Effect of Various Types of Heat Processing on the Content and Retention of Fat-Soluble Vitamins and Cholesterol in Goose Breast Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Heat Processing
2.3. Determination of Cooking Loss (CL)
2.4. Chemical Analysis
2.5. Determination of Retention Factor
2.6. Statistical Analysis
3. Results and Discussion
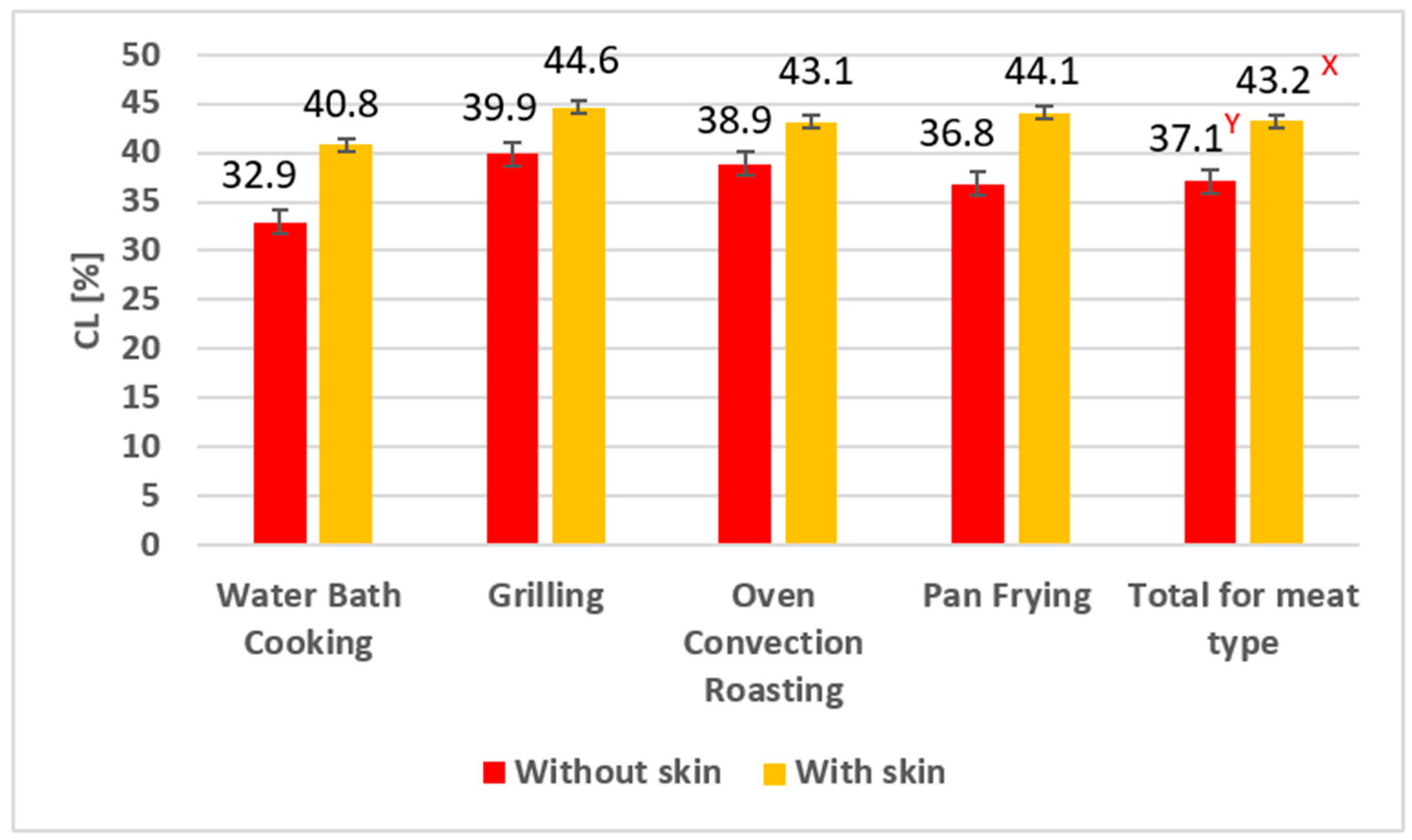
3.1. Cooking Loss
3.2. Fat-Soluble Vitamins and Cholesterol Content in Goose Breast Meat After Heat Processing
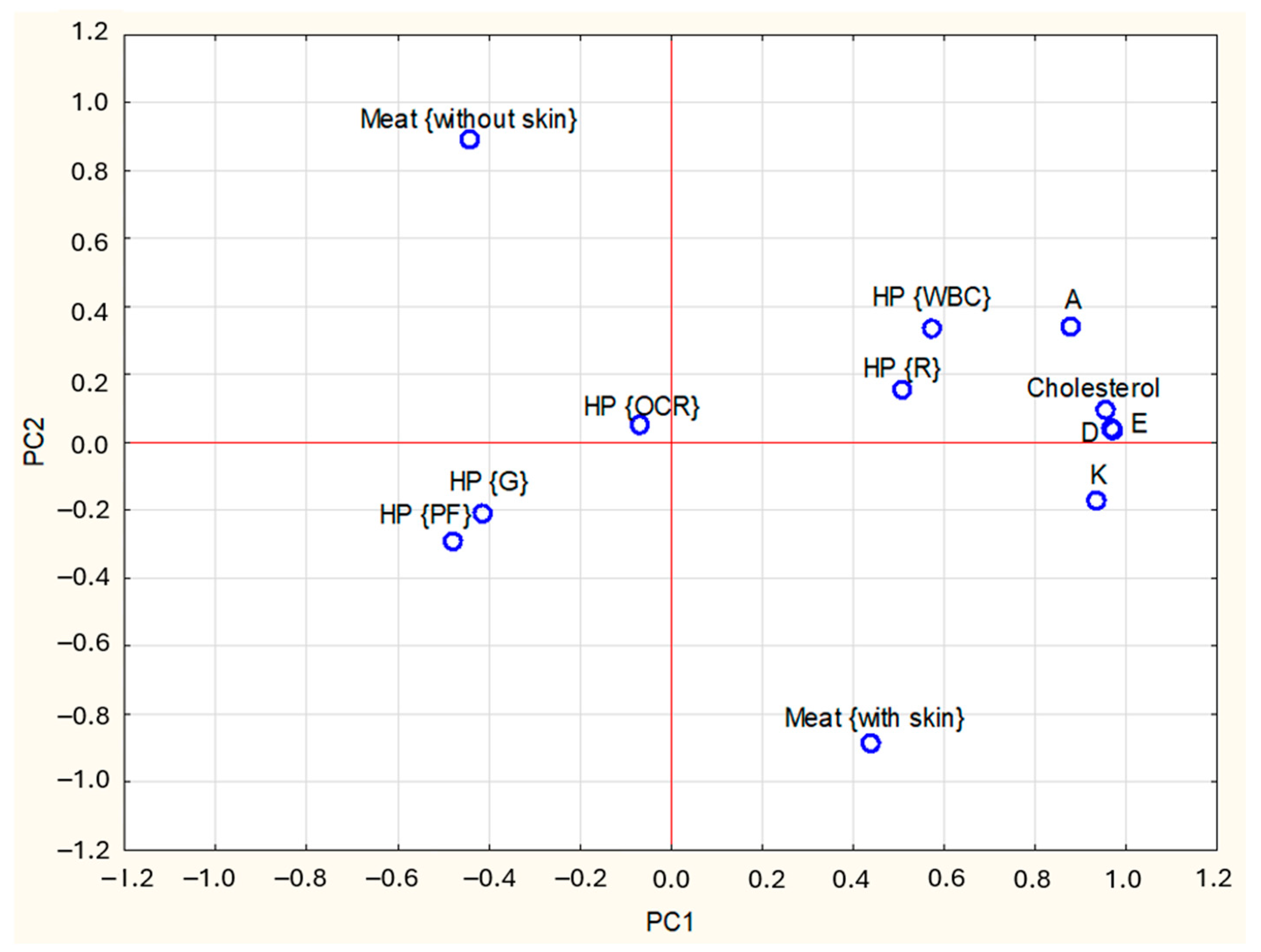
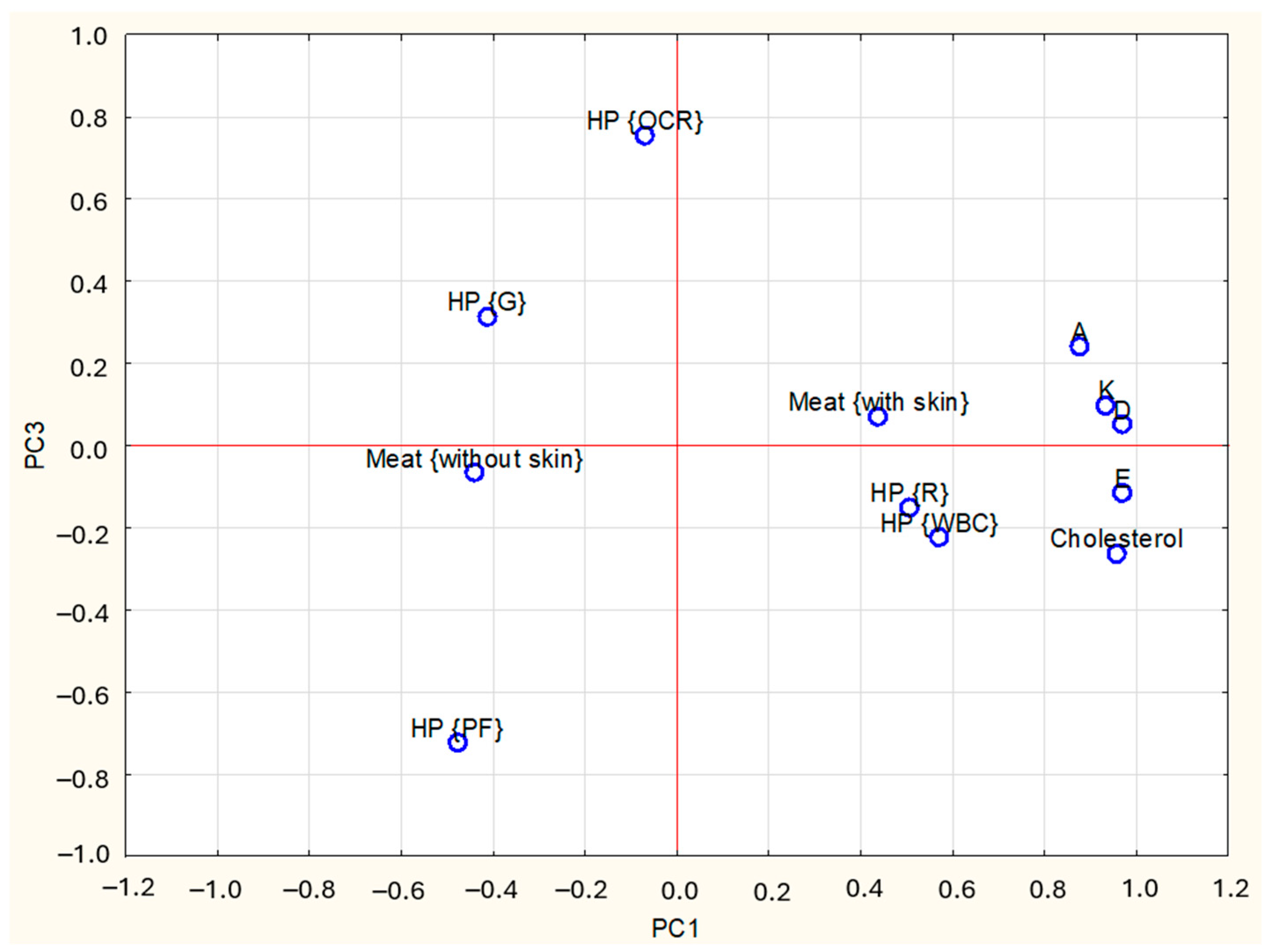
3.3. Principal Component Analysis
3.4. Fat-Soluble Vitamins and Cholesterol Retention in Goose Meat After Heat Processing
3.5. Goose Meat and Nutrient Reference Values (NRV) for Adults
3.6. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Adequate daily Intake |
| CL | Cooking loss |
| EFSA | European Food Safety Authority |
| HAA | Heterocyclic Aromatic Amines |
| HP | Heat processing |
| FAO | Food and Agriculture Organization of the United Nations |
| G | Grilling |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| NRV | Nutrient Reference Values |
| OCR | Oven Convection Roasting |
| OECD | Organization for Economic Co-operation and Development |
| PAH | Polycyclic Aromatic Hydrocarbons |
| PCA | Principal Component Analysis |
| PF | Pan Frying |
| UL | Upper Level |
| USDA | United States Department of Agriculture |
| WBC | Water Bath Cooking |
| WHO | World Health Organization |
References
- Leroy, F.; Smith, N.W.; Adesogan, A.T.; Beal, T.; Iannotti, L.; Moughan, P.J.; Mann, N. The Role of Meat in the Human Diet: Evolutionary Aspects and Nutritional Value. Anim. Front. 2023, 13, 11–18. [Google Scholar] [CrossRef]
- Cocking, C.; Walton, J.; Kehoe, L.; Cashman, K.D.; Flynn, A. The Role of Meat in the European Diet: Current State of Knowledge on Dietary Recommendations, Intakes and Contribution to Energy and Nutrient Intakes and Status. Nutr. Res. Rev. 2020, 33, 181–189. [Google Scholar] [CrossRef]
