Cadmium in the Soil–Tea–Infusion Continuum of Selenium-Enriched Gardens: Implications for Food Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling of Soil and Tea Tree Organs
2.3. Measurement of Soil and Tea Tree Indicators
2.4. Research Methods and Indicators
2.4.1. Single-Factor Pollution Index
2.4.2. Activated Rate of Cd (Se)
2.4.3. Enrichment and Transport Coefficients
2.4.4. Health Risk Evaluation
2.5. Quality Assurance and Quality Control
2.6. Statistical Analysis
3. Results
3.1. Soil Cd and Se Contents
3.2. Accumulation of Cd in Soil and Tea Tree Organs
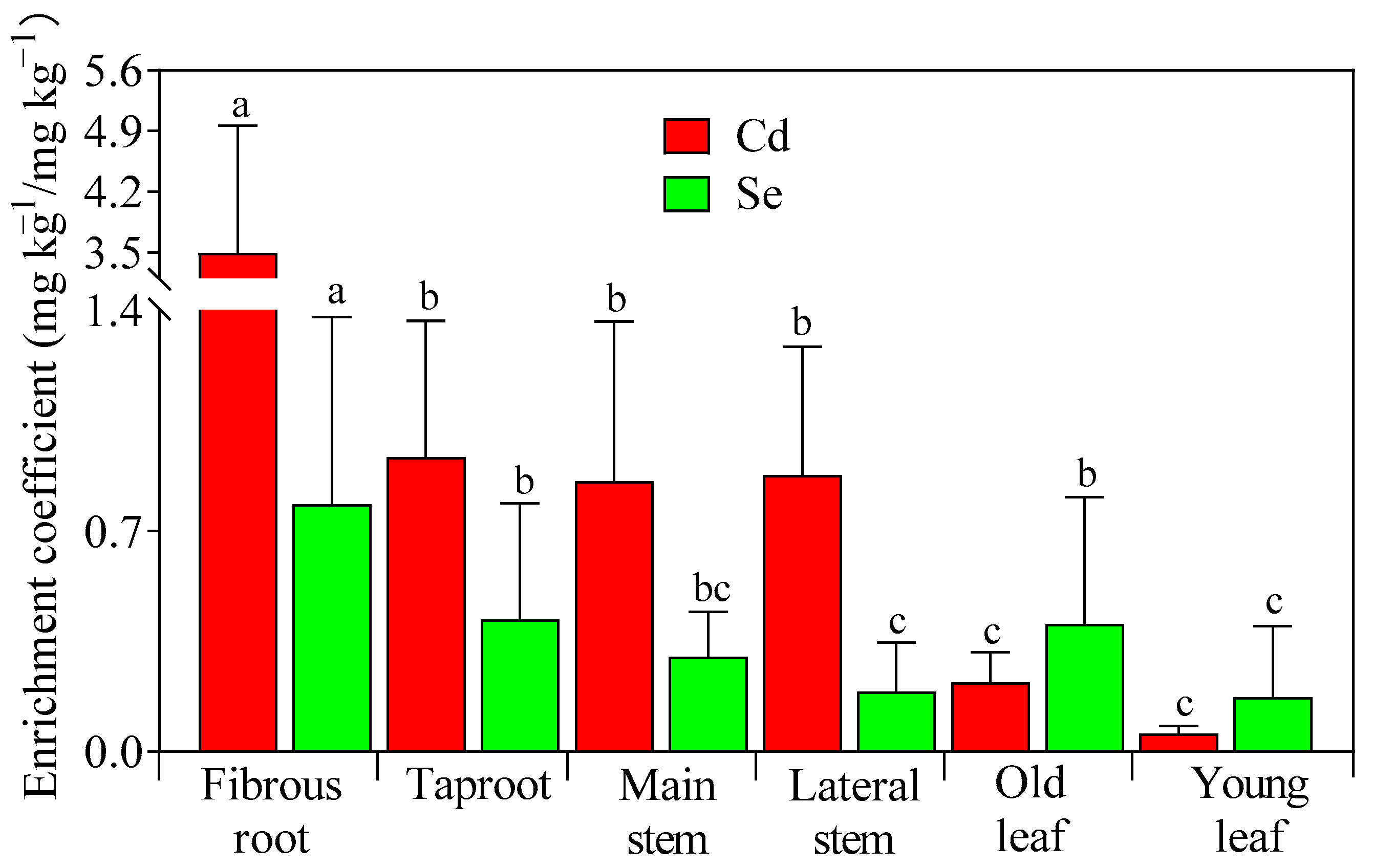
3.3. Enrichment and Translocation of Cd and Se in Tea Trees
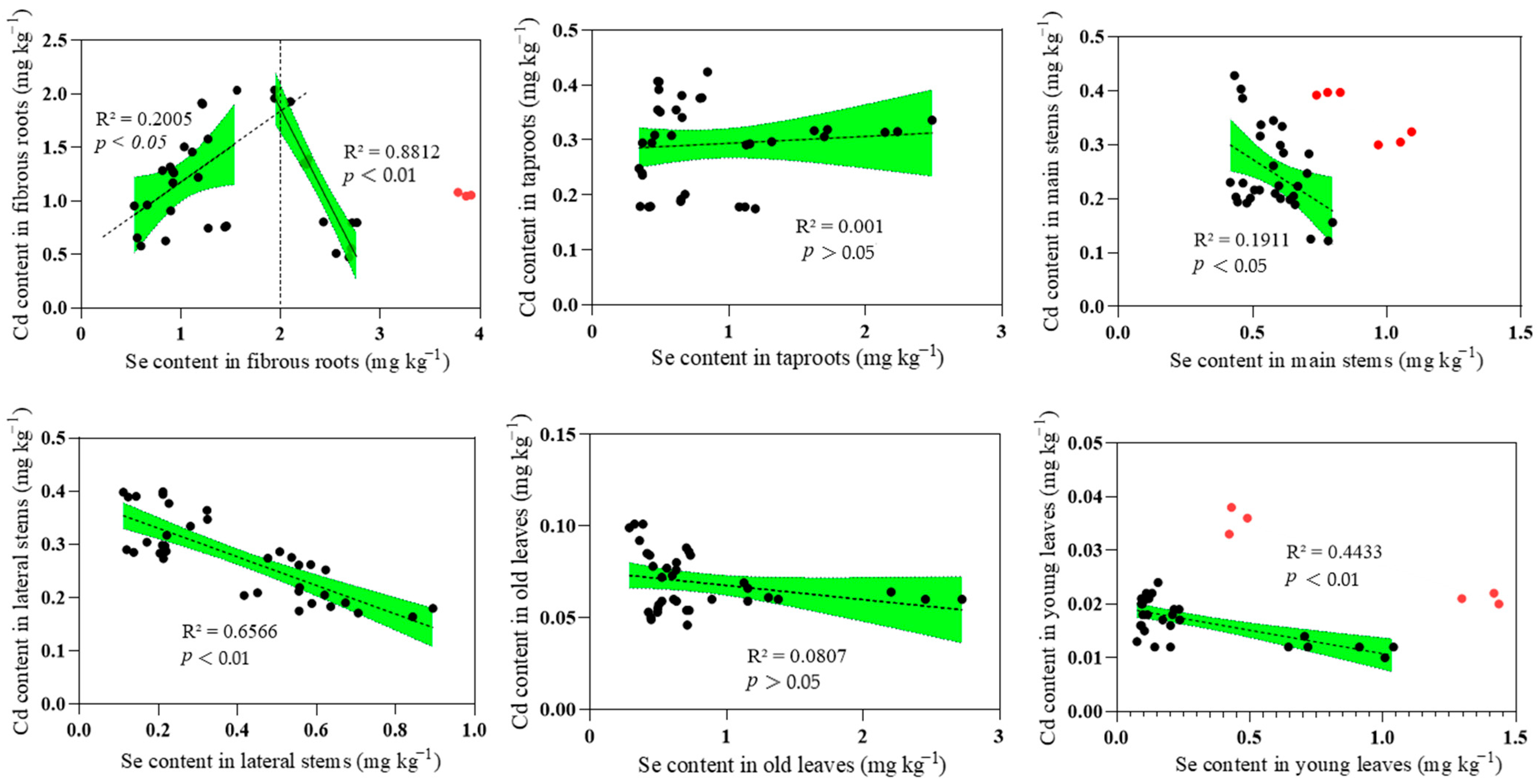
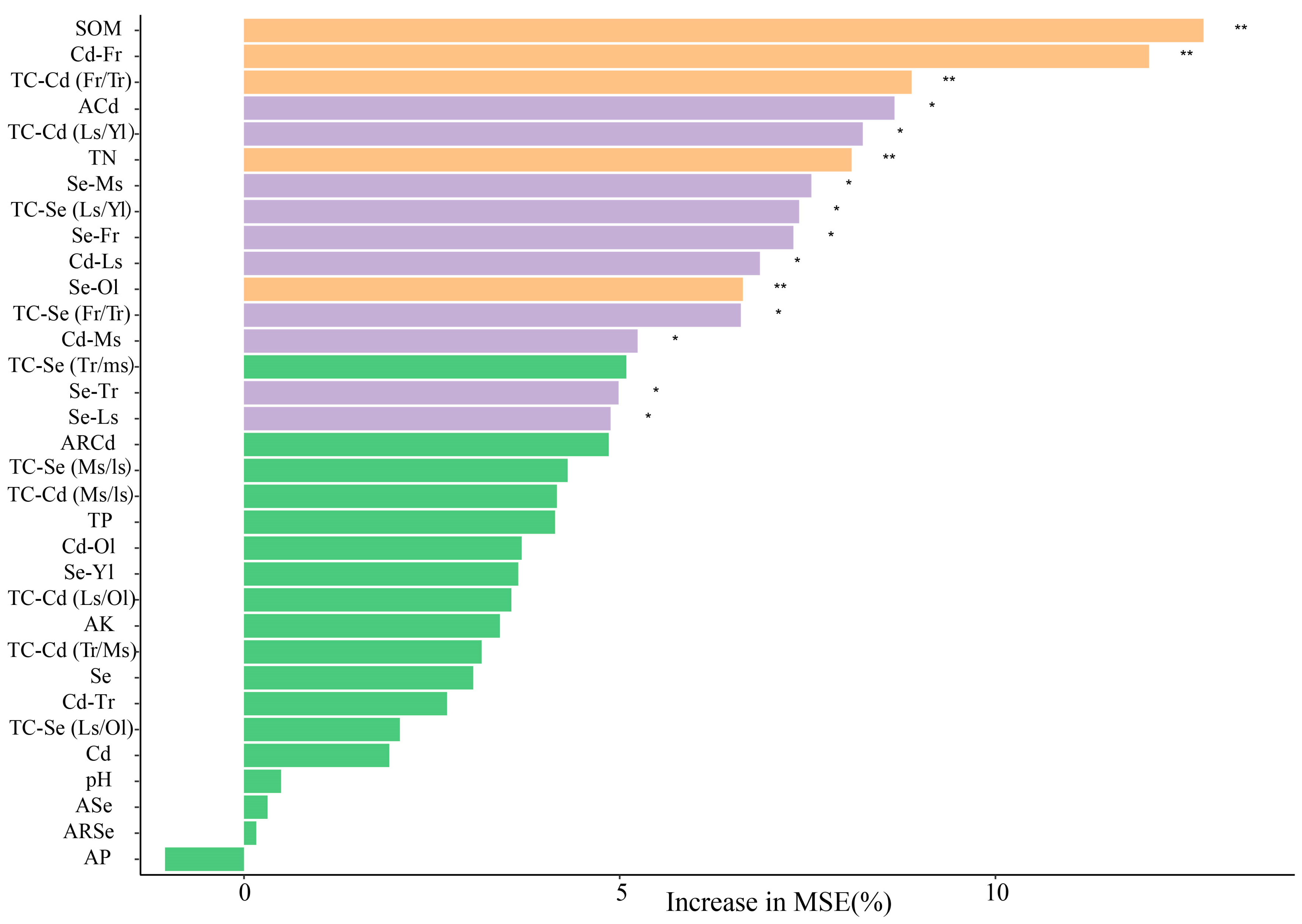
3.4. The Effect of Se on Cd in the Soil–Tea Tree System
3.5. Health Risk Assessment of Tea Infusion
4. Discussion
4.1. Cd and Its Availability in Se-Enriched Soil
4.2. Partitioning and Translocation of Cd in Tea Trees
4.3. Can Se Antagonize Cd in the Soil–Tea Tree System?
4.4. Health Risks of Cd Exposure in Tea Infusions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, F.; Hu, J.; Chen, L.; Wang, Z.; Sun, S.; Zhang, W.; Jiang, H.; Luo, Y.; Wang, L.; Zheng, Y.; et al. Microplastics may increase the environmental risks of Cd via promoting Cd uptake by plants: A meta-analysis. J. Hazard. Mater. 2023, 448, 130887. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.I.; ur Rehman, M.Z.; Rizwan, M.; Yousaf, B.; Ali, S.; ul Haq, M.A.; Anayat, A.; Waris, A.A. Efficiency of various silicon rich amendments on growth and cadmium accumulation in field grown cereals and health risk assessment. Chemosphere 2020, 244, 125481. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qu, J.; Sun, S.; Shi, Q.; Feng, H.; Zhang, Y.; Cao, S. Health risk assessment of total exposure from cadmium in South China. Chemosphere 2021, 269, 128673. [Google Scholar] [CrossRef]
- Zou, M.; Zhou, S.; Zhou, Y.; Jia, Z.; Guo, T.; Wang, J. Cadmium pollution of soil-rice ecosystems in rice cultivation dominated regions in China: A review. Environ. Pollut. 2021, 280, 116965. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Ayub, A.; Hussain, S.; Waraich, E.A.; El-Esawi, M.A.; Ishfaq, M.; Ahmad, M.; Ali, N.; Maqsood, M.F. Cadmium toxicity in plants: Recent progress on morpho-physiological effects and remediation strategies. J. Soil Sci. Plant Nutr. 2022, 22, 212–269. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Ohri, P.; Bhardwaj, R.; Ahmad, P. Agroecotoxicological aspect of Cd in soil–plant system: Uptake, translocation and amelioration strategies. Environ. Sci. Pollut. Res. 2022, 29, 30908–30934. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, Z.; Li, X.; Liu, H.; Li, N.; Wei, S. Mitigation of rice cadmium (Cd) accumulation by joint application of organic amendments and selenium (Se) in high-Cd-contaminated soils. Chemosphere 2020, 241, 125106. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Lin, Q.; Rashid, M.S.; He, Z.; Yang, X. Organic soil additives for the remediation of cadmium contaminated soils and their impact on the soil-plant system: A review. Sci. Total Environ. 2020, 707, 136121. [Google Scholar] [CrossRef]
