Structural Elucidation and Storage Stability of Novel Dietary Sulfur Compounds from Radish
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Extraction
2.3. Analytical Methods
2.3.1. High-Performance Liquid Chromatography (HPLC) Analysis
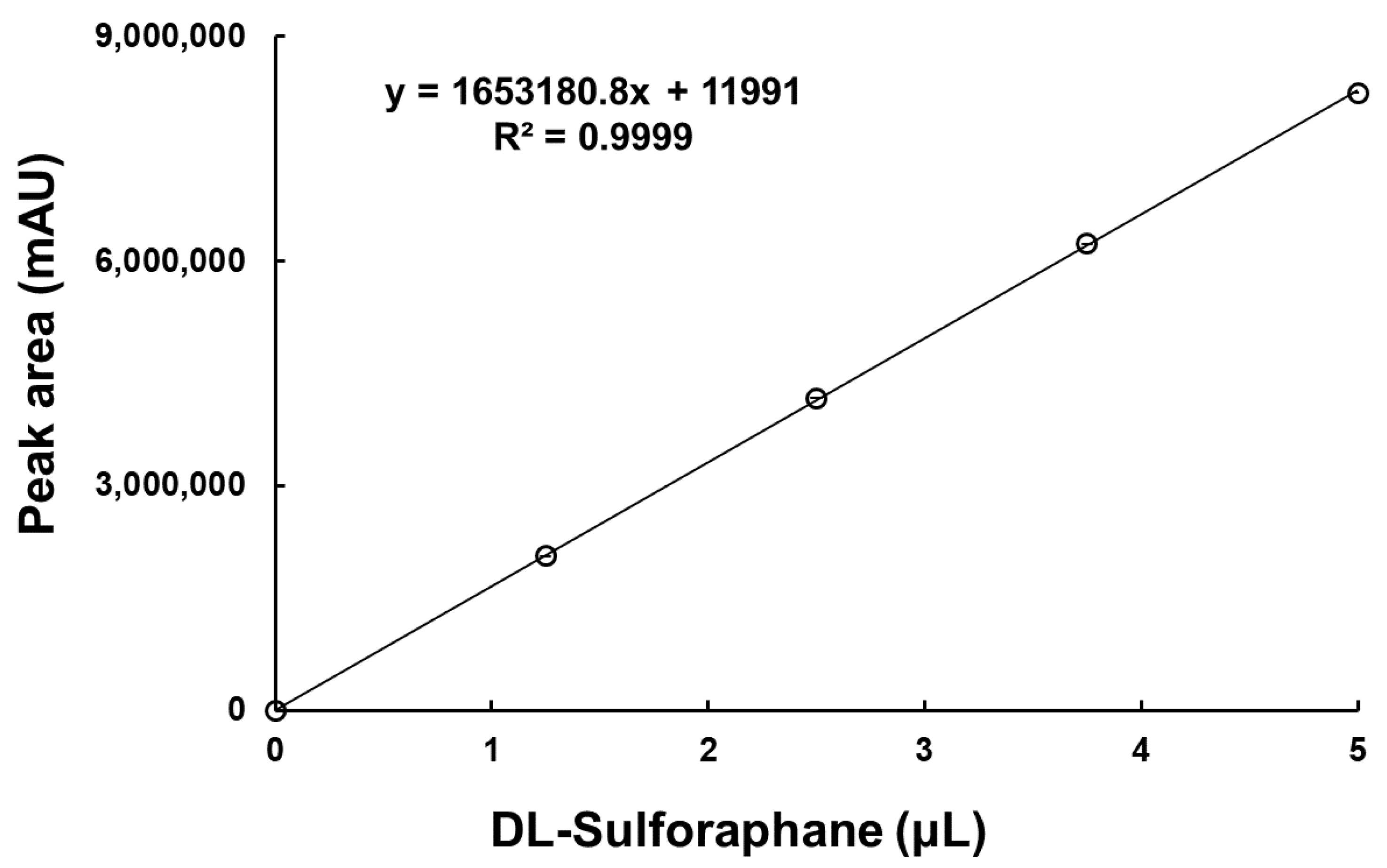
2.3.2. Standard Curve and Quantification for SFN
2.4. Separation and Purification
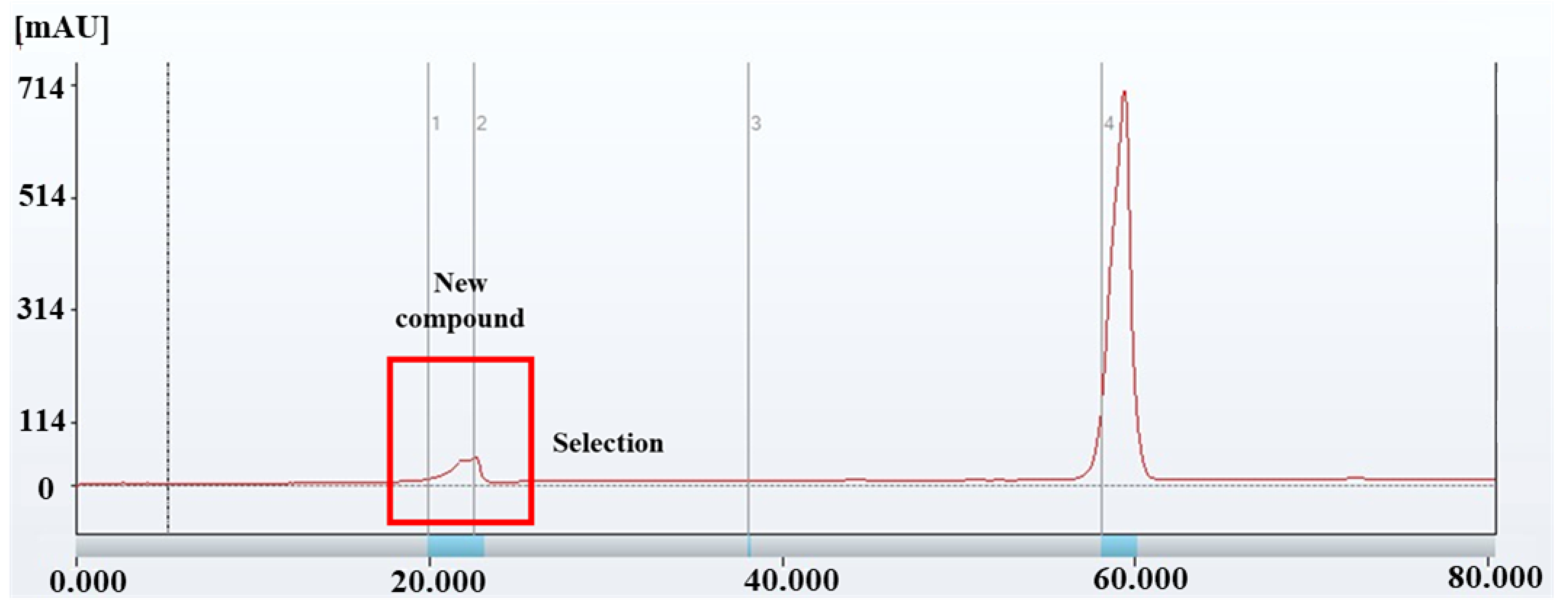
2.4.1. Isocratic and Gradient Elution (Recycling Preparative HPLC)
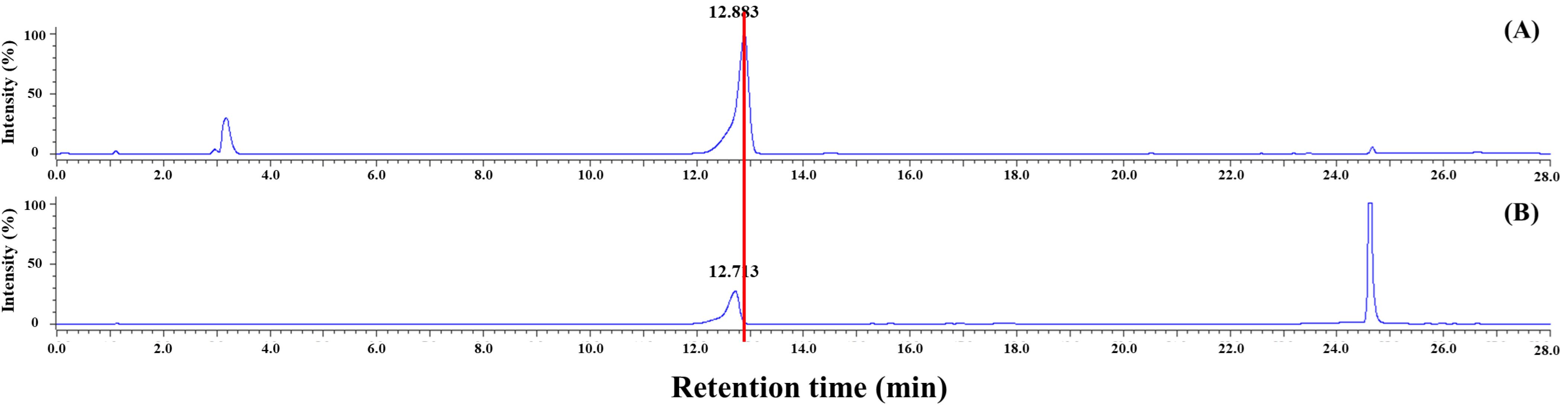
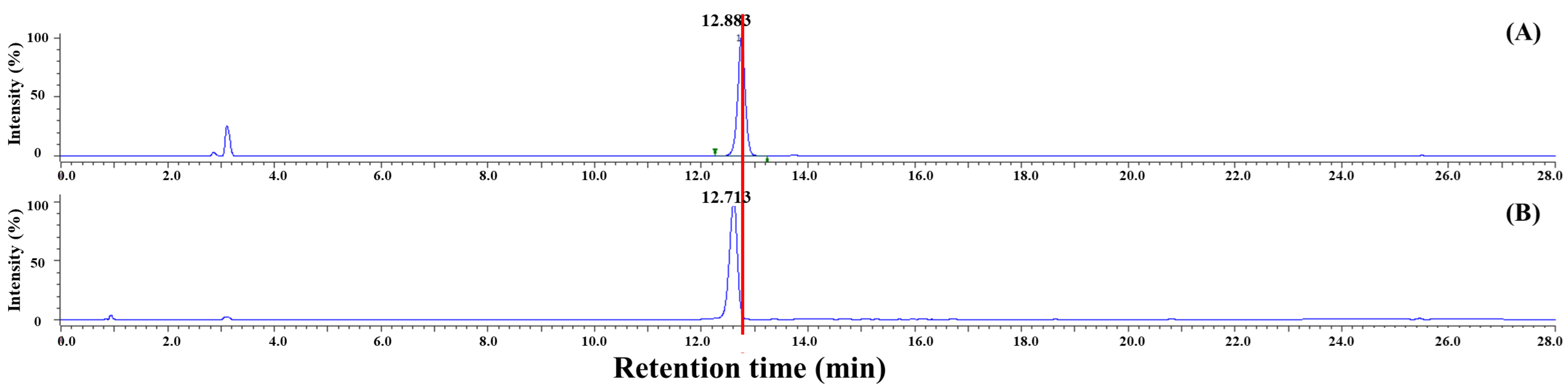
2.4.2. HPLC Confirmation of SFN-DX
2.5. Structure Identification
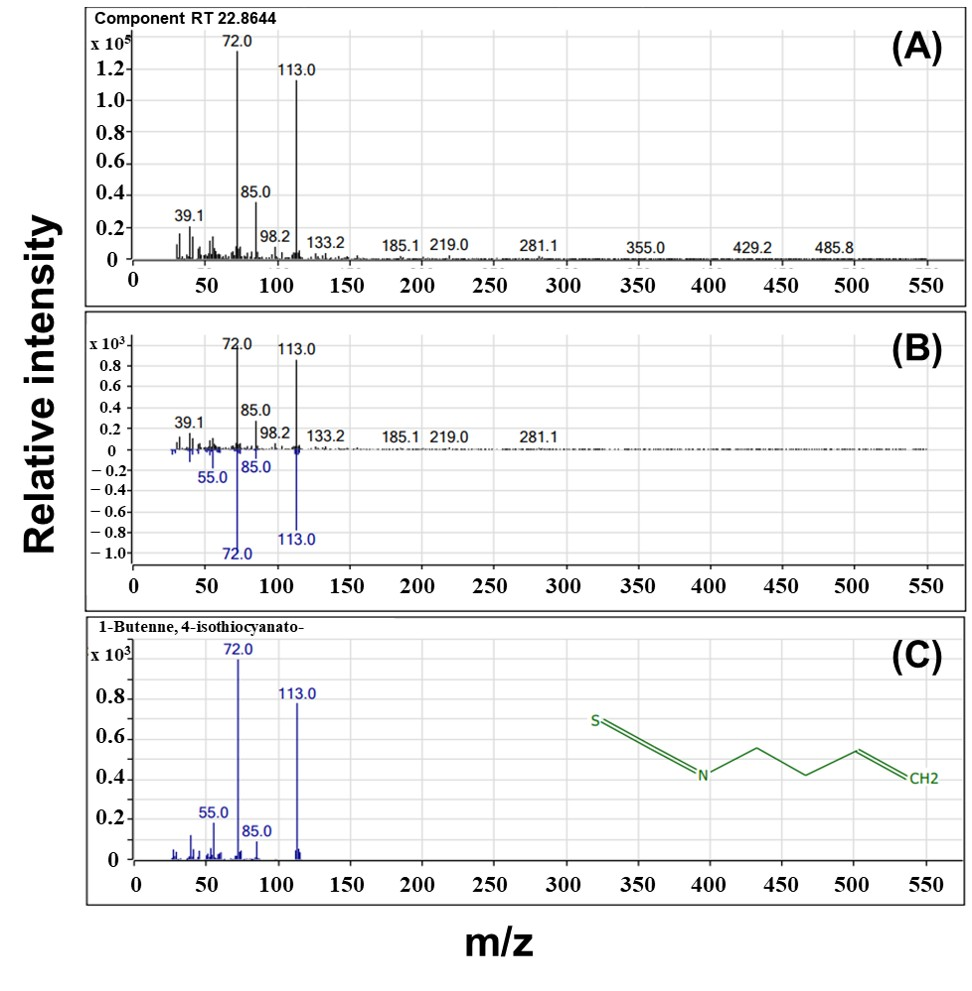
2.5.1. HS-SPME/GC-MS
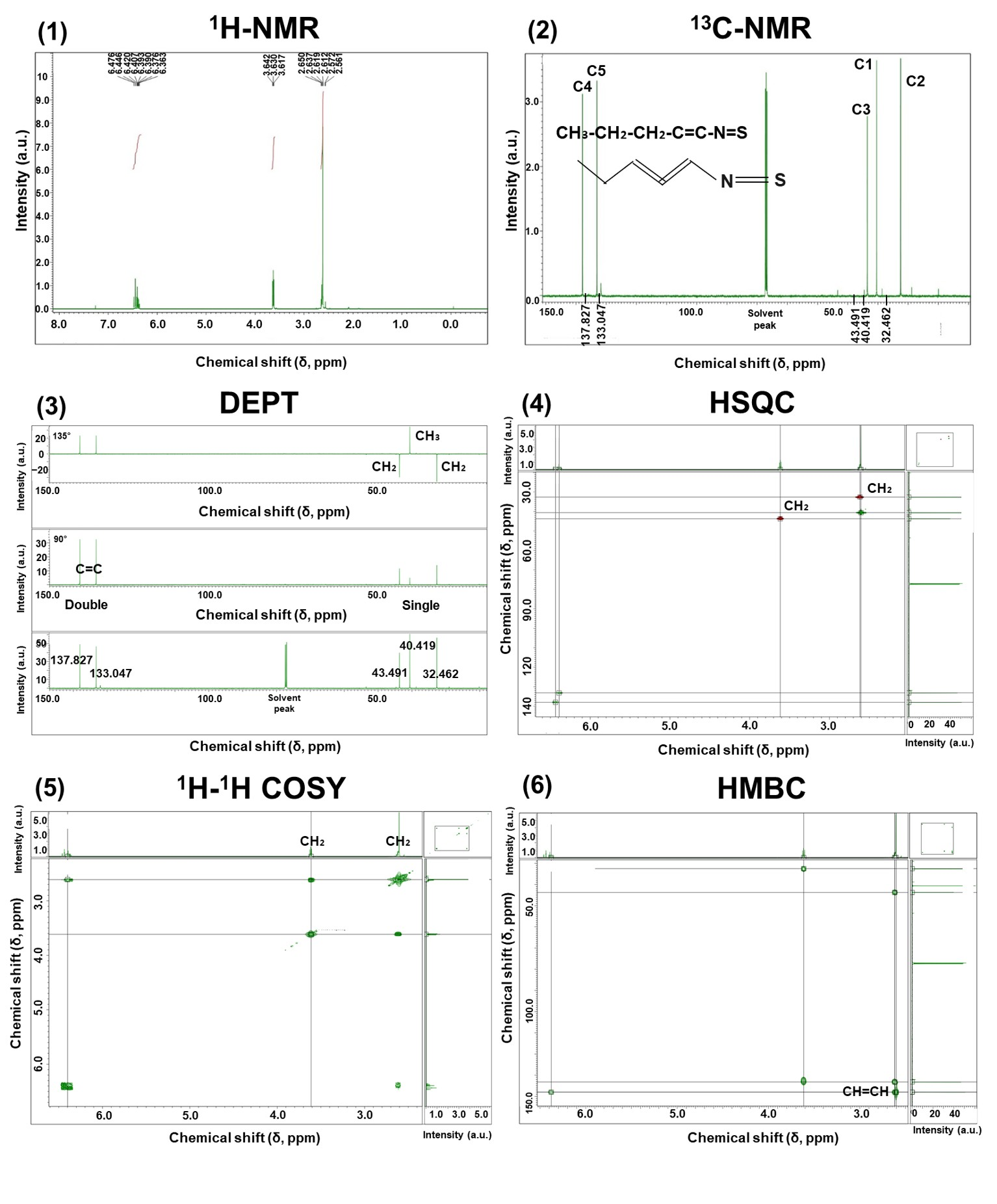
2.5.2. Nuclear Magnetic Resonance (NMR) Analysis
2.6. Storage Stability Evaluation
3. Results and Discussion
3.1. HPLC Analysis
3.1.1. Standard Curve of SFN
3.1.2. HPLC Analysis of SFN-DX Extracted from Radish
3.2. Separation, Purification, and Structural Elucidation
3.2.1. Isocratic and Gradient Elution
3.2.2. GC-MS
3.2.3. NMR
3.3. Stability of Purified SFN-DX at Different Temperatures and Storage Periods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SFN | sulforaphane |
| SFN-DX | SFN-derived compound X |
| RT | retention time |
| HPLC | high-performance liquid chromatography |
| DW | distilled water |
| ACN | acetonitrile |
| HS-SPME | headspace solid phase microextraction |
| GC-MS | gas chromatography–mass spectrometry system |
| NMR | nuclear magnetic resonance |
| DEPT | distortionless enhancement by polarization transfer |
| HSQC | heteronuclear single quantum coherence |
