Free and Immobilized Cells of Torulaspora delbrueckii and Lachancea thermotolerans in Sparkling Wine: Innovative Application in Secondary Bottle Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Immobilization Procedure
2.3. Elaboration of Sparkling Wine
2.4. Pressure and Biomass Evolution
2.5. Analytical Determination and the Quantification of the Main Volatile Compounds
2.6. Sensorial Analysis
2.7. Statistical Analysis
3. Results
3.1. Fermentation Kinetics and Biomass Evolution
3.2. The Main Analytical Characters
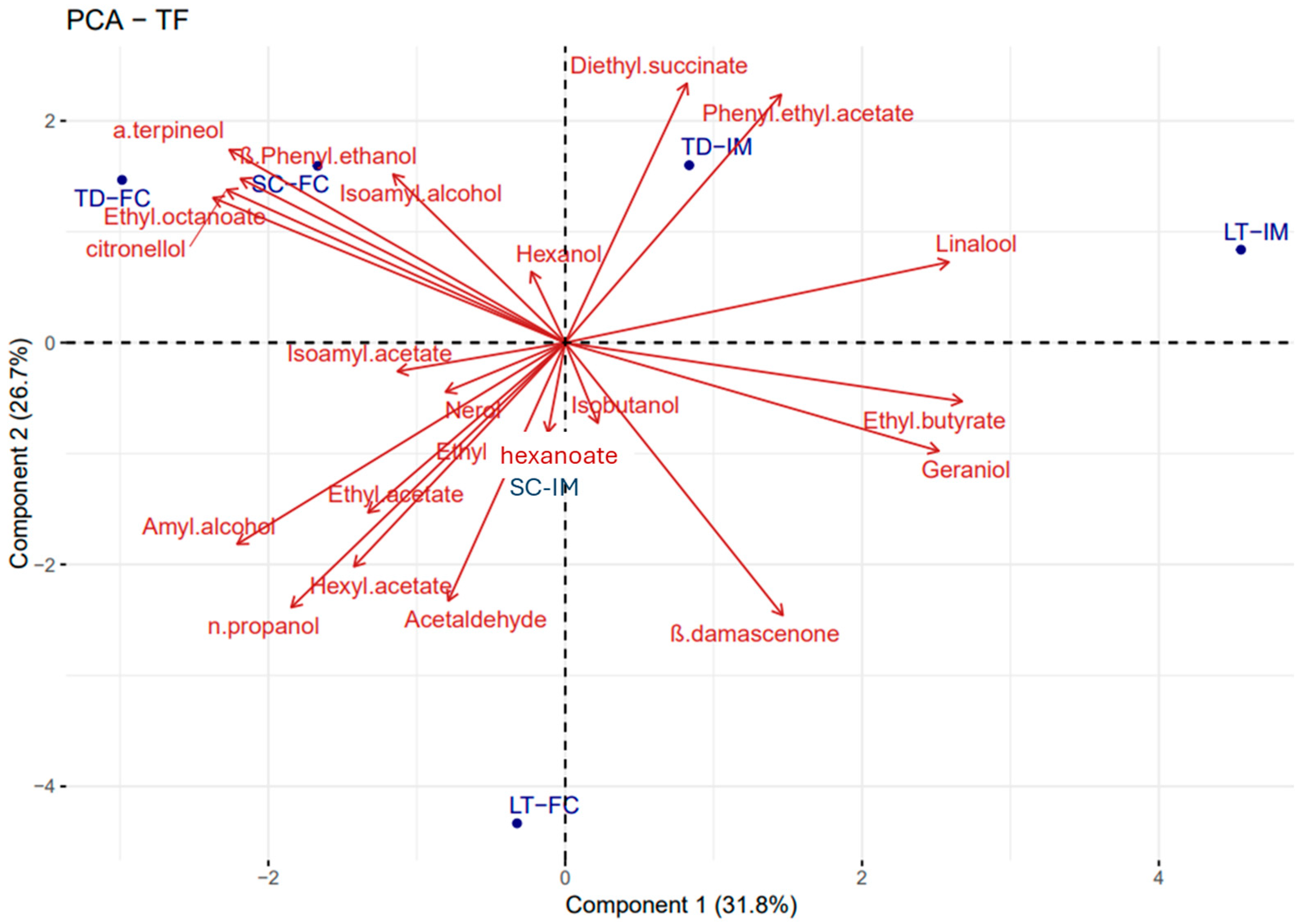
3.3. The Evolution of the Main Volatile Compounds
3.4. Sensorial Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexandre, H.; Guilloux-Benatier, M. Yeast Autolysis in Sparkling Wine—A Review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Bartra, E. Aspectos Microbiológicos de La Elaboración Del Cava. Microbiología 1995, 11, 43–50. [Google Scholar] [PubMed]
- Benucci, I.; Cerreti, M.; Maresca, D.; Mauriello, G.; Esti, M. Yeast Cells in Double Layer Calcium Alginate–Chitosan Microcapsules for Sparkling Wine Production. Food Chem. 2019, 300, 125174. [Google Scholar] [CrossRef]
- Maertinez-Rordriguez, A.J.; Polo, M.C. Enological aspects of yeast autolysis. Recent Res. Dev. Microbiol. 2000, 4, 285–301. [Google Scholar]
- Prokes, K.; Baron, M.; Mlcek, J.; Jurikova, T.; Adamkova, A.; Ercisli, S.; Sochor, J. The Influence of Traditional and Immobilized Yeast on the Amino-Acid Content of Sparkling Wine. Fermentation 2022, 8, 36. [Google Scholar] [CrossRef]
- Torresi, S.; Frangipane, M.T.; Anelli, G. Biotechnologies in Sparkling Wine Production. Interesting Approaches for Quality Improvement: A Review. Food Chem. 2011, 129, 1232–1241. [Google Scholar] [CrossRef]
- Karel, S.F.; Libicki, S.B.; Robertson, C.R. The Immobilization of Whole Cells: Engineering Principles. Chem. Eng. Sci. 1985, 40, 1321–1354. [Google Scholar] [CrossRef]
- Fernández-Fernández, E.; Rodríguez-Nogales, J.M.; Vila-Crespo, J.; Falqué-López, E. Application of Immobilized Yeasts for Improved Production of Sparkling Wines. Fermentation 2022, 8, 559. [Google Scholar] [CrossRef]
- Gòdia, F.; Casas, C.; Solàg, C. Application of Immobilized Yeast Cells to Sparkling Wine Fermentation. Biotechnol. Prog. 1991, 7, 468–470. [Google Scholar] [CrossRef]