- Miller, V.; Reedy, J.; Cudhea, F.; Zhang, J.; Shi, P.; Erndt-Marino, J.; Coates, J.; Micha, R.; Webb, P.; Mozaffarian, D.; et al. Global, Regional, and National Consumption of Animal-Source Foods between 1990 and 2018: Findings from the Global Dietary Database. Lancet Planet. Health 2022, 6, e243–e256. [Google Scholar] [CrossRef]
- FAO. Pathways Towards Lower Emissions—A Global Assessment of the Greenhouse Gas Emissions and Mitigation Options from Livestock Agrifood Systems; FAO: Rome, Italy, 2023; ISBN 978-92-5-138448-0. [Google Scholar] [CrossRef]
- Jia, J.; Wu, F.; Yu, H.; Chou, J.; Han, Q.; Cui, X. Global Meat Consumption Driver Analysis with Machine Learning Methods. Food Secur. 2024, 16, 829–843. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Varga, P.; Lehoczki, A.; Munkácsy, G.; Fekete, J.T.; Bianchini, G.; Ocana, A.; Buda, A.; Ungvari, A.; et al. Association between Red and Processed Meat Consumption and Colorectal Cancer Risk: A Comprehensive Meta-Analysis of Prospective Studies. GeroScience 2025, 47, 5123–5140. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.; De Smet, S.; Leroy, F.; Mente, A.; Stanton, A. Non-Communicable Disease Risk Associated with Red and Processed Meat Consumption—Magnitude, Certainty, and Contextuality of Risk? Anim. Front. 2023, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund International. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective: A Summary of the Third Expert Report; World Cancer Research Fund International: London, UK, 2018; ISBN 978-1-912259-46-5. [Google Scholar]
- Huang, Y.; Cao, D.; Chen, Z.; Chen, B.; Li, J.; Guo, J.; Dong, Q.; Liu, L.; Wei, Q. Red and Processed Meat Consumption and Cancer Outcomes: Umbrella Review. Food Chem. 2021, 356, 129697. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, S.; Chen, X.; Yang, J.; Zhou, Y.; Du, L.; Li, K. Red/Processed Meat Consumption and Non-Cancer-Related Outcomes in Humans: Umbrella Review. Br. J. Nutr. 2023, 130, 484–494. [Google Scholar] [CrossRef]