| COSY | 1H–1H correlation spectroscopy |
| HMBC | heteronuclear multiple bond correlation |
| EI-MS | electron ionization–mass spectrometry |
References
- Shimizu, T. Health claims on functional foods: The Japanese regulations and an international comparison. Nutr. Res. Rev. 2003, 16, 241–252. [Google Scholar] [CrossRef]
- Galanakis, C.M. Functionality of food components and emerging technologies. Foods 2021, 10, 128. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; García-Viguera, C.; Moreno, D.A. Evidence on the bioaccessibility of glucosinolates and breakdown products of cruciferous sprouts by simulated in vitro gastrointestinal digestion. Int. J. Mol. Sci. 2021, 22, 11046. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the value of cruciferous sprouts as sources of bioactive compounds for nutrition and health. Nutrients 2019, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Chiu, C.-H.; Hsieh, P.-C. Bioactive compounds and bioactivities of Brassica oleracea L. var. Italica sprouts and microgreens: An updated overview from a nutraceutical perspective. Plants 2020, 9, 946. [Google Scholar] [PubMed]
- Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Lee-Martínez, S.; Larrauri-Rodríguez, M.; Zaldívar-Lelo de Larrea, G.; Pérez-Serrano, R.M.; Camacho-Calderón, N. Isothiocyanate-rich extracts from cauliflower (Brassica oleracea var. Botrytis) and radish (Raphanus sativus) inhibited metabolic activity and induced ROS in selected human HCT116 and HT-29 colorectal cancer cells. Int. J. Environ. Res. Public Health 2022, 19, 14919. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Mastelić, J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.). Food Chem. 2009, 113, 96–102. [Google Scholar] [CrossRef]
