Effect of Osmotic Dehydration on Physico-Chemical Characteristics, Bioactive Compounds and Volatiles Profile of Diospyros kaki Subjected to Different Drying Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals, Reagents and Encapsulating Agent
2.3. Optimization of Osmotic Dehydration (OD) Process
2.4. Drying Experiments
2.4.1. Hot Air-Drying (HAD)
2.4.2. Freeze Drying (FD)
2.5. Physico-Chemical Analysis
2.6. Determination of Bioactive Compounds and Antioxidant Capacity
2.6.1. Total Carotenoids (TCs)
2.6.2. Preparation of the Extracts
2.6.3. Determination of Total Phenolic Compounds (TPCs)
2.6.4. Determination of Total Flavonoids (TFs)
2.6.5. Antioxidant Capacity Assays
2.7. Identification and Quantification of Organic Acids by HPLC-DAD
2.8. Determination of Volatile Compounds by Solid Phase Micro Extraction (SPME) and GC-MS
2.9. Quantification of Volatile Compounds
2.10. Statistical Analysis
3. Results and Discussion
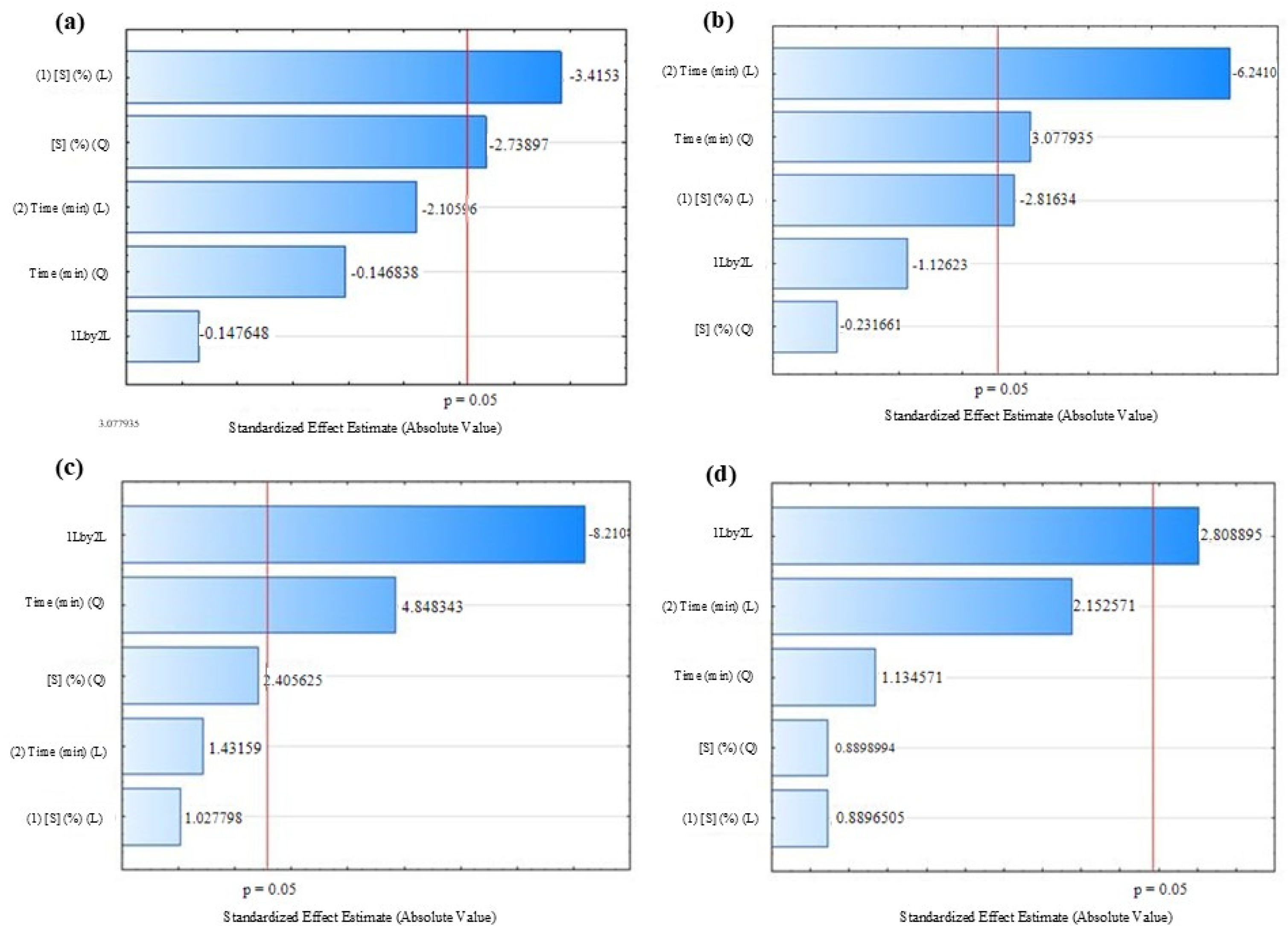
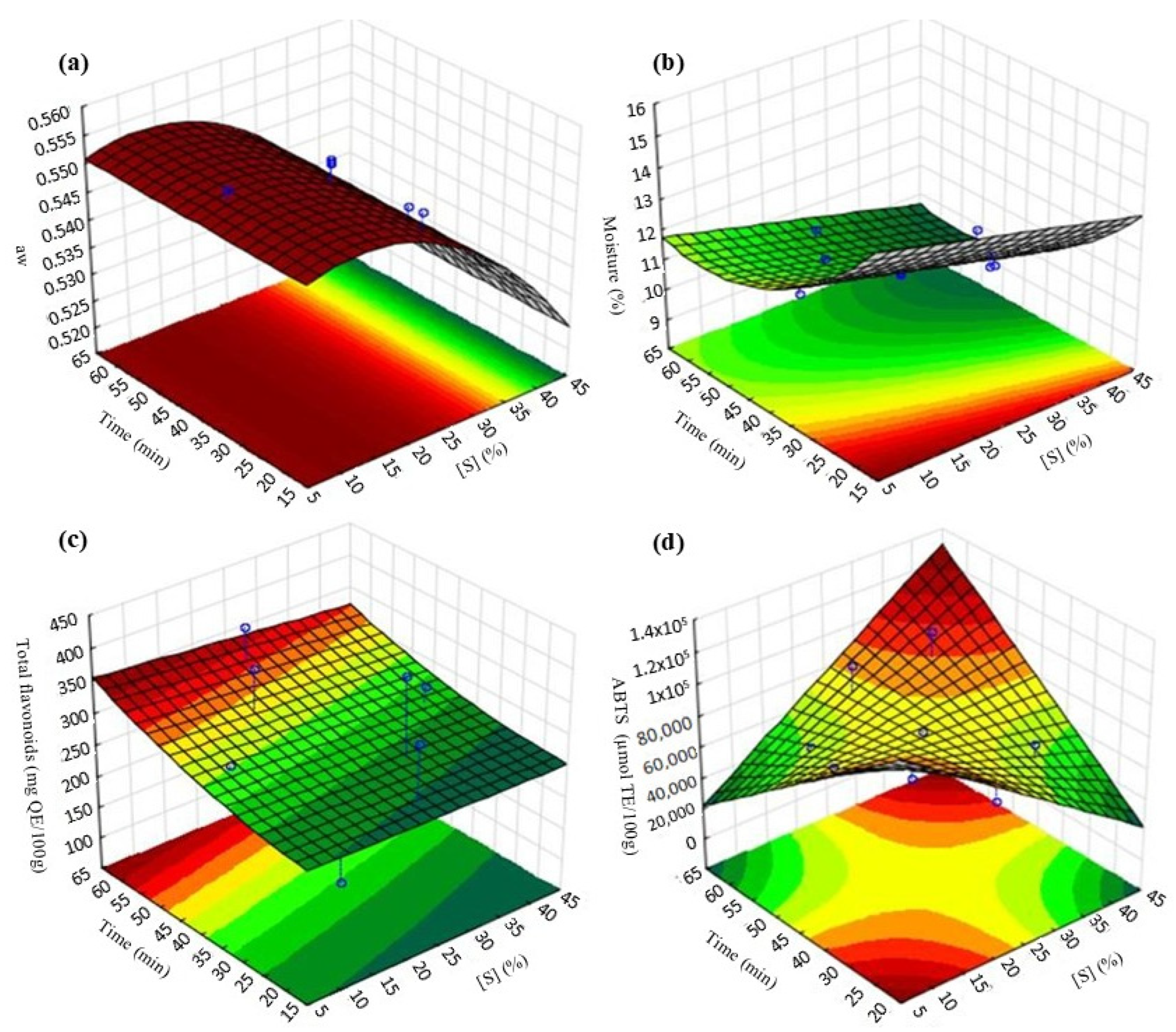
3.1. Optimization of Persimmon Osmotic Dehydration Process with Hot Air-Drying
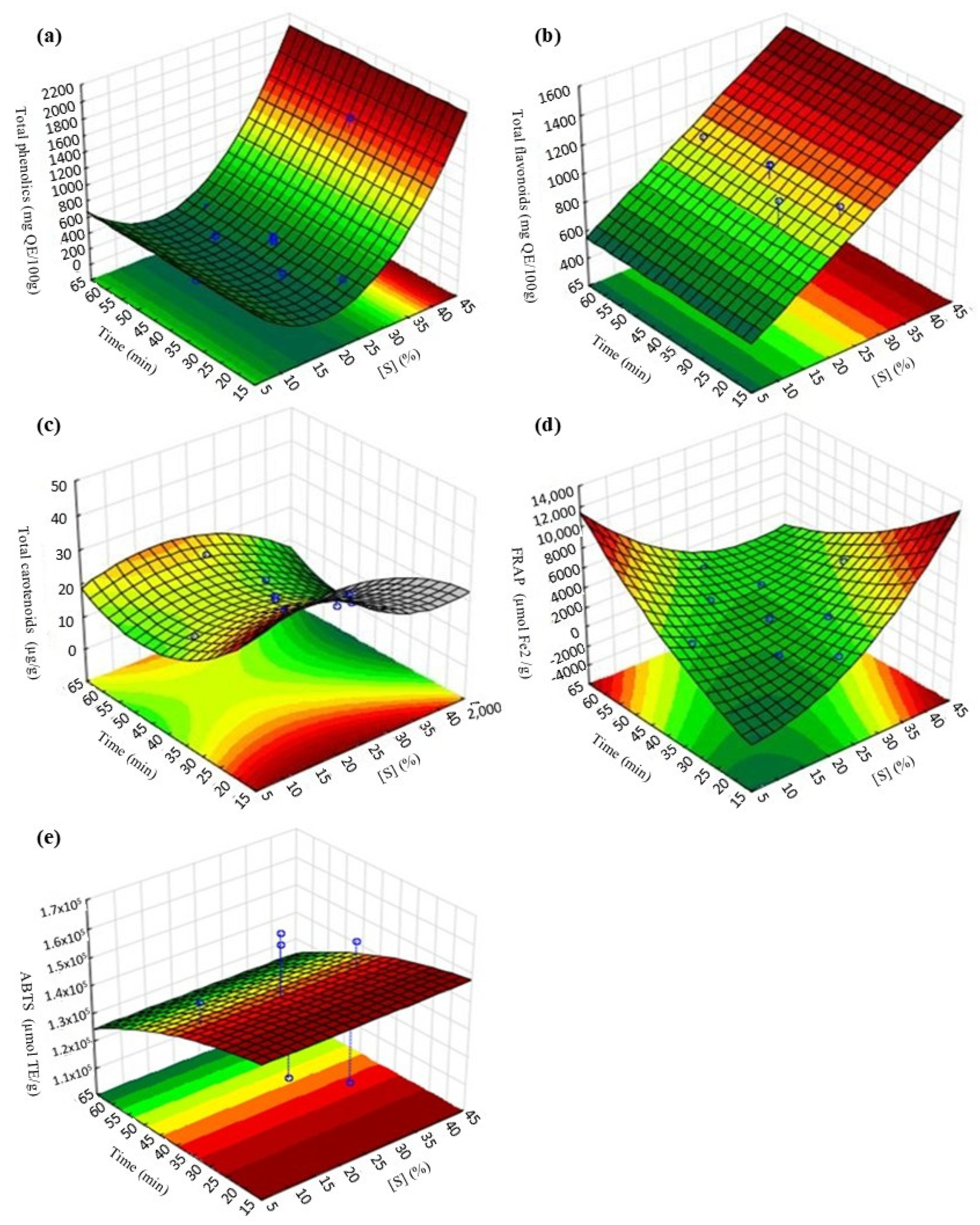
3.2. Optimization of Persimmon Osmotic Dehydration Process with Freeze-Drying
3.3. Desirability Function
3.4. Organic Acids in Dehydrated Persimmons
3.5. Volatile Compounds in Dehydrated Persimmons
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matheus, J.R.V.; Andrade, C.J.D.; Miyahir, R.F.; Fai, A.E.C. Persimmon (Diospyros kaki L.): Chemical properties, bioactive compounds and potential use in the development of new products—A review. Food Rev. Int. 2022, 38, 384–401. [Google Scholar] [CrossRef]
- Veberic, R.; Jurhar, J.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Comparative study of primary and secondary metabolites in 11 cultivars of persimmon fruit (Diospyros kaki L.). Food Chem. 2010, 119, 477–483. [Google Scholar] [CrossRef]
