Early Detection of Monilinia laxa in Yellow-Fleshed Peach Using a Non-Destructive E-Nose Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Volatile Compound Analysis
2.2.1. Volatile Extraction
2.2.2. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
2.3. E-Nose Analysis
2.3.1. E-Nose System
2.3.2. Measurement Process and Data Analysis
2.4. Statistical Analysis
3. Results and Discussion
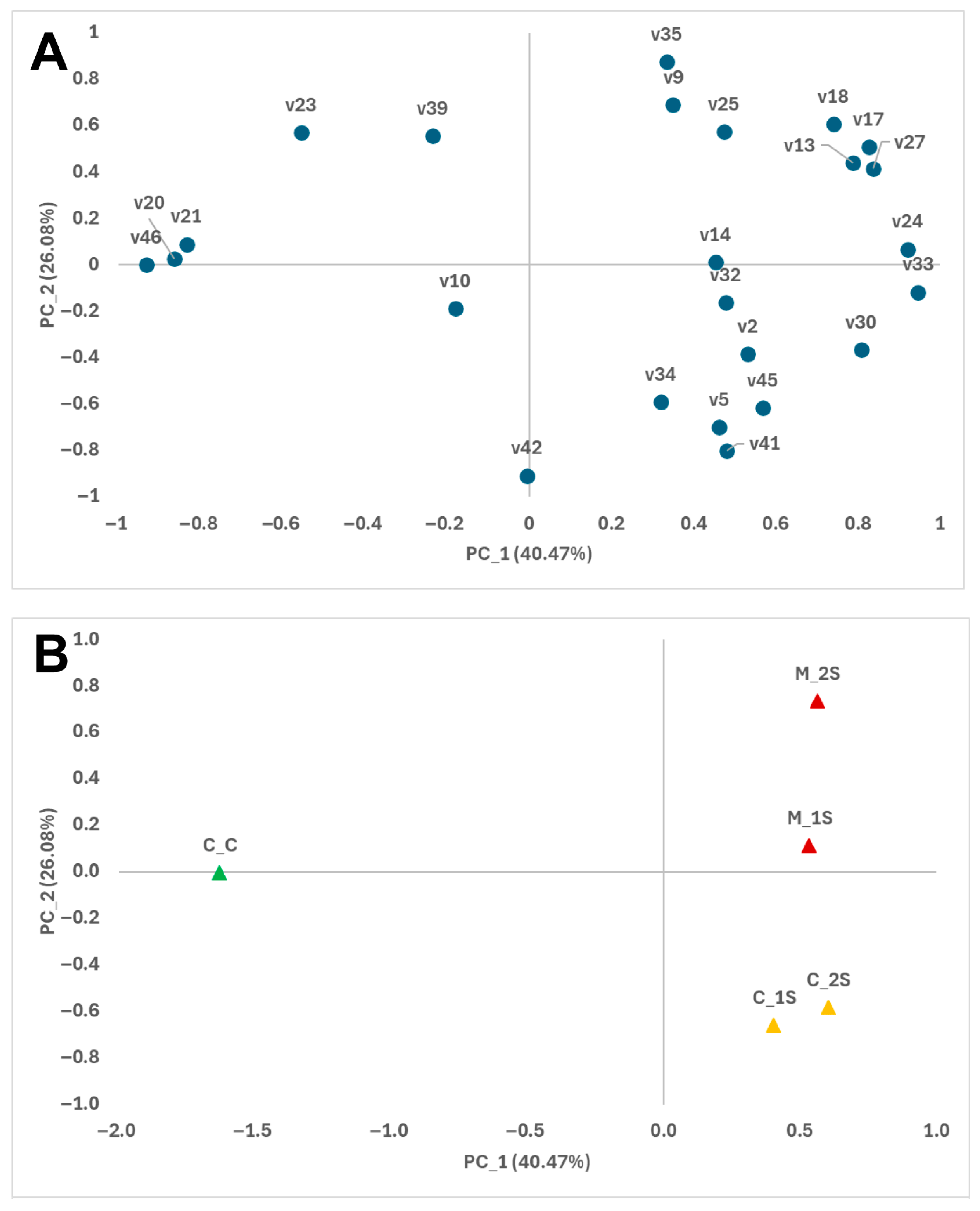
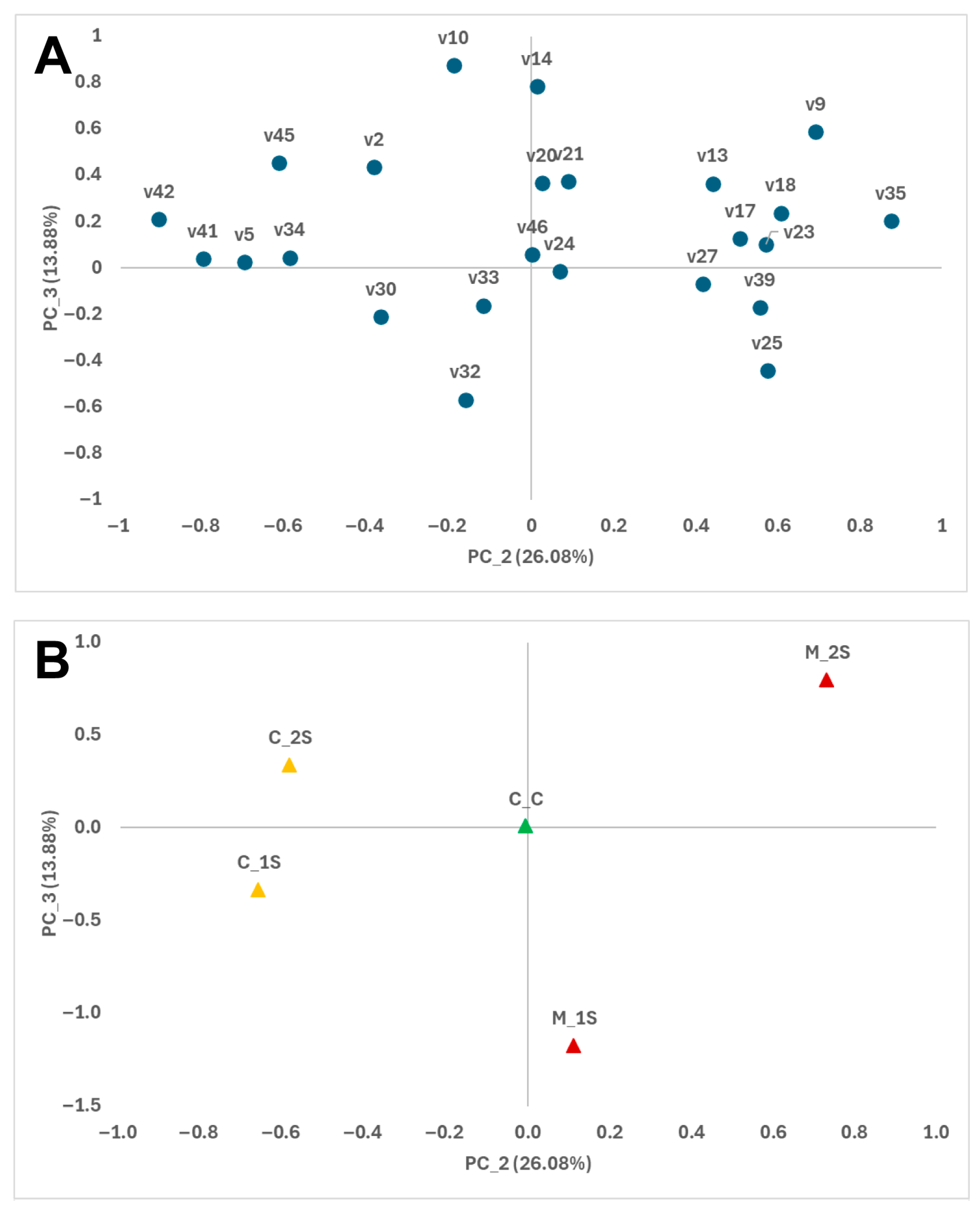
3.1. Volatile Compounds
3.2. Relationship Between VOCs and Signals from E-Nose Sensors
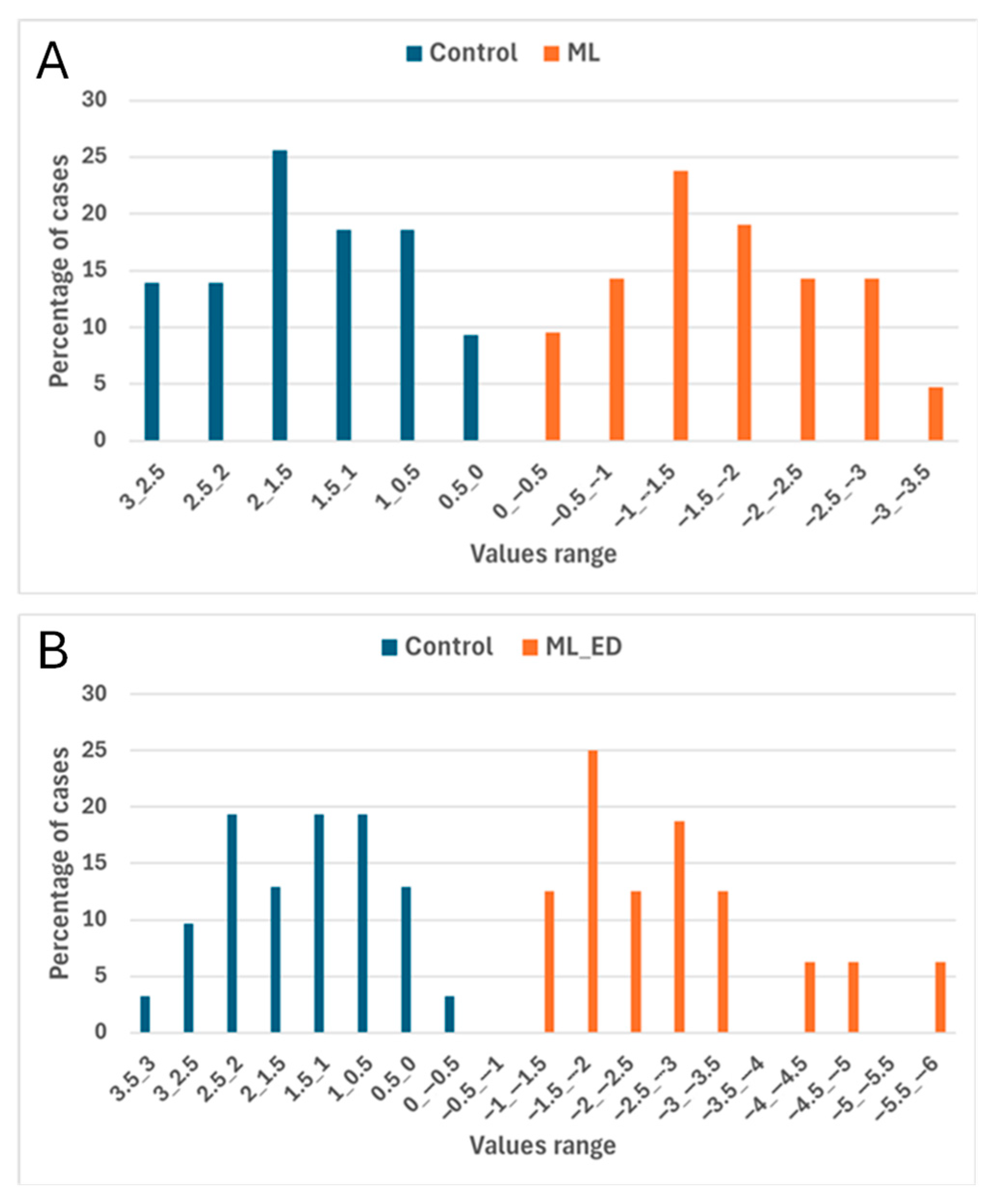
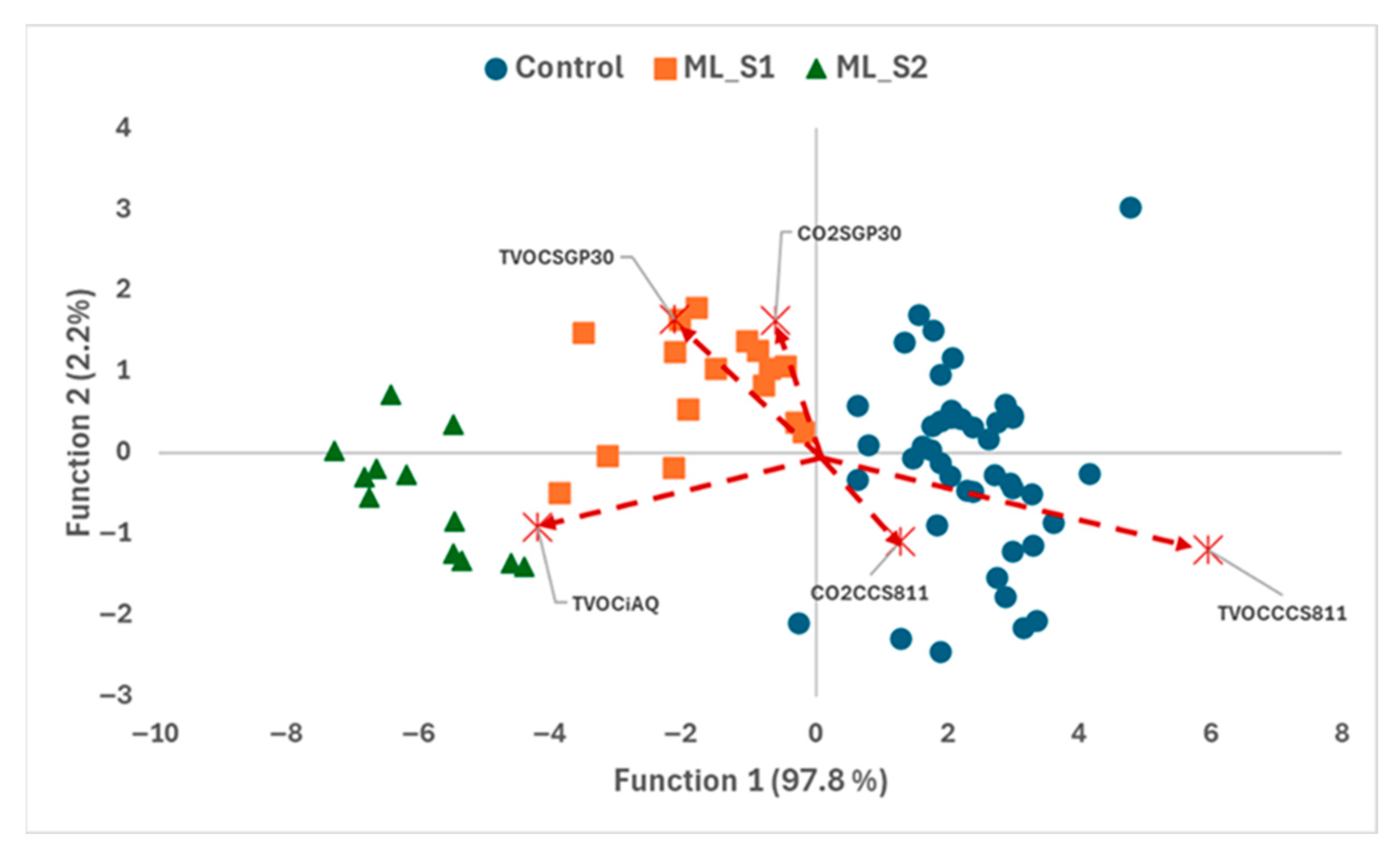
3.3. Determination of Incipient Fungal Decay of Peaches by E-Nose During Postharvest Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC/MS | Gas Chromatography/Mass Spectrometry |
| LDA | Linear Discriminant Analysis |
| PCA | Principal Component Analysis |
| NIR | Near-Infrared Spectroscopy |
| HSI | Hyperspectral imaging |
| VOCs | Volatile organic compounds |
| PLSR | Partial Least Squares Regression |
| SVM | Support Vector Machines |
| ANN | Artificial Neural Networks |
| MOX | Metal Oxide Sensors |
| E-nose | Electronic nose |
| HCA | Hierarchical Cluster Analysis |
| HS-SPME | Headspace Solid-Phase Microextraction |
References
- Zhang, S.; Zheng, Q.; Xu, B.; Liu, J. Identification of the fungal pathogens of postharvest disease on peach fruits and the control mechanisms of bacillus subtilis jk-14. Toxins 2019, 11, 322. [Google Scholar] [CrossRef]
- Lee, D.; Hassan, O.; Chang, T. Identification, characterization, and pathogenicity of Colletotrichum species causing anthracnose of peach in Korea. Mycobiology 2020, 48, 210–218. [Google Scholar] [CrossRef]
- Baltazar, E.; Rodrigues, S.; Ares, A.; Camelo, A.; Brandão, I.; Santo, C.; Trovão, J.; García, E.; Costa, J. Morphological, molecular and genomic identification and characterisation of Monilinia fructicola in Prunus persica from Portugal. Agronomy 2023, 13, 1493. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization (EPPO) Website. Available online: https://www.eppo.int/RESOURCES/eppo_databases/global_database (accessed on 23 March 2021).
- Luo, C.X.; Schnabel, G.; Hu, M.; De Cal, A. Global distribution and management of peach diseases. Phytopathol. Res. 2022, 4, 30–45. [Google Scholar] [CrossRef]