- Pawlik, A.; Wała, M.; Hać, A.; Felczykowska, A.; Herman-Antosiewicz, A. Sulforaphene, an isothiocyanate present in radish plants, inhibits proliferation of human breast cancer cells. Phytomedicine 2017, 29, 1–10. [Google Scholar] [CrossRef]
- Cheng, L.; Wan, K.; Liang, H.; Yuan, Q. Sulforaphane and sulforaphene: Two potential anticancer compounds from glucosinolates. In Glucosinolates: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 281–312. [Google Scholar]
- Ko, M.-O.; Kim, M.-B.; Lim, S.-B. Relationship between chemical structure and antimicrobial activities of isothiocyanates from cruciferous vegetables against oral pathogens. J. Microbiol. Biotechn. 2016, 26, 2036–2042. [Google Scholar] [CrossRef]
- Jadoun, J.; Yazbak, A.; Rushrush, S.; Rudy, A.; Azaizeh, H. Identification of a new antibacterial sulfur compound from Raphanus sativus seeds. Evid. Based Complement. Alternat. Med. 2016, 2016, 9271285. [Google Scholar] [CrossRef]
- Lu, Z.-L.; Liu, L.-W.; Li, X.-Y.; Gong, Y.-Q.; Hou, X.-L.; Zhu, X.-W.; Yang, J.-L.; Wang, L.-Z. Analysis and Evaluation of Nutritional Quality in Chinese Radish (Raphanus sativus L.). Agric. Sci. China 2008, 7, 823–830. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Salah-Abbès, J.B.; Abbès, S.; Zohra, H.; Oueslati, R. Tunisian radish (Raphanus sativus) extract prevents cadmium-induced immunotoxic and biochemical alterations in rats. J. Immunotoxicol. 2015, 12, 40–47. [Google Scholar] [CrossRef]