- Matsumura, Y.; Ito, T.; Yano, H.; Kita, E.; Mikasa, K.; Okada, M.; Furutani, A.; Murono, Y.; Shibata, M.; Nishii, Y.; et al. Antioxidant potential in non-extractable fractions of dried persimmon (Diospyros kaki Thunb.). Food Chem. 2016, 202, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.D.C.; Fonseca, F.A.; Dutra, L.M.; Santos, M.D.F.C.; Menezes, L.R.A.; Campos, F.R.; Nagata, N.; Ayub, R.; Barison, A. 1H HR-MAS NMR-based metabolomics study of different persimmon cultivars (Diospyros kaki) during fruit development. Food Chem. 2018, 239, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, S.A.O.; Ashaolu, T.J.; Babu, A.S. Food drying: A review. Agric. Rev. 2022, 46, 86–93. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Bennamoun, L.; Li, J. Drying process of food: Fundamental aspects and mathematical modeling. In Natural and Artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 29–82. [Google Scholar] [CrossRef]
- Park, Y.S.; Jung, S.T.; Kang, S.G.; Delgado-Licon, E.; Ayala, A.L.M.; Tapia, M.S.; Martín-Belloso, O.; Trakhtenberg, S.; Gorinstein, S. Drying of persimmons (Diospyros kaki L.) and the following changes in the studied bioactive compounds and the total radical scavenging activities. LWT-Food Sci. Technol. 2006, 39, 748–755. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ochiai, T. A mathematical model of multi-dimensional freeze drying for food products. J. Food Eng. 2015, 161, 55–67. [Google Scholar] [CrossRef]
- Karaman, S.; Toker, O.S.; Çam, M.; Hayta, M.; Doğan, M.; Kayacier, A. Bioactive and physicochemical properties of persimmon as affected by drying methods. Dry Technol. 2014, 32, 258–267. [Google Scholar] [CrossRef]
- Sagar, V.R.; Suresh Kumar, P. Recent advances in drying and dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2010, 47, 15–26. [Google Scholar] [CrossRef]
- Nowacka, M.; Dadan, M.; Tylewicz, U. Current applications of ultrasound in fruit and vegetables osmotic dehydration processes. Appl. Sci. 2021, 11, 1269. [Google Scholar] [CrossRef]
