Comparative Analysis of Three Different Types of Fermented Tea by Submerged Fermentation with Eurotium cristatum
Abstract
1. Introduction
2. Materials and Methods
2.1. E. cristatum
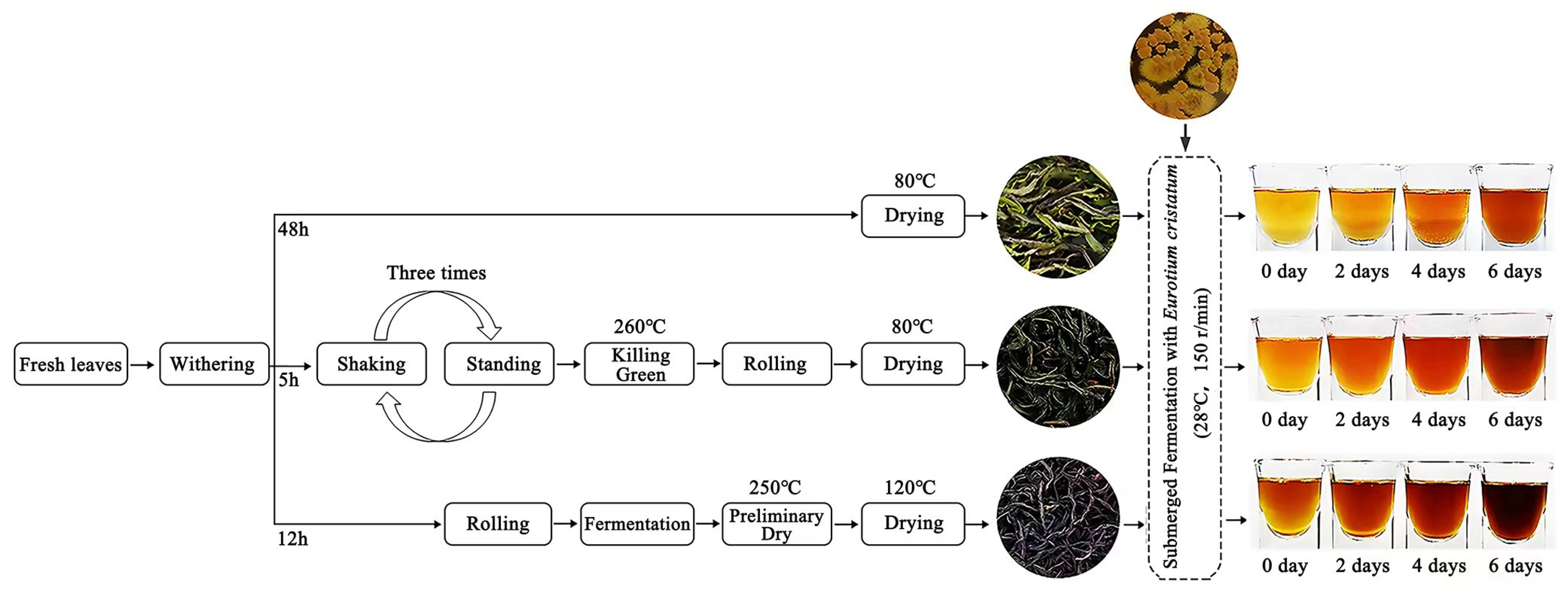
2.2. Preparation of Samples
2.3. Determination of Major Chemical Components Alteration
2.4. UHPLC-MS/MS Analysis
2.5. Antioxidant Capacity Analysis
2.5.1. DPPH Radical Scavenging Assay
2.5.2. ABTS Radical Scavenging Assay
2.5.3. Hydroxyl Radical Scavenging Assay
2.6. Network Pharmacology Analysis and Antioxidant Targets Screening
2.7. Molecular Docking
2.8. Sensory Evaluation
2.9. Data Analysis
3. Results and Discussion
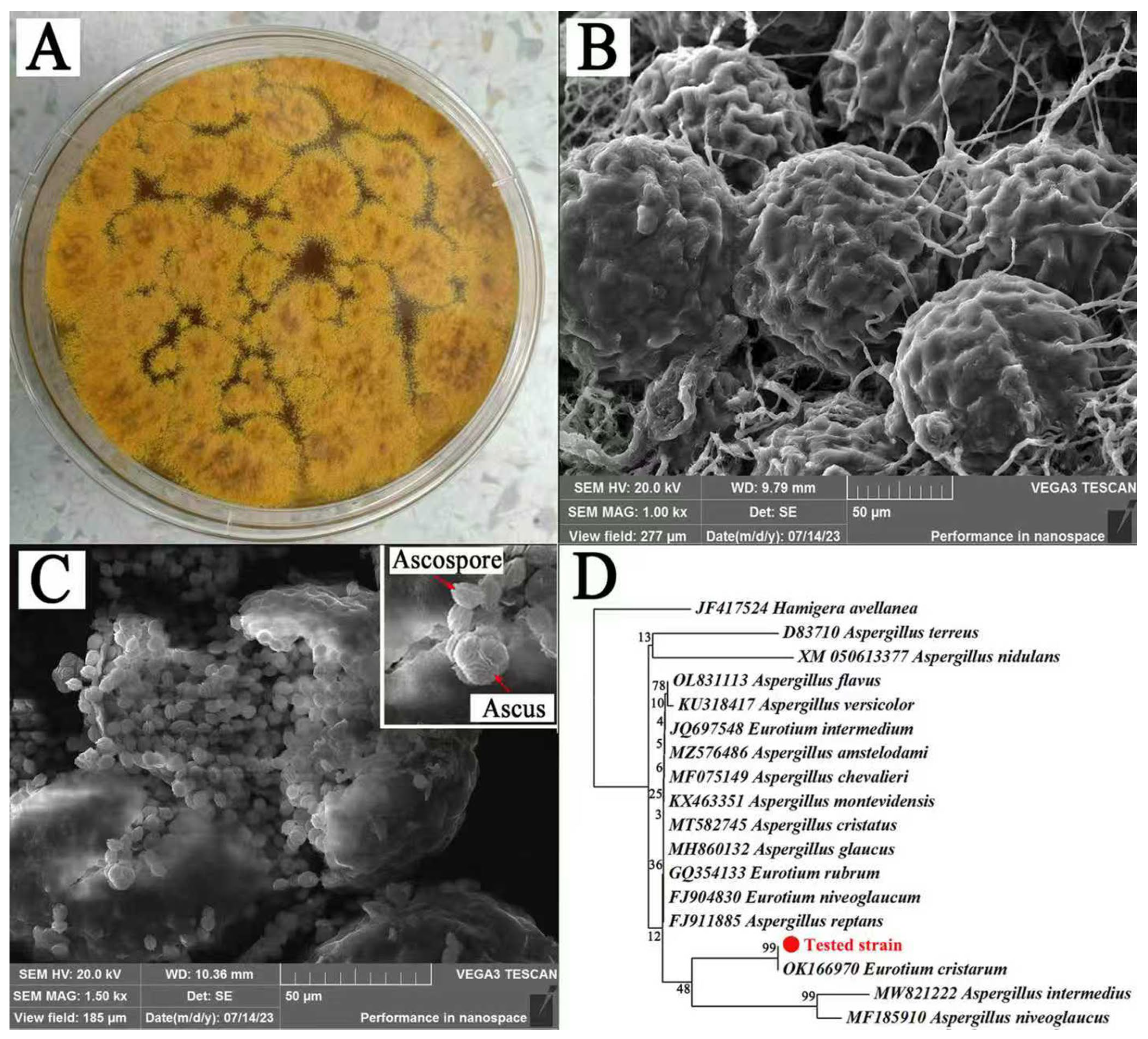
3.1. Identification and Purification of E. cristatum
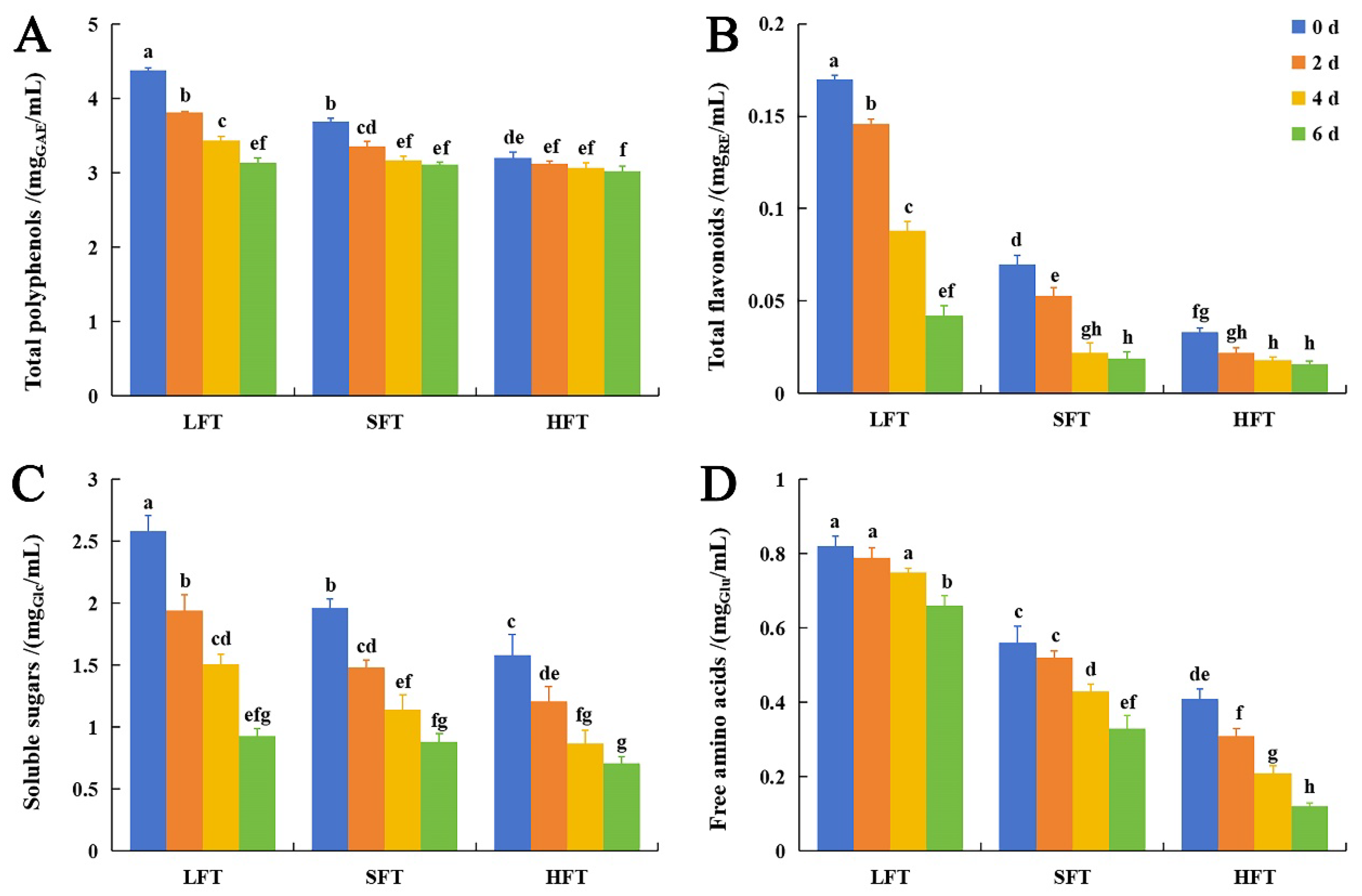
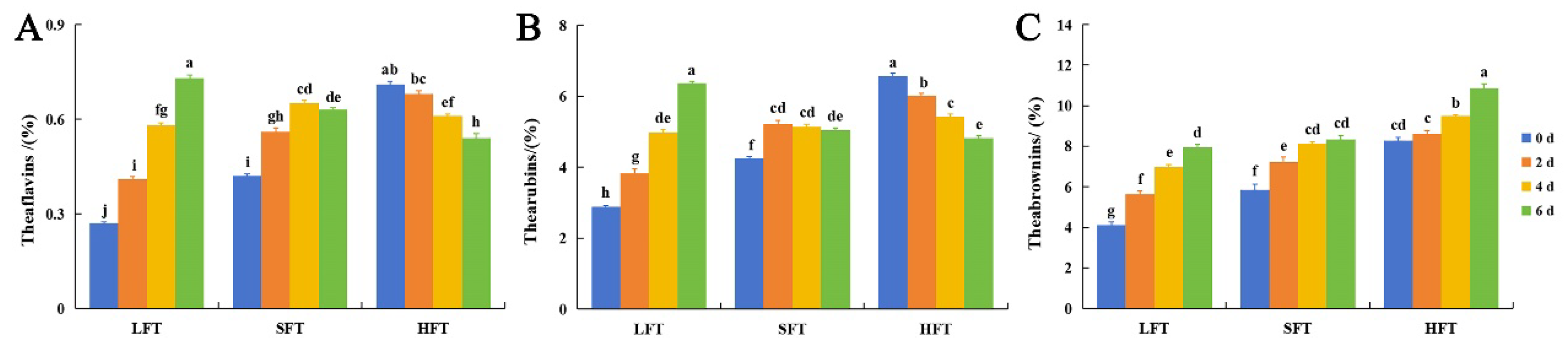
3.2. Major Chemical Components Comparison
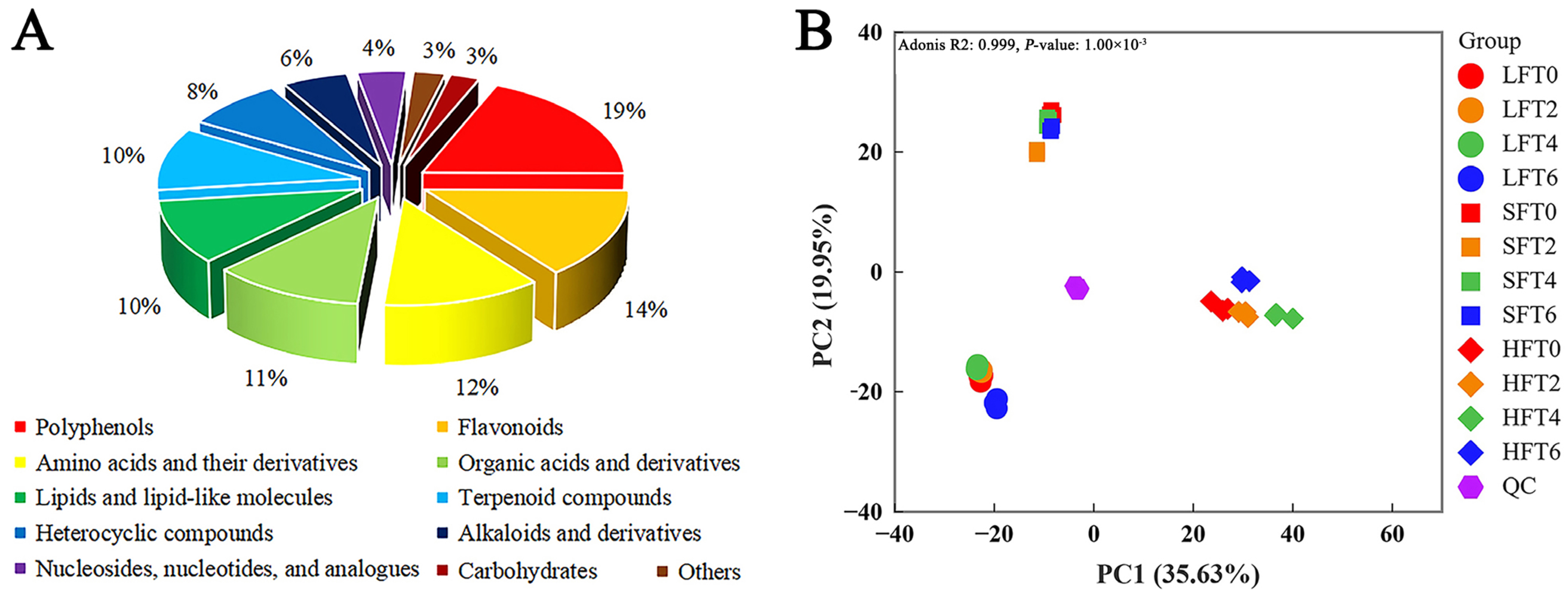
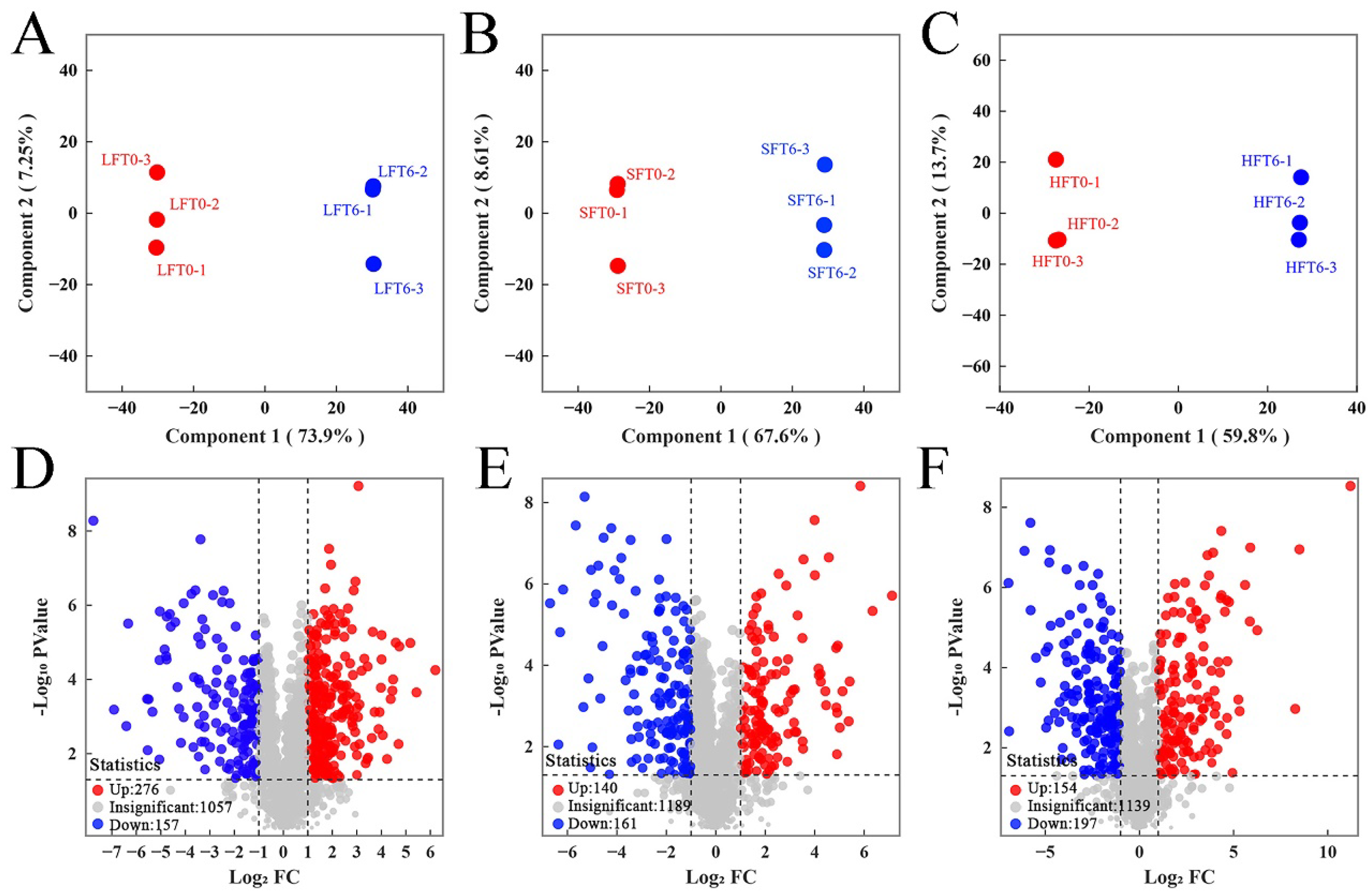
3.3. LC-MS/MS-Based Untargeted Metabolomic Analysis
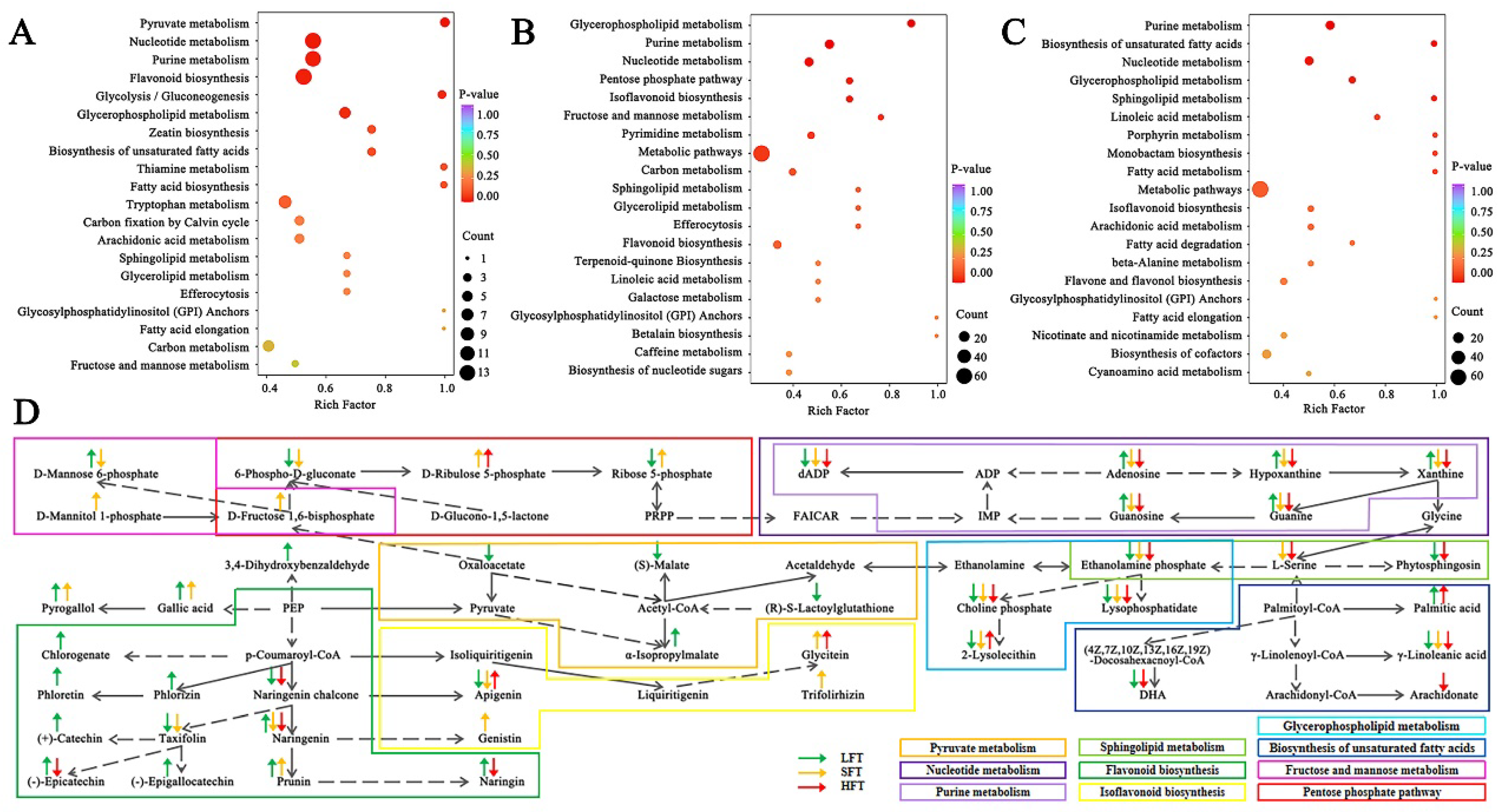
3.4. Complex Metabolites Alteration Comparison
3.5. Antioxidant Capacity and Metabolite Correlation Analysis
3.6. Prediction of Target Metabolites with Potential Health Benefit
3.6.1. Network Pharmacology-Based Antioxidant Targets Screening
3.6.2. Molecular Docking of the Key Antioxidant Metabolites and Core Antioxidant Targets
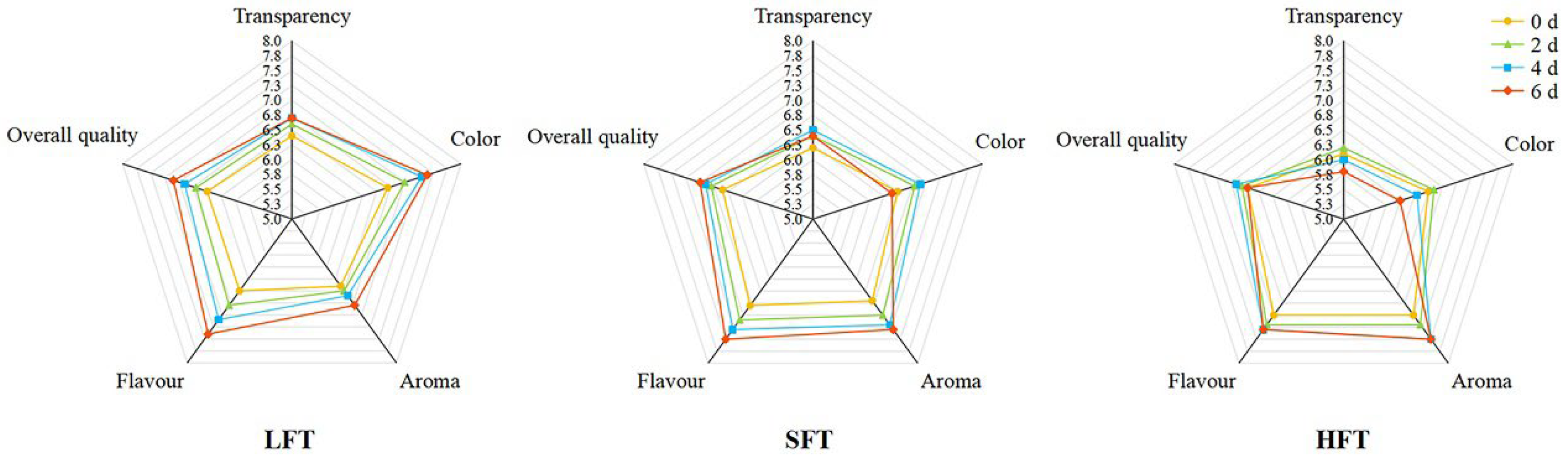
3.7. Comprehensive Evaluation of Sensory Attributes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hu, K.; Deng, W.; Zhu, Y.; Yao, K.; Li, J.; Liu, A.; Ao, X.; Zou, L.; Zhou, K.; He, L.; et al. Simultaneous degradation of β-cypermethrin and 3-phenoxybenzoic acid by Eurotium cristatum ET1, a novel “golden flower fungus” strain isolated from Fu Brick Tea. MicrobiologyOpen 2019, 8, e00776. [Google Scholar] [CrossRef]
- Li, J.; Xu, R.; Zong, L.; Brake, J.; Cheng, L.; Wu, J.; Wu, X. Dynamic Evolution and Correlation between Metabolites and Microorganisms during Manufacturing Process and Storage of Fu Brick Tea. Metabolites 2021, 11, 703. [Google Scholar] [CrossRef]
- An, T.; Shen, S.; Zu, Z.; Chen, M.; Wen, Y.; Chen, X.; Chen, Q.; Wang, Y.; Wang, S. Changes in the volatile compounds and characteristic aroma during submerged fermentation of instant dark tea by Eurotium cristatum. Food Chem. 2023, 410, 135462. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Yang, C.; Wu, Y.; Liu, Y.; Yang, X. Eurotium cristatum from Fu Brick Tea Promotes Adipose Thermogenesis by Boosting Colonic Akkermansia muciniphila in High-Fat-Fed Obese Mice. Foods 2023, 12, 3716. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, S.; Guo, H.; Hu, J.; Cao, Y.; Xu, Y.; Lin, X.; Tian, B.; Fan, F.; Gong, S.; et al. Golden-flower fungus (Eurotiwm Cristatum) presents fungal flower aroma as well as accelerates the aging of white tea (Shoumei). Food Chem. 2024, 451, 139452. [Google Scholar] [CrossRef]