- Pan, S.Y.; Nie, Q.; Tai, H.C.; Song, X.L.; Tong, Y.F.; Zhang, L.J.F.; Wu, X.W.; Lin, Z.H.; Zhang, Y.Y.; Ye, D.Y.; et al. Tea and tea drinking: China’s outstanding contributions to the mankind. Chin. Med. 2022, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Li, J.; Zhou, D.D.; Gan, R.Y.; Li, H.B. Molecular mechanisms underlying health benefits of tea compounds. Free Radic. Biol. Med. 2021, 172, 181–200. [Google Scholar] [CrossRef]
- Chen, D.I.; Dou, Q.P. Tea polyphenols and their roles in cancer prevention and chemotherapy. Int. J. Mol. Sci. 2008, 9, 1196–1206. [Google Scholar] [CrossRef]
- Podwika, W.; Kleszcz, K.; Krośniak, M.; Zagrodzki, P. Copper, manganese, zinc, and cadmium in tea leaves of different types and origin. Biol. Trace Elem. Res. 2018, 183, 389–395. [Google Scholar] [PubMed]
- Portugal, Ó.B.; Flores-Quispe, M. Concentrations of arsenic, cadmium, and lead in herbal infusion tea bags marketed in Tacna, Peru. Environ. Monit. Assess. 2022, 194, 534. [Google Scholar] [CrossRef]
- Pourramezani, F.; Akrami Mohajeri, F.; Salmani, M.H.; Dehghani Tafti, A.; Khalili Sadrabad, E. Evaluation of heavy metal concentration in imported black tea in Iran and consumer risk assessments. Food Sci. Nutr. 2019, 7, 4021–4026. [Google Scholar] [CrossRef]
- Li, W.; Cheng, H.; Mu, Y.; Xu, A.; Ma, B.; Wang, F.; Xu, P. Occurrence, accumulation, and risk assessment of trace metals in tea (Camellia sinensis): A national reconnaissance. Sci. Total Environ. 2021, 792, 148354. [Google Scholar]
- Zhang, J.; Yang, R.; Li, Y.C.; Peng, Y.; Wen, X.; Ni, X. Distribution, accumulation, and potential risks of heavy metals in soil and tea leaves from geologically different plantations. Ecotoxicol. Environ. Saf. 2020, 195, 110475. [Google Scholar] [CrossRef]
- Seenivasan, S.; Anderson, T.A.; Muraleedharan, N. Heavy metal content in tea soils and their distribution in different parts of tea plants, Camellia sinensis (L). O. Kuntze. Environ. Monit. Assess. 2016, 188, 428. [Google Scholar] [CrossRef]
- Wang, C.M.; Tang, Q.; Zhang, X.Q.; Zhang, D.C. Effects of high concentration cadmium stress on tea plant physiology and absorption and accumulation characteristics. J. Tea Sci. 2012, 32, 107–114, (In Chinese with English Abstract). [Google Scholar]
- Zhang, W.H.; Yan, Y.; Yu, R.L.; Hu, G.R. The sources-specific health risk assessment combined with APCS/MLR model for heavy metals in tea garden soils from south Fujian Province, China. Catena 2021, 203, 105306. [Google Scholar]