- Villarino, M.; Egüen, B.; Lamarca, N.; Segarra, J.; Usall, J.; Melgarejo, P.; De Cal, A. Occurrence of Monilinia laxa and M. fructigena after introduction of M. fructicola in peach orchards in Spain. Eur. J. Plant Pathol. 2013, 137, 835–845. [Google Scholar] [CrossRef]
- Zhou, D.; Wei, Y.; Peng, J.; Tu, S.; Wang, Z.; Tu, K. Carvacrol and eugenol inhibit postharvest soft rot disease by enhancing defense response in peaches during storage. J. Food Process. Preserv. 2019, 43, 14086–14097. [Google Scholar] [CrossRef]
- ElMasry, G.; Wang, N.; ElSayed, A.; Ngadi, M. Hyperspectral imaging for nondestructive determination of some quality attributes for strawberry. J. Food Eng. 2007, 81, 98–107. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, X.A.; Sun, D.W. Recent developments and applications of hyperspectral imaging for quality evaluation of agricultural products: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1744–1757. [Google Scholar] [CrossRef]
- Li, X.; Li, R.; Wang, M.; Liu, Y.; Zhang, B.; Zhou, J. Hyperspectral imaging and their applications in the nondestructive quality assessment of fruits and vegetables. In Hyperspectral Imaging in Agriculture, Food and Environment, 1st ed.; Luna, A., Rodríguez, H., Vidales, J., Eds.; IntechOpen: London, UK, 2018; pp. 27–63. [Google Scholar]
- Chandrasekaran, I.; Panigrahi, S.S.; Ravikanth, L.; Singh, C.B. Potential of near-infrared (NIR) spectroscopy and hyperspectral imaging for quality and safety assessment of fruits: An overview. Food Anal. Methods 2019, 12, 2438–2458. [Google Scholar] [CrossRef]
- Chandra, A.; Raj, H.; Ranjan, H.; Kumar, A.; Kumari, P. Isolation, characterization and sporulation of fungi from decaying vegetables and fruits of local vegetable market in Hazaribag India. Plant Arch. 2022, 22, 426–430. [Google Scholar] [CrossRef]
- Mailafia, S.; Okoh, G.; Olabode, O.; Osanupin, R. Isolation and identification of fungi associated with spoilt fruits vended in Gwagwalada market, Abuja, Nigeria. Vet. World 2016, 10, 393–397. [Google Scholar] [CrossRef]
- Chen, L.; Wu, C.; Chou, T.; Chiu, S.; Tang, K. Development of a dual MOS electronic nose/camera system for improving fruit ripeness classification. Sensors 2018, 18, 3256. [Google Scholar] [CrossRef]
- Huang, X.; Yu, S.; Xu, H.; Aheto, J.; Bonah, E.; Ma, M.; Wu, M.; Zhang, X. Rapid and nondestructive detection of freshness quality of postharvest spinaches based on machine vision and electronic nose. J. Food Saf. 2019, 39, 12708–12716. [Google Scholar] [CrossRef]