- Li, J.; Xie, B.; Yan, S.; Li, H.; Wang, Q. Extraction and determination of 4-methylthio-3-butenyl isothiocyanate in Chinese radish (Raphanus sativus L.) roots. LWT-Food Sci. Technol. 2015, 60, 1080–1087. [Google Scholar] [CrossRef]
- Son, S.-U.; Park, H.Y.; Suh, H.J.; Shin, K.-S. Evaluation of antitumor metastasis via immunostimulating activities of pectic polysaccharides isolated from radish leaves. J. Funct. Foods 2021, 85, 104639. [Google Scholar] [CrossRef]
- Wang, N.; Wang, W.; Huo, P.; Liu, C.-Q.; Jin, J.-C.; Shen, L.-Q. Mitochondria-mediated apoptosis in human lung cancer A549 cells by 4-methylsulfinyl-3-butenyl isothiocyanate from radish seeds. Asian Pac. J. Cancer Prev. 2014, 15, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Janczewski, Ł. Sulforaphane and its bifunctional analogs: Synthesis and biological activity. Molecules 2022, 27, 1750. [Google Scholar] [CrossRef]
- Mangla, B.; Javed, S.; Sultan, M.H.; Kumar, P.; Kohli, K.; Najmi, A.; Alhazmi, H.A.; Al Bratty, M.; Ahsan, W. Sulforaphane: A review of its therapeutic potentials, advances in its nanodelivery, recent patents, and clinical trials. Phytother. Ress 2021, 35, 5440–5458. [Google Scholar] [CrossRef]
- Kamal, M.M.; Akter, S.; Lin, C.-N.; Nazzal, S. Sulforaphane as an anticancer molecule: Mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch. Pharm. Res. 2020, 43, 371–384. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; Chang, N. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. Eur. J. Pharmacol. 2018, 824, 1–10. [Google Scholar] [CrossRef]