- Prosapio, V.; Norton, I. Influence of osmotic dehydration pre-treatment on oven drying and freeze drying performance. LWT-Food Sci. Technol. 2017, 80, 401–408. [Google Scholar] [CrossRef]
- Ahmed, I.; Qazi, I.M.; Jamal, S. Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov. Food Sci. Emerg. Technol. 2016, 34, 29–43. [Google Scholar] [CrossRef]
- Abrahão, F.R.; Corrêa, J.L.G. Osmotic dehydration: More than water loss and solid gain. Crit. Rev. Food Sci. Nutr. 2023, 63, 2970–2989. [Google Scholar] [CrossRef]
- Carmo, J.R.D.; Corrêa, J.L.G.; de Santos, A.A.D.L.; da Silva, C.N.; de Oliveira, C.R.; de Araújo, A.L.; Pena, R.D.S. Use of osmotic dehydration assisted by ultrasound to obtain dried mango slices enriched with isomaltulose. J. Food Sci. 2025, 90, e70223. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.D.; Talens, P.; Carot, J.M.; Chiralt, A.; Escriche, I. Volatile profile of mango (Mangifera indica L.), as affected by osmotic dehydration. Food Chem. 2007, 101, 219–228. [Google Scholar] [CrossRef]
- Zhao, J.H.; Liu, F.; Pang, X.L.; Xiao, H.W.; Wen, X.; Ni, Y.Y. Effects of different osmo-dehydrofreezing treatments on the volatile compounds, phenolic compounds and physicochemical properties in mango (Mangifera indica L.). Int. J. Food Sci. Technol. 2016, 51, 1441–1448. [Google Scholar] [CrossRef]
- Talens, P.; Escriche, I.; Martınez-Navarrete, N.; Chiralt, A. Influence of osmotic dehydration and freezing on the volatile profile of kiwi fruit. Food Res. Int. 2003, 36, 635–642. [Google Scholar] [CrossRef]
- Kroehnke, J.; Szadzińska, J.; Radziejewska-Kubzdela, E.; Biegańska-Marecik, R.; Musielak, G.; Mierzwa, D. Osmotic dehydration and convective drying of kiwifruit (Actinidia deliciosa)—The influence of ultrasound on process kinetics and product quality. Ultrason. Sonochem. 2021, 71, 105377. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.A.; Kumari, A.; Rafiq, M.; Siddeeg, A.; Manzoor, M.F.; Baloch, Z.; Ahmed, Z. Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason. Sonochem. 2019, 58, 104643. [Google Scholar] [CrossRef]