- Rohrmann, S.; Overvad, K.; Bueno-de-Mesquita, H.B.; Jakobsen, M.U.; Egeberg, R.; Tjønneland, A.; Nailler, L.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Krogh, V.; et al. Meat Consumption and Mortality—Results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013, 11, 63. [Google Scholar] [CrossRef]
- Abid, Z.; Cross, A.J.; Sinha, R. Meat, Dairy, and Cancer. Am. J. Clin. Nutr. 2014, 100, 386S–393S. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Nwachukwu, I.D. The Differential Effects of Cooking Methods on the Nutritional Properties and Quality Attributes of Meat from Various Animal Sources. Croat. J. Food Sci. Technol. 2020, 12, 37–47. [Google Scholar] [CrossRef]
- Orta-Aleman, D.; Thorne-Lyman, A.L.; Neff, R.; Wolfson, J.; Caulfield, L.E. Reduced Red and Processed Meat Consumption Is Associated with Lower Diet Costs in US Households: A National Analysis of Protein Substitutions. Public Health Nutr. 2024, 27, e205. [Google Scholar] [CrossRef] [PubMed]
- OECD; Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook 2023-2032; OECD-FAO Agricultural Outlook; OECD: Paris, France, 2023; ISBN 978-92-64-61933-3. [Google Scholar]
- Jia, J.; Dawson, T.P.; Wu, F.; Han, Q.; Cui, X. Global Meat Demand Projection: Quo Vadimus? J. Clean. Prod. 2023, 429, 139460. [Google Scholar] [CrossRef]
- Dumlu, B. The Global Goose Meat Production Quantity Forecast for the 2023–2027 Years. Selcuk. J. Agric. Food Sci. 2024, 38, 326–341. [Google Scholar] [CrossRef]
- Gąsior, R.; Wojtycza, K.; Majcher, M.A.; Bielińska, H.; Odrzywolska, A.; Bączkowicz, M.; Migdał, W. Key Aroma Compounds in Roasted White Kołuda Goose. J. Agric. Food Chem. 2021, 69, 5986–5996. [Google Scholar] [CrossRef]
- Minister of Agriculture and Development Rural National Support Centre for Agriculture. Available online: https://www.gov.pl/web/kowr/handel-zagraniczny-produktami-rolno-spozywczymi (accessed on 1 July 2025).