- Gong, T.-T.; Liu, X.-D.; Zhan, Z.-P.; Wu, Q.-J. Sulforaphane enhances the cisplatin sensitivity through regulating DNA repair and accumulation of intracellular cisplatin in ovarian cancer cells. Exp. Cell Res. 2020, 393, 112061. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.; Lee, C. Changes in the composition of dietary sulfur compounds in radish according to different drying methods. Food Eng. Prog. 2023, 27, 319–323. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kyung, K.H. Purification of S-Alk (en) yl alka/enethiosulfinates of garlic (Allium sativum L.) by using recycling preparative HPLC. Food Sci. Biotechnol. 2011, 20, 1167–1170. [Google Scholar] [CrossRef]
- Nakagawa, K.; Umeda, T.; Higuchi, O.; Tsuzuki, T.; Suzuki, T.; Miyazawa, T. Evaporative light-scattering analysis of sulforaphane in broccoli samples: Quality of broccoli products regarding sulforaphane contents. J. Agric. Food Chem. 2006, 54, 2479–2483. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Liu, Z.; Zhu, X.; Zhai, L.; Xu, L.; Yu, R.; Gong, Y.; Liu, L. De novo transcriptome sequencing of radish (Raphanus sativus L.) and analysis of major genes involved in glucosinolate metabolism. BMC Genom. 2013, 14, 836. [Google Scholar] [CrossRef]
- Li, R.; Song, D.; Vriesekoop, F.; Cheng, L.; Yuan, Q.; Liang, H. Glucoraphenin, sulforaphene, and antiproliferative capacity of radish sprouts in germinating and thermal processes. Eur. Food Res. Technol. 2017, 243, 547–554. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Im, J.-S.; Lee, E.-H.; Lee, J.-N.; Kim, K.-D.; Kim, H.-Y.; Kim, M.-J. Sulforaphane and total phenolics contents and antioxidant activity of radish according to genotype and cultivation location with different altitudes. Hortic. Sci. Technol. 2010, 28, 335–342. [Google Scholar]
