); IndustryARC: Hyderabad, India, 2021. [Google Scholar]
- Jędrejko, K.; Catlin, O.; Stewart, T.; Anderson, A.; Muszyńska, B.; Catlin, D.H. Unauthorized ingredients in “nootropic” dietary supplements: A review of the history, pharmacology, prevalence, international regulations, and potential as doping agents. Drug Test. Anal. 2023, 15, 803–839. [Google Scholar] [CrossRef]
- Chaudhari, K.S.; Tiwari, N.R.; Tiwari, R.R.; Sharma, R.S. Neurocognitive effect of nootropic drug Brahmi (Bacopa monnieri) in Alzheimer’s Disease. Ann. Neurosci. 2017, 24, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.; Amaral, J.S.; Oliveira, M.B.P.P. Adulteration of dietary supplements by the illegal addition of synthetic drugs: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.P.; Travadi, T.; Sharma, S.; Pandit, R.; Joshi, C.; Joshi, M. Comprehensive analysis using DNA metabarcoding, SCAR marker based PCR assay, and HPLC unveils the adulteration in Brahmi herbal products. Mol. Biol. Rep. 2023, 50, 7605–7618. [Google Scholar] [CrossRef]
- Xu, M.-R.; Lee, M.-S.; Yang, B.-C.; Chang, H.-C.; Kuo, C.-L.; Lin, C.-H.; Chen, H.-J.; Cheng, J.-H.; Sun, F.-C. Development of a specific and sensitive diagnostic PCR for rapid molecular authentication of the medicinal plant Portulaca oleracea. Mol. Cell. Probes 2023, 67, 101890. [Google Scholar] [CrossRef]
- Xu, M.-R.; Sun, F.-C.; Yang, B.-C.; Chen, H.-J.; Lin, C.-H.; Cheng, J.-H.; Lee, M.-S. Genetic authentication of the medicinal plant Portulaca oleracea using a quick, precise, and sensitive isothermal DNA amplification assay. Int. J. Mol. Sci. 2023, 24, 10730. [Google Scholar] [CrossRef]
- European Commission. EU Novel Food Status Catalogue: Bacopa monnieri (L.) Wettst. 2023. Available online: https://ec.europa.eu/food/food-feed-portal/screen/novel-food-catalogue/search (accessed on 30 May 2024).
- Morán, J.; Kilasoniya, A. Application of the “novel foods” regulation to botanicals in the European Union. Laws 2024, 13, 10. [Google Scholar] [CrossRef]
- FDA. FDA Takes Action Against 17 Companies for Illegally Selling Products Claiming to Treat Alzheimer’s Disease; FDA: Silver Spring, MD, USA, 2019.
- Grazina, L.; Amaral, J.S.; Mafra, I. Botanical origin authentication of dietary supplements by DNA-based approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1080–1109. [Google Scholar] [CrossRef]
- Fanelli, V.; Mascio, I.; Miazzi, M.M.; Savoia, M.A.; De Giovanni, C.; Montemurro, C. Molecular approaches to agri-food traceability and authentication: An updated review. Foods 2021, 10, 1644. [Google Scholar] [CrossRef]
- Yadav, A.; Ahmad, J.; Chaudhary, A.A.; Ahmad, A. Development of sequence characterized amplified region (SCAR) marker for the authentication of Bacopa monnieri (L.) Wettst. Eur. J. Med. Plants 2012, 2, 186–198. [Google Scholar] [CrossRef]
- Tungphatthong, C.; Somnuek, J.; Phadungcharoen, T.; Ingkaninan, K.; Denduangboripant, J.; Sukrong, S. DNA barcoding of species of Bacopa coupled with high-resolution melting analysis. Genome 2018, 61, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.V.; Tiwari, S.; Tripathi, N.; Tiwari, G. Molecular identification of medicinal plants with amplicon length polymorphism using universal DNA barcodes of the atpF–atpH, trnL and trnH–psbA regions. 3 Biotech 2019, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, B.; Carstensen, J.; Bhatia, H.S.; Huell, M.; Dietz, G.P.H.; Fiebich, B.L. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine 2018, 44, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Biltes, R.; Villa, C.; Costa, J.; Mafra, I. Molecular authentication of Centella asiatica products by a novel real-time PCR approach: Influence of plant matrix. Food Biosci. 2025, 68, 106395. [Google Scholar] [CrossRef]
- Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Effect of thermal processing on the performance of the novel single-tube nested real-time PCR for the detection of walnut allergens in sponge cakes. Food Res. Int. 2013, 54, 1722–1729. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Oliveira, M.B.; Mafra, I. Novel quantitative real-time PCR approach to determine safflower (Carthamus tinctorius) adulteration in saffron (Crocus sativus). Food Chem. 2017, 229, 680–687. [Google Scholar] [CrossRef]
- Fajardo, V.; Gonzalez, I.; Martin, I.; Rojas, M.; Hernandez, P.E.; Garcia, T.; Martin, R. Real-time PCR for detection and quantification of red deer (Cervus elaphus), fallow deer (Dama dama), and roe deer (Capreolus capreolus) in meat mixtures. Meat Sci. 2008, 79, 289–298. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- ENGL. Definition of Minimum Performance Requirements for Analytical Methods of GMO Testing. European Network of GMO Laboratories, Join Research Centre, EURL, 2015. Available online: https://gmo-crl.jrc.ec.europa.eu/doc/MPR%20Report%20Application%2020_10_2015.pdf (accessed on 30 May 2024).