- Sun, T.; Liu, Y.; Qin, X.; Aspridou, Z.; Zheng, J.; Wang, X.; Li, Z.; Dong, Q. The Prevalence and Epidemiology of Salmonella in Retail Raw Poultry Meat in China: A Systematic Review and Meta-Analysis. Foods 2021, 10, 2757. [Google Scholar] [CrossRef]
- Szosland-Fałtyn, A.M.; Bartodziejska, B. Influence of Campylobacter spp. on Putrescine Concentration in Different Types of Poultry Meat. Ann. Agric. Environ. Med. 2025, 32, 75–78. [Google Scholar] [CrossRef]
- Liu, W.; Luo, X.; Huang, Y.; Zhao, M.; Liu, T.; Wang, J.; Feng, F. Influence of Cooking Techniques on Food Quality, Digestibility, and Health Risks Regarding Lipid Oxidation. Food Res. Int. 2023, 167, 112685. [Google Scholar] [CrossRef]
- Dikeman, M.; Devine, C. Encyclopedia of Meat Sciences, 2nd ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-384734-8. [Google Scholar]
- Hassoun, A.; Aït-Kaddour, A.; Sahar, A.; Cozzolino, D. Monitoring Thermal Treatments Applied to Meat Using Traditional Methods and Spectroscopic Techniques: A Review of Advances over the Last Decade. Food Bioprocess Technol. 2021, 14, 195–208. [Google Scholar] [CrossRef]
- Sun, D.-W. (Ed.) Thermal Food Processing: New Technologies and Quality Issues; Food Science and Technology; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006; ISBN 978-1-57444-628-9. [Google Scholar]
- Khalid, W.; Maggiolino, A.; Kour, J.; Arshad, M.S.; Aslam, N.; Afzal, M.F.; Meghwar, P.; Zafar, K.-W.; De Palo, P.; Korma, S.A. Dynamic Alterations in Protein, Sensory, Chemical, and Oxidative Properties Occurring in Meat during Thermal and Non-Thermal Processing Techniques: A Comprehensive Review. Front. Nutr. 2023, 9, 1057457. [Google Scholar] [CrossRef]
- Sruthi, N.U.; Premjit, Y.; Pandiselvam, R.; Kothakota, A.; Ramesh, S.V. An Overview of Conventional and Emerging Techniques of Roasting: Effect on Food Bioactive Signatures. Food Chem. 2021, 348, 129088. [Google Scholar] [CrossRef]
- Wereńska, M.; Haraf, G.; Okruszek, A.; Marcinkowska, W.; Wołoszyn, J. The Effects of Sous Vide, Microwave Cooking, and Stewing on Some Quality Criteria of Goose Meat. Foods 2022, 12, 129. [Google Scholar] [CrossRef]
- Wereńska, M.; Haraf, G.; Wołoszyn, J.; Goluch, Z.; Okruszek, A.; Teleszko, M. Fatty Acid Profile and Health Lipid Indicies of Goose Meat in Relation to Various Types of Heat Treatment. Poult. Sci. 2021, 100, 101237. [Google Scholar] [CrossRef] [PubMed]
- Goluch, Z.; Król, B.K.; Haraf, G.; Wołoszyn, J.; Okruszek, A.; Wereńska, M. Impact of Various Types of Heat Processing on the Energy and Nutritional Values of Goose Breast Meat. Poult. Sci. 2021, 100, 101473. [Google Scholar] [CrossRef] [PubMed]
- Wołoszyn, J.; Wereńska, M.; Goluch, Z.; Haraf, G.; Okruszek, A.; Teleszko, M.; Król, B. The Selected Goose Meat Quality Traits in Relation to Various Types of Heat Treatment. Poult. Sci. 2020, 99, 7214–7224. [Google Scholar] [CrossRef] [PubMed]
- Więk, A.; Mozolewski, W.; Rybaczek, S.; Modzelewska-Kapituła, M. The Quality of Goose Breast Muscle Products Depending on the Cooking Method Used. Appl. Sci. 2024, 14, 3508. [Google Scholar] [CrossRef]
- Oz, F.; Celik, T. Proximate Composition, Color and Nutritional Profile of Raw and Cooked Goose Meat with Different Methods: Some Properties of Goose Meat. J. Food Process. Preserv. 2015, 39, 2442–2454. [Google Scholar] [CrossRef]
- Goluch, Z.; Czernecki, T.; Haraf, G.; Okruszek, A.; Wereńska, M. Impact of Various Types of Heat Processing on the Content of Selected Trace Elements of Goose Breast Meat. Appl. Sci. 2025, 15, 6795. [Google Scholar] [CrossRef]
- Goluch, Z.; Bąkowska, M.; Haraf, G.; Pilarczyk, B. Selenium Content of Goose Breast Meat Depending on the Type of Heat Processing. Appl. Sci. 2024, 14, 4693. [Google Scholar] [CrossRef]
- Marangoni, F.; Corsello, G.; Cricelli, C.; Ferrara, N.; Ghiselli, A.; Lucchin, L.; Poli, A. Role of Poultry Meat in a Balanced Diet Aimed at Maintaining Health and Wellbeing: An Italian Consensus Document. Food Nutr. Res. 2015, 59, 27606. [Google Scholar] [CrossRef]
- Stangierski, J.; Lesnierowski, G. Nutritional and Health-Promoting Aspects of Poultry Meat and Its Processed Products. World’s Poult. Sci. J. 2015, 71, 71–82. [Google Scholar] [CrossRef]
- Soriano-Santos, J. Chemical Composition and Nutritional Content of Raw Poultry Meat. In Handbook of Poultry Science and Technology; Wiley: Hoboken, NJ, USA, 2010; pp. 467–489. ISBN 978-0-470-18552-0. [Google Scholar]
- Wang, L.; Suderman, D.R. Application of Batters and Breadings to Various Substrates. In Batters and Breadings in Food Processing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 243–261. ISBN 978-1-891127-71-7. [Google Scholar]
- Bhat, Z.F.; Kumar, P.; Kumar, S. Effect of Skin, Enrobing and Refrigerated Storage on the Quality Characteristics of Chicken Meat Balls. J. Food Sci. Technol. 2013, 50, 890–899. [Google Scholar] [CrossRef]
- Cliche, S.; Amiot, J.; Avezard, C.; Gariepy, C. Extraction and Characterization of Collagen with or without Telopeptides from Chicken Skin. Poult. Sci. 2003, 82, 503–509. [Google Scholar] [CrossRef]
- Bhargavi, P.K.; Banerjee, R.; Md, R.; Maheswarappa, N.B.; Verma, A.K.; Govindaiah, P.M.; Lalthanmawii, J. Sustainable Gelatin Extraction from Poultry Skin-Head-Feet Blend: An Ultrasound-Assisted Approach. Poult. Sci. 2025, 104, 104975. [Google Scholar] [CrossRef]
- Andrès, E.; Lorenzo-Villalba, N.; Terrade, J.-E.; Méndez-Bailon, M. Fat-Soluble Vitamins A, D, E, and K: Review of the Literature and Points of Interest for the Clinician. J. Clin. Med. 2024, 13, 3641. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.A.; Dawoud, A.; ElTahtawy, O.; Farag, M.A. Fat-Soluble Vitamins: Updated Review of Their Role and Orchestration in Human Nutrition throughout Life Cycle with Sex Differences. Nutr. Metab. 2022, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Lodh, M.; Mukhopadhyay, R.; Jajodia, N.; Sen, D.; Roy, A. Adult Hypervitaminosis D-A Case Series. Int. J. Endocrinol. Metab. Disord. 2015, 1–3. [Google Scholar] [CrossRef]
- Fox, R.; Stace, N.; Wood, K.; French, C. Liver Toxicity from Vitamin A. JGH Open 2020, 4, 287–288. [Google Scholar] [CrossRef]
- Kaye, A.D.; Thomassen, A.S.; Mashaw, S.A.; MacDonald, E.M.; Waguespack, A.; Hickey, L.; Singh, A.; Gungor, D.; Kallurkar, A.; Kaye, A.M.; et al. Vitamin E (α-Tocopherol): Emerging Clinical Role and Adverse Risks of Supplementation in Adults. Cureus 2025, 17, e78679. [Google Scholar] [CrossRef]
- European Food Safety Authority. Overview on Tolerable Upper Intake Levels as Derived by the Scientific Committee on Food (SCF) and the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); European Food Safety Authority: Parma, Italy, 2024. [Google Scholar]
- U.S. Department of Agriculture Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 1 July 2025).