- López de Lerma Extremera, N.; Peinado Amores, R.A.; Puig-Pujol, A.; García Mauricio, J.C.; Moreno Vigara, J.J.; García-Martínez, T. Influence of Two Yeast Strains in Free, Bioimmobilized or Immobilized with Alginate Forms on the Aromatic Profile of Long Aged Sparkling Wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef]
- Călugăr, P.C.; Coldea, T.E.; Pop, C.-R.; Stan, L.; Gal, E.; Ranga, F.; Mihai, M.; Hegheș, S.C.; Geană, E.-I.; Mudura, E. Impact of Encapsulated Saccharomyces Cerevisiae Yeasts on the Chemical and Sensory Profiles of Sparkling Cider Produced by the Champenoise Method. Agronomy 2024, 14, 1036. [Google Scholar] [CrossRef]
- Catrileo, D.; Acuña-Fontecilla, A.; Godoy, L. Adaptive Laboratory Evolution of Native Torulaspora delbrueckii YCPUC10 With Enhanced Ethanol Resistance and Evaluation in Co-Inoculated Fermentation. Front. Microbiol. 2020, 11, 595023. [Google Scholar] [CrossRef]
- Guzzon, R.; Nardin, T.; Larcher, R. The Controversial Relationship between Chitosan and the Microorganisms Involved in the Production of Fermented Beverages. Eur. Food Res. Technol. 2022, 248, 751–765. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii for Secondary Fermentation in Sparkling Wine Production. Food Microbiol. 2018, 74, 100–106. [Google Scholar] [CrossRef]
- Ivit, N.N.; Kemp, B. The Impact of Non-Saccharomyces Yeast on Traditional Method Sparkling Wine. Fermentation 2018, 4, 73. [Google Scholar] [CrossRef]
- Medina-Trujillo, L.; González-Royo, E.; Sieczkowski, N.; Heras, J.; Canals, J.M.; Zamora, F. Effect of Sequential Inoculation (Torulaspora delbrueckii/Saccharomyces cerevisiae) in the First Fermentation on the Foaming Properties of Sparkling Wine. Eur. Food Res. Technol. 2017, 243, 681–688. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Dominance and Influence of Selected Saccharomyces cerevisiae Strains on the Analytical Profile of Craft Beer Refermentation: Dominance and Influence of Selected S. Cerevisiae Strains. J. Inst. Brew. 2014, 120, 262–267. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Recycled Brewer’s Spent Grain (BSG) and Grape Juice: A New Tool for Non-Alcoholic (NAB) or Low-Alcoholic (LAB) Craft Beer Using Non-Conventional Yeasts. Foods 2024, 13, 505. [Google Scholar] [CrossRef]
- Compendium of International Methods of Wine and Must Analysis|OIV. Available online: https://www.oiv.int/standards/compendium-of-international-methods-of-wine-and-must-analysis (accessed on 17 July 2025).
