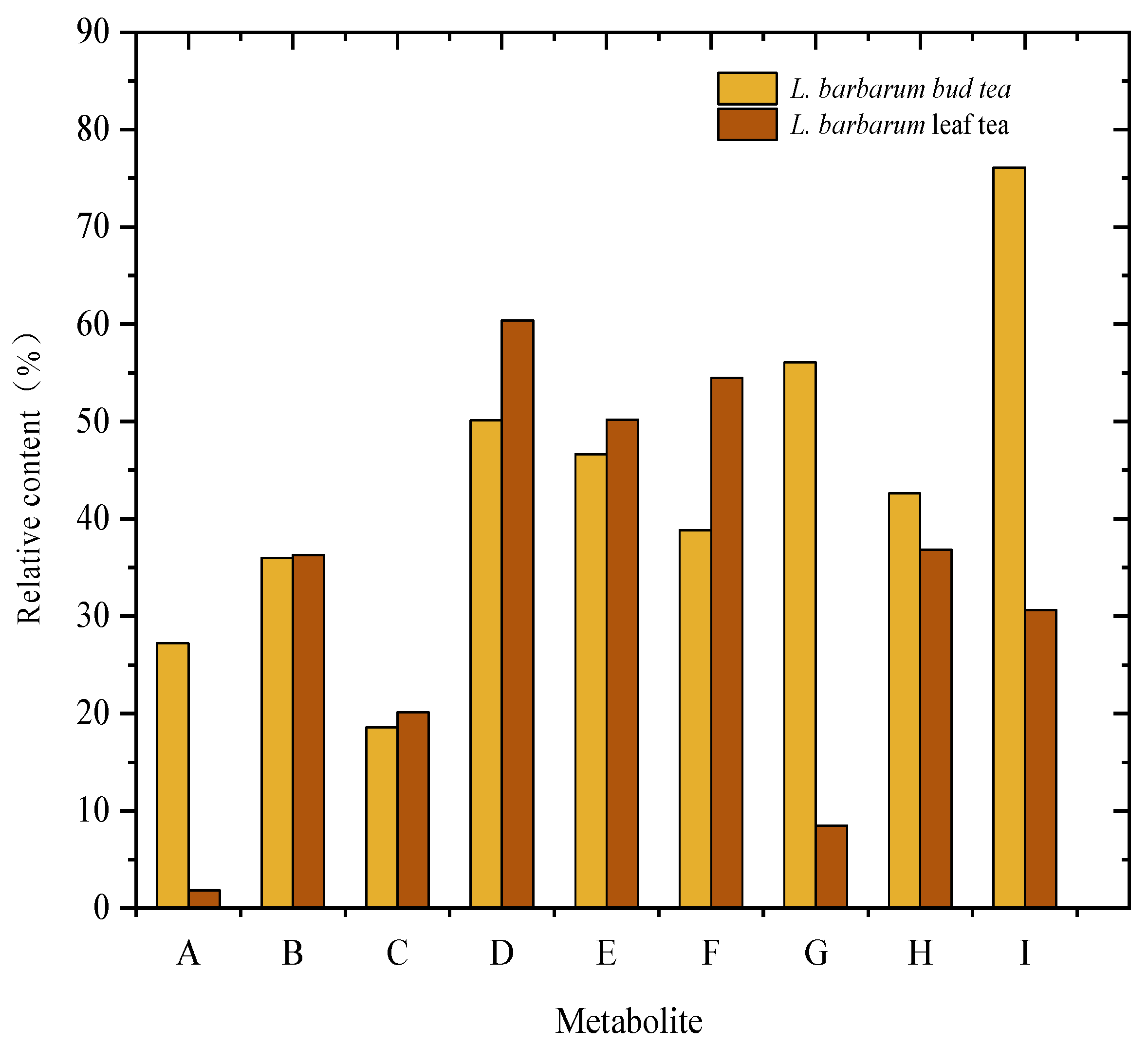
3.1. Contents of Total Polyphenols, Total Flavonoids, and Chlorogenic Acid in L. barbarum Bud Tea and Leaf Tea
The total polyphenol, total flavonoid, and chlorogenic acid contents in bud tea were 36.09 ± 1.97 mg/g, 7.44 ± 0.31 mg/g, and 4.18 ± 0.10 mg/g, respectively, 1.66-, 1.35-, and 1.23-fold higher than those in leaf tea (
Figure 1). Both bud tea and leaf tea exhibited significantly higher total polyphenol content relative to their total flavonoids and chlorogenic acid levels. Furthermore, the total polyphenol, flavonoid, and chlorogenic acid contents in bud tea were significantly greater than those in leaf tea processed under the same conditions, indicating that bud tea contains a richer profile of phenolic compounds compared to leaf tea. Paiva et al. [
27] reported that the buds of Azorean
Camellia sinensis, along with the first and second leaves, exhibited superior antioxidant activity and higher total phenolic content (TPC). This enhancement might be attributed to the higher concentration of polyphenols (particularly catechins) in these specific tea plant tissues. Consistent findings were reported by Rusaczonek et al. [