- Guo, J.; Chen, S.; Zhang, Y.; Liu, J.; Jiang, L.; Hu, L.; Yao, K.; Yu, Y.; Chen, X. Cholesterol Metabolism: Physiological Regulation and Diseases. MedComm 2024, 5, e476. [Google Scholar] [CrossRef]
- Leong, W.; Ngiam, J.; Tan, R.; Lim, S.; Poh, K. Controversies and Discrepancies in the Effect of Dietary Fat and Cholesterol on Cardiovascular Risk. Singap. Med. J. 2021, 62, 56–62. [Google Scholar] [CrossRef]
- Li, C.; Yang, L.; Zhang, D.; Jiang, W. Systematic Review and Meta-Analysis Suggest That Dietary Cholesterol Intake Increases Risk of Breast Cancer. Nutr. Res. 2016, 36, 627–635. [Google Scholar] [CrossRef]
- Wojciechowski, J. (Ed.) Breeding and Rearing of White Kołudzka Geese in the Realities of the 21st Century; Kuyavian-Pomeranian Agricultural Advisory Center: Minikowo, Poland, 2016; ISBN 978-83-65181-10-7. [Google Scholar]
- Buzała, M.; Adamski, M.; Janicki, B. Characteristics of Performance Traits and the Quality of Meat and Fat in Polish Oat Geese. World’s Poult. Sci. J. 2014, 70, 531–542. [Google Scholar] [CrossRef]
- European Commission. COMMISSION IMPLEMENTING REGULATION (EU) 2019/627 of 15 March 2019 Laying down Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin Intended for Human Consumption in Accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and Amending Commission Regulation (EC) No 2074/2005 as Regards Official Controls 2019. Available online: https://eur-lex.europa.eu/eli/reg_impl/2019/627/oj/eng (accessed on 17 September 2025).
- Sami, R.; Li, Y.; Qi, B.; Wang, S.; Zhang, Q.; Han, F.; Ma, Y.; Jing, J.; Jiang, L. HPLC Analysis of Water-Soluble Vitamins (B2, B3, B6, B12, and C) and Fat-Soluble Vitamins (E, K, D, A, and β-Carotene) of Okra (Abelmoschus esculentus). J. Chem. 2014, 2014, 831357. [Google Scholar] [CrossRef]
- Stewart, G.; Gosselin, C.; Pandian, S. Selected Ion Monitoring of Tert-Butyldimethylsilyl Cholesterol Ethers for Determination of Total Cholesterol Content in Foods. Food Chem. 1992, 44, 377–380. [Google Scholar] [CrossRef]
- Lešková, E.; Kubíková, J.; Kováčiková, E.; Košická, M.; Porubská, J.; Holčíková, K. Vitamin Losses: Retention during Heat Treatment and Continual Changes Expressed by Mathematical Models. J. Food Compos. Anal. 2006, 19, 252–276. [Google Scholar] [CrossRef]
- Murphy, R.Y.; Johnson, E.R.; Duncan, L.K.; Clausen, E.C.; Davis, M.D.; March, J.A. Heat Transfer Properties, Moisture Loss, Product Yield, and Soluble Proteins in Chicken Breast Patties During Air Convection Cooking. Poult. Sci. 2001, 80, 508–514. [Google Scholar] [CrossRef]
- Yu, J.; Yang, H.M.; Lai, Y.Y.; Wan, X.L.; Wang, Z.Y. The Body Fat Distribution and Fatty Acid Composition of Muscles and Adipose Tissues in Geese. Poult. Sci. 2020, 99, 4634–4641. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, M.; Qi, S.; Xu, X.; Liu, W.; Liu, L.; Bao, Q.; Zhang, Y.; Xu, Q.; Zhao, W.; et al. Lipidomics Reveals Lipid Changes in the Intramuscular Fat of Geese at Different Growth Stages. Poult. Sci. 2024, 103, 103172. [Google Scholar] [CrossRef]
- Wereńska, M. Comparative Study on the Effects of Sous-Vide, Microwave Cooking, and Stewing on Functional Properties and Sensory Quality of Goose Meat. Poult. Sci. 2023, 102, 103064. [Google Scholar] [CrossRef]
- Kunachowicz, I.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tables of Composition and Nutritional Value of Food; PZWL Wydawnictwo Lekarskie: Warsaw, Poland, 2020; ISBN 978-83-200-6258-8. [Google Scholar]
- Ministry of Agriculture and Rural and Development of the Slovak Republic the Slovak Food Composition Database (SFCDB). Available online: http://www.pbd-online.sk/en (accessed on 10 July 2025).
- Food Composition Database for Epidemiological Studies in Italy. Available online: https://bda.ieo.it/?page_id=690&lang=en (accessed on 1 July 2025).
- National Institute of Nutrition and Seafood Research—NIFES Seafood Data Norwegian Food Composition Table. Available online: https://www.matvaretabellen.no/en/ (accessed on 10 July 2025).
- Technical University of Denmark the Danish Food Composition Database. Available online: https://frida.fooddata.dk/?lang=en (accessed on 1 July 2025).
- Public Health England McCance and Widdowson’s the Composition of Foods Integrated Dataset 2021. Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid (accessed on 10 July 2025).