- Kang, T.S. Basic principles for developing real-time PCR methods used in food analysis: A review. Trends Food Sci. Technol. 2019, 91, 574–585. [Google Scholar] [CrossRef]
- Behr, M.; Garlant, L.; Pietretti, D.; Pellegrin, C.; Lievens, A.; Sanfeliu, A.B.; Maquet, A.; Alvarellos, L. A robust set of qPCR methods to evaluate adulteration in major spices and herbs. Food Control 2024, 165, 110623. [Google Scholar] [CrossRef]
- Franz, C.; Chizzola, R.; Novak, J.; Sponza, S. Botanical species being used for manufacturing plant food supplements (PFS) and related products in the EU member states and selected third countries. Food Funct. 2011, 2, 720–730. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Li, C.; Sun, J.; Zuo, Y.; Shi, S.; Cheng, T.; Guo, J.; Zhou, S. ycf1, the most promising plastid DNA barcode of land plants. Sci. Rep. 2015, 5, 8348. [Google Scholar] [CrossRef]
- Cantsilieris, S.; Western, P.S.; Baird, P.N.; White, S.J. Technical considerations for genotyping multi-allelic copy number variation (CNV), in regions of segmental duplication. BMC Genom. 2014, 15, 329. [Google Scholar] [CrossRef]


| Common Name | Species | Qualitative PCR a | ||
|---|---|---|---|---|
| 18S rRNA | Flag | Ycf1 | ||
| Bacopa | Bacopa monnieri b | +++ | +++ | +++ |
| Bacopa | B. monnieri c | +++ | +++ | +++ |
| House cricket | Acheta domestica | +++ | - | - |
| Onion | Allium cepa | +++ | - | - |
| Garlic | Allium sativum | +++ | - | - |
| Lemon verbena | Aloysia citrodora | +++ | - | - |
| Ananas | Ananas comosus | +++ | - | - |
| Daisy-leaved toadflax | Anarrhinum bellidifolium | +++ | - | - |
| Carniolan honeybee | Apis mellifera carnica | +++ | - | - |
| Celery | Apium graveolens | +++ | - | - |
| Common bearberry | Arctostaphylos uva-ursi | +++ | - | - |
| Argan | Argania spinosa L. | +++ | - | - |
| Spirulina | Arthrospira platensis | +++ | - | - |
| Cow | Bos taurus | +++ | - | - |
| Green tea | Camellia sinensis | +++ | - | - |
| Spanish chestnut | Castanea sativa | +++ | - | - |
| Gotu kola | Centella asiatia | +++ | - | - |
| Bitter orange | Citrus aurantium | +++ | - | - |
| Borututu | Cochlospermum angolense | +++ | - | - |
| Coriander | Coriandrum sativum | +++ | - | - |
| Oysters | Crassostrea angulata | +++ | - | - |
| Hawthorn | Crataegus monogyna | +++ | - | - |
| Turmeric | Curcuma longa | +++ | - | - |
| Lemongrass | Cymbopogon citratus | +++ | - | - |
| Artichoke | Cynara cardunculus var. scolymus | +++ | - | - |
| Scotch broom | Cytisus scoparius | +++ | - | - |
| Truncate donax | Donax trunculus | +++ | - | - |
| Siberian ginseng | Eleutherococcus senticosus | +++ | - | - |
| Common horsetail | Equisetum arvense | +++ | - | - |
| Messmate stringybark | Eucalyptus obliqua L’Hér | +++ | - | - |
| Common fennel | Foeniculum vulgare | +++ | - | - |
| Cod | Gadus morhua | +++ | - | - |
| Chicken | Gallus gallus domesticus | +++ | - | - |