- Amami, E.; Khezami, W.; Mezrigui, S.; Badwaik, L.S.; Bejar, A.K.; Perez, C.T.; Kechaou, N. Effect of ultrasound-assisted osmotic dehydration pretreatment on the convective drying of strawberry. Ultrason. Sonochem. 2017, 36, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Cheraghi, R.; Rasouli, M. Mass transfer kinetics (soluble solids gain and water loss) of ultrasound-assisted osmotic dehydration of apple slices. Sci. Rep. 2022, 12, 15392. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Wu, X.Y.; Cui, C.B.; Bi, J.F. Effect of osmotic dehydration combined with vacuum freeze-drying treatment on characteristic aroma components of peach slices. Food Chem. X 2024, 22, 101337. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, J.L.G.; Lopes, F.J.; de Mello Júnior, R.E.; de Jesus Junqueira, J.R.; de Mendonça, K.S.; Macedo, L.L.; Salvio, L.G.A. Drying of persimmon fruit (Diospyros kaki L.) pretreated by different osmotic processes. J. Food Process. Eng. 2021, 44, e13809. [Google Scholar] [CrossRef]
- Demiray, E.; Tulek, Y. The effect of pretreatments on air drying characteristics of persimmons. Heat Mass Transf. 2017, 53, 99–106. [Google Scholar] [CrossRef]
- Bozkir, H.; Ergün, A.R.; Serdar, E.; Metin, G.; Baysal, T. Influence of ultrasound and osmotic dehydration pretreatments on drying and quality properties of persimmon fruit. Ultrason. Sonochem. 2019, 54, 135–141. [Google Scholar] [CrossRef]
- IAL—Instituto Adolfo Lutz. Métodos Físico-Químicos para Análise de Alimentos. 4th ed. São Paulo, Brasil. 2008. Available online: http://www.ial.sp.gov.br/resources/editorinplace/ial/2016_3_19/analisedealimentosial_2008.pdf (accessed on 15 April 2019).