- Chan, L.Y.; Takahashi, M.; Lim, P.J.; Aoyama, S.; Makino, S.; Ferdinandus, F.; Ng, S.Y.C.; Arai, S.; Fujita, H.; Tan, H.C.; et al. Eurotium cristatum Fermented Okara as a Potential Food Ingredient to Combat Diabetes. Sci. Rep. 2019, 9, 17536. [Google Scholar] [CrossRef]
- Zou, M.; Zhang, W.; Dong, Q.; Tang, C.; Cao, F.; Su, E. Submerged fermentation of GINKGO BILOBA seed powder using Eurotium cristatum for the development of ginkgo seeds fermented products. J. Sci. Food Agric. 2021, 101, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, X.; Yao, X.; Chen, Y.; Ho, C.T.; He, C.; Li, Z.; Wang, Y. Metabolite profiling, antioxidant and α-glucosidase inhibitory activities of buckwheat processed by solid-state fermentation with Eurotium cristatum YL-1. Food Res. Int. 2021, 143, 110262. [Google Scholar]
- Yang, X.; Liu, Z.; Zhang, Y.; Zhao, S.; Yan, S.; Zhu, L.; Zhou, Q.; Chen, L. Effects of Fermentation with Eurotium cristatum on Sensory Properties and Flavor Compounds of Mulberry Leaf Tea. Foods 2024, 13, 2347. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; He, C.; Chen, Y.; Ho, C.T.; Wu, X.; Huang, Y.; Gao, Y.; Hou, A.; Li, Z.; Wang, Y.; et al. UPLC–QQQ–MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chem. 2022, 378, 131999. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, M.; Liu, Y.; Xu, S.; Zhong, K.; Wu, Y.; Gao, H. The effect of Eurotium cristatum (MF800948) fermentation on the quality of autumn green tea. Food Chem. 2021, 358, 129848. [Google Scholar] [CrossRef]
- Tan, Z.; Yu, P.; Zhu, H.; Gao, J.; Han, N.; Yang, C.; Shen, Z.; Gao, C.; Yang, X. Differential characteristics of chemical composition, fermentation metabolites and antioxidant effects of polysaccharides from Eurotium cristatum and Fu-brick tea. Food Chem. 2024, 461, 140934. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shi, K.; Xu, Y.; Zhang, P.; Zhang, H.; Pan, S. Integrated metabolomics and network pharmacology to reveal antioxidant mechanisms and potential pharmacological ingredients of citrus herbs. Food Res. Int. 2023, 174, 113514. [Google Scholar] [CrossRef]
- Wu, F.; Lin, B.; Chen, J.; Zheng, F.; Yang, Y.; Rasheed, U.; Chen, G. Mechanistic Insights into the Antioxidant Potential of Sugarcane Vinegar Polyphenols: A Combined Approach of DPPH-UPLC-MS, Network Pharmacology and Molecular Docking. Foods 2024, 13, 3379. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, Y.; Fan, X.; Zeng, X. Effects of solid-state fermentation with Aspergillus cristatus (MK346334) on the dynamics changes in the chemical and flavor profile of dark tea by HS-SPME-GC–MS, HS-GC-IMS and electronic nose. Food Chem. 2024, 455, 139864. [Google Scholar] [CrossRef]
- Shen, S.; Fu, J.; Fan, R.; Zhang, J.; Sun, H.; Wang, Y.; Ning, J.; Yue, P.; Zhang, L.; Gao, X. Changes in the key odorants of loose-leaf dark tea fermented by Eurotium cristatum during aging for one year: Focus on the stale aroma. Food Res. Int. 2024, 197, 115244. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Ma, F.; Chen, H.; Fei, Q.; Tao, G.; Wu, S.; Shi, D.; Deng, J.; Zhao, D.; Dong, X.; et al. Dynamic changes in flavor characteristics of black tea during solid-state fermentation with Eurotium cristatum. Food Chem. 2025, 465, 142028. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yuan, X.; Luo, Z.; Cao, Y.; Liu, S.; Liu, Y. Metabolomic and transcriptomic analyses reveal comparisons against submerged fermentation of primary dark tea, green tea and white tea by Aspergillus cristatus. Food Res. Int. 2023, 172, 113115. [Google Scholar] [CrossRef]