- Zhu, R.; Zheng, Z.; Li, T.; He, S.; Zhang, X.; Wang, Y.; Liu, T. Effect of tea plantation age on the distribution of glomalin-related soil protein in soil water-stable aggregates in southwestern China. Environ. Sci. Pollut. Res. 2019, 26, 1973–1982. [Google Scholar]
- Sun, J.; Hu, G.; Yu, R. Bioavailability of heavy metals in soil-tea plant system of Tieguanyin tea garden. Environ. Chem. 2020, 39, 2765–2776. [Google Scholar]
- Wang, K.; Fang, Q.; He, P.; Tu, Y.; Liu, Z.; Li, B. Unveiling the potential of selenium-enriched tea: Compositional profiles, physiological activities, and health benefits. Trends Food Sci. Technol. 2024, 145, 104356. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, L.; Qi, S.; Yin, X. The threshold effect between the soil bioavailable molar Se: Cd ratio and the accumulation of Cd in corn (Zea mays L.) from natural Se-Cd rich soils. Sci. Total Environ. 2019, 688, 1228–1235. [Google Scholar] [CrossRef]
- Chen, J.; He, W.; Zhu, X.; Yang, S.; Yu, T.; Ma, W. Epidemiological study of kidney health in an area with high levels of soil cadmium and selenium: Does selenium protect against cadmium-induced kidney injury? Sci. Total Environ. 2020, 698, 134106. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; He, Y.; Luo, L.; Zhu, M.; Zan, S.; Guo, F.; Wang, B.; Yang, B. The interaction between selenium and cadmium in the soil-rice-human continuum in an area with high geological background of selenium and cadmium. Ecotoxicol. Environ. Saf. 2021, 222, 112516. [Google Scholar] [PubMed]
- Zhao, W.; Song, Y.; Guan, D.; Ma, Q.; Guo, C.; Wen, Y.; Ji, J. Pollution status and bioavailability of heavy metals in soils of a typical black shale area. J. Agro-Environ. Sci. 2018, 37, 1332–1341, (In Chinese with English Abstract). [Google Scholar]
- Chang, C.; Yin, R.; Wang, X.; Shao, S.; Chen, C.; Zhang, H. Selenium translocation in the soil-rice system in the Enshi seleniferous area, Central China. Sci. Total Environ. 2019, 669, 83–90. [Google Scholar]
- Huang, Y.; Wang, Q.; Gao, J.; Lin, Z.; Banuelos, G.S.; Yuan, L.; Yin, X. Daily dietary selenium intake in a high selenium area of Enshi, China. Nutrients 2013, 5, 700–710. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology: Revised Edition; Springer: Dordrecht, The Netherlands, 2012; pp. 375–416. [Google Scholar]
- Yang, L.; Huang, Q.S.; Liu, Y.; Zheng, S.A.; Liu, H.; Rensing, C.; Fan, Z.; Feng, R. Selenate regulates the activity of cell wall enzymes to infuence cell wall component concentration and thereby affects the uptake and translocation of Cd in the roots of Brassica rapa L. Sci. Total Environ. 2022, 821, 153156. [Google Scholar]