28] who observed greater antioxidant capacity and TPC in the bud–leaf complexes of several herbal teas compared to their stem and petiole components. Furthermore, the results revealed that the combination B + 1st + 2nd L + I (buds, first leaves, second leaves with internodes) showed marginally better values across all evaluated parameters than the B + 1st + 2nd L combination (bud–leaf complex without internodes), suggesting a potential contributory role of internodal tissues in green tea quality enhancement.
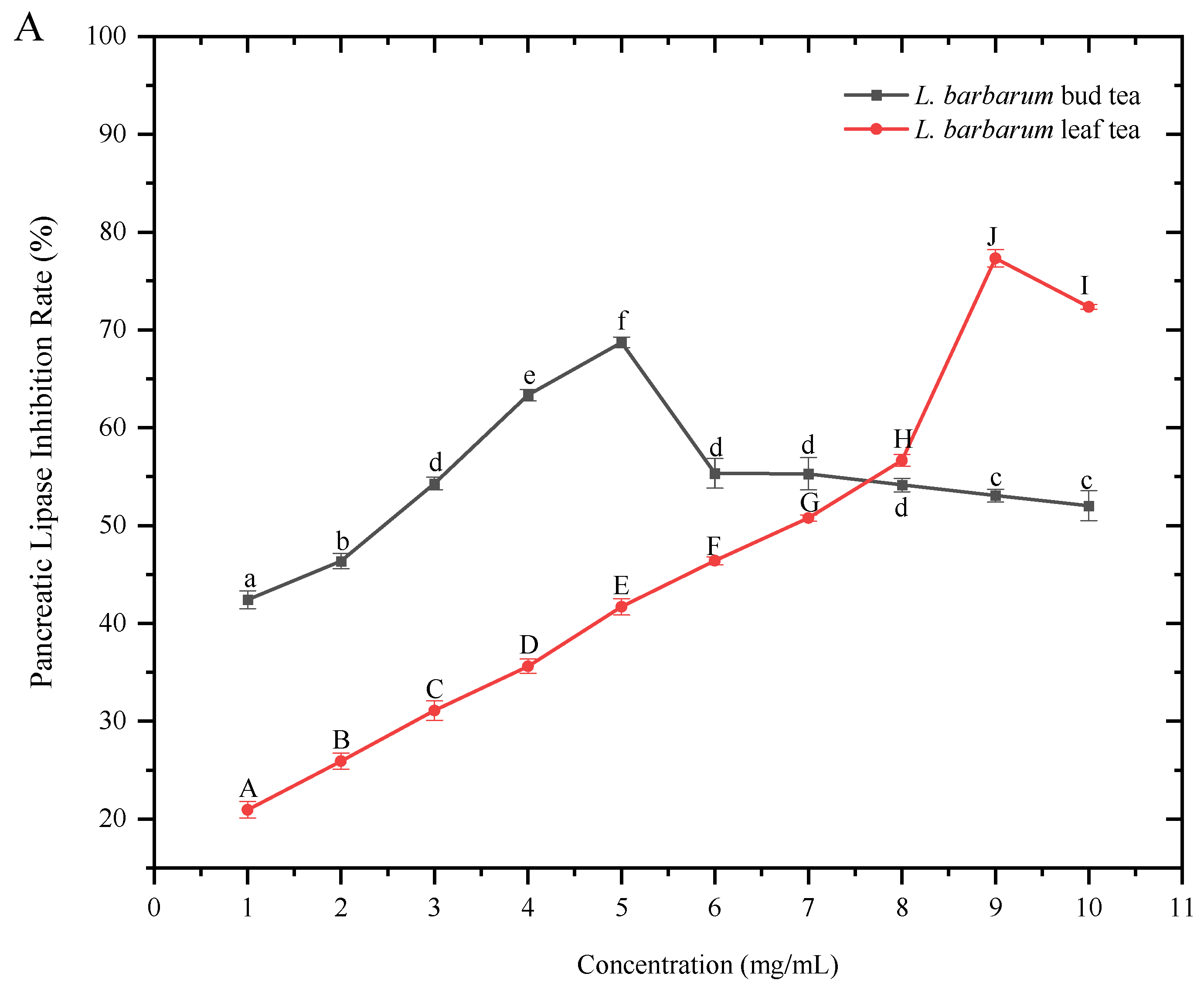
3.3. Influence of Water Extracts of L. barbarum Bud Tea and L. barbarum Leaf Tea on the Inhibitory Rate of Pancreatic Lipase and α-Amylase
As shown in (
Figure 6A), (
Table 4)the inhibitory rate of pancreatic lipase by the extracts of
L. barbarum bud tea and
L. barbarum leaf tea first increased and then decreased with the increase in the extract concentration, indicating that both
L. barbarum bud tea and leaf tea had a good inhibitory effect on pancreatic lipase. When the concentration of the tea extract was in the range of 0–7 mg/mL, the inhibitory effect of
L. barbarum bud tea was much higher than that of
L. barbarum leaf tea. However, when the concentration was greater than 8 mg/mL, the effect of
L. barbarum leaf tea increased significantly and reached the highest inhibitory rate (77.33 ± 0.88%) at 9 mg/mL. While the
L. barbarum bud tea reached the highest inhibitory rate (68.71 ± 0.54%) at 5 mg/mL, and then the inhibitory effect decreased with the increase in the concentration. This showed that the water extracts of both