| Herb-robert | Geranium robertianium | +++ | - | - |
| Ginkgo | Ginkgo biloba | +++ | - | - |
| Soybean | Glycine max L. | +++ | - | - |
| St. John’s wort | Hypericum perforatum | +++ | - | - |
| Laurel | Laurus nobilis | +++ | - | - |
| White leg shrimp | Litopenaeus vannamei | +++ | - | - |
| Chamomile | Matricaria chamomilla | +++ | - | - |
| Lemon balm | Melissa officinalis | +++ | - | - |
| Peppermint | Mentha piperita L. | +++ | - | - |
| Common ling | Molva molva | +++ | - | - |
| Incense | Pittosporum undulatum Vent. | +++ | - | - |
| Olive | Olea europaea L. | +++ | - | - |
| Domestic sheep | Ovis aries | +++ | - | - |
| Asian ginseng | Panax ginseng | +++ | - | - |
| Passion fruit | Passiflora edulis | +++ | - | - |
| Avocado | Persea americana | +++ | - | - |
| Parsley | Petroselinum crispum | +++ | - | - |
| Forkbeard | Phycis phycis | +++ | - | - |
| Stone pine | Pinus pinea | +++ | - | - |
| Common plum | Prunus domestica | +++ | - | - |
| Guava | Psidium guajava | +++ | - | - |
| Sea radish | Raphanus raphanistrum subsp. maritimus | +++ | - | - |
| Blackberry | Rubus fruticosus L. | +++ | - | - |
| Sage | Salvia officinalis | +++ | - | - |
| Rosemary | Salvia rosmarinus | +++ | - | - |
| Atlantic chub mackerel | Scomber colias | +++ | - | - |
| Egyptian senna | Senna alexandrina | +++ | - | - |
| Common cuttlefish | Sepia officinalis | +++ | - | - |
| White mustard | Sinapis alba | +++ | - | - |
| Tomato | Solanum lycopersicum | +++ | - | - |
| Arizona necklacepod | Sophora arizonica | +++ | - | - |
| Common dandelion | Taraxacum officinale | +++ | - | - |
| Yellow mealworm | Tenebrio molitor | +++ | - | - |
| White garden snail | Theba pisana | +++ | - | - |
| European white lime | Tilia tomentosa | +++ | - | - |
| Hare’s-foot clover | Trifolium arvense | +++ | - | - |
| Trefoil | Trifolium sp. | +++ | - | - |
| Broad bean | Vicia faba | +++ | - | - |
| Grape vine | Vitis vinífera L. | +++ | - | - |
| Maize | Zea mays | +++ | - | - |
| Ginger | Zingiber officinale | +++ | - | - |
| Target Gene | Primers | Sequence (5→3) | Amplicon | Annealing Temperature | Concentration (nM) | NCBI Accession/ Reference |
|---|---|---|---|---|---|---|
| Flavonoid glucosyltransferase | BMflagA-F BMflagA-R | CGATTAAGGTTGTCGCTGCG TATCCCTGTTCCAGCTCCTCA | 100 bp | 58 °C | 200 | FJ586246.1 |
| Ycf1 photosystem I assembly protein | BMYcf1-F BMYcf1-R | ACGAATACGACCGATCCACC ATTTTACGCCTTTGAGCTCGT | 110 bp | 62 °C | 200 | NC_047469.1 |
| BMYcf1S-F BMYcf1S-R | ATCAGGAGAACGTCAAGAAGATGTA TGATTCTTTTGTCCCTACCCAATTTTG | 370 bp | 60 °C | 200 | NC_047469.1 | |
| Nuclear 18S rRNA | 18SRG-F 18SRG-R | CTGCCCTATCAACTTTCGATGGTA TTGGATGTGGTAGCCGTTTCTCA | 113 bp | 65 °C | 240 | [29] |
| EG-F EG-R | TCGATGGTAGGATAGTGGCCTACT TGCTGCCTTCCTTGGATGTGGTA | 109 bp | 63 °C | 240 | [30] | |
| 18SEU-F 18SEU-R | TCTGCCCTATCAACTTTCGATGG TAATTTGCGCGCCTGCTG | 140 bp | 60 °C | 240 | [31] |