- Alfonzo, A.; Francesca, N.; Mercurio, V.; Prestianni, R.; Settanni, L.; Spanò, G.; Naselli, V.; Moschetti, G. Use of Grape Racemes from Grillo Cultivar to Increase the Acidity Level of Sparkling Base Wines Produced with Different Saccharomyces cerevisiae Strains. Yeast 2020, 37, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Di Gianvito, P.; Perpetuini, G.; Tittarelli, F.; Schirone, M.; Arfelli, G.; Piva, A.; Patrignani, F.; Lanciotti, R.; Olivastri, L.; Suzzi, G.; et al. Impact of Saccharomyces cerevisiae Strains on Traditional Sparkling Wines Production. Food Res. Int. 2018, 109, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Berbegal, C.; Grieco, F.; Tufariello, M.; Spano, G.; Capozzi, V. Selection of Indigenous Yeast Strains for the Production of Sparkling Wines from Native Apulian Grape Varieties. Int. J. Food Microbiol. 2018, 285, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Vigentini, I.; Gonzalez, R.; Tronchoni, J. Genetic Improvement of Wine Yeasts. In Yeasts in the Production of Wine; Romano, P., Ciani, M., Fleet, G.H., Eds.; Springer: New York, NY, USA, 2019; pp. 315–342. ISBN 978-1-4939-9782-4. [Google Scholar]
- Martínez-Rodríguez, A.; Carrascosa, A.V.; Barcenilla, J.M.; Angeles Pozo-Bayón, M.; Carmen Polo, M. Autolytic Capacity and Foam Analysis as Additional Criteria for the Selection of Yeast Strains for Sparkling Wine Production. Food Microbiol. 2001, 18, 183–191. [Google Scholar] [CrossRef]
- Guzzon, R.; Paolini, M.; Malacarne, M.; Roman, T.; Naselli, V.; Francesca, N.; Larcher, R. Use of Non-Saccharomyces Yeasts in the Prise de Mousse of Lambrusco. Microbial Evolution through Alcoholic Fermentation and Effect on Wine Volatile Profile. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Voce, S.; Bortolini, A.; Tat, L.; Natolino, A.; Comuzzo, P. Use of Emerging Technologies and Non-Saccharomyces Spp. for Tailoring the Composition of Yeast Derivatives: Effect on White Wine Aging. Foods 2025, 14, 652. [Google Scholar] [CrossRef] [PubMed]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological Consequences of Sequential Inoculation with Non-Saccharomyces Yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in Base Wine for Sparkling Wine Production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Rossetti, A.P.; Gaggiotti, S.; Piva, A.; Olivastri, L.; Cichelli, A.; Compagnone, D.; Arfelli, G. Impact of Saccharomyces cerevisiae and Non-Saccharomyces Yeasts to Improve Traditional Sparkling Wines Production. Food Microbiol. 2022, 108, 104097. [Google Scholar] [CrossRef]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile Profile of Reduced Alcohol Wines Fermented with Selected Non-Saccharomyces Yeasts under Different Aeration Conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef]
- Ogawa, M.; Carmona-Jiménez, P.; García-Martínez, T.; Jorrín-Novo, J.V.; Moreno, J.; Rey, M.D.; Moreno-García, J. Use of Yeast Biocapsules as a Fungal-Based Immobilized Cell Technology for Indian Pale Ale-Type Beer Brewing. Appl. Microbiol. Biotechnol. 2022, 106, 7615–7625. [Google Scholar] [CrossRef]
- Puig-Pujol, A.; Bertran, E.; García-Martínez, T.; Capdevila, F.; Mínguez, S.; Mauricio, J.C. Application of a New Organic Yeast Immobilization Method for Sparkling Wine Production. Am. J. Enol. Vitic. 2013, 64, 386–394. [Google Scholar] [CrossRef]
- Fumi, M.D.; Trioli, G.; Colombi, M.G.; Colagrande, O. Immobilization of Saccharomyces cerevisiae in Calcium Alginate Gel and Its Application to Bottle-Fermented Sparkling Wine Production. Am. J. Enol. Vitic. 1988, 39, 267–272. [Google Scholar] [CrossRef]
- Moreno-García, J.; García-Martínez, T.; Mauricio, J.C.; Moreno, J. Yeast Immobilization Systems for Alcoholic Wine Fermentations: Actual Trends and Future Perspectives. Front. Microbiol. 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed]
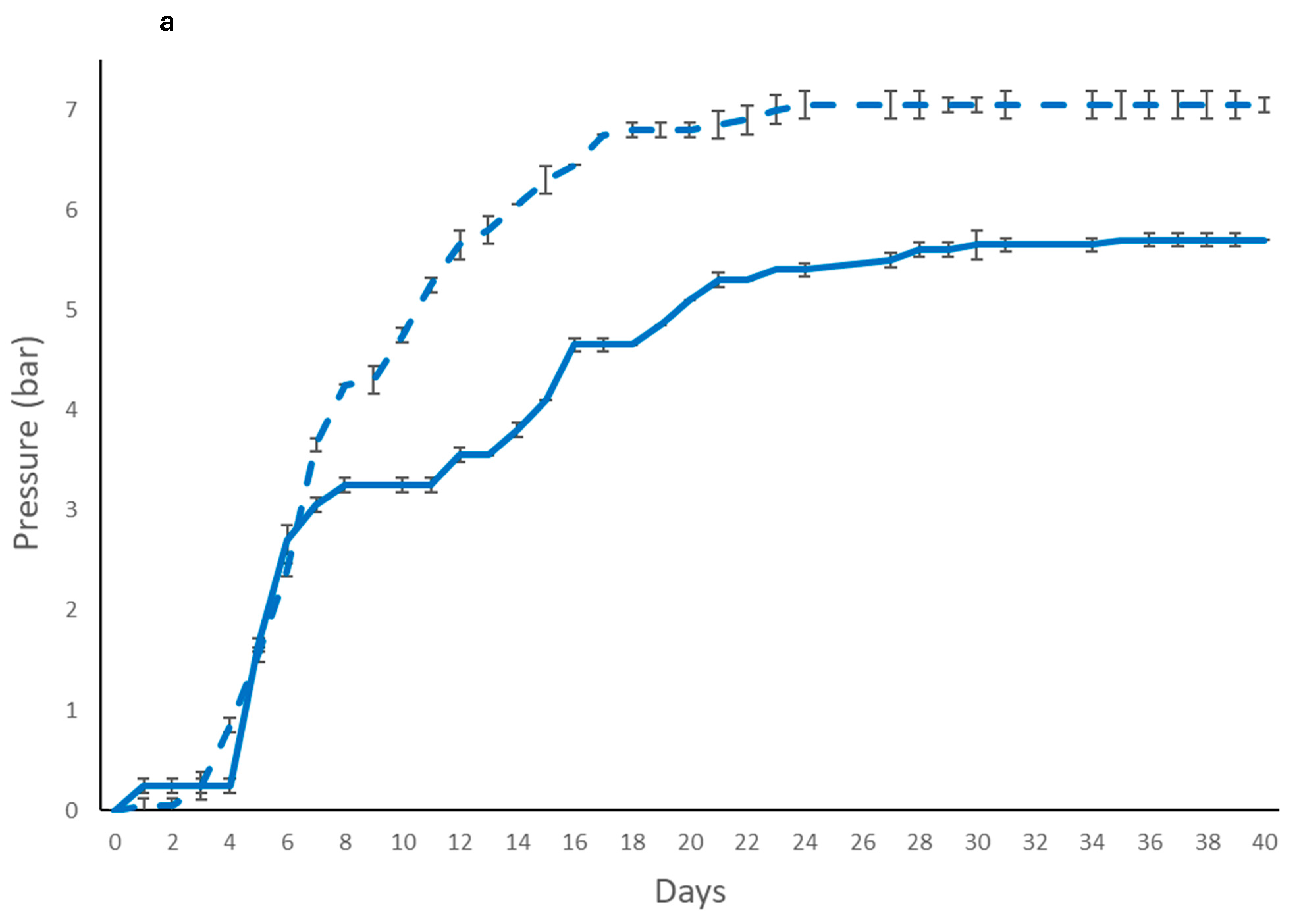
 ) and immobilized (
) and immobilized (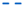 ); T. delbrueckii DiSVA 130: (b) free(
); T. delbrueckii DiSVA 130: (b) free( ) and immobilized (
) and immobilized ( ); and L. thermotolerans DiSVA 322: (c) free (
); and L. thermotolerans DiSVA 322: (c) free ( ) and immobilized (
) and immobilized (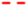 ).