- Jia, W.; Liang, G.; Tian, H.; Sun, J.; Wan, C. Electronic nose-based technique for rapid detection and recognition of moldy apples. Sensors 2019, 19, 1526. [Google Scholar] [CrossRef]
- Guo, Z.; Guo, C.; Chen, Q.; Ouyang, Q.; Shi, J.; El-Seedi, H.; Zou, X. Classification for Penicillium expansum spoilage and defect in apples by electronic nose combined with chemometrics. Sensors 2020, 20, 2130. [Google Scholar] [CrossRef]
- Martínez, A.; Hernández, A.; Arroyo, P.; Lozano, J.S.; de Guía Córdoba, M.; Martín, A. E-Nose Detection of Changes in Volatile Profile Associated with Early Decay of ‘Golden Delicious’ Apple by Penicillium expansum. Food Control 2024, 168, 110907–110919. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, W.; Zhu, N.; Mao, S.; Tu, K. Early detection and classification of pathogenic fungal disease in post-harvest strawberry fruit by electronic nose and gas chromatography–mass spectrometry. Food Res. Int. 2014, 62, 162–168. [Google Scholar] [CrossRef]
- Amari, A.; El Bari, N.; Bouchikhi, B. Electronic nose for anchovy freshness monitoring based on sensor array and pattern recognition methods: Principal components analysis, linear discriminant analysis and support vector machine. Int. J. Comput. 2014, 6, 61–67. [Google Scholar] [CrossRef]
- Paredes-Doig, A.; Sun-Kou, M.; Doig-Camino, E.; Picasso, G.; Gómez, A.L.R.T. Aplicación de métodos multivariados para la diferenciación de vinos peruanos. Infoanalítica 2022, 10, 85–99. [Google Scholar]
- Yang, C.; Wu, D.; Ma, L.; Jia, R. Discrimination and characterization of different intensities of goaty flavor in goat milk by means of an electronic nose. J. Dairy Sci. 2015, 98, 55–67. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, J.; Gao, L. Discrimination and characterization of strawberry juice based on electronic nose and tongue: Comparison of different juice processing approaches by LDA, PLSR, RF, and SVM. J. Agric. Food Chem. 2014, 62, 6426–6434. [Google Scholar] [CrossRef]