| Sample | Cq a (Mean ± SD) | B. monnieri (% w/w) | SD | CV b (%) | Bias c (%) | |
|---|---|---|---|---|---|---|
| Actual |
Mean
Predicted | |||||
| A | 20.92 ± 0.32 | 20.0 | 17.7 | 2.10 | 11.8 | −11.4 |
| B | 21.09 ± 0.15 | 15.0 | 14.3 | 1.00 | 7.0 | −4.7 |
| C | 21.92 ± 0.26 | 7.5 | 8.19 | 1.00 | 12.2 | 9.2 |
| D | 23.06 ± 0.23 | 3.75 | 3.87 | 0.59 | 15.3 | 3.2 |
| E | 24.17 ± 0.31 | 2.0 | 1.83 | 0.32 | 17.5 | −8.4 |
| F | 27.09 ± 0.58 | 0.375 | 0.45 | 0.08 | 18.8 | 19.3 |
| G | 26.37 ± 0.37 | 0.20 | 0.25 | 0.06 | 24.6 | 23.8 |
| Sample | Origin | Relevant Label Information | Qualitative PCR a | Real-Time PCR | ||
|---|---|---|---|---|---|---|
| EG-F/ EG-R | BMYcf1-F/ BMYcf1-R | BMYcf1-F/BMYcf1-R (Cq ± SD) b | Estimated Content (w/w, %) (Mean ± SD) c | |||
| P1 | Germany | 100% Organically grown Bacopa powder | + | + | 18.72 ± 0.07 | >25% |
| P2 | India | 100% B. monnieri leaf powder | + | + | 19.95 ± 0.01 | >25% |
| P3 | India | 100% B. monnieri powder | + | + | 31.64 ± 0.15 | <LOD |
| P4 | India | 100% Bacopa leaf powder | + | − | ND d | |
| P5 | India | 100% Brahmi | + | + | 22.94 ± 0.09 | 4.19 ± 0.22 |
| P6 | India | 100% Brahmi whole plant powder | + | − | ND | |
| P7 | EU | 25% Gotu kola (Hydrocotyle asiatica) | + | − | ND | |
| P8 | Portugal | 100% Centella asiatica leaves | + | − | ND | |
| P9 | India | 30% Gotu Kola (C. asiatica) | + | − | ND | |
| P10 | Portugal | 10% C. asiatica aerial parts | + | − | ND | |
| P11 | Portugal | 15% C. asiatica aerial parts | + | − | ND | |
| P12 | Portugal | 100% C. asiatica leaves | + | − | ND | |
| P13 | India | 100% C. asiatica leaves | + | − | ND | |
| P14 | Portugal | 100% C. asiatica leaves | + | − | ND | |
| P15 | India | 10% C. asiatica leaves | + | − | ND | |
| P16 | India | 6.76% Brahmi (C. asiatica) leaves | + | − | ND | |
| P17 | Portugal | 100% C. asiatica leaves | + | − | ND | |
| P18 | Portugal | 15% Ginkgo biloba leaves | + | − | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biltes, R.; Villa, C.; Costa, J.; Mafra, I. Exploiting Marker Genes for Reliable Botanical Authentication of Bacopa monnieri Products. Foods 2025, 14, 3275. https://doi.org/10.3390/foods14183275
Biltes R, Villa C, Costa J, Mafra I. Exploiting Marker Genes for Reliable Botanical Authentication of Bacopa monnieri Products. Foods. 2025; 14(18):3275. https://doi.org/10.3390/foods14183275
Chicago/Turabian StyleBiltes, Rita, Caterina Villa, Joana Costa, and Isabel Mafra. 2025. "Exploiting Marker Genes for Reliable Botanical Authentication of Bacopa monnieri Products" Foods 14, no. 18: 3275. https://doi.org/10.3390/foods14183275
APA StyleBiltes, R., Villa, C., Costa, J., & Mafra, I. (2025). Exploiting Marker Genes for Reliable Botanical Authentication of Bacopa monnieri Products. Foods, 14(18), 3275. https://doi.org/10.3390/foods14183275