- Warren, M.F.; Livingston, K.A. Implications of Vitamin D Research in Chickens Can Advance Human Nutrition and Perspectives for the Future. Curr. Dev. Nutr. 2021, 5, nzab018. [Google Scholar] [CrossRef]
- Clausen, I.; Jakobsen, J.; Leth, T.; Ovesen, L. Vitamin D3 and 25-Hydroxyvitamin D3 in Raw and Cooked Pork Cuts. J. Food Compos. Anal. 2003, 16, 575–585. [Google Scholar] [CrossRef]
- Nowicka, K. Variability in Nutritional Value of Traditional Goose Meat Product. Anim. Sci. Pap. Rep. 2018, 36, 405–420. [Google Scholar]
- Schurgers, L.J.; Vermeer, C. Determination of Phylloquinone and Menaquinones in Food. Pathophysiol. Haemost. Thromb. 2000, 30, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Łoś-Kuczera, M.; Piekarska, J. (Eds.) Composition and Nutritional Value of Food Products. Part II–VII; Państ. Zakład Wydawnictw Lekarskich: Warszawa, Poland, 1988; ISBN 978-83-200-1110-4. [Google Scholar]
- Czech Food Composition Database. Available online: http://www.nutridatabaze.cz/ (accessed on 1 July 2025).
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Godoy, H.T.; Amaya-Farfan, J.; Rodriguez-Amaya, D.B. Degradation of Vitamins. In Chemical Changes During Processing and Storage of Foods; Elsevier: Amsterdam, The Netherlands, 2021; pp. 329–383. ISBN 978-0-12-817380-0. [Google Scholar]
- Sobral, M.M.C.; Cunha, S.C.; Faria, M.A.; Ferreira, I.M. Domestic Cooking of Muscle Foods: Impact on Composition of Nutrients and Contaminants. Compr. Rev. Food Sci. Food Saf. 2018, 17, 309–333. [Google Scholar] [CrossRef]
- Kumar, S.; Aalbersberg, B. Nutrient Retention in Foods after Earth-Oven Cooking Compared to Other Forms of Domestic Cooking. J. Food Compos. Anal. 2006, 19, 311–320. [Google Scholar] [CrossRef]
- Gerber, N.; Scheeder, M.R.L.; Wenk, C. The Influence of Cooking and Fat Trimming on the Actual Nutrient Intake from Meat. Meat Sci. 2009, 81, 148–154. [Google Scholar] [CrossRef]
- Bosco, A.D.; Castellini, C.; Bernardini, M. Nutritional Quality of Rabbit Meat as Affected by Cooking Procedure and Dietary Vitamin E. J. Food Sci. 2001, 66, 1047–1051. [Google Scholar] [CrossRef]
- European Parrlament. REGULATION (EU) No 1169/2011 of the European Parrlament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, 2011. Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj/eng?locale=pl (accessed on 17 September 2025).
- Lewis, J. Codex Nutrient Reference Values; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2019; ISBN 978-92-5-131957-4. [Google Scholar]
- Deutsche Gesellschaft für Ernährung (DGE); Österreichische Gesellschaft für Ernährung (ÖGE). Referenzwerte für die Nährstoffzufuhr; Deutsche Gesellschaft für Ernährung (DGE), Österreichische Gesellschaft für Ernährung (ÖGE): Bonn, Germany, 2025; ISBN 978-3-88749-261-8. [Google Scholar]
- Health Council of the Netherlands Dietary Reference Values for Vitamins and Minerals for Adults. 2018. Available online: https://www.healthcouncil.nl/documents/2018/09/18/dietary-reference-values-for-vitamins-and-minerals-for-adults (accessed on 17 September 2025).
- Blomhoff, R.; Andersen, R.; Arnesen, E.K.; Christensen, J.J.; Eneroth, H.; Erkkola, M.; Gudanaviciene, I.; Halldórsson, Þ.I.; Høyer-Lund, A.; Lemming, E.W.; et al. Nordic Nutrition Recommendations 2023; Nordic Council of Ministers: Copenhagen, Denmark, 2023; ISBN 978-92-893-7534-4. [Google Scholar]
- EFSA Scientific Panel NDA. Scientific Opinion on Dietary Reference Values for Vitamin A. EFSA J. 2015, 13, 4028. [Google Scholar] [CrossRef]
- Agence Nationale De Sécurité Sanitaire. Les Références Nutritionnelles en Vitamines et Minéraux; ANSES: Maisons-Alfort: Paris, France, 2021. [Google Scholar]
- Rychlik, E.; Stoś, K.; Woźniak, A.; Mojskiej, H. Nutritional Standards for the Polish Population; National Institute of Public Health-National Institute of Hygiene: Warsaw, Poland, 2024; ISBN 978-83-65870-78-0. [Google Scholar]
- Fernandez, M.L.; Calle, M. Revisiting Dietary Cholesterol Recommendations: Does the Evidence Support a Limit of 300 Mg/d? Curr. Atheroscler. Rep. 2010, 12, 377–383. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Cholesterol and the Lack of Evidence in Cardiovascular Disease. Nutrients 2018, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, L.G.; De Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020–2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr. Today 2021, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Iammarino, M.; Marino, R.; Nardelli, V.; Ingegno, M.; Albenzio, M. Red Meat Heating Processes, Toxic Compounds Production and Nutritional Parameters Changes: What about Risk–Benefit? Foods 2024, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Kızıl, M.; Çelık, T. Effects of Different Cooking Methods on the Formation of Heterocyclic Aromatic Amines in Goose Meat: Goose and HCAs. J. Food Process. Preserv. 2016, 40, 1047–1053. [Google Scholar] [CrossRef]