- Liu, X.; Yan, Y.; Zhao, P.; Song, J.; Yu, X.; Wang, Z.; Xia, J.; Wang, X. Oil crop wastes as substrate candidates for enhancing erythritol production by modified Yarrowia lipolytica via one-step solid state fermentation. Bioresour. Technol. 2019, 294, 122194. [Google Scholar] [CrossRef]
- He, Y.; Tan, M.; Cao, Q.; Linghu, X.; Yang, Z.; Meng, Q.; Fu, S. The Liquid Fermentation Process for Mycelia of Poria cocos (Agaricomycetes) by Single-Factor Experimentation and Response Surface Methodology. Int. J. Med. Mushrooms 2024, 26, 41–51. [Google Scholar] [CrossRef]
- Liu, M.; Tian, X.; He, L.; Li, C.; Tao, H.; Wang, X.; Qiao, S.; Zeng, X. Effects of tandem fermentation of edible mushroom and L. plantarum on sensory, polysaccharide, vitamin C, and γ-aminobutyric acid of Rosa roxburghii Tratt and coix seed beverage. Food Chem. X 2023, 20, 101041. [Google Scholar] [CrossRef]
- Wang, X.; Hu, K.; Chen, Y.; Lai, J.; Zhang, M.; Li, J.; Li, Q.; Zhao, N.; Liu, S. Effect of Lactiplantibacillus plantarum fermentation on the physicochemical, antioxidant activity and immunomodulatory ability of polysaccharides from Lvjian okra. Int. J. Biol. Macromol. 2024, 257, 128649. [Google Scholar] [CrossRef]
- Ho, C.T.; Lin, J.K.; Shahidi, F. (Eds.) Tea and Tea Products: Chemistry and Health-Promoting Properties; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Mander, L.; Liu, H.W. Comprehensive Natural Products II: Chemistry and Biology | NHBS Academic & Professional Books; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Zhu, M.Z.; Li, N.A.; Zhou, F.; Ouyang, J.; Lu, D.M.; Xu, W.; Li, J.; Lin, H.Y.; Zhang, Z.; Xiao, J.B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Z.; Asir, Y. Assessment of instrumental and sensory quality characteristics of the bread products enriched with Kombucha tea. Int. J. Gastron. Food Sci. 2022, 29, 100562. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, J.; Cui, D.; Zhao, D.; Li, Y.; Wan, X.; Zhao, J. Transcriptome and Metabolic Profiling Unveiled Roles of Peroxidases in Theaflavin Production in Black Tea Processing and Determination of Tea Processing Suitability. J. Agric. Food Chem. 2020, 68, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, S.; Alam, S.; Saleem, M.; Ahmad, W.; Bibi, R.; Hamid, F.S.; Shah, H.U. Withering timings affect the total free amino acids and mineral contents of tea leaves during black tea manufacturing. Arab. J. Chem. 2019, 12, 2411–2417. [Google Scholar] [CrossRef]
- Jiang, Y.; Hua, J.; Wang, B.; Yuan, H.; Ma, H. Effects of Variety, Season, and Region on Theaflavins Content of Fermented Chinese Congou Black Tea. J. Food Qual. 2018, 2018, 5427302. [Google Scholar] [CrossRef]