).
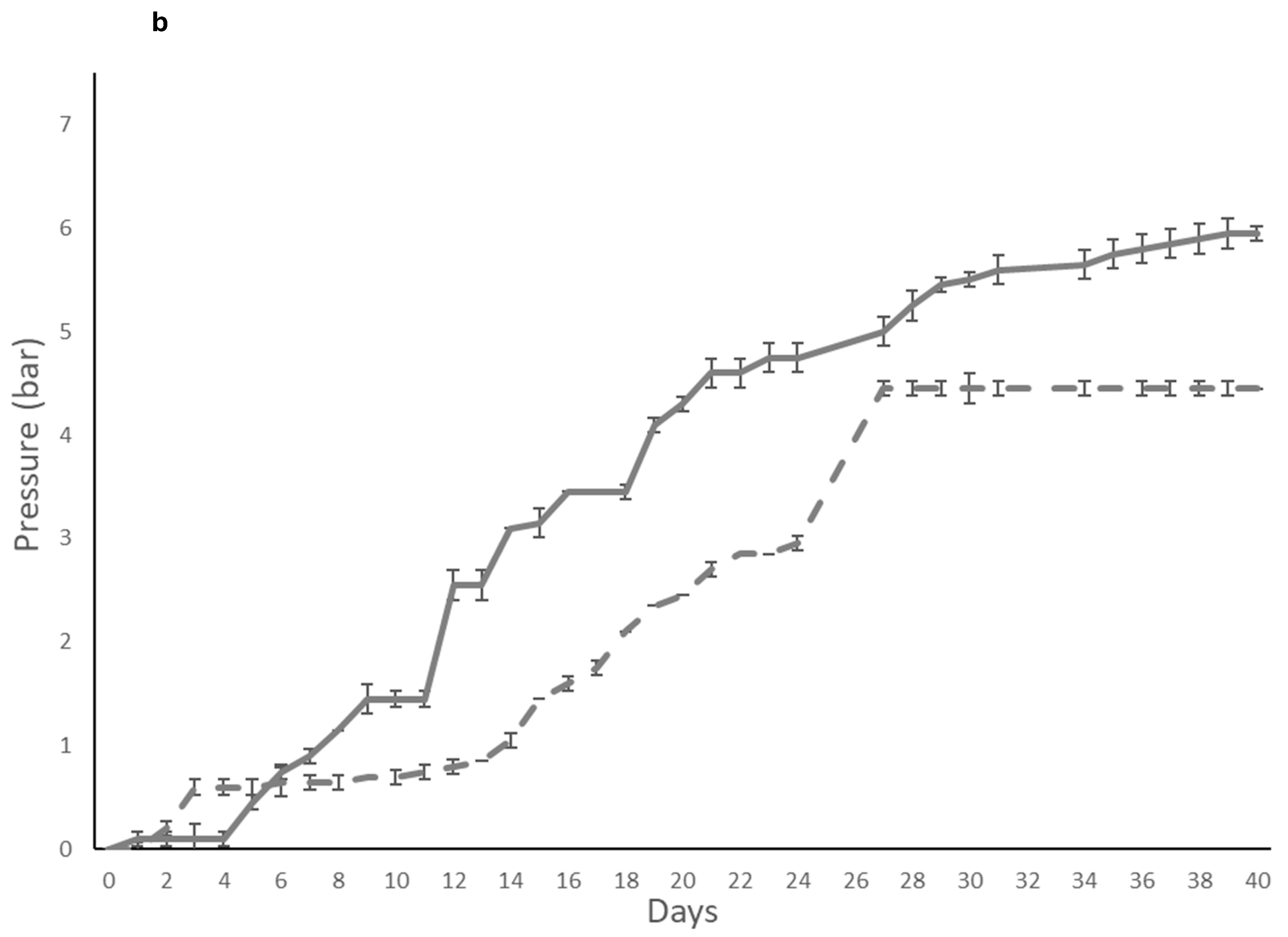
 ) and immobilized (
) and immobilized (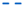 ); T. delbrueckii DiSVA 130: (b) free(
); T. delbrueckii DiSVA 130: (b) free( ) and immobilized (
) and immobilized ( ); and L. thermotolerans DiSVA 322: (c) free (
); and L. thermotolerans DiSVA 322: (c) free ( ) and immobilized (
) and immobilized (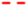 ).