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; Horwitz, G., Latimer, Eds.; AOAC International: Washington, DC, USA, 2012. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidante activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia científica: Determinação da atividade antioxidante total em frutas pela captura do radical ABTS+. Comun. Tec. 2007, 128, 1–4. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPAT/10225/1/Cot_128.pdf (accessed on 20 August 2019).
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia científica: Determinação da atividade antioxidante total em frutas pelo método de redução do ferro (FRAP). Comun. Tec. 2006, 125, 1–4. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/664098/1/cot125.pdf (accessed on 20 August 2019).
- Lee, H.S. HPLC method for separation and determination of nonvolatile organic acids in orange juice. J. Agric. Food Chem. 1993, 41, 1991–1993. [Google Scholar] [CrossRef]
- Hung, S.F.; Roan, S.F.; Chang, T.L.; King, H.B.; Chen, I.Z. Analysis of aroma compounds and nutrient contents of mabolo (Diospyros blancoi A. DC.), an ethnobotanical fruit of Austronesian Taiwan. J. Food Drug Anal. 2016, 24, 83–89. [Google Scholar] [CrossRef]
- Cheong, K.W.; Tan, C.P.; Mirhosseini, H.; Hamid, N.S.A.; Osman, A.; Basri, M. Equilibrium headspace analysis of volatile flavor compounds extracted from soursop (Annona muricata) using solid-phase microextraction. Food Res. Int. 2010, 43, 1267–1276. [Google Scholar] [CrossRef]
- Feng, X.; Sun, J.; Liu, B.; Zhou, X.; Jiang, L.; Jiang, W. Effect of gradient concentration pre-osmotic dehydration on keeping air-dried apricot antioxidant activity and bioactive compounds. J. Food Process. Preserv. 2022, 46, e16688. [Google Scholar] [CrossRef]
- Pan, Y.K.; Zhao, L.J.; Zhang, Y.; Che, G.; Mujumdar, A.S. Osmotic dehydration pretreatment in drying of fruits and vegetables. Dry Technol. 2003, 21, 1101–1114. [Google Scholar] [CrossRef]
- Chandra, S.; Kumari, D. Recent development in osmotic dehydration of fruit and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Novillo, P.; Besada, C.; Tian, L.; Bermejo, A.; Salvador, A. Nutritional composition of ten persimmon cultivars in the “ready-to-eat crisp” stage. Effect of deastringency treatment. Food Nutr. Sci. 2015, 6, 1296. [Google Scholar] [CrossRef][Green Version]
- Persic, M.; Jakopic, J.; Hudina, M. The effect of post-harvest technologies on selected metabolites in persimmon (Diospyros kaki Thunb.) fruit. J. Sci. Food. Agric. 2019, 99, 854–860. [Google Scholar] [CrossRef]
- Gökmen, V.; Acar, J. Fumaric acid in apple juice: A potential indicator of microbial spoilage of apples used as raw material. Food Addit. Contam. 2004, 21, 626–631. [Google Scholar] [CrossRef]
- Nguyễn, H.V.H.; Savage, G.P. Oxalate content of New Zealand grown and imported fruits. J. Food Compost. Anal. 2013, 31, 180–184. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.B.; Seo, W.D.; Kang, S.T.; Lim, J.W.; Cho, K.M. Comparative studies of antioxidant activities and nutritional constituents of persimmon juice (Diospyros kaki L. cv. Gapjubaekmok). Prev. Nutr. Food Sci. 2012, 17, 141. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical stability of ascorbic acid integrated into commercial products: A review on bioactivity and delivery technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Senter, S.D.; Chapman, G.W.; Forbus, W.R., Jr.; Payne, J.A. Sugar and nonvolatile acid composition of persimmons during maturation. J. Food Sci. 1991, 56, 989–991. [Google Scholar] [CrossRef]