- Want, E.J.; O’Maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, S.A.; Siuzdak, G. Solvent-Dependent Metabolite Distribution, Clustering, and Protein Extraction for Serum Profiling with Mass Spectrometry. Anal. Chem. 2006, 78, 743–752. [Google Scholar] [CrossRef]
- Yuan, H.; Xie, Q.; Liang, L.; Luo, J.; Jiang, S.; Peng, C.; Wang, W. An Efficient Workflow for Quality Control Marker Screening and Metabolite Discovery in Dietary Herbs by LC-Orbitrap-MS/MS and Chemometric Methods: A Case Study of Chrysanthemum Flowers. Foods 2024, 13, 1008. [Google Scholar] [CrossRef]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef]
- Yin, X.L.; Peng, Z.X.; Pan, Y.; Lv, Y.; Long, W.; Gu, H.W.; Fu, H.; She, Y. UHPLC-QTOF-MS-based untargeted metabolomic authentication of Chinese red wines according to their grape varieties. Food Res. Int. 2024, 178, 113923. [Google Scholar] [CrossRef] [PubMed]
- Krauss, M.; Singer, H.; Hollender, J. LC-high resolution MS in environmental analysis: From target screening to the identification of unknowns. Anal. Bioanal. Chem. 2010, 397, 943–951. [Google Scholar] [CrossRef]
- Oppermann, M.; Damoc, N.E.; Crone, C.; Moehring, T.; Muenster, H.; Hornshaw, M. High precision measurement and fragmentation analysis for metabolite identification. In Plant Metabolomics: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2012; Volume 860, pp. 145–156. [Google Scholar]
- Peng, C.Y.; Ren, Y.F.; Ye, Z.H.; Zhu, H.Y.; Liu, X.Q.; Chen, X.T.; Hou, R.Y.; Granato, D.; Cai, H.M. A comparative UHPLC-Q/TOF-MS-based metabolomics approach coupled with machine learning algorithms to differentiate Keemun black teas from narrow-geographic origins. Food Res. Int. 2022, 158, 111512. [Google Scholar] [CrossRef]
- Wu, C.T.; Wang, W.H.; Lin, W.S.; Hu, S.Y.; Chen, C.Y.; Chang, M.Y.; Lin, Y.S.; Li, C.P. Effects of Different Chenopodium formosanum Parts on Antioxidant Capacity and Optimal Extraction Analysis by Taguchi Method. Materials 2021, 14, 4679. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Poklar Ulrih, N.; Cigić, B. The Methodology Applied in DPPH, ABTS and Folin-Ciocalteau Assays Has a Large Influence on the Determined Antioxidant Potential. Acta Chim. Slov. 2017, 64, 491–499. [Google Scholar] [CrossRef]
- Tong, T.; Liu, Y.J.; Kang, J.; Zhang, C.M.; Kang, S.G. Antioxidant Activity and Main Chemical Components of a Novel Fermented Tea. Molecules 2019, 24, 2917. [Google Scholar] [CrossRef]
- Zhang, Y.; Sanner, M.F. AutoDock CrankPep: Combining folding and docking to predict protein–peptide complexes. Bioinformatics 2019, 35, 5121–5127. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, X.; Gao, Y.; Jin, Z.; Guo, S.; Li, Z.; Wang, M.; Zhao, R.; Zhou, W.; Wu, J. Study on the mechanism of Shujin Tongluo granules in treating cervical spondylosis based on network pharmacology and molecular docking. Medicine 2023, 102, e34030. [Google Scholar] [CrossRef] [PubMed]