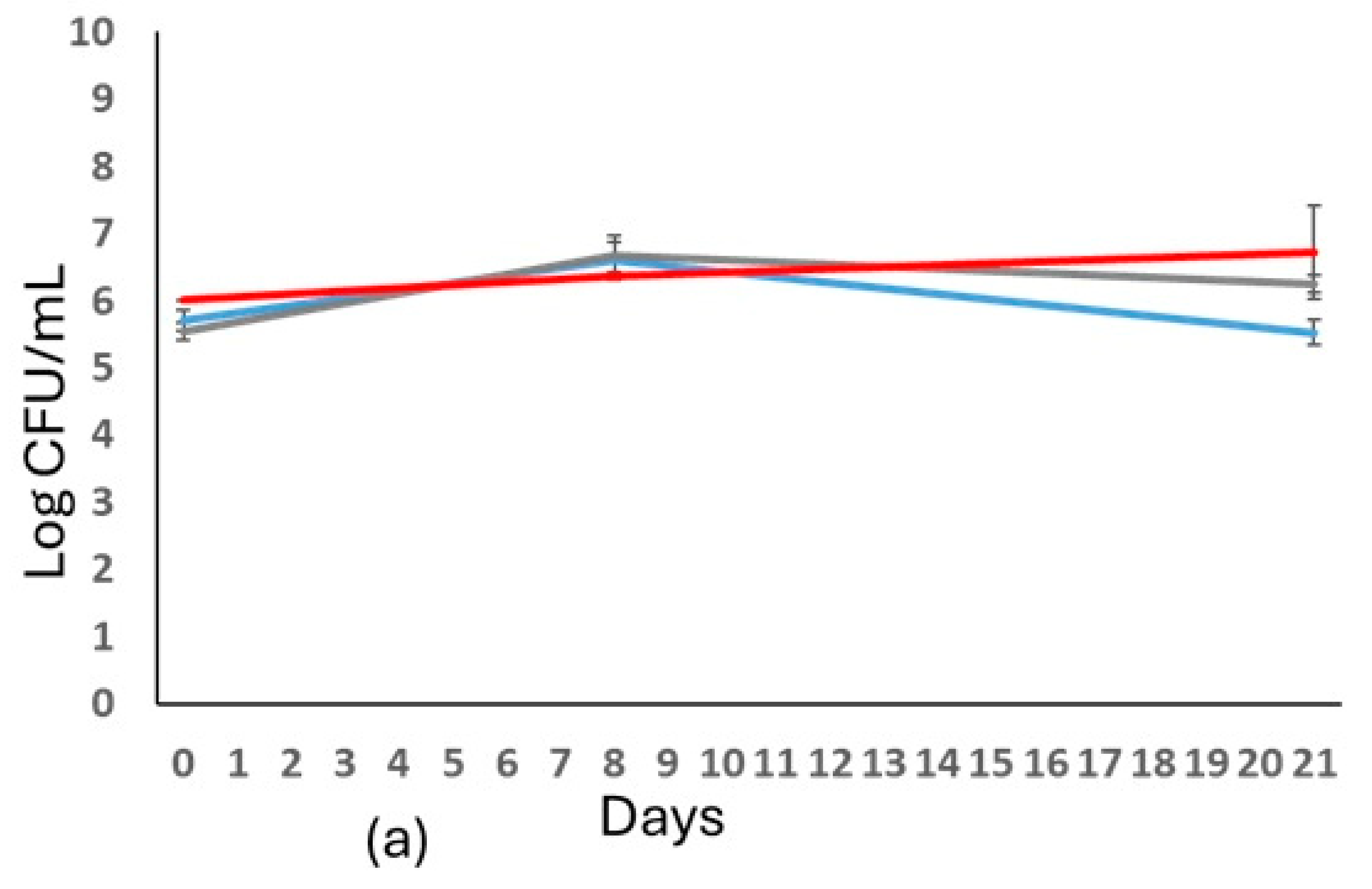
).
 ); T. delbrueckii (
); T. delbrueckii ( ); L. thermotolerans (
); L. thermotolerans ( ). In (b): S. cerevisiae DiSVA 554 cell released (
). In (b): S. cerevisiae DiSVA 554 cell released ( ); T. delbrueckii DiSVA 130 cell released (
); T. delbrueckii DiSVA 130 cell released ( ); and L. thermotolerans DiSVA 322 cell released (
); and L. thermotolerans DiSVA 322 cell released ( ). S. cerevisiae immobilized (
). S. cerevisiae immobilized (  ); T. delbrueckii immobilized (
); T. delbrueckii immobilized ( ); L. thermotolerans immobilized (
); L. thermotolerans immobilized ( ).