- Zhou, Q.; Zheng, Z.; Li, L.; Gao, J.; Wu, Y.; Yang, F.; Zhong, K.; Gao, H. Effects of variety on quality and taste of spontaneous fermented dried radish. Food Sci. Technol. 2023, 43, e125322. [Google Scholar] [CrossRef]
- Baek, S.-H.; Park, M.; Suh, J.-H.; Choi, H.-S. Protective effects of an extract of young radish (Raphanus sativus L) cultivated with sulfur (sulfur-radish extract) and of sulforaphane on carbon tetrachloride-induced hepatotoxicity. Biosci. Biotechnol. Biochem. 2008, 72, 1176–1182. [Google Scholar] [CrossRef]
- Lim, S.; Lee, E.J.; Kim, J. Decreased sulforaphene concentration and reduced myrosinase activity of radish (Raphanus sativus L.) root during cold storage. Postharvest Biol. Technol. 2015, 100, 219–225. [Google Scholar] [CrossRef]
- Sangthong, S.; Weerapreeyakul, N. Simultaneous quantification of sulforaphene and sulforaphane by reverse phase HPLC and their content in Raphanus sativus L. var. caudatus Alef extracts. Food Chem. 2016, 201, 139–144. [Google Scholar] [CrossRef]
- Chiang, W.C.; Pusateri, D.J.; Leitz, R.E. Gas chromatography/mass spectrometry method for the determination of sulforaphane and sulforaphane nitrile in broccoli. J. Agric. Food Chem. 1998, 46, 1018–1021. [Google Scholar] [CrossRef]
