Microstructural and Enzymatic Contributions to Texture in High Pressure Processed Fruits and Vegetables
Abstract
1. Introduction
1.1. Physicochemical Aspects of Plant Cells That Influence Texture
1.1.1. Cell Wall Structure
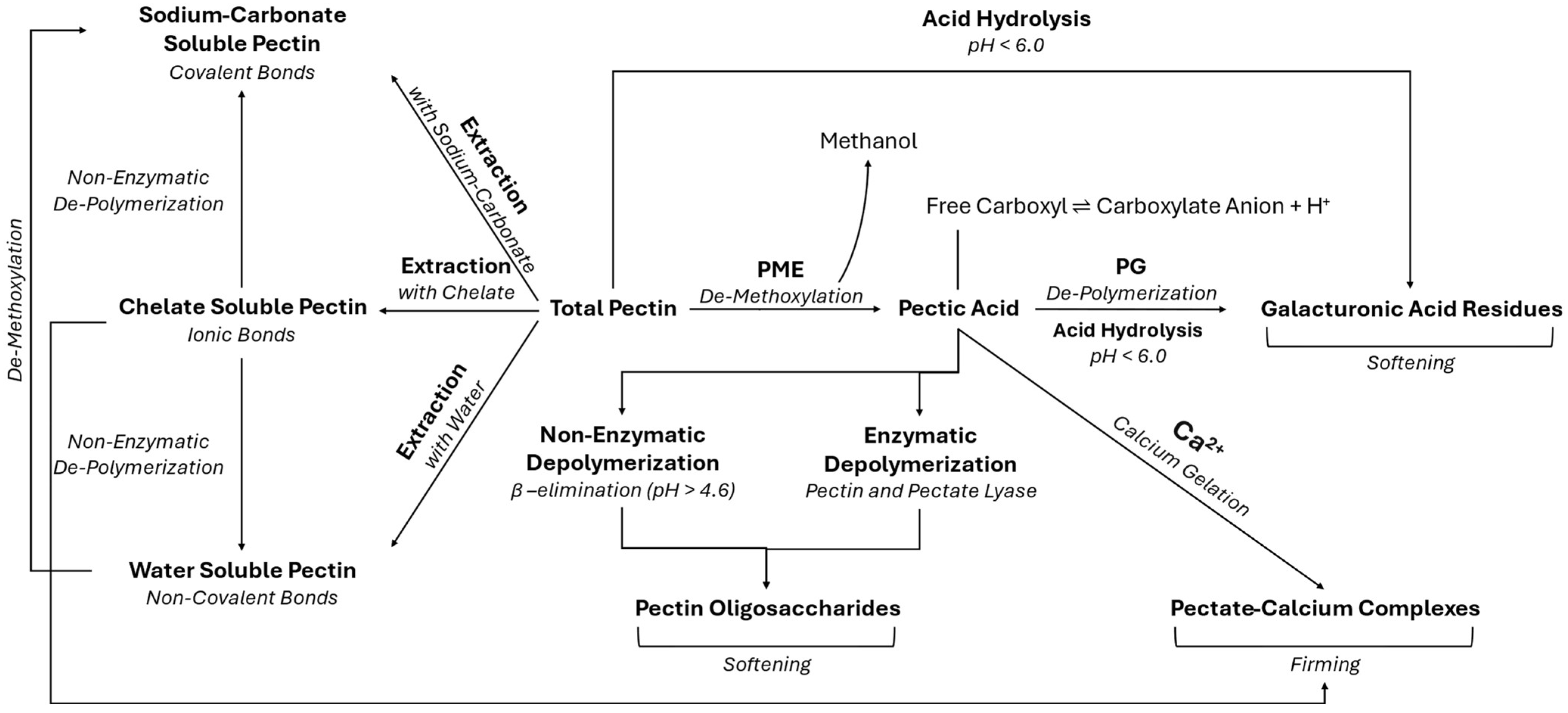
1.1.2. Pectin Methylesterase and Polygalacturonase-Induced Changes
2. Enzymatic Changes Induced by High Pressure
2.1. Effects of High-Pressure Processing Parameters on Enzyme Activity
2.1.1. Pressure and Holding Time: Polygalacturonase
2.1.2. Pressure and Holding Time: Pectin Methylesterase
2.2. Synergetic Effects of High-Pressure Processing with Temperature
2.3. Relationship Between Enzymatic Activity and Texture
2.4. Changes to Pectin Composition and Degree of Methylesterification After High Pressure Processing
3. Nonenzymatic Changes Induced by High Pressure
3.1. Microstructure
3.2. Texture
3.2.1. Hardness
3.2.2. Displacement Distance
3.2.3. Springiness, Chewiness, Cohesiveness, and Resilience
3.3. Recovery and Shelf Life
4. Enzyme Infusion and Added Calcium
5. Isozyme Stability and Matrix Composition
6. Research Needs and Improvement Opportunities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Matrix | HPP Conditions | Control Conditions | Texture | Microstructure | |||
|---|---|---|---|---|---|---|---|
| Effects Compared to Control(s) | Analysis Method(s) | Effects Compared to Control(s) | Analysis Method(s) | Reference | |||
| Apple | 100 and 200 MPa, 15–60 min | Untreated | Initial pulse softening increased with pressure; Increased holding time increased firmness at 100 MPa | Single compression | n.d. | n.d. | [55] |
| 600 MPa, 1–5 min, 18–22 °C | Granny smith apple: Maximum force increased with holding time; Pink lady apple: no difference | Puncture | n.d. | n.d. | [27] | ||
| Asparagus | 10–600 MPa, 0.5–30 min, 20–38 °C | Untreated | Slight (≤100 MPa) to significant firmness loss (400–600 MPa); Increased holding time led to recovery | Puncture | Altered cell shapes and disorganization; 400 and 600 MPa showed signs of cell rupture | Scanning Electron Microscope (SEM) | [38] |
| Blueberry | 200 and 600 MPa, 5–60 min, 3 °C | Untreated | Hardness decreased, except at 600 MPa, 60 min which was the same; 600 MPa treated samples were firmer than control after one week | Double compression | n.d. | n.d. | [71] |
| Carrot | 100–400 MPa, 5–60 min | Untreated | Initial pulse softening increased with pressure; Increased holding time increased firmness (100 MPa) | Single compression | n.d. | n.d. | [55] |
| Pre-treatment: 0.1–500 MPa, 15 min, 20–60 °C Treatment: 90–110 °C, 0–80 min | 90–110 °C | Texture degradation rate decreased with increased pre-treatment temperature and pressure; Increased processing time led to the same final hardness; Calcium soaking increased hardness | Single compression | n.d. | n.d. | [36] | |
| 100–550 MPa, 2–30 min, ≤39 °C | Untreated | Hardness decreased, with no further losses > 300 MPa; Longer holding time (300–500 MPa, 30 min) increased hardness; Displacement distance and force to cut increased | Single compression; Cut | Cell to cell contact decreased and cell wall deformation, buckling, folding, and elongation increased with pressure | Light Microscope (LM) | [25] | |
| Pre-treatment: 400 MPa, 60 °C, 15 min Treatment: 90–110 °C, ~0–140 min | 90–110 °C, ~0–120 min | Thermo-softening rate constants decreased; Higher residual hardness values | Single compression; Cut | Less cell-wall swelling | LM | [37] | |
| 600 MPa, 2 min | Untreated | Hardness decreased, except day 14 which was comparable to control; Peak cutting force was slightly higher; Displacement distance was greater | Single compression; Cut; Three-point bend | Less compact and less organized cells | Cryo-SEM | [54] | |
| Sous-vide, 90–95 °C, 5 min | Hardness decreased and less recovery over 14 days; Peak cutting force was significantly higher | Single compression; Cut; Three-point bend | Similar | Cryo-SEM | |||
| 100 °C, 20 min | Increased hardness over 14 days; Peak cutting force was significantly higher | Single compression; Cut | Less damage, smaller gaps in the cells, and less cell separation | Cryo-SEM | |||
| 600 MPa, 5 min, 25 °C | Untreated | Hardness, crunchiness index, and force-deformation curve slope decreased; Max shear force increased | Puncture, Compression, Shear | n.d. | n.d. | [72] | |
| 105 °C, 5 min | Hardness, crunchiness index, and force-deformation curve slope were greater | Puncture, Compression, Shear | n.d. | n.d. | |||
| 85 °C, 23 min | Same hardness, crunchiness index, and force-deformation curve slope | Puncture, Compression, Shear | n.d. | n.d. | |||
| Pre-treatment: 600 MPa, 5 min, 25 °C Treatment: 105 °C, 5 min | Untreated & 85 °C, 23 min | Hardness, crunchiness index, and force-deformation curve slope decreased | Puncture, Compression, Shear | n.d. | n.d. | ||