- Guo, Y.; Mao, K.; Cao, H.; Ali, W.; Lei, D.; Teng, D.; Chang, C.; Yang, X.; Yang, Q.; Niazi, N.K.; et al. Exogenous selenium (cadmium) inhibits the absorption and transportation of cadmium (selenium) in rice. Environ. Pollut. 2021, 268, 115829. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, Y.; Li, F. Selenium reduces cadmium uptake into rice suspension cells by regulating the expression of lignin synthesis and cadmium-related genes. Sci. Total Environ. 2018, 644, 602–610. [Google Scholar] [CrossRef]
- Mozafariyan, M.; Shekari, L.; Hawrylak-Nowak, B.; Kamelmanesh, M.M. Protective role of selenium on pepper exposed to cadmium stress during reproductive stage. Biol. Trace Elem. Res. 2014, 160, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wang, L.; Yang, J.; Zhao, P.; Zhu, Y.; Li, Y.; Yu, Y.; Liu, H.; Rensing, C.; Wu, Z.; et al. Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J. Hazard. Mater. 2021, 402, 123570. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fu, P.; Huang, Q.; Zhang, J.; Li, H. Accumulation, subcellular distribution, and oxidative stress of cadmium in Brassica chinensis supplied with selenite and selenate at different growth stages. Chemosphere 2019, 216, 331–340. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Zhou, H.; Li, F.; Wang, Y.; Du, X. The Regulatory Effect of Se-Cd Interaction on Tea Plants (Camellia sinensis (L.) O. Kuntze) Under Cadmium Stress. Agronomy 2025, 15, 246. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Ma, J.; Wu, F.; Wang, L.; Sun, L.; Zhang, P.; Wang, W.; Xu, J. Comparative physiological and soil microbial community structural analysis revealed that selenium alleviates cadmium stress in Perilla frutescens. Front. Plant Sci. 2022, 13, 1022935. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, T.; Wang, T.; Yang, P. Effect of Se-enriched foliar fertilizer on Se-enrichment and Cd-reduction in tea in region of Wanyuan. Hans J. Agric. Sci. 2020, 10, 623–627, (In Chinese with English Abstract). [Google Scholar]
- Yang, B.B.; Yang, C.; Shao, Z.Y.; Wang, H.; Zan, S.T.; Zhu, M.; Zhou, S.B.; Yang, R.Y. Selenium (Se) does not reduce cadmium (Cd) uptake and translocation in rice (Oryza sativa L.) in naturally occurred Se-rich paddy fields with a high geological background of Cd. Bull. Environ. Contam. Toxicol. 2019, 103, 127–132. [Google Scholar] [CrossRef]
- Long, Z.; Yuan, L.; Hou, Y.; Bañuelos, G.S.; Liu, Y.; Pan, L.; Liu, X.; Yin, X. Spatial variations in soil selenium and residential dietary selenium intake in a selenium-rich county, Shitai, Anhui, China. J. Trace Elem. Med. Biol. 2018, 50, 111–116. [Google Scholar] [CrossRef]
- GB5413.21—2010; National Food Safety Standard: Determination of Multiple Elements in Foods. National Health and Family Planning Commission of the People’s Republic of China and the China Food and Drug Administration: Beijing, China, 2016.