L. barbarum bud tea and leaf tea had a good inhibitory effect on pancreatic lipase.
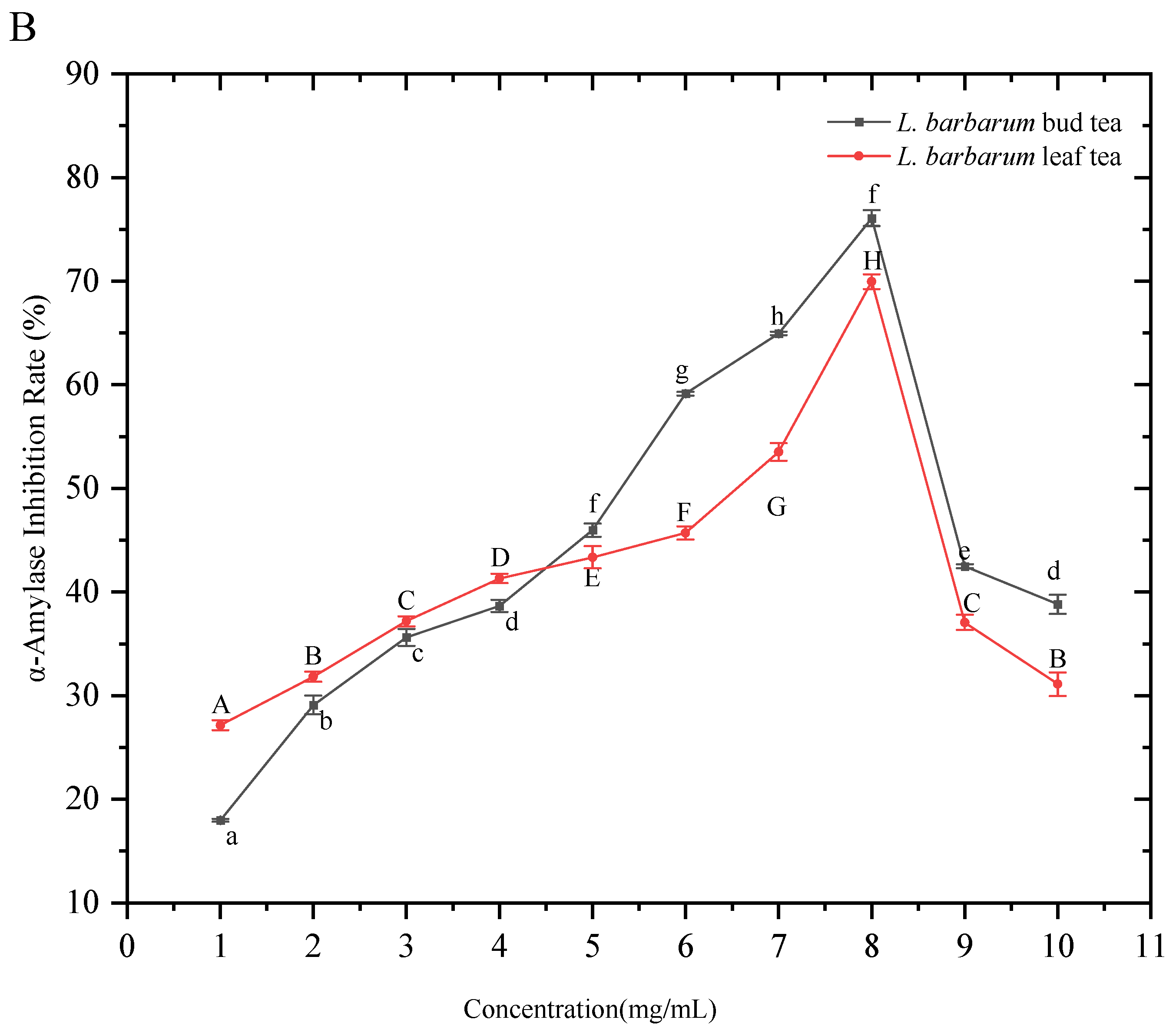
The inhibitory rate of
α-amylase by the extracts of
L. barbarum bud tea and
L. barbarum leaf tea first increased and then decreased with the increase in the extract concentration, and the inhibitory trends were basically the same (
Figure 6B), indicating that both
L. barbarum bud tea and leaf tea had a good inhibitory effect on
α-Amylase. The
L. barbarum bud tea reached the highest inhibitory rate of 76.08 ± 0.77% at 8 mg/mL, and the
L. barbarum leaf tea reached the highest inhibitory rate of 69.96 ± 0.71% at the same concentration, indicating that the water extracts of both
L. barbarum bud tea and leaf tea had a good inhibitory effect on the activity of
α-Amylase.
It is noteworthy that “when the concentration of
L. barbarum bud tea extract exceeds 8 mg/mL and
L. barbarum leaf tea extract exceeds 9 mg/mL, the activity of pancreatic lipase begins to decline.” Observations on α-amylase activity revealed that “when the concentrations of both
L. barbarum bud tea and leaf tea extracts reach 8 mg/mL, the activity of α-amylase starts to decrease. This phenomenon is caused by multiple factors. First of all, from the aggregation of compounds, it is speculated that at higher concentrations, active ingredients such as polyphenols may aggregate due to intermolecular interactions. The formed aggregates may not effectively bind to the active site of enzymes, resulting in decreased inhibitory activity. Reference analysis under similar research background. For example, SUN et al. found that: in high concentrations, tea polyphenols (e.g., EGCG) form aggregates with α-amylase, resulting in the shielding of enzyme active sites and a decrease in catalytic efficiency despite increased binding [
39]. However, high concentrations of polyphenols may trigger the feedback regulation mechanism of enzymes and thus reduce the inhibitory activity. High concentrations of flavan-3-ols (e.g., catechins) form aggregates in ethanol solution through hydrogen bonding and hydrophobic interactions, resulting in reduced α-Amylase inhibitory activity. The ionic strength and ethanol content significantly affect the degree of aggregation, which is analogous to the behavior of polyphenols in high concentrations in bud tea [
40]. Zhang et al. also reported that the inhibitory activity of stearic acid on α-amylase decreased with the increase in substrate (starch) concentration, which indicated that substrate competitive interference also reduced the rate of enzymatic reaction and weakened the overall inhibitory effect [
41].