| 105 °C, 5 min | Hardness, crunchiness index, and force-deformation curve slope were greater | Puncture, Compression, Shear | n.d. | n.d. | |||
| Pre-treatment: 85 °C, 23 min Treatment: Stepwise compression to 600 MPa to match the thermal history of 105 °C, 5 min | |||||||
| Untreated | Hardness, crunchiness index, and force-deformation curve slope decreased | Puncture, Compression, Shear | n.d. | n.d. | |||
| 85 °C, 23 min | Hardness and crunchiness index decreased; Force-deformation curve slope was the same | Puncture, Compression, Shear | n.d. | n.d. | |||
| Celery | 100–400 MPa, 5–60 min | Untreated | Initial pulse softening increased with pressure; Texture loss was most pronounced >200 MPa; Firmness increased with holding time | Single compression | n.d. | n.d. | [55] |
| Cocoyam | 600 MPa, 5 and 30 min, 8–29 °C | Untreated | Maximum cutting force decreased and had a negative relationship with holding time | Cut | 600 MPa for 5 min had undefined cells | LM | [57] |
| Green bean | 500 MPa, 1 min | Untreated | Retained up to 60% firmness of control at day 0 and higher than control after 31 days | Cut | n.d. | n.d. | [58] |
| Pre-treatments: 75–90 °C, 2–4 min; Treatments: Canned (118 °C, 30 min); Pulsed-HPP (1000 MPa, 80 s, 75 °C; 30 s rest; 1000 MPa); Forced air blanched (10 min) and freeze-thawed | Significantly firmer and maintained firmness over 31 days | Cut | n.d. | n.d. | |||
| Jicama | 600 MPa, 5 min, 25 °C | Untreated | Hardness was the same; Force-deformation curve slope and crunchiness index decreased | Puncture, Compression, Shear | n.d. | n.d. | [72] |
| 105 °C, 5 min | Hardness, crunchiness index, and force-deformation curve slope were greater | Puncture, Compression, Shear | n.d. | n.d. | |||
| 85 °C, 23 min | Hardness increased; Force-deformation curve slope decreased; Crunchiness index was the same | Puncture, Compression, Shear | n.d. | n.d. | |||
| Pre-treatment: 600 MPa, 5 min, 25 °C Treatment: 105 °C, 5 min | Untreated | Hardness, crunchiness index, and force-deformation curve slope decreased | Puncture, Compression, Shear | n.d. | n.d. | ||
| 105 °C, 5 min | Hardness, crunchiness index, and force-deformation curve slope were greater | Puncture, Compression, Shear | n.d. | n.d. | |||
| 85 °C, 23 min | No change in hardness, force-deformation curve slope, and crunchiness index | Puncture, Compression, Shear | n.d. | n.d. | |||
| Pre-treatment: 85 °C, 23 min Treatment: 600 MPa, 105 °C, 5 min | Untreated | Hardness, crunchiness index, and force-deformation curve slope decreased | Puncture, Compression, Shear | n.d. | n.d. | ||
| 105 °C, 5 min | Hardness, crunchiness index, and force-deformation curve slope were greater | Puncture, Compression, Shear | n.d. | n.d. | |||
| 85 °C, 23 min | Hardness and crunchiness index were the same; Force-deformation curve slope was lower | Puncture, Compression, Shear | n.d. | n.d. | |||
| Pre-treatment: 85 °C, 23 min Treatment: Stepwise compression to 600 MPa to match the thermal history of 105 °C, 5 min | Untreated | Hardness was the same; Force-deformation curve slope and crunchiness index decreased | Puncture, Compression, Shear | n.d. | n.d. | ||
| 105 °C, 5 min | Hardness, crunchiness index, and force-deformation curve slope were greater | Puncture, Compression, Shear | n.d. | n.d. | |||
| 85 °C, 23 min | Hardness increased; No change in force-deformation curve slope and crunchiness index | Puncture, Compression, Shear | n.d. | n.d. | |||
| Kohlrabi (Pickled) | 400 MPa, 10–30 min | Untreated | Hardness, springiness, and chewiness decreased and had a negative relationship with holding time | Double compression | Similar cell size, shape, and intercellular spaces as control, but the middle lamella was scattered and loose | Transmission electron microscope (TEM) | [44] |
| 90 °C, 10–30 min | Increased hardness and springiness; No difference in chewiness | Double compression | Smaller intercellular spaces and more regular cell shapes | ||||
| Mango | 300–600 MPa, 5 min, ≤30 °C | Untreated | Increates hardness; Adding calcium increased hardness | Puncture | n.d. | n.d. | [63] |
| Olive | 400 and 600 MPa, 5 and 10 min, 14 °C | Untreated | All HPP samples were insignificantly different and maintained firmness for 186 days; Increasing storage temperature (from 15–22 °C to 30 °C) led to greater softening for all samples | Cut | n.d. | n.d. | [73] |
| Onion | 50–600 MPa, 5 min, 20–35 °C, Vacuum packed | Untreated, With and without vacuum packing | Tissue stiffness decreased >200 MPa | Puncture | 50 MPa had similar cell viability; Air spaces decreased with increasing pressure and no air or viable cells were present ≥300 MPa | LM | [74] |
| 40–90 °C, 30 min | 300–600 MPa had similar visoelastic initial response to control at 60–90 °C but less than that at 40–50 °C | Puncture | No viable cells or air spaces were present ≥60 °C and ≥300 MPa | LM | |||