- Gordillo, D.; Castellanos, M.; Barrera, D.; Escobar, J.; Sanchez, J.; Leon-Medina, J. Kullback–Leibler importance estimation procedure to improve gas quantification in an electronic nose. Chemosensors 2022, 10, 538. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Villalobos, M.C.; Calle, A.; Serradilla, M.J.; Córdoba, M.G.; Hernández, A. Yeasts isolated from figs (Ficus carica L.) as biocontrol agents of postharvest fruit diseases. Food Microbiol. 2016, 57, 45–53. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Hernández, A.; López-Corrales, M.; de Guía Córdoba, M. Physicochemical and sensorial characterisation of four sweet cherry cultivars grown in Jerte Valley (Spain). Food Chem. 2012, 133, 1551–1559. [Google Scholar] [CrossRef]
- Arroyo, P.; Meléndez, F.; Suárez, J.I.; Herrero, J.L.; Rodríguez, S.; Lozano, J. Electronic nose with digital gas sensors connected via Bluetooth to a smartphone for air quality measurements. Sensors 2020, 20, 786. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.Y.; Wei, W.W.; Xi, W.P.; Xu, C.J.; Ferguson, I.; Chen, K. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef]
- Muto, A.; Müller, C.T.; Bruno, L.; McGregor, L.; Ferrante, A.; Chiappetta, A.A.C.; Bitonti, M.B.; Rogers, H.J.; Spadafora, N.D. Fruit volatilome profiling through GC× GC-ToF-MS and gene expression analyses reveal differences amongst peach cultivars in their response to cold storage. Sci. Rep. 2020, 10, 18333–18349. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Ma, R.; Yu, M. Comparison of aroma trait of the white-fleshed peach ‘Hu Jing Mi Lu’ and the yellow-fleshed peach ‘Jin Yuan’ based on odor activity value and odor characteristics. Horticulturae 2022, 8, 245. [Google Scholar] [CrossRef]
- Sánchez, G.; Besada, C.; Badenes, M.; Monforte, A.; Granell, A. A non-targeted approach unravels the volatile network in peach fruit. PLoS ONE 2012, 7, e38992. [Google Scholar] [CrossRef]
- Xin, R.; Liu, X.; Wei, C.; Yang, C.; Liu, H.; Cao, X.; Wu, D.; Zhang, B.; Chen, K. E-nose and GC-MS reveal a difference in the volatile profiles of white- and red-fleshed peach fruit. Sensors 2018, 18, 765. [Google Scholar] [CrossRef]
- Cano-Salazar, J.; Echeverría, G.; Crisosto, C.H.; Lopez, L. Cold-storage potential of four yellow-fleshed peach cultivars defined by their volatile compounds emissions, standard quality parameters, and consumer acceptance. J. Agric. Food Chem. 2012, 60, 1266–1282. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Jia, L.; Yu, W.; Wang, D.; Wei, W.; Li, S.; Tian, S.; Wu, D. Rapid and non-destructive detection of compression damage of yellow peach using an electronic nose and chemometrics. Sensors 2020, 20, 1866. [Google Scholar] [CrossRef]