- Canzoneri, F.; Leoni, V.; Rosso, G.; Risso, D.; Menta, R.; Poli, G. Oxysterols as Reliable Markers of Quality and Safety in Cholesterol Containing Food Ingredients and Products. Front. Nutr. 2022, 9, 853460. [Google Scholar] [CrossRef]
- Molognoni, L.; Daguer, H.; Motta, G.E.; Merlo, T.C.; Lindner, J.D.D. Interactions of Preservatives in Meat Processing: Formation of Carcinogenic Compounds, Analytical Methods, and Inhibitory Agents. Food Res. Int. 2019, 125, 108608. [Google Scholar] [CrossRef]
| Nutrient | LOD [μg/mL] | LOQ [μg/mL] | Precision [%] | Recovery [%] | Uncertainty [%] |
|---|---|---|---|---|---|
| A | 0.44 | 1.35 | 0.37 | 99.91 | 0.09 |
| D3 | 0.31 | 0.94 | 2.04 | 101.0 | 0.01 |
| E | 0.94 | 2.86 | 0.60 | 99.98 | 1.98 |
| K1 | 0.29 | 0.88 | 0.27 | 99.97 | 0.02 |
| Cholesterol | 0.72 | 2.17 | 0.28 | 100.4 | 0.23 |
Item | Meat Type | Raw | Heat Processing | Total for Meat Type | SEM | Level of Significance | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Bath Cooking (WBC) | Grilled (G) | Oven Convection Roasting (OCR) | Pan Fried (PF) | Meat Type (M) | Heat Processing (HP) | M × HP | |||||
| Vitamin A (µg) | without skin | 21.2 A | 20.7 A | 13.6 B | 19.0 A | 11.3 B | 16.7 Y | 0.98 | 0.001 | 0.001 | 0.001 |
| with skin | 22.5 B | 24.6 A | 15.2 D | 16.2 C | 12.6 E | 17.7 X | 1.11 | ||||
| Total for HP | 21.9 A | 22.7 A | 14.4 C | 17.6 B | 12.0 D | 17.2 | 0.74 | ||||
| SEM | 0.38 | 0.75 | 0.60 | 0.54 | 0.23 | ||||||
| Vitamin D (µg) | without skin | 0.33 A | 0.27 B | 0.17 D | 0.25 C | 0.18 D | 0.23 Y | 0.013 | 0.001 | 0.001 | 0.004 |
| with skin | 0.38 A | 0.34 B | 0.22 D | 0.28 C | 0.28 D | 0.28 X | 0.013 | ||||
| Total for HP | 0.35 A | 0.30 B | 0.19 D | 0.26 C | 0.20 D | 0.25 | 0.010 | ||||
| SEM | 0.013 | 0.012 | 0.010 | 0.006 | 0.009 | ||||||
| Vitamin E (µg) | without skin | 289.5 A | 260.4 B | 187.6 E | 202.8 C | 196.6 D | 220.5 Y | 8.82 | 0.001 | 0.001 | 0.001 |
| with skin | 323.7 A | 303.6 B | 210.5 E | 249.9 C | 214.8 D | 253.5 X | 10.4 | ||||
| Total for HP | 306.6 A | 282.0 B | 199.1 E | 226.4 C | 205.7 D | 337.0 | 7.29 | ||||
| SEM | 9.92 | 8.21 | 4.36 | 8.91 | 3.46 | ||||||
| Vitamin K (µg) | without skin | 5.61 A | 5.18 B | 4.30 D | 4.98 C | 4.17 E | 4.77 y | 0.12 | 0.028 | 0.013 | 0.002 |
| with skin | 6.76 A | 6.34 B | 5.53 C | 5.00 D | 4.82 E | 5.57 x | 0.17 | ||||
| Total for HP | 6.19 A | 5.76 B | 4.92 D | 4.99 C | 4.50 E | 5.17 | 0.12 | ||||
| SEM | 0.33 | 0.22 | 0.23 | 0.02 | 0.12 | ||||||
| Cholesterol (mg) | without skin | 72.3 A | 69.4 B | 41.3 E | 45.8 C | 45.3 D | 52.9 Y | 3.03 | 0.001 | 0.001 | 0.001 |
| with skin | 87.2 A | 79.4 B | 50.2 E | 52.3 C | 51.5 D | 61.6 X | 3.55 | ||||
| Total for HP | 79.8 A | 74.4 B | 45.8 E | 49.0 C | 48.4 D | 57.2 | 2.41 | ||||
| SEM | 4.30 | 1.90 | 1.69 | 1.23 | 1.17 | ||||||
| Items | PC1 1 | PC2 1 | PC3 1 | |
|---|---|---|---|---|
| Fat-soluble vitamins contents: | A | 0.88 | 0.37 | 0.24 |
| D | 0.97 | 0.07 | 0.05 | |
| E | 0.96 | 0.05 | −0.11 | |
| K | 0.94 | −0.04 | 0.10 | |
| Cholesterol | 0.96 | 0.09 | −0.26 | |
| Type of meat (M): | M {without skin} | −0.47 | 0.87 | −0.07 |
| M {with skin} | 0.47 | −0.87 | 0.07 | |
| Kind of heat processing (HP): | HP {raw} | 0.49 | 0.16 | −0.15 |
| HP {WBC} | 0.54 | 0.33 | −0.23 | |
| HP {G} | −0.39 | −0.22 | 0.31 | |
| HP {OCR} | −0.00 | 0.10 | 0.75 | |
| HP {PF} | −0.51 | −0.33 | 0.73 |
Item | Meat Type | Raw | Heat Processing | Total for Meat Type | SEM | Level of Significance | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Bath Cooking (WBC) | Grilled (G) | Oven Convection Roasting (OCR) | Pan Fried (PF) | Meat Type (M) | Heat Processing (HP) | M × HP | |||||
| Vitamin A | without skin | - | 63.7 A | 37.5 Bb | 51.4 Aa | 32.7 B | 46.3 | 3.50 | 0.415 | 0.001 | 0.079 |
| with skin | - | 66.8 A | 38.6 B | 42.1 B | 32.1 C | 44.9 | 3.44 | ||||
| Total for HP | - | 65.3 A | 38.0 Ca | 46.7 B | 32.4 Cb | 45.6 | 2.42 | ||||
| SEM | - | 2.89 | 1.60 | 1.78 | 0.94 | ||||||
| Vitamin D | without skin | - | 52.4 A | 28.8 B | 41.8 A | 32.3 B | 38.8 Y | 2.74 | 0.005 | 0.001 | 0.697 |
| with skin | - | 56.4 A | 34.7 C | 44.8 B | 36.1 C | 43.0 X | 2.35 | ||||
| Total for HP | - | 54.4 A | 31.8 C | 43.3 B | 34.2 C | 40.9 | 1.81 | ||||
| SEM | - | 2.94 | 1.54 | 1.06 | 1.00 | ||||||