| 40–90 °C, 30 min, Vacuum packed | Stiffness was similar at 50 MPa and ≤50 °C, and decreased ≥60 °C and ≥300 MPa, but pressure treatments decreased more | Puncture | n.d. | n.d. | [52] | ||
| Orange | 100 and 200 MPa, 15–60 min | Untreated | Initial pulse softening increased with pressure; Firmness increased with holding time | Single compression | n.d. | n.d. | [55] |
| Peach | 600 MPa, 5–30 min | 90 °C, 20 min | Hardness was greater and maintained better with increased holding time, but decreased over shelf-life | Double compression | Structure was similar but with less damage; Extracellular spaces decreased as holding time increased | LM | [46] |
| 500 MPa, 5 min, 20 °C, Vacuum packed | Untreated | Decreased hardness and chewiness at day 0, but higher at day 21; Increased cohesiveness at day 14 and beyond; No difference in springiness | Double compression | n.d. | n.d. | [60] | |
| Untreated, Vacuum packed | Insignificantly different hardness, cohesiveness, and springiness; Increased chewiness at day 21 | Double compression | n.d. | n.d. | |||
| 600 MPa, 5 min, 22–38 °C | Untreated | n.d. | n.d. | Irregular cells with smaller intercellular spaces and little or no cytoplasmic material; Cell wall hydrated, swelled, and unfolded | LM; TEM | [47] | |
| 400–600 MPa, 1–9 min, 21–38 °C | Increased pressure was associated with a greater deformation distance but did not effect other parameters; Hardness and chewiness decreased as holding time increased | Double compression | Greater cell membrane lysis; Holding time > 5 min experienced cell wall breakdown | LM | [53] | ||
| Pear | 100 and 200 MPa, 15–60 min | Untreated | Initial pulse softening increased with pressure; Firmness increased with holding time | Single compression | n.d. | n.d. | [55] |
| Pepper (Fermented) | 500 MPa, 5 min, 20 and 50 °C | Untreated | Firmness decreased but better maintained over 12 weeks | Single compression | n.d. | n.d. | [75] |
| 83 °C, 15 min | Increased firmness and maintained better over 12 weeks | Single compression | n.d. | n.d. | |||
| 500 MPa, 5 min, 50 °C | 80 °C, 15 min | Significantly harder; Hardness increased during storage, then decreased, whereas control sample’s hardness decreased | Water-soluble pectin was more linear; Chelate-soluble pectin fractions were similarly long branched, but more stable over 30 days; Sodium-carbonate-soluble pectin fractions were smaller, but grew over time | Atomic force microscope | [76] | ||
| Pepper (Green and Red) | 100–400 MPa, 5–60 min | Untreated | Initial pulse softening increased with pressure; Texture loss was most pronounced >200 MPa; Increased holding time increased firmness for all pressures (red pepper) or only at 100 MPa (green pepper) | Single compression | n.d. | n.d. | [55] |
| 100 and 200 MPa, 10 and 20 min, 18–26 °C | Insignificant difference from control | Puncture | n.d. | n.d. | [35] | ||
| 70–98 °C, 1 and 2.5 min | No difference in flesh firmness; Green pepper had firmer skin than the most intense blanching treatment; Red pepper skin was firmer than all treatments, except 98 °C, 1 min, which was the same | Puncture | n.d. | n.d. | |||
| 100–500 MPa, 15 min, 25 °C | Untreated | Firmness, hardness, cohesiveness, chewiness, gumminess, and shear force decreased, with 500 MPa decreasing the least; Springiness was insignificantly different, except endocarp treated with 100 and 300 MPa, which increased | Double compression; Cut | Cell wall swelling and separation, cell elongating, middle lamella dissolution, and cell membrane rupture, leakage, and withdrawal, especially at higher pressures | LM; TEM | [49] | |
| 70 °C, 10 min | Similar results as compared to untreated except no differences in springiness and cohesiveness decreased, except endocarp treated with 500 MPa, which was insignificantly different | Double compression; Cut | More parenchymal tissue breakdown; Less cell wall structure; Similar cell membrane breakdown | LM; TEM | |||
| Persimmon (Astringent) | 200 MPa, 6 min, 25 °C | Untreated | Decrease in firmness, cohesiveness, and shear force | Double compression; Cut | Cell structure compacted, deformed, and spread; Middle lamella thickened and broke; Cell-cell separation; Cell membrane remained intact; Solute leakage and tannin precipitation | LM; TEM; Cryo-SEM | [59] |
| 70 °C, 15 min | Decrease in firmness, cohesiveness, and shear force | Double compression; Cut | Larger cells and less deformation; Thicker cell walls; Both resulted in tannin precipitation; Less withdrawal from cell wall | LM; TEM; Cryo-SEM | |||
| Peruvian Carrot | 600 MPa, 5 and 30 min, 8–29 °C | Untreated | Maximum cutting force decreased but was not affected by holding time | Cut | Starch granules gelatinized, birefringence was lost, and Maltese crosses reduced as holding time increased | LM | [57] |