- Hidayat, W.; Shakaff, A.; Ahmad, M.; Adom, A. Classification of agarwood oil using an electronic nose. Sensors 2010, 10, 4675. [Google Scholar] [CrossRef]
- Li, Z.; Suslick, K. Portable optoelectronic nose for monitoring meat freshness. ACS Sens. 2016, 1, 1330–1335. [Google Scholar] [CrossRef]
- Baldwin, E.; Bai, J.; Plotto, A.; Dea, S. Electronic noses and tongues: Applications for the food and pharmaceutical industries. Sensors 2011, 11, 4744. [Google Scholar] [CrossRef]
- Nakano, R.; Akimoto, H.; Fukuda, F.; Kawai, T.; Ushijima, K.; Fukamatsu, Y.; Kubo, Y.; Fujii, Y.; Hirano, K.; Morinaga, K.; et al. Nondestructive detection of split pit in peaches using an acoustic vibration method. Hortic. J. 2018, 87, 281–287. [Google Scholar] [CrossRef]
- Keerthana, S.; Santhi, B. Survey on applications of electronic nose. J. Comput. Sci. 2020, 16, 314–320. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, J.; Zhu, P. An electronic nose system for the monitoring of water cane shoots quality with swarm clustering algorithm. J. Food Saf. 2020, 41, 12860–12869. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Lv, Z.; Zeng, Q.; Fu, X.; Chen, Q.; Lou, Z.; Lou, C.; Wang, D.; Zhang, W. Analysis of the volatile profiles of kiwifruits experiencing soft rot using E-nose and HS-SPME/GC–MS. LWT 2023, 173, 114405–114413. [Google Scholar] [CrossRef]
- Nouri, B.; Mohtasebi, S.S.; Rafiee, S. Quality detection of pomegranate fruit infected with fungal disease. Int. J. Food Prop. 2020, 23, 9–21. [Google Scholar] [CrossRef]
- Guohua, H.; Yuling, W.; Dandan, Y.; Wenwen, D.; Linshan, Z.; Lvye, W. Study of peach freshness predictive method based on electronic nose. Food Control 2012, 28, 25–32. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Y.; Wu, D.; Wei, Z.; Chen, K. Rapid and non-destructive detection of decay in peach fruit at the cold environment using a self-developed handheld electronic-nose system. Food Anal. Methods 2018, 11, 2990–3004. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, N.; Zhou, D.; Sun, Y.; Sun, K.; Pan, L.; Tu, K. Discrimination and growth tracking of fungi contamination in peaches using electronic nose. Food Chem. 2018, 262, 226–234. [Google Scholar] [CrossRef]
| RT 1 | CD 2 | Volatile Compounds | ID 3 | IK 4 | Mean 5 | RSD (%) 6 | p Values 7 | ||
|---|---|---|---|---|---|---|---|---|---|
| AAU | % | Ps | Pi | ||||||
| Hydrocarbons | 7820 | 2.56 | |||||||