| Vitamin E | without skin | - | 57.4 A | 37.0 B | 39.3 B | 40.5 B | 43.5 | 2.56 | 0.158 | 0.001 | 0.271 |
| with skin | - | 58.8 A | 38.3 Bb | 46.4 Ba | 39.1 Bb | 45.7 | 2.25 | ||||
| Total for HP | - | 58.1 A | 37.6 B | 42.8 B | 39.8 B | 44.6 | 1.69 | ||||
| SEM | - | 2.95 | 1.30 | 1.48 | 0.96 | ||||||
| Vitamin K | without skin | - | 57.0 | 42.3 | 48.1 | 43.8 | 47.5 | 2.36 | 0.211 | 0.001 | 0.457 |
| with skin | - | 61.2 Aa | 50.2 | 46.2 b | 43.7 B | 50.3 | 2.17 | ||||
| Total for HP | - | 59.1 Aa | 46.2 B | 47.2 b | 43.2 B | 48.9 | 1.60 | ||||
| SEM | - | 3.67 | 2.64 | 1.51 | 1.22 | ||||||
| Cholesterol | without skin | - | 59.3 A | 31.5 B | 34.3 B | 36.0 B | 40.2 | 3.36 | 0.220 | 0.001 | 0.811 |
| with skin | - | 59.5 A | 35.3 B | 37.5 B | 36.2 B | 42.1 | 2.80 | ||||
| Total for HP | - | 59.4 A | 33.4 B | 35.9 B | 36.1 B | 41.2 | 2.16 | ||||
| SEM | - | 3.70 | 1.72 | 1.14 | 0.95 | ||||||
| Item | NRV 1 (μg) | Meat Type | Raw | Heat Processing | Total for Meat Type | SEM | Level of Significance | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Bath Cooking (WBC) | Grilled (G) | Oven Convection Roasting (OCR) | Pan Fried (PF) | Meat Type (M) | Heat Processing (HP) | M × HP | ||||||
| Vitamin A | 800 | without skin | 2.65 A | 2.59 A | 1.70 B | 2.37 A | 1.42 B | 2.09 Y | 0.122 | 0.006 | 0.001 | 0.001 |
| with skin | 2.82 B | 3.08 A | 1.90 D | 2.02 C | 1.57 E | 2.22 X | 0.139 | |||||
| Total for HP | 2.79 A | 2.83 A | 1.80 C | 2.20 B | 1.49 D | 2.15 | 0.092 | |||||
| SEM | 0.048 | 0.094 | 0.075 | 0.067 | 0.029 | |||||||
| Vitamin D | 5 | without skin | 6.63 A | 5.47 B | 3.37 Db | 4.97 C | 3.62 Da | 4.61 Y | 0.268 | 0.001 | 0.001 | 0.005 |
| with skin | 7.50 Aa | 6.70 Ab | 4.42 C | 5.55 B | 4.57 C | 5.55 X | 0.270 | |||||
| Total for HP | 7.07 A | 6.08 B | 3.89 Db | 5.26 C | 4.09 Da | 5.08 | 0.204 | |||||
| SEM | 0.260 | 0.240 | 0.205 | 0.115 | 0.187 | |||||||
| Vitamin E | 9000 | without skin | 3.22 A | 2.89 B | 2.08 E | 2.25 C | 2.18 D | 2.45 Y | 0.098 | 0.001 | 0.001 | 0.001 |
| with skin | 3.60 A | 3.37 B | 2.34 E | 2.78 C | 2.39 D | 2.82 X | 0.116 | |||||
| Total for HP | 3.41 A | 3.13 B | 2.21 E | 2.52 C | 2.29 D | 2.63 | 0.081 | |||||
| SEM | 0.110 | 0.091 | 0.048 | 0.099 | 0.038 | |||||||
| Vitamin K | 60 | without skin | 5.36 A | 4.82 B | 3.47 E | 3.76 C | 3.64 D | 4.08 Y | 0.163 | 0.001 | 0.001 | 0.001 |
| with skin | 5.99 A | 5.62 B | 3.90 E | 4.63 C | 3.98 D | 4.69 X | 0.193 | |||||
| Total for HP | 5.68 A | 5.22 B | 3.69 E | 4.19 C | 3.81 D | 4.39 | 0.135 | |||||
| SEM | 0.184 | 0.152 | 0.081 | 0.165 | 0.064 | |||||||
| Institution | A [μg of Retinol Equivalent/Day] | D [μg/Day] | E [mg of α-Tocopherol Equivalent/Day] | K [μg/Day] | ||||
|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | |
| Nutrition Societies in Germany and Austria and Switzerland [83] | 700 1) | 800–850 1) | 20 | 20 | 11–12 | 12–15 2) | 60–65 2) | 70–80 2) |
| The Health Council of the Netherland [84] | 600 | 800 | 20 | 20 | 11 | 13 2) | 70 2) | 70 2) |
| Nordic Nutrition Recommendations [85] | 650–700 4) | 750–900 4) | 10–20 3) | 10–20 3) | 9–10 3) | 11 3) | 60–65 2) | 70–75 2) |
| European Food Safety Authority [86] | 650 1) | 750 1) | 15 2) | 15 2) | 11 | 13 2) | 70 2) | 70 2) |
| French Agency for Food [87] | 650 1) | 750 1) | 15 2) | 15 2) | 12 2) | 12 2) | 79 2) | 79 2) |
| National Institute of Public Health- National Institute of Hygiene (Poland) [88] | 700 4) | 900 4) | 15 | 15 | 8 2) | 10 2) | 55 2) | 65 2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goluch, Z.; Stryjecka, M.; Haraf, G.; Okruszek, A. Effect of Various Types of Heat Processing on the Content and Retention of Fat-Soluble Vitamins and Cholesterol in Goose Breast Meat. Foods 2025, 14, 3266. https://doi.org/10.3390/foods14183266
Goluch Z, Stryjecka M, Haraf G, Okruszek A. Effect of Various Types of Heat Processing on the Content and Retention of Fat-Soluble Vitamins and Cholesterol in Goose Breast Meat. Foods. 2025; 14(18):3266. https://doi.org/10.3390/foods14183266
Chicago/Turabian StyleGoluch, Zuzanna, Małgorzata Stryjecka, Gabriela Haraf, and Andrzej Okruszek. 2025. "Effect of Various Types of Heat Processing on the Content and Retention of Fat-Soluble Vitamins and Cholesterol in Goose Breast Meat" Foods 14, no. 18: 3266. https://doi.org/10.3390/foods14183266
APA StyleGoluch, Z., Stryjecka, M., Haraf, G., & Okruszek, A. (2025). Effect of Various Types of Heat Processing on the Content and Retention of Fat-Soluble Vitamins and Cholesterol in Goose Breast Meat. Foods, 14(18), 3266. https://doi.org/10.3390/foods14183266