| Pineapple | 100 and 200 MPa, 15–60 min | Untreated | Initial pulse softening increased with pressure; Firmness increased with holding time | Single compression | n.d. | n.d. | [55] |
| Pre-treatment: 100–700 MPa, ≤35 °C Treatment: Same as control | Osmotic dehydration (40 °C, 50 °Bx) followed by vacuum oven drying (60 °C, 18 h) | Less force was needed to penetrate the same distance; Softening increased with pressure, but minimal additional softening > 300 MPa | Puncture | Increased cell permeability, decreased intercellular materials, water loss, and damaged cell walls; Minimal further changes > 300 MPa | LM | [51] | |
| 600 MPa, 3 min, 4 °C | Untreated | Decreased hardness and chewiness; No difference in cohesiveness, resilience, and springiness | Double compression | Relatively unchanged, with some signs of dehydration and cell wall thickening/swelling | LM | [34] | |
| 93.3 °C, 5 min | Increased hardness and chewiness; No difference in cohesiveness, resilience, and springiness | Double compression | n.d. | n.d. | |||
| Ohmic heating: ≤90 °C increase, 1900 L/h | No difference | Double compression | n.d. | n.d. | |||
| Pumpkin | 100–600 MPa, 2 min, 20–38 °C | Untreated | Hardness significantly decreased and pressures > 300 MPa were not significantly different from each other | Double compression | At 300 MPa, plasmosis, dissolving of middle lamella, and intercellular leakage; At 600 MPa, greater deformation of cell membrane and wall | TEM; LM | [41] |
| 100 °C, 2 min | Hardness, springiness, cohesiveness, resilience, and chewiness were all significantly higher | Double compression | n.d. | n.d. | |||
| Red Radish | 600 MPa, 5 min, 25 °C | Untreated | Increased hardness; Force-deformation curve slope and crunchiness index decreased | Puncture, Compression, Shear | n.d. | n.d. | [72] |
| 105 °C, 5 min | Hardness, force-deformation curve slope, and crunchiness index increased | Puncture, Compression, Shear | n.d. | n.d. | |||
| 85 °C, 23 min | Hardness and crunchiness index increased; Force-deformation curve slope was the same | Puncture, Compression, Shear | n.d. | n.d. | |||
| Pre-treatment: 600 MPa, 5 min, 25 °C Treatment: 105 °C, 5 min | 105 °C, 5 min | ||||||
| Untreated | Hardness, force-deformation curve slope, and crunchiness index decreased | Puncture, Compression, Shear | n.d. | n.d. | |||
| 85 °C, 23 min | |||||||
| Pre-treatment: 85 °C, 23 min Treatment: 600 MPa, 105 °C, 5 min OR Stepwise compression to 600 MPa to match the thermal history of 105 °C, 5 min | Untreated | ||||||
| 105 °C, 5 min | Hardness, force-deformation curve slope, and crunchiness index increased | Puncture, Compression, Shear | n.d. | n.d. | |||
| 85 °C, 23 min | Hardness decreased; Increased force-deformation curve slope; Crunchiness index was the same | Puncture, Compression, Shear | n.d. | n.d. | |||
| Strawberry | 550 MPa, 10–40 min, 25 and 70 °C | Untreated | Significant loss in firmness; Infusion with PME and calcium maintained or improved firmness; Treatment time did not effect firmness at 25 °C but significantly lowered it at 70 °C | Puncture | HPPed samples at 25 °C appeared similar to control, except some irregular shapes at longer processing time (40 min); HPP at 70 °C caused more damage to the cells | LM | [61] |
| 70 °C, 10–40 min | HPPed samples at 25 °C had higher firmness when infused with PME and calcium compared to controls and HPPed samples at 70 °C | Puncture | HPPed samples at 25 °C had less tissue disruption; HPP at 70 °C caused more damage to the cells | LM | |||
| Sweet Potato | 600 MPa, 5 and 30 min, 8–29 °C | Untreated | Maximum cutting force decreased and cutting force had a negative relationship with holding time | Cut | Starch granules gelatinized and agglomerated, birefringence was lost, and Maltese crosses reduced as holding time increased | LM | [57] |
| Tomato | 200–600 MPa, 20 min, 20 °C | Untreated | Softening with increased pressure ≤ 400 MPa; >400 MPa, firmness increased (but was still lower than control) | Single compression | Cell damage with large bubbles (200–300 MPa) and broad intercellular cavities (500–600 MPa); Cell rupture increased with pressure, but minimal differences > 300 MPa | LM; SEM | [22] |
| Zucchini | 400 and 600 MPa, 1 and 5 min | Untreated | Max force decreased; Displacement distance increased; Insignificant difference in first peak force (except 400 MPa, 1 min decreased) | Puncture | Cell wall swelling and dehydration increased with holding time; Cell wall lysis at 5 min | LM | [28] |
| 90 °C, 2 min | Insignificantly different max force, displacement distance, and first peak force (except 400 MPa, 1 min decreased) | Puncture | n.d. | n.d. | |||
References