- Bian, L.L.; You, S.Y.; Park, J.; Yang, S.J.; Chung, H.J. Characteristics of nutritional components in astringent persimmons according to growing region and cultivar. J. Korean Soc. Food Sci. Nutr. 2015, 44, 379–385. [Google Scholar] [CrossRef]
- Ardestani, S.B.; Sahari, M.A.; Barzegar, M. Effect of extraction and processing conditions on organic acids of barberry fruits. J. Food Biochem. 2015, 39, 554–565. [Google Scholar] [CrossRef]
- Wang, Y.; Hossain, D.; Perry, P.L.; Adams, B.; Lin, J. Characterization of volatile and aroma-impact compounds in persimmon (Diospyros kaki L., var. Triumph) fruit by GC-MS and GC-O analyses. Flavour Frag. J. 2012, 27, 141–148. [Google Scholar] [CrossRef]


| Independent Variables | Code | Unit | Factors Level | ||||
|---|---|---|---|---|---|---|---|
| −1.41 | −1 | 0 | 1 | 1.41 | |||
| Sucrose | X1 | % | 10 | 20 | 25 | 30 | 40 |
| Time | X2 | min | 20 | 30 | 40 | 50 | 60 |
| Assay | X1 | X2 | Y1 | Y2 | Y3 | Y4 |
|---|---|---|---|---|---|---|
| 1 | 20 | 30 | 0.5629 | 12.71 | 109.93 | 83,712.86 |
| 2 | 30 | 30 | 0.5530 | 12.7 | 391.52 | 72,183.33 |
| 3 | 20 | 50 | 0.5540 | 11.00 | 367.46 | 63,355.54 |
| 4 | 30 | 50 | 0.5405 | 10.07 | 207.81 | 106,302.92 |
| 5 | 10 | 40 | 0.5430 | 11.52 | 296.92 | 77,105.19 |
| 6 | 40 | 40 | 0.5302 | 9.91 | 299.46 | 72,103.09 |
| 7 | 25 | 20 | 0.5576 | 12.74 | 345.83 | 60,572.22 |
| 8 | 25 | 60 | 0.5453 | 10.77 | 375.56 | 92,622.05 |
| 9 | 25 | 40 | 0.5471 | 10.84 | 239.55 | 63,073.17 |
| 10 | 25 | 40 | 0.5467 | 10.94 | 247.66 | 70,945.61 |
| 11 | 25 | 40 | 0.5439 | 10.92 | 248.78 | 76,749.57 |
| Assay | X1 | X2 | Y1 | Y2 | Y3 | Y4 | Y5 |
|---|---|---|---|---|---|---|---|
| 1 | 20 | 30 | 391.27 | 1086.37 | 23.93 | 1171.07 | 12,065.89 |
| 2 | 30 | 30 | 377.68 | 1014.3 | 18.8 | 2986.58 | 142,921.42 |
| 3 | 20 | 50 | 354.85 | 767.59 | 14.45 | 2661.55 | 132,622.82 |
| 4 | 30 | 50 | 388.52 | 1045.84 | 14.98 | 2159.42 | 130,455.06 |
| 5 | 10 | 40 | 308.15 | 525.76 | 16.66 | 2430.94 | 148,051.56 |
| 6 | 40 | 40 | 1579.52 | 1339.91 | 7.61 | 4547.50 | 148,717.62 |
| 7 | 25 | 20 | 428.77 | 1111.95 | 31.70 | 1983.04 | 122,405.75 |
| 8 | 25 | 60 | 356.95 | 1055.75 | 19.81 | 2986.55 | 107,918.20 |
| 9 | 25 | 40 | 401.16 | 1122.85 | 16.35 | 1428.17 | 157,388.51 |
| 10 | 25 | 40 | 439.91 | 1134.07 | 17.95 | 1293.82 | 161,170.22 |
| 11 | 25 | 40 | 478.21 | 1126.55 | 19.06 | 1675.17 | 157,427.12 |
| Dependent Variables | Predicted Values | Experimental Values | Coefficient of Variation (%) |
|---|---|---|---|
| Hot air drying | |||
| Aw | 0.5428 | 0.5493 | 0.84 |
| Moisture (%) | 11.32 | 10.45 | 5.65 |
| Total flavonoids (mg QE/100 g) | 250.72 | 238.18 | 3.63 |
| ABTS (µmol TE/100 g) | 83,000 | 100,000 | 13.14 |
| Freeze drying | |||
| Total phenolics (mg GA/100 g) | 943.84 | 921.47 | 1.70 |
| Total flavonoid (mg QE/100 g) | 932.83 | 917.35 | 1.18 |
| Carotenoids (µg/g) | 19.66 | 17.93 | 6.51 |
| FRAP (µmol Fe2+/100 g) | 2859.30 | 2571.09 | 7.51 |
| ABTS (µmol TE/100 g) | 134,500 | 110,862 | 13.62 |
| Compounds | Dehydrated Persimmons—Concentrations (mg/100 g) | |||
|---|---|---|---|---|
| C-HAD | OD-HAD | C-FD | OD-FD | |