| 4.6 | v10 | Toluene | A | 756 | 814 | 0.24 | 73 | + | |
| 5.7 | v14 | Octane | A | 796 | 128 | 0.03 | 153 | +++ | |
| 8.4 | v20 | Ethylbenzene | A | 855 | 845 | 0.29 | 104 | −−− | |
| 8.7 | v21 | p-Xylene | A | 862 | 4446 | 1.49 | 89 | −−− | |
| 9.7 | v23 | Styrene | A | 883 | 638 | 0.20 | 64 | −− | ++ |
| 14.3 | v28 | Branched hydrocarbon | D | 983 | 682 | 0.19 | 33 | ||
| 36.2 | v46 | Heptadecane | A | 1700 | 267 | 0.12 | 194 | −−− | |
| Alcohols | 3301 | 1.05 | |||||||
| 2.8 | v3 | 1-pentene-3-ol | B | 683 | 159 | 0.05 | 182 | +++ | |
| 3.8 | v7 | 3-methylbutan-1-ol | A | 726 | 2590 | 0.84 | 193 | ||
| 3.9 | v8 | (s)-2-methylbutan-1-ol | A | 730 | 299 | 0.09 | 235 | +++ | +++ |
| 4.7 | v11 | (z)-2-penten-1-ol | B | 759 | 253 | 0.07 | 236 | +++ | +++ |
| Lactones | 3859 | 1.07 | |||||||
| 17 | v34 | γ-Hexanolactone | B | 1045 | 3859 | 1.07 | 28 | − | |
| Esters | 358,843 | 95.16 | |||||||
| 1.7 | v1 | Methyl acetate | A | 522 | 1058 | 0.28 | 94 | ++ | |
| 2.1 | v2 | Ethyl acetate | A | 608 | 39,442 | 8.71 | 106 | + | |
| 3.3 | v4 | Ethyl propionate | B | 707 | 693 | 0.17 | 202 | ||
| 3.4 | v5 | n-propyl acetate | B | 711 | 3160 | 0.89 | 27 | + | −− |
| 3.5 | v6 | Methyl butyrate | B | 715 | 680 | 0.18 | 332 | ||
| 4.4 | v9 | Ethyl 2-methylpropanoate | B | 748 | 220 | 0.06 | 187 | +++ | +++ |
| 4.9 | v12 | Isobutyl acetate | B | 767 | 1249 | 0.38 | 101 | ++ | +++ |
| 5.3 | v13 | Diethyl carbonate | B | 781 | 952 | 0.24 | 86 | +++ | ++ |
| 5.9 | v15 | Ethyl butanoate | A | 802 | 22,211 | 6.11 | 28 | ++ | + |
| 6.5 | v16 | Butyl acetate | A | 815 | 341 | 0.09 | 95 | ||
| 7.8 | v17 | Ethyl (E)-2-butenoate | B | 843 | 1854 | 0.47 | 85 | +++ | +++ |
| 8 | v18 | Ethyl 2-methylbutanoate | B | 847 | 2111 | 0.54 | 94 | +++ | +++ |
| 8.2 | v19 | Ethyl 3-methylbutanoate | B | 851 | 9757 | 2.84 | 38 | ++ | |
| 9.3 | v22 | 3-methylbutyl acetate | B | 874 | 4671 | 1.36 | 91 | ++ | +++ |
| 10.4 | v24 | Ethyl pentanoate | B | 898 | 536 | 0.14 | 25 | +++ | |
| 11 | v25 | Pentyl acetate | B | 911 | 1170 | 0.32 | 103 | +++ | |
| 12.2 | v27 | Ethyl (E)-2-methyl-2-butenoate | B | 937 | 3209 | 0.81 | 77 | +++ | +++ |
| 14.9 | v29 | Ethyl hexanoate | B | 996 | 25,282 | 6.77 | 13 | +++ | −− |
| 15.2 | v30 | Ethyl (Z)-3-hexenoate | B | 1002 | 18,525 | 4.54 | 50 | +++ | |
| 15.5 | v31 | Hexyl acetate | B | 1010 | 917 | 0.24 | 66 | ++ | −− |
| 15.6 | v32 | (Z)-2-hexenyl acetate | B | 1012 | 1800 | 0.44 | 97 | ||