- Wichchukit, S.; O’Mahony, M. The 9-point hedonic scale and hedonic ranking in food science: Some reappraisals and alternatives. J. Sci. Food Agric. 2015, 95, 2167–2178. [Google Scholar] [CrossRef]
- Peng, Z.X.; Gu, H.W.; Pan, Y.; Wang, Y.; Yan, J.; Long, W.; Fu, H.; She, Y. Revealing the key antioxidant compounds and potential action mechanisms of Chinese Cabernet Sauvignon red wines by integrating UHPLC-QTOF-MS-based untargeted metabolomics, network pharmacology and molecular docking approaches. Food Chem. 2024, 460, 140540. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, S.; Zhou, X.; Liu, Z.; Li, T.; Yu, S.; Zhang, X.; Xu, Z. Morphological variation and expressed sequence tags-simple sequence repeats-based genetic diversity of Aspergillus cristatus in Chinese dark tea. Front. Microbiol. 2024, 15, 1390030. [Google Scholar] [CrossRef]
- Xie, G.; Yan, J.; Lu, A.; Kun, J.; Wang, B.; Song, C.; Tong, H.; Meng, Q. Characterizing relationship between chemicals and in vitro bioactivities of teas made by six typical processing methods using a single Camellia sinensis cultivar, Meizhan. Bioengineered 2021, 12, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wu, P.; Zhan, J.; Wang, W.; Shen, J.; Ho, C.T.; Li, S. Influencing Factors on the Physicochemical Characteristics of Tea Polysaccharides. Molecules 2021, 26, 3457. [Google Scholar] [CrossRef]
- Tang, M.; Liao, X.; Xu, M.; Zhang, J.; Wu, X.; Wei, M.; Jin, S.; Zheng, Y.; Ye, N. Comprehensive investigation on the flavor difference in five types of tea from JMD (Camellia sinensis ‘Jinmudan’). J. Sci. Food Agric. 2025, 105, 990–1002. [Google Scholar]
- Zhao, X.; Wang, P.; Li, M.; Wang, Y.; Jiang, X.; Cui, L.; Qian, Y.; Zhuang, J.; Gao, L.; Xia, T. Functional Characterization of a New Tea (Camellia sinensis) Flavonoid Glycosyltransferase. J. Agric. Food Chem. 2017, 65, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Trovò, L.; Fuchs, C.; De Rosa, R.; Barbiero, I.; Tramarin, M.; Ciani, E.; Rusconi, L.; Kilstrup-Nielsen, C. The green tea polyphenol epigallocatechin-3-gallate (EGCG) restores CDKL5-dependent synaptic defects in vitro and in vivo. Neurobiol. Dis. 2020, 138, 104791. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, C.; Luo, X.; Yao, H.; Zhang, D.; Zhang, T. Revealing the influence of microbiota on the quality of Pu-erh tea during fermentation process by shotgun metagenomic and metabolomic analysis. Food Microbiol. 2018, 76, 405–415. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, M.; Chen, M.; Li, M.; Zhang, H.; Song, P.; An, T.; Yue, P.; Gao, X. Influence of Eurotium cristatum and Aspergillus niger individual and collaborative inoculation on volatile profile in submerged fermentation of instant dark teas. Food Chem. 2021, 350, 129234. [Google Scholar] [CrossRef]
- Feng, L.; Liu, P.; Zheng, P.; Zhang, L.; Zhou, J.; Gong, Z.; Yu, Y.; Gao, S.; Zheng, L.; Wang, X.; et al. Chemical profile changes during pile fermentation of Qingzhuan tea affect inhibition of α-amylase and lipase. Sci. Rep. 2020, 10, 3489. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Yuan, H.; Ouyang, W.; Li, J.; Hua, J.; Jiang, Y. Effects of Fermentation Temperature and Time on the Color Attributes and Tea Pigments of Yunnan Congou Black Tea. Foods 2022, 11, 1845. [Google Scholar] [CrossRef]