- Fahey, J.; Talalay, P. Antioxidant functions of sulforaphane: A potent inducer of Phase II detoxication enzymes. Food Chem. Toxicol. 1999, 37, 973–979. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Kore, A.M.; Spencer, G.F.; Wallig, M.A. Purification of the. omega.-(methylsulfinyl) alkyl glucosinolate hydrolysis products: 1-isothiocyanato-3-(methylsulfinyl) propane, 1-isothiocyanato-4-(methylsulfinyl) butane, 4-(methylsulfinyl) butanenitrile, and 5-(methylsulfinyl) pentanenitrile from broccoli and Lesquerella fendleri. J. Agric. Food Chem. 1993, 41, 89–95. [Google Scholar]
- Zhang, Y.; Talalay, P.; Cho, C.-G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-I. Optimization of extraction conditions for garlic oleoresin and changes in the quality characteristics of oleoresin during storage. Korean J. Food Nutr. 2006, 19, 219–226. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, G.A.; Lee, I.; Sung, J.; Lee, C.J. Structural Elucidation and Storage Stability of Novel Dietary Sulfur Compounds from Radish. Foods 2025, 14, 3254. https://doi.org/10.3390/foods14183254
Jeong GA, Lee I, Sung J, Lee CJ. Structural Elucidation and Storage Stability of Novel Dietary Sulfur Compounds from Radish. Foods. 2025; 14(18):3254. https://doi.org/10.3390/foods14183254
Chicago/Turabian StyleJeong, Gyeong A., Inae Lee, Jeehye Sung, and Chang Joo Lee. 2025. "Structural Elucidation and Storage Stability of Novel Dietary Sulfur Compounds from Radish" Foods 14, no. 18: 3254. https://doi.org/10.3390/foods14183254
APA StyleJeong, G. A., Lee, I., Sung, J., & Lee, C. J. (2025). Structural Elucidation and Storage Stability of Novel Dietary Sulfur Compounds from Radish. Foods, 14(18), 3254. https://doi.org/10.3390/foods14183254