- Shaheen, S.M.; Ali, R.A.; Abowaly, M.E.; Rabie, A.E.M.A.; El Abbasy, N.E.; Rinklebe, J. Assessing the mobilization of As, Cr, Mo, and Se in Egyptian lacustrine and calcareous soils using sequential extraction and biogeochemical microcosm techniques. J. Geochem. Explor. 2018, 191, 28–42. [Google Scholar] [CrossRef]
- Xiang, J.; Rao, S.; Chen, Q.; Zhang, W.; Cheng, S.; Cong, X.; Zhang, Y.; Yang, X.; Xu, F. Research progress on the effects of selenium on the growth and quality of tea plants. Plants 2022, 11, 2491. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.K. Soil Agricultural Chemistry Analysis; Chinese Agriculture and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Liu, X.; Chen, S.; Yan, X.; Liang, T.; Yang, X.; El-Naggar, A.; Liu, J.; Chen, H. Evaluation of potential ecological risks in potential toxic elements contaminated agricultural soils: Correlations between soil contamination and polymetallic mining activity. J. Environ. Manag. 2021, 300, 113679. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (USEPA). Exposure Factors Handbook—Update, External Review Draft; EPA/600/R-09/052A; U.S. Environmental Protection Agency: Washington, DC, USA, 2009.
- Tao, C.; Song, Y.; Chen, Z.; Zhao, W.; Ji, J.; Shen, N.; Ayoko, G.A.; Frost, R.L. Geological load and health risk of heavy metals uptake by tea from soil: What are the significant influencing factors? Catena 2021, 204, 105419. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Zhu, Y.G.; Li, H.Y.; Smith, S.E.; Smith, F.A. Effects of forms and rates of potassium fertilizers on cadmium uptake by two cultivars of spring wheat (Triticum aestivum, L.). Environ. Int. 2004, 29, 973–978. [Google Scholar] [CrossRef]
- Huang, R.; Dong, M.; Mao, P.; Zhuang, P.; Paz-Ferreiro, J.; Li, Y.; Li, Y.; Hu, X.; Netherway, P.; Li, Z. Evaluation of phytoremediation potential of five Cd (hyper) accumulators in two Cd contaminated soils. Sci. Total Environ. 2020, 721, 137581. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, J.; Tao, L.; Cao, Z.; Tang, W.; Zhang, J.; Yu, X.; Fu, G.; Zhang, X.; Lu, Y. Regulatory mechanisms of nitrogen (N) on cadmium (Cd) uptake and accumulation in plants: A review. Sci. Total Environ. 2020, 708, 135186. [Google Scholar] [CrossRef]
- Duan, H.Q.; Qin, Q.; Lu, W.G.; Xue, Y.; Sun, L.J.; Song, K. Effects of Long-term Application of Organic Manure on Contents of Total and Available Cadmium in Greenhouse Soil. Acta Pedol. Sin. 2021, 58, 1486–1495, (In Chinese with English Abstract). [Google Scholar]
- Li, C.; Yu, Z.; Lin, J.; Meng, M.; Zhao, Y.; Jia, Z.; Peng, X.; Liu, X.; Zhang, J. Forest conversion and soil depth can modify the contributions of organic and inorganic colloids to the stability of soil aggregates. Forests 2022, 13, 546. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- Hong, C.O.; Lee, D.K.; Kim, P.J. Feasibility of phosphate fertilizer to immobilize cadmium in a field. Chemosphere 2008, 70, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Carne, G.; Leconte, S.; Sirot, V.; Breysse, N.; Badot, P.M.; Bispo, A.; Deportes, I.Z.; Dumat, C.; Rivière, G.; Crépet, A. Mass balance approach to assess the impact of cadmium decrease in mineral phosphate fertilizers on health risk: The case-study of French agricultural soils. Sci. Total Environ. 2021, 760, 143374. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Wu, Y.; Xu, B.; Zhang, S.; Deng, X.; Zhang, Y.; Siddique, K.H.; Chen, Y. Nitrogen supply improved plant growth and Cd translocation in maize at the silking and physiological maturity under moderate Cd stress. Ecotoxicol. Environ. Saf. 2022, 230, 113137. [Google Scholar] [CrossRef]