- Chen, H.; Shurlknight, K.; Leung, T.; Sang, S. Structural Identification of Theaflavin Trigallate and Tetragallate from Black Tea Using Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2012, 43, 10850–10857. [Google Scholar]
- Chang, M.Y.; Lin, Y.Y.; Chang, Y.C.; Huang, W.Y.; Lin, W.S.; Chen, C.Y.; Huang, S.L.; Lin, Y.S. Effects of Infusion and Storage on Antioxidant Activity and Total Phenolic Content of Black Tea. Appl. Sci. 2020, 10, 2685. [Google Scholar]
- Jiménez-Zamora, A.; Delgado-Andrade, C.; Rufián-Henares, J.A. Antioxidant capacity, total phenols and color profile during the storage of selected plants used for infusion. Food Chem. 2016, 199, 339–346. [Google Scholar] [CrossRef]
- Li, Q.; Chai, S.; Li, Y.; Huang, J.; Luo, Y.; Xiao, L.; Liu, Z. Biochemical Components Associated With Microbial Community Shift During the Pile-Fermentation of Primary Dark Tea. Front. Microbiol. 2018, 9, 1509. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.; Duan, Y.; Zhao, Y.; Zhang, D.; Zang, L.; Ya, H. Widely Targeted Metabolomics Analysis of Different Parts of Salsola collina Pall. Molecules 2021, 26, 1126. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhou, X.; Xie, S.; Huang, J.A.; Li, J.; Yao, Y.; Li, M.; Bo, J.; Xiao, L.; Liu, Z.; et al. Unlocking the healing power of Jinhua white tea fermented by Eurotium cristatum using the unique “flowering” technology: A promising ally against inflammatory bowel disease. Food Res. Int. 2025, 213, 116555. [Google Scholar] [CrossRef]
- Zhao, F.; Qiu, X.; Ye, N.; Qian, J.; Wang, D.; Zhou, P.; Chen, M. Hydrophilic interaction liquid chromatography coupled with quadrupole-orbitrap ultra high resolution mass spectrometry to quantitate nucleobases, nucleosides, and nucleotides during white tea withering process. Food Chem. 2018, 266, 343–349. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Niu, L.; Yuan, H.; Shan, X.; Zhang, Q.; Feng, Y.; Zhou, Q.; Jiang, Y.; Li, J. New insights into the role of lipids in aroma formation during black tea processing revealed by integrated lipidomics and volatolomics. Curr. Res. Food Sci. 2024, 9, 100910. [Google Scholar] [CrossRef]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U. Fungal Morphogenesis, from the Polarized Growth of Hyphae to Complex Reproduction and Infection Structures. Microbiol. Mol. Biol. Rev. 2018, 82, e00068-17. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, T.; Iwama, R.; Fukuda, R.; Horiuchi, H. Phosphatidylcholine levels regulate hyphal elongation and differentiation in the filamentous fungus Aspergillus oryzae. Sci. Rep. 2024, 14, 11729. [Google Scholar] [CrossRef]
- Iwama, R. Phospholipid dynamics in Aspergillus species: Relations between biological membrane composition and cellular morphology. Biosci. Biotechnol. Biochem. 2025, 89, 515–522. [Google Scholar] [CrossRef]