| Oxalic acid | 14.42 c ± 0.18 | 15.06 b ± 0.03 | 14.21 c ± 0.01 | 16.73 a ± 0.05 |
| Quinic acid | 15.62 c ± 0.03 | 17.46 b ± 0.28 | 15.79 c ± 0.01 | 18.57 a ± 0.08 |
| L-ascorbic acid | nd | nd | 0.15 b ± 0.00 | 2.27 a ± 0.21 |
| Acetic acid | 1.80 b ± 0.06 | 1.56 c ± 0.00 | 2.09 a ± 0.01 | 2.13 a ± 0.01 |
| Citric acid | 1.49 a ± 0.09 | nd | 1.27 b ± 0.00 | 1.50 a ± 0.02 |
| Succinic acid | 2.13 c ± 0.18 | 8.38 a ± 0.02 | 1.26 d ± 0.02 | 5.96 b ± 0.02 |
| Fumaric acid | 0.24 b ± 0.00 | 0.25 a ± 0.00 | nd | nd |
| N°. | Compounds | CAS | LRIE | LRIL | Dehydrated Persimmons—Concentrations (µg/kg) * | Odor | |||
|---|---|---|---|---|---|---|---|---|---|
| C-HAD | OD-HAD | C-FD | OD-FD | ||||||
| Aldehydes | |||||||||
| 1 | (E)-2-Hexenal | 6728-26-3 | 859 | 856 | nd | nd | 141.33 ± 2.29 | 187.82 ± 6.52 | Green |
| 2 | Heptanal | 111-71-7 | 901 | 903 | nd | 143.91 ± 19.67 | nd | nd | Green, fresh |
| 3 | Benzaldehyde | 100-52-7 | 956 | 960 | 226.30 ± 22.30 | 148.31 ± 10.03 | 199.23 ± 6.52 | 212.68 ± 4.85 | Sweet, cherry |
| 4 | Octanal | 124-13-0 | 1002 | 1004 | nd | 238.73 ± 6.78 | 230.51 ± 4.18 | 260.15 ± 11.56 | Green, citrus |
| 5 | (E)-2-Octenal | 2548-87-0 | 1056 | 1056 | nd | 278.74 ± 4.18 | 239.25 ± 4.82 | 301.56 ± 0.40 | Green, citrus |
| 6 | Nonanal | 124-19-6 | 1103 | 1104 | 569.70 ± 13.56 | 707.30 ± 25.91 | 578.22 ± 2.40 | 759.12 ± 13.15 | Rose, fresh |
| 7 | (E)-2-Nonenal | 18829-56-6 | 1158 | 1158 | nd | 145.31 ± 1.74 | 71.36 ± 2.11 | 142.49 ± 4.72 | Green, cucumber |
| 8 | Benzaldehyde, 2.5-dimethyl- | 5779-94-2 | 1169 | 1164 | 116.14 ± 5.98 | 103.62 ± 7.33 | 97.71 ± 3.80 | 107.40 ± 3.32 | |
| 9 | Decanal | 112-31-2 | 1199 | 1200 | 239.03 ± 4.17 | 494.57 ± 24.03 | 503.82 ± 14.10 | 543.00 ± 7.09 | Green, cucumber |
| 10 | Dodecanal | 112-54-9 | 1407 | 1407 | 40.64 ± 0.73 | 92.23 ± 6.89 | 111.07 ± 0.51 | 149.31 ± 4.46 | Citrus, floral |
| Subtotal | 1191.81 | 2352.71 | 2172.51 | 2663.5 | |||||
| Alcohols | |||||||||
| 11 | Phenylethyl Alcohol | 60-12-8 | 1112 | 1112 | 191.21 ± 7.52 | nd | 260.12 ± 39.53 | nd | Floral, sweet, rose |
| Subtotal | 191.21 | 0.00 | 260.12 | 0.00 | |||||
| Ketones | |||||||||
| 12 | 6-Methyl-5-hepten-2-one | 110-93-0 | 984 | 982 | 603.88 ± 22.00 | 770.91 ± 34.70 | 535.40 ± 21.54 | 1065.92 ± 270.67 | Citrus, fruity |
| 13 | 2,6,6-Trimethylcyclohexanone | 2408-37-9 | 1030 | 1034 | 365.83 ± 4.51 | 467.18 ± 7.75 | nd | nd | Pungent |
| 14 | 3,5-Octadien-2-one | 38284-27-4 | 1090 | 1074 | nd | 221.80 ± 0.08 | 160.58 ± 5.15 | 197.03 ± 3.15 | Fruity |
| 15 | 2-Cyclopentylcyclopentanone | 4884-24-6 | 1276 | - | 72.76 ± 0.13 | 120.70 ± 5.97 | 105.41 ± 3.31 | 138.69 ± 3.40 | Fruity, mint |
| Subtotal | 1042.48 | 1580.59 | 801.39 | 1401.63 | |||||
| Terpenes and terpenoids | |||||||||
| 16 | D-Limonene | 5989-27-5 | 1024 | - | nd | 162.63 ± 4.61 | nd | nd | Citrus, orange, sweet |
| 17 | Citronellal | 106-23-0 | 1151 | 1149 | nd | 286.80 ± 5.68 | nd | nd | Rose, green |
| 18 | Safranal | 116-26-7 | 1193 | 1195 | 180.82 ± 9.04 | 239.73 ± 8.47 | 345.02 ± 9.79 | 387.43 ± 4.66 | Aldehydic, sweet |
| 19 | Verbenone | 80-57-9 | 1208 | 1206 | nd | nd | 147.04 ± 8.24 | 151.18 ± 2.03 | Menthol |
| 20 | β-Cyclocitral | 432-25-7 | 1214 | 1208 | 257.08 ± 13.73 | 172.40 ± 36.79 | 224.75 ± 10.46 | 163.00 ± 17.07 | Herbal. saffron |
| 21 | β-Citral | 106-26-3 | 1231 | 1240 | 24.44 ± 1.02 | 38.65 ± 0.73 | nd | 44.01 ± 1.92 | Citrus, sweet |