- Ali, E.; Maodzeka, A.; Hussain, N.; Shamsi, I.H.; Jiang, L. The alleviation of cadmium toxicity in oilseed rape (Brassica napus) by the application of salicylic acid. Plant Growth Regul. 2015, 75, 641–655. [Google Scholar] [CrossRef]
- Borgo, L.; Rabêlo, F.H.S.; Rossi, M.L.; Dos Santos, F.H.; Nogueira, M.L.G.; Alleoni, L.R.F.; Linhares, F.S.; Vangronsveld, J.; Lavres, J. Effect of selenium and soil pH on cadmium phytoextraction by Urochloa decumbens grown in Oxisol. J. Hazard. Mater. 2023, 447, 130771. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Dai, H.; Cao, F.; Zhang, G.; Wu, F. Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J. Hazard. Mater. 2012, 235, 343–351. [Google Scholar] [CrossRef]
- Saidi, I.; Chtourou, Y.; Djebali, W. Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 2014, 171, 85–91. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Pilon-Smits, E.A.; Zhao, F.J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Huang, B.; Xin, J.; Dai, H.; Zhou, W. Effects of interaction between cadmium (Cd) and selenium (Se) on grain yield and Cd and Se accumulation in a hybrid rice (Oryza sativa) system. J. Agric. Food Chem. 2017, 65, 9537–9546. [Google Scholar] [CrossRef]
- Yu, Y.; Yuan, S.; Zhuang, J.; Wan, Y.; Wang, Q.; Zhang, J.; Li, H. Effect of selenium on the uptake kinetics and accumulation of and oxidative stress induced by cadmium in Brassica chinensis. Ecotoxicol. Environ. Saf. 2018, 162, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Li, X.; Guo, L.; Wang, L.; Hao, X.; Zeng, J. Integrative transcriptome and proteome analysis reveals the absorption and metabolism of selenium in tea plants [Camellia sinensis (L.) O. Kuntze]. Front. Plant Sci. 2022, 13, 848349. [Google Scholar] [CrossRef] [PubMed]
- Shanker, K.; Mishra, S.; Srivastava, S. Effect of selenite and selenate on plant uptake of cadmium by maize (Zea mays). Bull. Environ. Contam. Toxicol. 1996, 56, 419–424. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, S.; Dai, H.; Jia, G. The alleviating effects of selenium on cadmium-induced toxicity in tea leaves. J. Nanjing For. Univ. 2020, 44, 200–204, (In Chinese with English Abstract). [Google Scholar]
- Wang, F.; Shan, R.; Chen, Y.; Lin, D.; Zang, C.; Chen, C.; You, Z.; Yu, W. A case study of cadmium distribution in soil-tea plant-tea soup system in Central Fujian Province and relative health risk assessment. J Tea Sci. 2018, 38, 537–546. [Google Scholar]
- Wei, J.; Cen, K. Content and dietary exposure of cadmium among residents in Northeast China: A case study based on the 5th China Total Diet Study. Environ. Sci. Pollut. Res. 2020, 27, 8224–8230. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Malik, A.; Zhang, Q.; Sadeghpour, A.; Zhu, Y.; Li, Q. Improving Cd risk managements of rice cropping system by integrating source-soil-rice-human chain for a typical intensive industrial and agricultural region. J. Clean. Prod. 2021, 313, 127883. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Ruan, J.Y.; Ma, L.F.; Han, W.Y.; Wang, F. Accumulation and distribution of arsenic and cadmium by tea plants. J. Zhejiang Univ. B 2008, 9, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Gong, C.; Zhang, Y.; Guo, K.; Bai, J. Lead, cadmium, arsenic, mercury and copper levels in Chinese Yunnan Pu’er tea. Food Addit. Contam. Part B 2011, 4, 28–33. [Google Scholar] [CrossRef]
- Yao, Q.; Lin, Q.; Yan, S.A.; Huang, M.; Chen, L. Dietary risk assessment of fluoride, lead, chromium, and cadmium through consumption of Tieguanyin tea and white tea. Food Sci. Technol. 2021, 41, 782–789. [Google Scholar] [CrossRef]


| Sampling Point | Cd (mg/kg) | ACd (μg/kg) | ARCd (%) | Se (mg/kg) | ASe (μg/kg) | ARSe (%) | Pollution Index |