- Arntzen, M.Ø.; Bengtsson, O.; Várnai, A.; Delogu, F.; Mathiesen, G.; Eijsink, V.G.H. Quantitative comparison of the biomass-degrading enzyme repertoires of five filamentous fungi. Sci. Rep. 2020, 10, 20267. [Google Scholar] [CrossRef]
- Li, T.; Wei, Y.; Lu, M.; Wu, Y.; Jiang, Y.; Ke, H.; Shao, A.; Ning, J. Exploring microbial and moist-heat effects on Pu-erh tea volatiles and understanding the methoxybenzene formation mechanism using molecular sensory science. Food Chem. X 2024, 23, 101553. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in Food and Their Health Benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, B.; Long, F.; Wei, J.; Zhang, Y.; Cui, Y.; Yuan, Y.; Yue, T. Comparison of chemical constituents of Eurotium cristatum-mediated pure and mixed fermentation in summer-autumn tea. LWT 2021, 143, 111132. [Google Scholar] [CrossRef]
- Lai, X.; Wang, X.; Hu, Y.; Su, S.; Li, W.; Li, S. Editorial: Network Pharmacology and Traditional Medicine. Front. Pharmacol. 2020, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Li, Y.; Wang, Y.; Yan, S.; Xu, S.; Deng, Z.; Yang, X.; Xie, H.; Li, J. Bioinformatics and Network Pharmacology Identify the Therapeutic Role and Potential Mechanism of Melatonin in AD and Rosacea. Front. Immunol. 2021, 12, 756550. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Zhang, T.; Zhang, Y.; Xie, Z.; Lu, Y.; Yao, Y.; Yang, Y.; Wu, H.; Liu, B. Reveals of candidate active ingredients in Justicia and its anti-thrombotic action of mechanism based on network pharmacology approach and experimental validation. Sci. Rep. 2021, 11, 17187. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar]
- Huang, M.; Yan, Y.; Deng, Z.; Zhou, L.; She, M.; Yang, Y.; Zhang, M.; Wang, D. Saikosaponin A and D attenuate skeletal muscle atrophy in chronic kidney disease by reducing oxidative stress through activation of PI3K/AKT/Nrf2 pathway. Phytomedicine 2023, 114, 154766. [Google Scholar] [CrossRef]
- Fan, Z.; Qing, Q.; Yin, J.; Fan, Z.; Qing, Q.; Yin, J.; He, C.; Hu, Y.; Chen, Y.; Ren, Y.; et al. Metabolites of epigallocatechin gallate and changes in antioxidant activity through biotransformation with Eurotium cristatum during submerged fermentation. Food Chem. X 2025, 29, 102618. [Google Scholar]
- Wang, Y.; Kan, Z.; Thompson, H.J.; Ling, T.; Ho, C.T.; Li, D.; Wan, X. Impact of Six Typical Processing Methods on the Chemical Composition of Tea Leaves Using a Single Camellia sinensis Cultivar, Longjing 43. J. Agric. Food Chem. 2019, 67, 5423–5436. [Google Scholar] [CrossRef]
- Guo, X.; Chen, F.; Liu, J.; Shao, Y.; Wang, X.; Zhou, Y. Genome Mining and Analysis of PKS Genes in Eurotium cristatum E1 Isolated from Fuzhuan Brick Tea. J. Fungi. 2022, 8, 193. [Google Scholar] [CrossRef]
- Wang, H.; Xu, W. Mito-methyl coumarin, a novel mitochondria-targeted drug with great antitumor potential was synthesized. Biochem. Biophys. Res. Commun. 2017, 489, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Li, H.Z.; Li, A.L.; Li, Y.R.; Wang, X.N.; Ren, D.M. Homoeriodictyol protects human endothelial cells against oxidative insults through activation of Nrf2 and inhibition of mitochondrial dysfunction. Vasc. Pharmacol. 2018, 109, 72–82. [Google Scholar] [CrossRef]