| 16.7 | v33 | Ethyl 2-hexenoate | B | 1038 | 1169 | 0.28 | 65 | +++ | |
| 18.4 | v35 | Unidentified Ester | D | 1079 | 331 | 0.10 | 195 | +++ | +++ |
| 18.8 | v36 | Ethyl (E)-4-heptenoate | B | 1088 | 1854 | 0.51 | 38 | ++ | +++ |
| 19 | v37 | Ethyl heptanoate | B | 1093 | 15,849 | 4.13 | 19 | +++ | −−− |
| 19.6 | v38 | Methyl (Z)-4-octenoate | B | 1115 | 567 | 0.16 | 46 | +++ | |
| 20 | v39 | Methyl octanoate | B | 1132 | 1515 | 0.46 | 59 | ++ | |
| 22.4 | v40 | Ethyl (Z)-4-octenoate | B | 1187 | 46,281 | 12.23 | 19 | +++ | −−− |
| 22.8 | v41 | Ethyl octanoate | B | 1199 | 142,677 | 39.63 | 21 | +++ | −−− |
| 24.5 | v42 | Ethyl 2-octenoate | B | 1243 | 1117 | 0.32 | 50 | −−− | |
| 26.2 | v43 | Ethyl nonanoate | B | 1319 | 1866 | 0.46 | 47 | +++ | −−− |
| 29 | v44 | Ethyl (E)-4-decenoate | C | 1385 | 3057 | 0.71 | 105 | − | |
| 29.5 | v45 | Ethyl decanoate | B | 1391 | 2722 | 0.59 | 101 | +++ | −−− |
| Phenol | 608 | 0.19 | |||||||
| 11.2 | v26 | Methoxyphenyl-oxime | C | 915 | 608 | 0.19 | 163 | +++ | |
| Cluster | MOX 1,2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VOCs | M_1 | M_2 | M_3 | M_4 | M_5 | M_6 | M_7 | M_8 | M_9 | M_10 | M_11 | |
| 1 | Toluene | - | - | - | + | - | - | |||||
| 1 | Methyl octanoate | + | - | - | + | + | - | |||||
| 1 | n-Propyl acetate | - | + | - | - | + | - | -- | ||||
| 2 | Diethyl carbonate | ++ | - | -- | + | + | -- | -- | ++ | ++ | ||
| 2 | Ethyl (E)-2-butenoate | ++ | - | -- | + | + | -- | -- | ++ | + | ||
| 2 | Ethyl 2-methylbutanoate | ++ | - | -- | + | -- | -- | ++ | + | |||
| 2 | Ethyl pentanoate | ++ | - | + | - | -- | ++ | |||||
| 2 | Ethyl (E)-2-methyl-2-butenoate | ++ | - | + | -- | - | ++ | + | ||||
| 2 | Ethyl (Z)-3-hexenoate | + | - | ++ | ++ | -- | ++ | - | - | ++ | ||
| 2 | Ethyl 2-hexenoate | ++ | -- | -- | ++ | ++ | -- | -- | ++ | - | - | ++ |
| 3 | Octane | - | ||||||||||
| 3 | Ethyl acetate | - | + | - | - | |||||||
| 3 | Pentyl acetate | + | ||||||||||
| 3 | (Z)-2-Hexenyl acetate | - | - | |||||||||
| 3 | Unidentified Ester | - | ||||||||||
| 3 | Ethyl decanoate | + | + | |||||||||
| 3 | Ethyl 2-methylpropanoate | - | ||||||||||
| 4 | Ethylbenzene | -- | ++ | + | -- | -- | ++ | + | -- | ++ | ++ | -- |
| 4 | p-Xylene | -- | ++ | + | -- | -- | ++ | + | -- | ++ | ++ | -- |
| 4 | Styrene | - | + | + | -- | -- | ++ | -- | ++ | ++ | -- | |
| 4 | γ-Hexanolactone | -- | + | ++ | - | - | + | ++ | -- | + | + | |