Pancreatic lipase, a key enzyme in dietary fat digestion and absorption, exhibits inhibitory effects that reduce lipid hydrolysis and absorption, thereby exerting anti-obesity and lipid-lowering effects. Lower IC
50 values indicate stronger inhibitory potency. Data reveal significant differences in pancreatic lipase inhibition activity among substances: Edgeworthia gardneri tea extract (IC
50 = 23.16 ± 0.79 μg/mL) and purple tea extract (IC
50 = 67.4 μg/mL) demonstrated the strongest inhibitory effects [
42];
L. barbarum bud tea (IC
50 = 0.284 ± 0.121 mg/mL) (
Table 5) ranked second, outperforming tea polyphenols (IC
50 = 0.41 mg/mL) and Bacillus spore-fermented green tea (IC
50 = 0.48 mg/mL) [
43];
L. barbarum leaf tea (IC
50 = 0.831 ± 0.108 mg/mL) showed relatively weak inhibition, while. Chinese black tea extract (BTE) (IC
50 = 1.016 mg/mL) exhibited the weakest effect. These variations may relate to the types and concentrations of active components [
44]. Edgeworthia gardneri tea extract and purple tea likely contain high concentrations of specific polyphenols or flavonoids with strong affinity for pancreatic lipase [
45], whereas
L. barbarum bud tea demonstrates superior inhibitory efficacy compared to leaf tea due to its higher concentration of active components (e.g., polyphenols and saponins) in the bud structure.
α-Amylase is the key enzyme in carbohydrate digestion. Inhibiting its activity delays carbohydrate hydrolysis, which helps control postprandial blood glucose levels. IC
50 Lower inhibitory values indicate stronger effects. Comparative data shows: Green tea extract (EGCG) (IC
50 = 0.350 mg/mL) exhibits the strongest inhibition of α-amylase, followed by oolong tea polyphenols (IC
50 = 0.375 mg/mL) and EGCG methyl derivatives (IC
50 = 0.572 mg/mL) [
46];
L. barbarum bud tea (IC
50 = 0.765 ± 0.009 mg/mL) (
Table 5) demonstrates better inhibitory effects than
L. barbarum leaf tea (IC
50 = 0.864 ± 0.113 mg/mL); Pu’er tea extracts show significant IC
50 fluctuations (0.1–10 mg/mL) due to variations in fermentation degree and extraction methods. These differences mainly stem from active component specificity: EGCG, as the core component of tea polyphenols, may have higher affinity for α-amylase’s active site [
47]; the difference between
L. barbarum bud and leaf teas likely arises from structural adaptations of bud polyphenols and other active components to enzyme binding sites; while the IC
50 heterogeneity in Pu’er tea confirms the conclusion that “extraction methods and material composition affect enzymatic inhibition” [
48].
Overall, L. barbarum bud tea outperforms L. barbarum leaf tea in inhibiting pancreatic lipase and α-Amylase, closely related to the types, concentrations, and structural characteristics of active components. The differences among substances reflect the critical impact of component specificity on enzymatic inhibition. It should be emphasized that this experiment is a preliminary exploratory study, and no positive control was set up. Therefore, the above conclusions are still speculative and need further verification. It is particularly emphasized that the results of enzyme activity should be interpreted with caution, as the universality of the results may need to be verified by different nurseries and harvest periods.