- De Roeck, A.; Duvetter, T.; Fraeye, I.; der Plancken, I.V.; Sila, D.N.; Loey, A.V.; Hendrickx, M. Effect of High-Pressure/High-Temperature Processing on Chemical Pectin Conversions in Relation to Fruit and Vegetable Texture. Food Chem. 2009, 115, 207–213. [Google Scholar] [CrossRef]
- Noguerol, A.T.; Pagán, M.J.; García-Segovia, P.; Varela, P. Green or Clean? Perception of Clean Label Plant-Based Products by Omnivorous, Vegan, Vegetarian and Flexitarian Consumers. Food Res. Int. 2021, 149, 110652. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, V.M.; Barbosa-Cánovas, G.V.; Lelieveld, H.L.M. (Eds.) High Pressure Processing of Food: Principles, Technology and Applications; Food Engineering Series; Springer: New York, NY, USA, 2016; ISBN 978-1-4939-3233-7. [Google Scholar]
- Tonello, C. Case Studies on High-Pressure Processing of Foods. In Nonthermal Processing Technologies for Food; Zhang, H.Q., Barbosa-Cánovas, G.V., Balasubramaniam, V.M., Dunne, C.P., Farkas, D.F., Yuan, J.T.C., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 36–50. ISBN 978-0-470-95836-0. [Google Scholar]
- Oey, I.; Lille, M.; Van Loey, A.; Hendrickx, M. Effect of High-Pressure Processing on Colour, Texture and Flavour of Fruit- and Vegetable-Based Food Products: A Review. Trends Food Sci. Technol. 2008, 19, 320–328. [Google Scholar] [CrossRef]
- Rodrigo, D.; Cortés, C.; Clynen, E.; Schoofs, L.; Loey, A.V.; Hendrickx, M. Thermal and High-Pressure Stability of Purified Polygalacturonase and Pectinmethylesterase from Four Different Tomato Processing Varieties. Food Res. Int. 2006, 39, 440–448. [Google Scholar] [CrossRef]
- Chen, H.; Cao, S.; Fang, X.; Mu, H.; Yang, H.; Wang, X.; Xu, Q.; Gao, H. Changes in Fruit Firmness, Cell Wall Composition and Cell Wall Degrading Enzymes in Postharvest Blueberries during Storage. Sci. Hortic. 2015, 188, 44–48. [Google Scholar] [CrossRef]
- Elfalleh, W.; Guo, L.; He, S.; Wang, P.; Cui, J.; Ma, Y. Characteristics of Cell Wall Structure of Green Beans During Controlled Freezing Point Storage. Int. J. Food Prop. 2015, 18, 1756–1772. [Google Scholar] [CrossRef]
- Terefe, N.S.; Buckow, R.; Versteeg, C. Quality-Related Enzymes in Fruit and Vegetable Products: Effects of Novel Food Processing Technologies, Part 1: High-Pressure Processing. Crit. Rev. Food Sci. Nutr. 2014, 54, 24–63. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaushik, N.; Rao, P.S.; Mishra, H.N. High-Pressure Inactivation of Enzymes: A Review on Its Recent Applications on Fruit Purees and Juices. Compr. Rev. Food Sci. Food Saf. 2014, 13, 578–596. [Google Scholar] [CrossRef]
- Gokul Nath, K.; Pandiselvam, R.; Sunil, C.K. High-Pressure Processing: Effect on Textural Properties of Food- A Review. J. Food Eng. 2023, 351, 111521. [Google Scholar] [CrossRef]
- Peng, J.; Bi, J.; Yi, J.; Lyu, J.; Zhao, Y.; Xu, Y.; Yu, Y. Characterization of Tissue-Specific Differences in Cell Wall Pectic Polysaccharides of Carrot Root. J. Food Process. Preserv. 2021, 45, e15331. [Google Scholar] [CrossRef]
- Constenla, D.; Lozano, J. Kinetic Model of Pectin Demethylation. Lat. Am. Appl. Res. 2003, 33, 91–95. [Google Scholar]
- Christiaens, S.; Van Buggenhout, S.; Vandevenne, E.; Jolie, R.; Van Loey, A.M.; Hendrickx, M.E. Towards a Better Understanding of the Pectin Structure–Function Relationship in Broccoli during Processing: Part II—Analyses with Anti-Pectin Antibodies. Food Res. Int. 2011, 44, 2896–2906. [Google Scholar] [CrossRef]
- Brummell, D.A. Cell Wall Disassembly in Ripening Fruit. Funct. Plant Biol. 2006, 33, 103. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.V.; Anthon, G.E.; Barrett, D.M. Nonenzymatic Degradation of Citrus Pectin and Pectate during Prolonged Heating: Effects of pH, Temperature, and Degree of Methyl Esterification. J. Agric. Food Chem. 2007, 55, 5131–5136. [Google Scholar] [CrossRef] [PubMed]
- Krall, S.M.; McFeeters, R.F. Pectin Hydrolysis: Effect of Temperature, Degree of Methylation, pH, and Calcium on Hydrolysis Rates. J. Agric. Food Chem. 1998, 46, 1311–1315. [Google Scholar] [CrossRef]
- Sila, D.N.; Smout, C.; Elliot, F.; Loey, A.V.; Hendrickx, M. Non-Enzymatic Depolymerization of Carrot Pectin: Toward a Better Understanding of Carrot Texture During Thermal Processing. J. Food Sci. 2006, 71, E1–E9. [Google Scholar] [CrossRef]
- Sila, D.N.; Van Buggenhout, S.; Duvetter, T.; Fraeye, I.; De Roeck, A.; Van Loey, A.; Hendrickx, M. Pectins in Processed Fruits and Vegetables: Part II—Structure–Function Relationships. Compr. Rev. Food Sci. Food Saf. 2009, 8, 86–104. [Google Scholar] [CrossRef]
- Fraeye, I.; Knockaert, G.; Buggenhout, S.V.; Duvetter, T.; Hendrickx, M.; Loey, A.V. Enzyme Infusion and Thermal Processing of Strawberries: Pectin Conversions Related to Firmness Evolution. Food Chem. 2009, 114, 1371–1379. [Google Scholar] [CrossRef]