| 22 | β-Cyclohomocitral | 472-66-2 | 1246 | 1251 | 216.33 ± 8.00 | 150.20 ± 8.74 | 120.37 ± 3.70 | 223.25 ± 4.01 | Citrus, woody |
| 23 | Citral | 5392-40-5 | 1263 | 1239 | 60.65 ± 4.45 | 54.24 ± 1.94 | nd | nd | Citrus, sweet |
| 24 | α-Ionone | 127-41-3 | 1414 | 1412 | 122.24 ± 0.11 | 89.08 ± 8.17 | 134.20 ± 4.88 | 220.54 ± 1.67 | Floral, violet |
| 25 | Geranyl acetone | 3796-70-1 | 1442 | 1447 | 348.42 ± 17.92 | 248.23 ± 14.76 | 315.57 ± 13.31 | 353.25 ± 5.53 | Floral, fruity |
| 26 | β-Ionone | 14901-07-6 | 1468 | 1480 | 531.16 ± 33.48 | 638.59 ± 16.17 | 533.29 ± 27.48 | 748.56 ± 46.78 | Floral, fruity |
| 27 | β-Ionone epoxide | 23267-57-4 | 1471 | 1455 | 65.80 ± 3.19 | nd | 247.53 ± 6.81 | 138.32 ± 3.66 | Fruity, sweet |
| Subtotal | 1806.94 | 2080.56 | 2067.77 | 2429.53 | |||||
| Esters | |||||||||
| 28 | Methyl octanoate | 111-11-5 | 1123 | 1123 | nd | 166.40 ± 6.08 | nd | nd | Green, fruity |
| 29 | Ethyl octanoate | 106-32-1 | 1196 | 1196 | 51.53 ± 4.43 | 262.72 ± 7.68 | 129.14 ± 1.89 | 312.93 ± 5.35 | Sweet, wine |
| 30 | Ethyl nonanoate | 123-29-5 | 1295 | 1294 | nd | 32.01 ± 0.02 | nd | nd | Fruity, wine |
| 31 | Ethyl decanoate | 110-38-3 | 1394 | 1394 | nd | 116.98 ± 6.73 | nd | 164.11 ± 6.70 | Fruity, sweet |
| 32 | Methyl hexadecanoate | 112-39-0 | 1924 | 1926 | nd | 63.59 ± 6.29 | nd | nd | Oily, greasy |
| 33 | Ethyl 9-hexadecenoate | 54546-22-4 | 1970 | 1975 | nd | 92.32 ± 2.73 | nd | 92.46 ± 3.83 | |
| 34 | Ethyl hexadecanoate | 628-97-7 | 1992 | 1994 | 18.26 ± 1.12 | 67.95 ± 9.98 | 52.21 ± 1.80 | 85.93 ± 1.35 | Fermented, fruity |
| Subtotal | 69.79 | 801.97 | 181.35 | 655.42 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, C.M.S.; de Jesus, M.S.; da Silva, A.d.S.; Gualberto, N.C.; Araujo, H.C.S.; Sandes, R.D.D.; dos Santos, R.A.R.; Leite Neta, M.T.S.; Narain, N. Effect of Osmotic Dehydration on Physico-Chemical Characteristics, Bioactive Compounds and Volatiles Profile of Diospyros kaki Subjected to Different Drying Methods. Foods 2025, 14, 1727. https://doi.org/10.3390/foods14101727
Matos CMS, de Jesus MS, da Silva AdS, Gualberto NC, Araujo HCS, Sandes RDD, dos Santos RAR, Leite Neta MTS, Narain N. Effect of Osmotic Dehydration on Physico-Chemical Characteristics, Bioactive Compounds and Volatiles Profile of Diospyros kaki Subjected to Different Drying Methods. Foods. 2025; 14(10):1727. https://doi.org/10.3390/foods14101727
Chicago/Turabian StyleMatos, Cecília Morais Santana, Mônica Silva de Jesus, Augusto de Souza da Silva, Nayjara Carvalho Gualberto, Hannah Caroline Santos Araujo, Rafael Donizete Dutra Sandes, Raquel Anne Ribeiro dos Santos, Maria Terezinha Santos Leite Neta, and Narendra Narain. 2025. "Effect of Osmotic Dehydration on Physico-Chemical Characteristics, Bioactive Compounds and Volatiles Profile of Diospyros kaki Subjected to Different Drying Methods" Foods 14, no. 10: 1727. https://doi.org/10.3390/foods14101727
APA StyleMatos, C. M. S., de Jesus, M. S., da Silva, A. d. S., Gualberto, N. C., Araujo, H. C. S., Sandes, R. D. D., dos Santos, R. A. R., Leite Neta, M. T. S., & Narain, N. (2025). Effect of Osmotic Dehydration on Physico-Chemical Characteristics, Bioactive Compounds and Volatiles Profile of Diospyros kaki Subjected to Different Drying Methods. Foods, 14(10), 1727. https://doi.org/10.3390/foods14101727