|---|---|---|---|---|---|---|---|
| no. 1 | 0.34 ± 0.01 bcd | 87.21 ± 16.10 ab | 25.82 ± 4.43 bc | 2.26 ± 0.39 cd | 62.50 ± 42.25 abcde | 2.62 ± 1.38 abc | 1.12 ± 0.03 bcd |
| no. 2 | 0.44 ± 0.03 ab | 111.75 ± 8.08 a | 25.67 ± 0.30 bc | 2.19 ± 0.44 cd | 53.10 ± 26.72 bcde | 2.44 ± 1.07 bc | 1.45 ± 0.11 ab |
| no. 3 | 0.48 ± 0.03 ab | 98.32 ± 9.76 ab | 20.59 ± 3.60 cd | 2.58 ± 1.07 bcd | 31.27 ± 17.26 cde | 1.48 ± 1.01 c | 1.60 ± 0.11 ab |
| no. 4 | 0.18 ± 0.01 d | 46.56 ± 1.12 bcd | 25.86 ± 1.44 bc | 1.76 ± 0.02 cd | 58.87 ± 16.85 abcde | 3.36 ± 0.99 ab | 0.60 ± 0.03 d |
| no. 5 | 0.20 ± 0.01 d | 47.84 ± 3.57 bcd | 24.41 ± 1.50 bc | 2.93 ± 0.93 bcd | 99.20 ± 40.50 a | 3.33 ± 0.68 ab | 0.65 ± 0.02 d |
| no. 6 | 0.54 ± 0.30 a | 96.54 ± 93.01 ab | 15.66 ± 7.26 de | 2.04 ± 1.12 cd | 34.57 ± 21.16 bcde | 2.08 ± 1.76 bc | 1.81 ± 0.99 a |
| no. 7 | 0.40 ± 0.10 abc | 25.19 ± 10.23 d | 6.69 ± 3.15 f | 3.16 ± 1.02 bc | 44.03 ± 22.86 bcde | 1.55 ± 0.98 bc | 1.33 ± 0.34 abc |
| no. 8 | 0.48 ± 0.09 ab | 29.77 ± 2.35 cd | 6.44 ± 1.74 f | 1.46 ± 0.37 d | 22.30 ± 14.39 de | 1.43 ± 0.58 c | 1.59 ± 0.32 ab |
| no. 9 | 0.23 ± 0.03 cd | 81.34 ± 2.62 abc | 36.22 ± 3.15 a | 3.99 ± 1.12 b | 75.00 ± 6.10 abc | 1.99 ± 0.62 bc | 0.75 ± 0.09 cd |
| no. 10 | 0.55 ± 0.04 a | 110.84 ± 6.76 a | 20.11 ± 2.36 cd | 5.50 ± 1.10 a | 78.07 ± 16.14 ab | 1.43 ± 0.19 c | 1.85 ± 0.14 a |
| no. 11 | 0.47 ± 0.05 ab | 61.85 ± 26.94 abcd | 13.07 ± 4.88 e | 1.49 ± 0.38 d | 18.53 ± 5.76 e | 1.31 ± 0.60 c | 1.56 ± 0.17 ab |
| no. 12 | 0.18 ± 0.02 d | 53.55 ± 4.77 bcd | 30.6 ± 4.81 ab | 1.54 ± 0.07 d | 65.27 ± 7.09 abcd | 4.25 ± 0.65 a | 0.59 ± 0.08 d |
| Mean | 0.37 ± 0.16 | 70.90 ± 38.17 | 20.93 ± 9.36 | 2.58 ± 1.31 | 53.56 ± 29.90 | 2.27 ± 1.20 | 1.24 ± 0.54 |
| Sampling Site | Fibrous Root (mg kg−1) | Taproot (mg kg−1) | Main Stem (mg kg−1) | Lateral Stem (mg kg−1) | Old Leaf (mg kg−1) | Young Leaf (mg kg−1) | |
|---|---|---|---|---|---|---|---|
| no. 1 | 0.62 ± 0.04 h | 0.30 ± 0.01 de | 0.33 ± 0.01 b | 0.29 ± 0.01 cd | 0.05 ± 0.00 ef | 0.02 ± 0.00 cd | |
| no. 2 | 1.95 ± 0.07 a | 0.34 ± 0.03 bc | 0.27 ± 0.02 d | 0.20 ± 0.01 f | 0.05 ± 0.00 f | 0.02 ± 0.00 cd | |
| no. 3 | 1.97 ± 0.06 a | 0.19 ± 0.01 g | 0.41 ± 0.02 a | 0.21 ± 0.01 f | 0.07 ± 0.00 c | 0.02 ± 0.00 d | |
| no. 4 | 0.80 ± 0.01 g | 0.29 ± 0.00 e | 0.40 ± 0.00 a | 0.28 ± 0.01 d | 0.06 ± 0.00 de | 0.01 ± 0.00 e | |
| no. 5 | 1.06 ± 0.02 e | 0.32 ± 0.01 cd | 0.20 ± 0.01 e | 0.17 ± 0.01 g | 0.06 ± 0.00 d | 0.02 ± 0.00 bc | |
| no. 6 | 0.94 ± 0.03 f | 0.18 ± 0.00 g | 0.22 ± 0.00 e | 0.39 ± 0.01 a | 0.09 ± 0.01 b | 0.04 ± 0.00 a | |
| Cd | no. 7 | 1.29 ± 0.02 c | 0.40 ± 0.01 a | 0.20 ± 0.02 e | 0.35 ± 0.02 b | 0.10 ± 0.00 a | 0.02 ± 0.00 b |
| no. 8 | 1.51 ± 0.06 b | 0.24 ± 0.01 f | 0.20 ± 0.01 e | 0.39 ± 0.00 a | 0.09 ± 0.00 b | 0.02 ± 0.00 bc | |
| no. 9 | 1.36 ± 0.03 c | 0.31 ± 0.01 cde | 0.31 ± 0.01 bc | 0.30 ± 0.02 c | 0.08 ± 0.00 c | 0.02 ± 0.00 d | |
| no. 10 | 1.22 ± 0.05 d | 0.39 ± 0.03 a | 0.30 ± 0.04 cd | 0.28 ± 0.01 cd | 0.05 ± 0.00 ef | 0.02 ± 0.00 bc | |
| no. 11 | 0.76 ± 0.01 g | 0.36 ± 0.02 b | 0.22 ± 0.02 e | 0.18 ± 0.01 g | 0.07 ± 0.00 d | 0.01 ± 0.00 e | |
| no. 12 | 0.51 ± 0.04 i | 0.18 ± 0.00 g | 0.13 ± 0.02 f | 0.26 ± 0.01 e | 0.06 ± 0.00 de | 0.01 ± 0.00 e | |
| Mean | 1.17 ± 0.47 | 0.29 ± 0.08 | 0.27 ± 0.08 | 0.28 ± 0.07 | 0.07 ± 0.02 | 0.02 ± 0.01 | |
| no. 1 | 0.69 ± 0.18 i | 0.40 ± 0.04 fg | 0.57 ± 0.09 d | 0.22 ± 0.01 e | 0.41 ± 0.03 ef | 0.45 ± 0.04 d | |
| no. 2 | 1.14 ± 0.12 fg | 0.36 ± 0.01 g | 0.45 ± 0.02 e | 0.13 ± 0.02 f | 0.72 ± 0.02 d | 0.11 ± 0.00 g | |
| no. 3 | 1.00 ± 0.14 gh | 0.81 ± 0.03 d | 0.60 ± 0.02 cd | 0.21 ± 0.00 e | 0.46 ± 0.04 ef | 0.10 ± 0.01 g | |