- Saharan, R.; Sharma, K.P. Production, Purification and Characterization of Pectin Lyase from Bacillus subtilis Isolated from Moong Beans Leaves (Vigna radiata). Biocatal. Agric. Biotechnol. 2019, 21, 101306. [Google Scholar] [CrossRef]
- Tangwongchai, R.; Ledward, D.A.; Ames, J.M. Effect of High-Pressure Treatment on the Texture of Cherry Tomato. J. Agric. Food Chem. 2000, 48, 1434–1441. [Google Scholar] [CrossRef]
- Rodríguez-Garayar, M.; Martín-Cabrejas, M.A.; Esteban, R.M. High Hydrostatic Pressure in Astringent and Non-Astringent Persimmons to Obtain Fiber-Enriched Ingredients with Improved Functionality. Food Bioprocess Technol. 2017, 10, 854–865. [Google Scholar] [CrossRef]
- Sila, D.N.; Smout, C.; Satara, Y.; Truong, V.; Loey, A.V.; Hendrickx, M. Combined Thermal and High Pressure Effect on Carrot Pectinmethylesterase Stability and Catalytic Activity. J. Food Eng. 2007, 78, 755–764. [Google Scholar] [CrossRef]
- Trejo Araya, X.I.; Hendrickx, M.; Verlinden, B.E.; Van Buggenhout, S.; Smale, N.J.; Stewart, C.; John Mawson, A. Understanding Texture Changes of High Pressure Processed Fresh Carrots: A Microstructural and Biochemical Approach. J. Food Eng. 2007, 80, 873–884. [Google Scholar] [CrossRef]
- Balogh, T.; Smout, C.; Nguyen, B.L.; Van Loey, A.M.; Hendrickx, M.E. Thermal and High-Pressure Inactivation Kinetics of Carrot Pectinmethylesterase: From Model System to Real Foods. Innov. Food Sci. Emerg. Technol. 2004, 5, 429–436. [Google Scholar] [CrossRef]
- Perera, N.; Gamage, T.V.; Wakeling, L.; Gamlath, G.G.S.; Versteeg, C. Colour and Texture of Apples High Pressure Processed in Pineapple Juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 39–46. [Google Scholar] [CrossRef]
- Paciulli, M.; Ganino, T.; Meza, I.G.M.; Rinaldi, M.; Rodolfi, M.; Morbarigazzi, M.; Chiavaro, E. High Pressure and Thermal Processing on the Quality of Zucchini Slices. Eur. Food Res. Technol. 2021, 247, 475–484. [Google Scholar] [CrossRef]
- Ly-Nguyen, B.; Van Loey, A.M.; Smout, C.; ErenÖzcan, S.; Fachin, D.; Verlent, I.; Truong, S.V.; Duvetter, T.; Hendrickx, M.E. Mild-Heat and High-Pressure Inactivation of Carrot Pectin Methylesterase: A Kinetic Study. J. Food Sci. 2003, 68, 1377–1383. [Google Scholar] [CrossRef]
- Verlent, I.; Hendrickx, M.; Verbeyst, L.; Van Loey, A. Effect of Temperature and Pressure on the Combined Action of Purified Tomato Pectinmethylesterase and Polygalacturonase in Presence of Pectin. Enzym. Microb. Technol. 2007, 40, 1141–1146. [Google Scholar] [CrossRef]
- Ünal, M.Ü.; Şener, A. Extraction and Characterization of Pectin Methylesterase from Alyanak Apricot (Prunus armeniaca L.). J. Food Sci. Technol. 2015, 52, 1194–1199. [Google Scholar] [CrossRef][Green Version]
- Balny, C.; Masson, P. Effects of High Pressure on Proteins. Food Rev. Int. 1993, 9, 611–628. [Google Scholar] [CrossRef]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. High Pressure Effects on Protein Structure and Function. Proteins 1996, 24, 81–91. [Google Scholar] [CrossRef]
- Rinaldi, M.; Littardi, P.; Ganino, T.; Aldini, A.; Rodolfi, M.; Barbanti, D.; Chiavaro, E. Comparison of Physical, Microstructural, Antioxidant and Enzymatic Properties of Pineapple Cubes Treated with Conventional Heating, Ohmic Heating and High-Pressure Processing. LWT 2020, 134, 110207. [Google Scholar] [CrossRef]
- Castro, S.M.; Saraiva, J.A.; Lopes-da-Silva, J.A.; Delgadillo, I.; Loey, A.V.; Smout, C.; Hendrickx, M. Effect of Thermal Blanching and of High Pressure Treatments on Sweet Green and Red Bell Pepper Fruits (Capsicum annuum L.). Food Chem. 2008, 107, 1436–1449. [Google Scholar] [CrossRef]
- Sila, D.N.; Smout, C.; Vu, T.S.; Hendrickx, M.E. Effects of High-Pressure Pretreatment and Calcium Soaking on the Texture Degradation Kinetics of Carrots during Thermal Processing. J. Food Sci. 2004, 69, E205–E211. [Google Scholar] [CrossRef]
- Sila, D.N.; Yue, X.; VanBuggenhout, S.; Smout, C.; Van Loey, A.; Hendrickx, M. The Relation between (Bio-)Chemical, Morphological, and Mechanical Properties of Thermally Processed Carrots as Influenced by High-Pressure Pretreatment Condition. Eur. Food Res. Technol. 2007, 226, 127–135. [Google Scholar] [CrossRef]
- Yi, J.; Feng, H.; Bi, J.; Zhou, L.; Zhou, M.; Cao, J.; Li, J. High Hydrostatic Pressure Induced Physiological Changes and Physical Damages in Asparagus Spears. Postharvest Biol. Technol. 2016, 118, 1–10. [Google Scholar] [CrossRef]
- Terefe, N.S.; Tepper, P.; Ullman, A.; Knoerzer, K.; Juliano, P. High Pressure Thermal Processing of Pears: Effect on Endogenous Enzyme Activity and Related Quality Attributes. Innov. Food Sci. Emerg. Technol. 2016, 33, 56–66. [Google Scholar] [CrossRef]
- De Roeck, A.; Sila, D.; Duvetter, T.; Vanloey, A.; Hendrickx, M. Effect of High Pressure/High Temperature Processing on Cell Wall Pectic Substances in Relation to Firmness of Carrot Tissue. Food Chem. 2008, 107, 1225–1235. [Google Scholar] [CrossRef]