- Jiang, C.; Zeng, Z.; Huang, Y.; Zhang, X. Chemical compositions of Pu’er tea fermented by Eurotium cristatum and their lipid-lowering activity. LWT 2018, 98, 204–211. [Google Scholar] [CrossRef]
- Wang, J.; Fei, Z.; Yang, C.; Zhang, R.; Xu, J.; Yuan, Y.; Tang, C.; Hu, Q.; Liu, Z.; Zhu, M.; et al. Influence of AcCdc42-overexpressing strain individual inoculation on volatile profile in fermentation of liquid-state malt yeast agar and dark tea soup. Food Chem. Mol. Sci. 2025, 11, 100262. [Google Scholar] [CrossRef]
- Deng, J.; Li, Y.; Yuan, Y.; Yin, F.; Chao, J.; Huang, J.; Liu, Z.; Wang, K.; Zhu, M. Secondary Metabolites from the Genus Eurotium and Their Biological Activities. Foods 2023, 12, 4452. [Google Scholar] [CrossRef]
- Ngure, F.M.; Wanyoko, J.K.; Mahungu, S.M.; Shitandi, A.A. Catechins depletion patterns in relation to theaflavin and thearubigins formation. Food Chem. 2009, 115, 8–14. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, Z.; He, J.; Zhao, S.; Ruan, S.; Liu, X.; Yu, S.; Sun, Y. Analysis of aroma precursors in Jinmudan fresh tea leaves and dynamic change of fatty acid volatile during black tea processing. Food Chem. X 2024, 21, 101155. [Google Scholar]
- Wang, T.; Li, R.Y.; Liu, K.Y.; Chen, Q.Y.; Bo, N.G.; Wang, Q.; Xiao, Y.Q.; Sha, G.; Chen, S.Q.; Lei, X. Changes in sensory characteristics, chemical composition and microbial succession during fermentation of ancient plants Pu-erh tea. Food Chem. X 2023, 20, 101003. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, Y.; Tong, H. Amino acids and flavonoids analysis reveals quality constituents difference among different albino tea resources. Food Chem. 2024, 449, 139200. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, J.; Xie, M.; Li, X.; Zhao, J.; Li, Z.; Yang, T.; Wang, J.; Li, J.; Li, L.; Le, J.; et al. Comparative Analysis of Three Different Types of Fermented Tea by Submerged Fermentation with Eurotium cristatum. Foods 2025, 14, 3241. https://doi.org/10.3390/foods14183241
Lan J, Xie M, Li X, Zhao J, Li Z, Yang T, Wang J, Li J, Li L, Le J, et al. Comparative Analysis of Three Different Types of Fermented Tea by Submerged Fermentation with Eurotium cristatum. Foods. 2025; 14(18):3241. https://doi.org/10.3390/foods14183241
Chicago/Turabian StyleLan, Jiahong, Mingwei Xie, Xudong Li, Jialin Zhao, Zhenyu Li, Tong Yang, Jinping Wang, Junda Li, Linkai Li, Jie Le, and et al. 2025. "Comparative Analysis of Three Different Types of Fermented Tea by Submerged Fermentation with Eurotium cristatum" Foods 14, no. 18: 3241. https://doi.org/10.3390/foods14183241
APA StyleLan, J., Xie, M., Li, X., Zhao, J., Li, Z., Yang, T., Wang, J., Li, J., Li, L., Le, J., Fan, L., & Li, L. (2025). Comparative Analysis of Three Different Types of Fermented Tea by Submerged Fermentation with Eurotium cristatum. Foods, 14(18), 3241. https://doi.org/10.3390/foods14183241