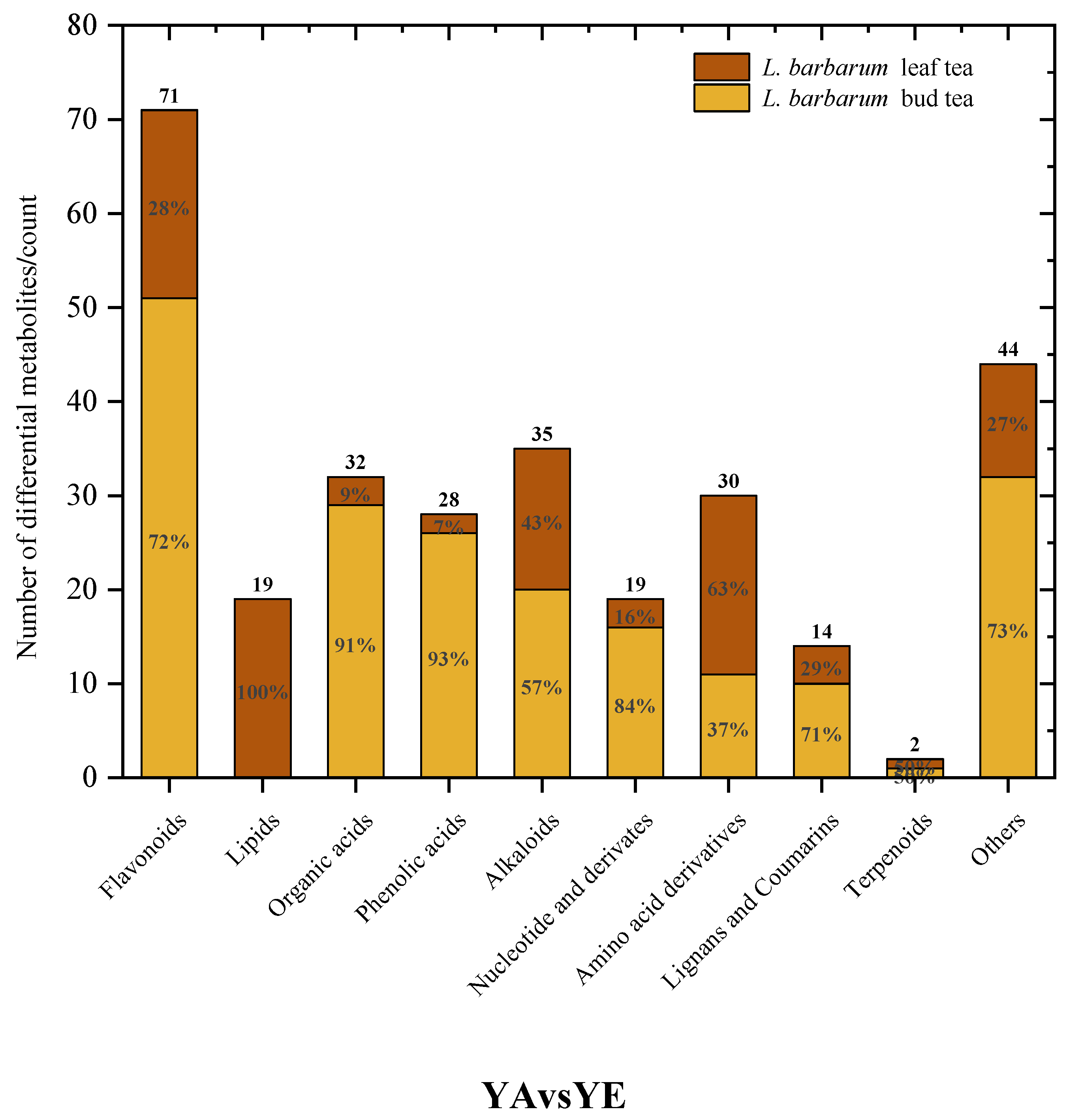
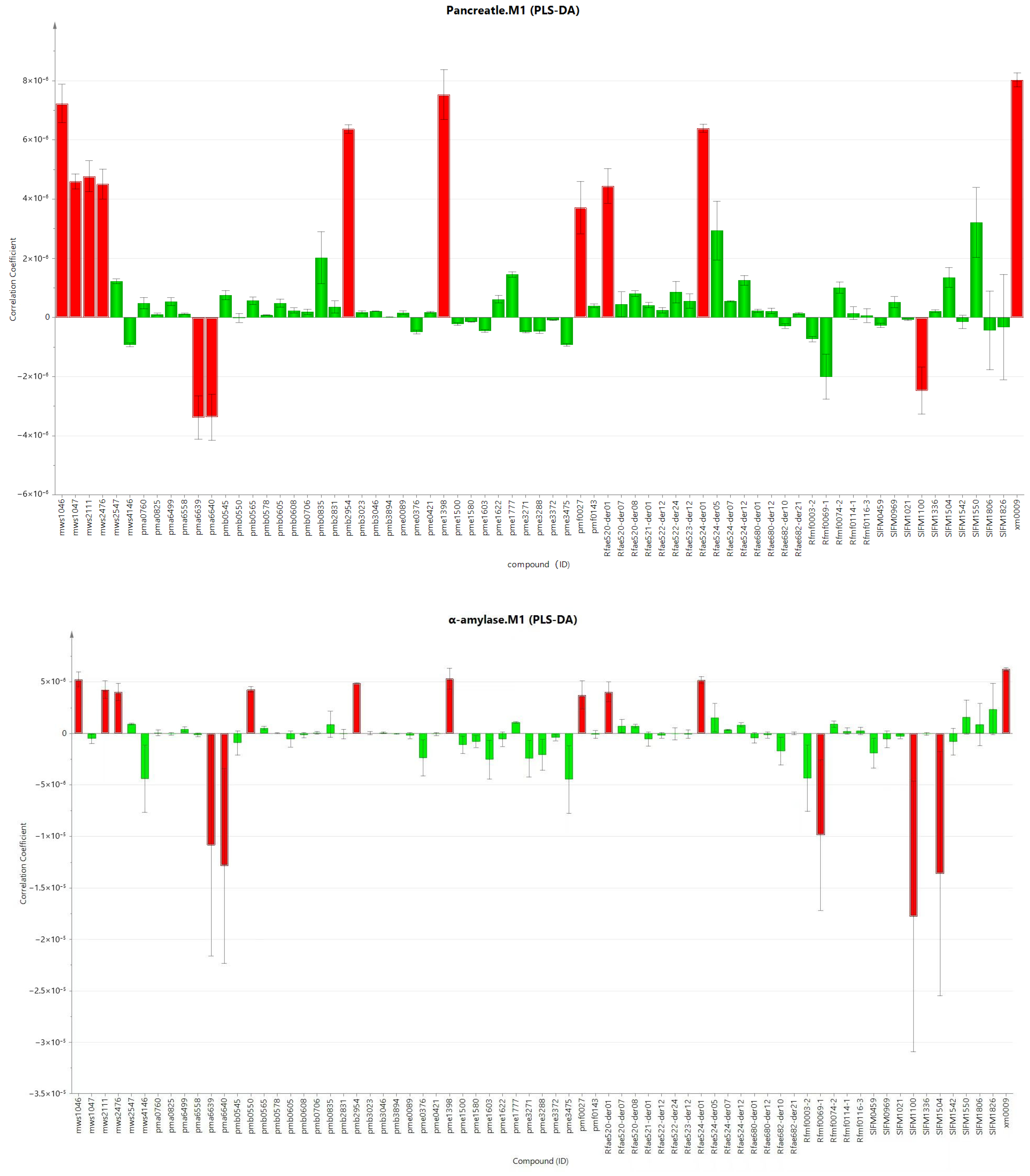
3.4. Correlation of the Chemical Components in L. barbarum Bud Tea and L. barbarum Leaf Tea and Their Inhibitory Activities on Pancreatic Lipase and α-Amylase
As shown in
Figure 7,
Table 6 and
Table 7, among the 72 significantly different flavonoid compounds, 13 compounds with a VIP value greater than 1 and contributing to the inhibitory activity of pancreatic lipase were screened out, and there was a good correlation between the independent variable and the dependent variable (R2 = 0.987,
p < 0.05). Among them, there were 10 bioactive compounds responsible for activity with a positive correlation, including delphinidin-3-O-galactoside, cyanidin-3-O-glucoside, cyanidin-3-glucoside, cyanidin galactoside, cyanidin-O-hexoside, delphinidin-3-O-galactoside, delphinidin-O-hexoside, delphinidin diglucoside, delphinidin-3-sophoroside-5-rhamnoside and delphinidin-3,5-diglucoside. Fifteen compounds with a VIP value greater than 1 and contributing to the inhibitory activity of
α-amylase were screened out, and there was a good correlation between the independent variable and the dependent variable (R2 = 0.910,
p < 0.05). Among them, there were 10 bioactive compounds responsible for activity with a positive correlation, including delphinidin-3-O-galactoside, cyanidin-3-O-glucoside, cyanidin-3-glucoside, cyanidin-3-galactoside, cyanidin-O-hexoside, delphinidin-3-O-glucoside, cyanidin-3-O-galactoside, cyanidin