- Hu, X.; Ma, T.; Ao, L.; Kang, H.; Hu, X.; Song, Y.; Liao, X. Effect of High Hydrostatic Pressure Processing on Textural Properties and Microstructural Characterization of Fresh-Cut Pumpkin (Cucurbita pepo). J. Food Process Eng. 2020, 43, e13379. [Google Scholar] [CrossRef]
- Howard, L.R.; Buescher, R.W. Cell Wall Characteristics and Firmness of Fresh Pack Cucumber Pickles Affected by Pasteurization and Calcium Chloride. J. Food Biochem. 1990, 14, 31–43. [Google Scholar] [CrossRef]
- Wan, L.; Wang, H.; Zhu, Y.; Pan, S.; Cai, R.; Liu, F.; Pan, S. Comparative Study on Gelling Properties of Low Methoxyl Pectin Prepared by High Hydrostatic Pressure-Assisted Enzymatic, Atmospheric Enzymatic, and Alkaline de-Esterification. Carbohydr. Polym. 2019, 226, 115285. [Google Scholar] [CrossRef]
- Yang, Z.; Duan, X.; Yang, J.; Wang, H.; Liu, F.; Xu, X.; Pan, S. Effects of High Hydrostatic Pressure and Thermal Treatment on Texture Properties of Pickled Kohlrabi. LWT 2022, 157, 113078. [Google Scholar] [CrossRef]
- Gosavi, N.S.; Polunas, M.; Martin, D.; Karwe, M.V. Effect of Food Microstructure on Calcium Infusion Under High Pressure. Food Eng. Rev. 2021, 13, 36–53. [Google Scholar] [CrossRef]
- Zhang, F.; Dong, P.; Feng, L.; Chen, F.; Wu, J.; Liao, X.; Hu, X. Textural Changes of Yellow Peach in Pouches Processed by High Hydrostatic Pressure and Thermal Processing During Storage. Food Bioprocess Technol. 2012, 5, 3170–3180. [Google Scholar] [CrossRef]
- Denoya, G.I.; Nanni, M.S.; Apóstolo, N.M.; Vaudagna, S.R.; Polenta, G.A. Biochemical and Microstructural Assessment of Minimally Processed Peaches Subjected to High-Pressure Processing: Implications on the Freshness Condition. Innov. Food Sci. Emerg. Technol. 2016, 36, 212–220. [Google Scholar] [CrossRef]
- Préstamo, G.; Arroyo, G. High Hydrostatic Pressure Effects on Vegetable Structure. J. Food Sci. 1998, 63, 878–881. [Google Scholar] [CrossRef]
- Hernández-Carrión, M.; Hernando, I.; Quiles, A. High Hydrostatic Pressure Treatment as an Alternative to Pasteurization to Maintain Bioactive Compound Content and Texture in Red Sweet Pepper. Innov. Food Sci. Emerg. Technol. 2014, 26, 76–85. [Google Scholar] [CrossRef]
- Préstamo, G.; Arroyo, G. Protective Effect of Ascorbic Acid against the Browning Developed in Apple Fruit Treated with High Hydrostatic Pressure. J. Agric. Food Chem. 1999, 47, 3541–3545. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Niranjan, K. Enhanced Mass Transfer During Osmotic Dehydration of High Pressure Treated Pineapple. J. Food Sci. 1998, 63, 508–511. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Anthon, G.E.; Barrett, D.M. Onion Cells After High Pressure and Thermal Processing: Comparison of Membrane Integrity Changes Using Different Analytical Methods and Impact on Tissue Texture. J. Food Sci. 2010, 75, E426–E432. [Google Scholar] [CrossRef]
- Denoya, G.I.; Polenta, G.A.; Apóstolo, N.M.; Budde, C.O.; Sancho, A.M.; Vaudagna, S.R. Optimization of High Hydrostatic Pressure Processing for the Preservation of Minimally Processed Peach Pieces. Innov. Food Sci. Emerg. Technol. 2016, 33, 84–93. [Google Scholar] [CrossRef]
- Trejo Araya, X.I.; Smale, N.; Zabaras, D.; Winley, E.; Forde, C.; Stewart, C.M.; Mawson, A.J. Sensory Perception and Quality Attributes of High Pressure Processed Carrots in Comparison to Raw, Sous-Vide and Cooked Carrots. Innov. Food Sci. Emerg. Technol. 2009, 10, 420–433. [Google Scholar] [CrossRef]
- Basak, S.; Ramaswamy, H.S. Effect of High Pressure Processing on the Texture of Selected Fruits and Vegetables. J. Texture Stud. 1998, 29, 587–601. [Google Scholar] [CrossRef]
- Cheftel, J.C. Review: High-Pressure, Microbial Inactivation and Food Preservation. Food Sci. Technol. Int. 1995, 1, 75–90. [Google Scholar] [CrossRef]
- de Oliveira, M.M.; Tribst, A.A.L.; de Castro Leite Júnior, B.R.; de Oliveira, R.A.; Cristianini, M. Effects of High Pressure Processing on Cocoyam, Peruvian Carrot, and Sweet Potato: Changes in Microstructure, Physical Characteristics, Starch, and Drying Rate. Innov. Food Sci. Emerg. Technol. 2015, 31, 45–53. [Google Scholar] [CrossRef]
- Krebbers, B.; Matser, A.M.; Koets, M.; Van den Berg, R.W. Quality and Storage-Stability of High-Pressure Preserved Green Beans. J. Food Eng. 2002, 54, 27–33. [Google Scholar] [CrossRef]
- Vázquez-Gutiérrez, J.L.; Hernández-Carrión, M.; Quiles, A.; Hernando, I.; Pérez-Munuera, I. Impact of High Hydrostatic Pressures on the Structure, Diffusion of Soluble Compounds and Textural Properties of Persimmon ‘Rojo Brillante’. Food Res. Int. 2012, 47, 218–222. [Google Scholar] [CrossRef]
- Denoya, G.I.; Vaudagna, S.R.; Polenta, G. Effect of High Pressure Processing and Vacuum Packaging on the Preservation of Fresh-Cut Peaches. LWT Food Sci. Technol. 2015, 62, 801–806. [Google Scholar] [CrossRef]