| no. 4 | 2.26 ± 0.02 c | 1.68 ± 0.05 b | 1.04 ± 0.06 a | 0.22 ± 0.00 e | 0.55 ± 0.09 de | 0.10 ± 0.01 g | |
| no. 5 | 0.87 ± 0.05 hi | 0.48 ± 0.01 f | 0.62 ± 0.03 cd | 0.31 ± 0.02 d | 0.33 ± 0.04 f | 0.14 ± 0.02 fg | |
| no. 6 | 3.85 ± 0.07 a | 2.29 ± 0.18 a | 0.63 ± 0.04 cd | 0.81 ± 0.10 a | 2.46 ± 0.26 a | 1.38 ± 0.08 a | |
| Se | no. 7 | 2.65 ± 0.08 b | 1.13 ± 0.06 c | 0.77 ± 0.04 b | 0.59 ± 0.03 b | 1.28 ± 0.11 b | 0.99 ± 0.07 b |
| no. 8 | 1.39 ± 0.10 e | 0.64 ± 0.02 e | 0.46 ± 0.04 e | 0.59 ± 0.04 b | 1.06 ± 0.15 c | 0.14 ± 0.06 fg | |
| no. 9 | 2.64 ± 0.18 b | 1.20 ± 0.10 c | 0.78 ± 0.04 b | 0.51 ± 0.03 c | 0.59 ± 0.06 de | 0.69 ± 0.04 c | |
| no. 10 | 1.99 ± 0.09 d | 0.66 ± 0.02 e | 0.45 ± 0.02 e | 0.48 ± 0.07 c | 0.59 ± 0.05 de | 0.20 ± 0.02 ef | |
| no. 11 | 0.67 ± 0.15 i | 0.42 ± 0.05 fg | 0.55 ± 0.03 d | 0.14 ± 0.03 ef | 0.48 ± 0.03 ef | 0.23 ± 0.01 e | |
| no. 12 | 1.33 ± 0.20 ef | 0.52 ± 0.05 f | 0.68 ± 0.05 c | 0.62 ± 0.06 b | 0.72 ± 0.01 d | 0.09 ± 0.00 g | |
| Mean | 1.71 ± 0.96 | 0.88 ± 0.58 | 0.63 ± 0.17 | 0.40 ± 0.22 | 0.80 ± 0.58 | 0.38 ± 0.41 |
| Sampling Point | Cd in Tea Infusion (µg L−1) | Cd Leaching Rate (%) | Se in Tea Infusion (µg L−1) | Se Leaching Rate (%) | Personal Annual Health Risks (a−1) | Total Personal Annual Health Risks (a−1) |
|---|---|---|---|---|---|---|
| no. 1 | 0.163 ± 0.019 ab | 11.34 ± 1.89 cd | 7.06 ± 0.04 d | 25.66 ± 1.49 abcd | 2.95 × 10−8 | 2.60 × 10−7 |
| no. 2 | 0.148 ± 0.006 bc | 9.70 ± 1.47 de | 2.31 ± 0.10 gh | 17.47 ± 2.05 cde | 2.53 × 10−8 | 2.61 × 10−7 |
| no. 3 | 0.175 ± 0.022 a | 13.56 ± 2.13 bc | 2.42 ± 0.06 gh | 21.55 ± 0.90 bcde | 3.29 × 10−8 | 2.43 × 10−7 |
| no. 4 | 0.131 ± 0.005 cd | 18.39 ± 0.78 a | 1.93 ± 0.04 h | 12.25 ± 1.34 e | 3.30 × 10−8 | 1.79 × 10−7 |
| no. 5 | 0.130 ± 0.007 cd | 10.99 ± 0.97 cd | 2.81 ± 0.13 fg | 21.99 ± 2.33 bcde | 3.27 × 10−8 | 2.97 × 10−7 |
| no. 6 | 0.138 ± 0.007 bcd | 6.98 ± 0.82 e | 11.17 ± 1.06 b | 14.18 ± 1.03 de | 3.51 × 10−8 | 5.03 × 10−7 |
| no. 7 | 0.147 ± 0.002 bc | 12.14 ± 0.51 bcd | 12.29 ± 0.19 a | 22.78 ± 0.95 bcde | 3.84 × 10−8 | 3.16 × 10−7 |
| no. 8 | 0.127 ± 0.021 cd | 11.60 ± 2.13 cd | 3.31 ± 0.13 f | 35.56 ± 16.05 a | 3.39 × 10−8 | 2.92 × 10−7 |
| no. 9 | 0.138 ± 0.008 bcd | 15.15 ± 1.36 b | 8.46 ± 0.36 c | 20.69 ± 1.17 bcde | 3.50 × 10−8 | 2.31 × 10−7 |
| no. 10 | 0.148 ± 0.004 bc | 12.71 ± 0.35 bcd | 4.14 ± 0.11 e | 28.94 ± 1.49 abc | 3.79 × 10−8 | 2.98 × 10−7 |
| no. 11 | 0.134 ± 0.001 cd | 19.35 ± 0.83 a | 3.49 ± 0.01 ef | 18.93 ± 0.74 cde | 3.38 × 10−8 | 1.75 × 10−7 |
| no. 12 | 0.116 ± 0.007 d | 18.05 ± 1.87 a | 2.79 ± 0.16 fg | 31.39 ± 2.94 ab | 2.88 × 10−8 | 1.60 × 10−7 |
| Mean | 0.141 ± 0.019 | 13.33 ± 3.86 | 5.18 ± 3.50 | 22.62 ± 8.19 | 3.30 × 10−8 | 2.68 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhang, D.; Jie, X.; Liu, S.; Wang, D. Cadmium in the Soil–Tea–Infusion Continuum of Selenium-Enriched Gardens: Implications for Food Safety. Foods 2025, 14, 3156. https://doi.org/10.3390/foods14183156
Wu H, Zhang D, Jie X, Liu S, Wang D. Cadmium in the Soil–Tea–Infusion Continuum of Selenium-Enriched Gardens: Implications for Food Safety. Foods. 2025; 14(18):3156. https://doi.org/10.3390/foods14183156
Chicago/Turabian StyleWu, Haizhong, Dengxiao Zhang, Xiaolei Jie, Shiliang Liu, and Daichang Wang. 2025. "Cadmium in the Soil–Tea–Infusion Continuum of Selenium-Enriched Gardens: Implications for Food Safety" Foods 14, no. 18: 3156. https://doi.org/10.3390/foods14183156
APA StyleWu, H., Zhang, D., Jie, X., Liu, S., & Wang, D. (2025). Cadmium in the Soil–Tea–Infusion Continuum of Selenium-Enriched Gardens: Implications for Food Safety. Foods, 14(18), 3156. https://doi.org/10.3390/foods14183156