hexoside, delphinidin-O-hexoside and delphinidin diglucoside). There were 6 compounds that showed a significant positive correlation with the inhibitory activities of
L. barbarum bud tea and
L. barbarum leaf tea on pancreatic lipase and
α-amylase (delphinidin-3-O-galactoside, cyanidin-3-glucoside, cyanidin-3-O-glucoside, cyanidin-O-hexoside, delphinidin-O-hexoside and delphinidin diglucoside). It is worth noting that in this study, the six compounds with significant positive correlation were all anthocyanins and their derivatives.
Flavonoid compounds are an important class of substances for reducing blood lipids and blood sugar [
49]. Anthocyanins are a type of flavonoid compound. Due to the different substituents at the R1 and R2 carbon positions in the structure of anthocyanins, various types of anthocyanins are formed, and they rarely exist in a free state in nature, usually in the form of glycosides bound to sugars [
50]. These structural features endow anthocyanins with a variety of biological activities, including antioxidant, anti-inflammatory, hypoglycemic and lipid-lowering effects. Inoue et al. [
51] reported that delphinidin-3-O-galactoside mainly inhibited the generation of reactive oxygen species induced by oxidized low-density lipoprotein, and the activity and expression of nuclear transcription factor NF-κB p65. This mechanism of action indicates that this compound has antioxidant and anti-inflammatory effects. Asaki et al. [
52] found that cyanidin-3-glucoside can reduce the expression of retinol-binding protein 4 (RBP4) in type 2 diabetic mice, thus improving hyperglycemia and insulin sensitivity. Cyanidin-3-O-glucoside, a typical anthocyanin pigment, was found to stimulate the activation of AMPK in HepG2 cells through CAMKK, reduce the level of malonyl-CoA, leading to enhanced fatty acid β-oxidation, and inhibition of lipid accumulation in HepG2 cells [
53,
54]. Liu et al. [
55] reported that delphinidin-3,5-di glucoside exerts hypoglycemic and lipid-lowering effects by reducing the level of cleaved caspase-3 and increasing the phosphorylation level of AMP-activated protein kinase
α at Thr172, thereby activating the AMPK signaling pathway to promote glucose uptake and fatty acid β-oxidation while inhibiting hepatic lipid synthesis. delphinidin-O-hexoside and cyanidin-O-hexoside exerted hypoglycemic and lipid-lowering activities by inhibiting the digestion and absorption of sugars and fats, regulating lipid metabolism, improving insulin sensitivity, and exhibited antioxidant and anti-inflammatory effects [
56]. Its polysaccharide glycoside structure may affect its bioavailability, but it can still exert significant effects under the action of intestinal microorganisms.