- Fraeye, I.; Knockaert, G.; Van Buggenhout, S.; Duvetter, T.; Hendrickx, M.; Van Loey, A. Enzyme Infusion Prior to Thermal/High Pressure Processing of Strawberries: Mechanistic Insight into Firmness Evolution. Innov. Food Sci. Emerg. Technol. 2010, 11, 23–31. [Google Scholar] [CrossRef]
- Gosavi, N.S.; Salvi, D.; Karwe, M.V. High Pressure-Assisted Infusion of Calcium into Baby Carrots Part I: Influence of Process Variables on Calcium Infusion and Hardness of the Baby Carrots. Food Bioprocess Technol. 2019, 12, 255–266. [Google Scholar] [CrossRef]
- Perdomo Lamilla, C.; Vaudagna, S.R.; Cap, M.; Rodriguez, A. Application of High Pressure-Assisted Infusion Treatment to Mango Pieces: Effect on Quality Properties. Innov. Food Sci. Emerg. Technol. 2020, 64, 102431. [Google Scholar] [CrossRef]
- Katsaros, G.J.; Alexandrakis, Z.S.; Taoukis, P.S. Kinetic Assessment of High Pressure Inactivation of Different Plant Origin Pectinmethylesterase Enzymes. Food Eng. Rev. 2017, 9, 170–189. [Google Scholar] [CrossRef]
- Goodner, J.K.; Braddock, R.J.; Parish, M.E. Inactivation of Pectinesterase in Orange and Grapefruit Juices by High Pressure. J. Agric. Food Chem. 1998, 46, 1997–2000. [Google Scholar] [CrossRef]
- Duvetter, T.; Sila, D.N.; Van Buggenhout, S.; Jolie, R.; Van Loey, A.; Hendrickx, M. Pectins in Processed Fruit and Vegetables: Part I—Stability and Catalytic Activity of Pectinases. Compr. Rev. Food Sci. Food Saf. 2009, 8, 75–85. [Google Scholar] [CrossRef]
- Wicker, L.; Ackerley, J.L.; Corredig, M. Clarification of Juice by Thermolabile Valencia Pectinmethylesterase Is Accelerated by Cations. J. Agric. Food Chem. 2002, 50, 4091–4095. [Google Scholar] [CrossRef]
- Cacace, F.; Bottani, E.; Rizzi, A.; Vignali, G. Evaluation of the Economic and Environmental Sustainability of High Pressure Processing of Foods. Innov. Food Sci. Emerg. Technol. 2020, 60, 102281. [Google Scholar] [CrossRef]
- Badwaik, L.S.; Gautam, G.; Deka, S.C. Influence of Blanching on Antioxidant, Nutritional and Physical Properties of Bamboo Shoot. J. Agric. Sci. Sri Lanka 2015, 10, 140–150. [Google Scholar] [CrossRef]
- Tadapaneni, R.K.; Daryaei, H.; Krishnamurthy, K.; Edirisinghe, I.; Burton-Freeman, B.M. High-Pressure Processing of Berry and Other Fruit Products: Implications for Bioactive Compounds and Food Safety. J. Agric. Food Chem. 2014, 62, 3877–3885. [Google Scholar] [CrossRef]
- Scheidt, T.B.; Silva, F.V.M. High Pressure Processing and Storage of Blueberries: Effect on Fruit Hardness. High Press. Res. 2018, 38, 80–89. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Tay, A.; Balasubramaniam, V.M.; Legan, J.D.; Turek, E.J.; Gupta, R. Evaluating the Impact of Thermal and Pressure Treatment in Preserving Textural Quality of Selected Foods. LWT Food Sci. Technol. 2010, 43, 525–534. [Google Scholar] [CrossRef]
- Pradas, I.; del Pino, B.; Peña, F.; Ortiz, V.; Moreno-Rojas, J.M.; Fernández-Hernández, A.; García-Mesa, J.A. The Use of High Hydrostatic Pressure (HHP) Treatments for Table Olives Preservation. Innov. Food Sci. Emerg. Technol. 2012, 13, 64–68. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Jernstedt, J.A.; Slaughter, D.C.; Barrett, D.M. Influence of Cell Integrity on Textural Properties of Raw, High Pressure, and Thermally Processed Onions. J. Food Sci. 2010, 75, E409–E416. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Liu, H.; Li, R.; Wang, Y.; Liao, X. Fermented Minced Pepper by High Pressure Processing, High Pressure Processing with Mild Temperature and Thermal Pasteurization. Innov. Food Sci. Emerg. Technol. 2016, 36, 34–41. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Y.; Wang, Y.; Ding, S.; Qin, Y.; Jiang, L.; Wang, R. High Pressure Processing Improves the Texture Quality of Fermented Minced Pepper by Maintaining Pectin Characteristics during Storage. J. Food Sci. 2022, 87, 2427–2439. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heaney, D.; Padilla-Zakour, O.I. Microstructural and Enzymatic Contributions to Texture in High Pressure Processed Fruits and Vegetables. Foods 2025, 14, 3267. https://doi.org/10.3390/foods14183267
Heaney D, Padilla-Zakour OI. Microstructural and Enzymatic Contributions to Texture in High Pressure Processed Fruits and Vegetables. Foods. 2025; 14(18):3267. https://doi.org/10.3390/foods14183267
Chicago/Turabian StyleHeaney, Danielle, and Olga I. Padilla-Zakour. 2025. "Microstructural and Enzymatic Contributions to Texture in High Pressure Processed Fruits and Vegetables" Foods 14, no. 18: 3267. https://doi.org/10.3390/foods14183267
APA StyleHeaney, D., & Padilla-Zakour, O. I. (2025). Microstructural and Enzymatic Contributions to Texture in High Pressure Processed Fruits and Vegetables. Foods, 14(18), 3267. https://doi.org/10.3390/foods14183267