| 4 | Ethyl octanoate | -- | ++ | ++ | -- | -- | ++ | ++ | -- | ++ | ++ | -- |
| 4 | Ethyl 2-octenoate | -- | ++ | + | -- | -- | ++ | ++ | -- | + | + | -- |
| 4 | Heptadecane | -- | ++ | ++ | -- | -- | ++ | ++ | -- | ++ | ++ | -- |
| Samples | Selected Variable | Count | Peach Batches | Total | |
|---|---|---|---|---|---|
| Control | Monilinia | ||||
| Early stage | TVOCCCS811 | n | 31 | 16 | 47 |
| TVOCiAQ | Computed classes | ||||
| TVOCSGP30 | Class 1 | 31 | 16 | 47 | |
| CO2CCS811 | % 2 | 100 | 100 | 100 | |
| CO2SGP30 | Predicted classes | ||||
| Class 1 | 31 | 16 | 47 | ||
| % 2 | 100 | 100 | 100 | ||
| All | CO2CCS811 | n | 43 | 28 | 71 |
| CO2iAQ | Computed classes | ||||
| TVOCCCS811 | Class 1 | 43 | 26 | 69 | |
| CO2SGP30 | % 2 | 100 | 92.9 | 97.2 | |
| Predicted classes | |||||
| Class 1 | 43 | 26 | 69 | ||
| %2 | 100 | 92.9 | 97.2 | ||
| Samples | Selected Variable | Count | Peach Batches | Total | ||
|---|---|---|---|---|---|---|
| Control | Monilinia | |||||
| S_1 | S_2 | |||||
| All | TVOCCCS811 | n | 43 | 16 | 12 | 71 |
| TVOCiAQ | Computed classes | |||||
| TVOCSGP30 | Class 1 | 43 | 15 | 12 | 70 | |
| CO2CCS811 | % 2 | 100 | 93.8 | 100 | 98.6 | |
| CO2SGP30 | Predicted classes | |||||
| Class 1 | 42 | 15 | 12 | 69 | ||
| % 2 | 97.7 | 93.8 | 100 | 97.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, A.; Hernández, A.; Arroyo, P.; Lozano, J.; Córdoba, M.d.G.; Martín, A. Early Detection of Monilinia laxa in Yellow-Fleshed Peach Using a Non-Destructive E-Nose Approach. Foods 2025, 14, 3155. https://doi.org/10.3390/foods14183155
Martínez A, Hernández A, Arroyo P, Lozano J, Córdoba MdG, Martín A. Early Detection of Monilinia laxa in Yellow-Fleshed Peach Using a Non-Destructive E-Nose Approach. Foods. 2025; 14(18):3155. https://doi.org/10.3390/foods14183155
Chicago/Turabian StyleMartínez, Ana, Alejandro Hernández, Patricia Arroyo, Jesús Lozano, María de Guía Córdoba, and Alberto Martín. 2025. "Early Detection of Monilinia laxa in Yellow-Fleshed Peach Using a Non-Destructive E-Nose Approach" Foods 14, no. 18: 3155. https://doi.org/10.3390/foods14183155
APA StyleMartínez, A., Hernández, A., Arroyo, P., Lozano, J., Córdoba, M. d. G., & Martín, A. (2025). Early Detection of Monilinia laxa in Yellow-Fleshed Peach Using a Non-Destructive E-Nose Approach. Foods, 14(18), 3155. https://doi.org/10.3390/foods14183155