).
 ); T. delbrueckii (
); T. delbrueckii ( ); L. thermotolerans (
); L. thermotolerans ( ). In (b): S. cerevisiae DiSVA 554 cell released (
). In (b): S. cerevisiae DiSVA 554 cell released ( ); T. delbrueckii DiSVA 130 cell released (
); T. delbrueckii DiSVA 130 cell released ( ); and L. thermotolerans DiSVA 322 cell released (
); and L. thermotolerans DiSVA 322 cell released ( ). S. cerevisiae immobilized (
). S. cerevisiae immobilized (  ); T. delbrueckii immobilized (
); T. delbrueckii immobilized ( ); L. thermotolerans immobilized (
); L. thermotolerans immobilized ( ).
).
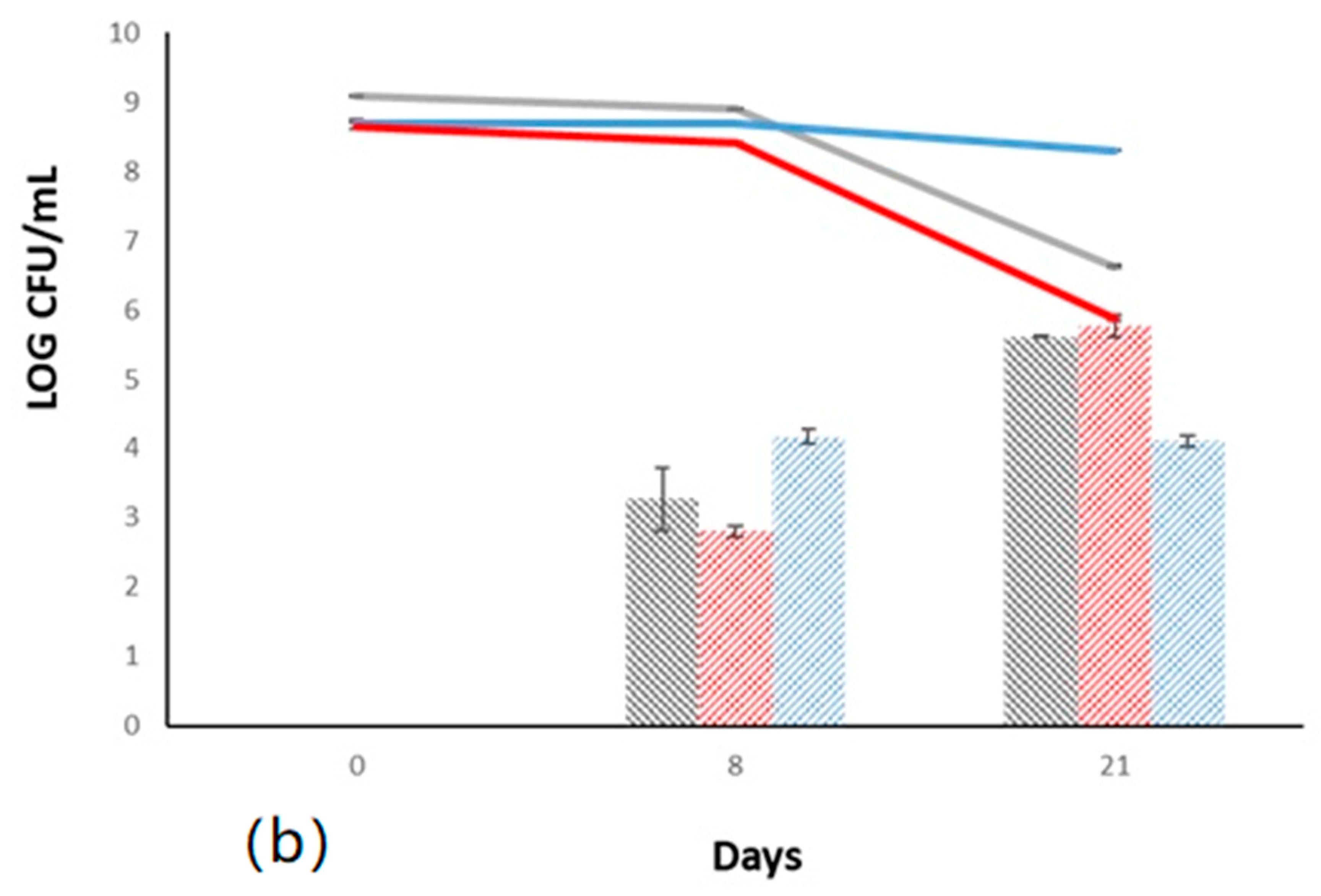
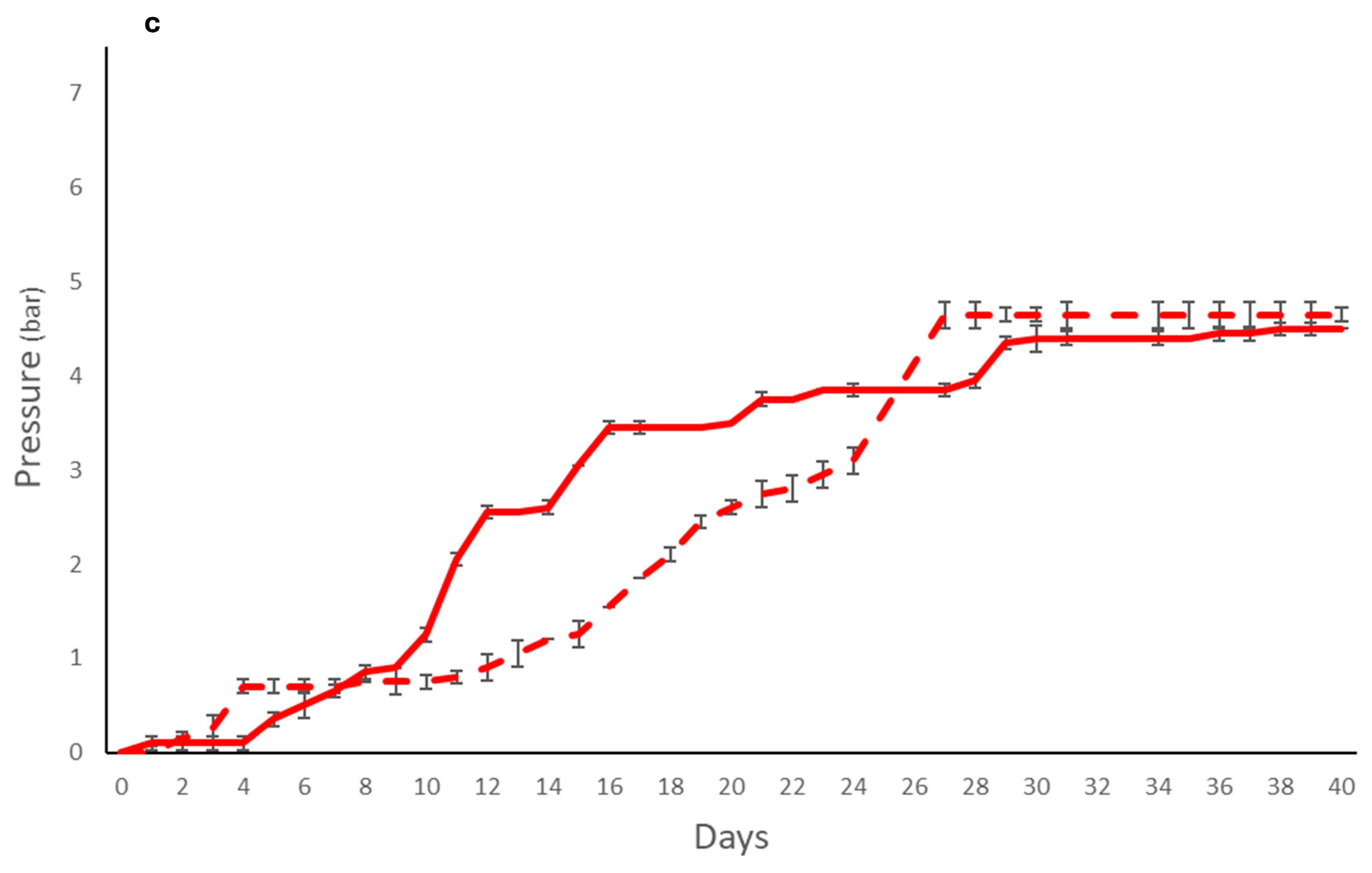
 ) and immobilized (
) and immobilized ( ); T. delbrueckii DiSVA, 130 free (
); T. delbrueckii DiSVA, 130 free ( ) and immobilized (
) and immobilized ( ); L. thermotolerans DiSVA 322, free (
); L. thermotolerans DiSVA 322, free ( ) and immobilized (
) and immobilized ( ). Note: * significantly different (Fisher ANOVA; p-value 0.05).
). Note: * significantly different (Fisher ANOVA; p-value 0.05).
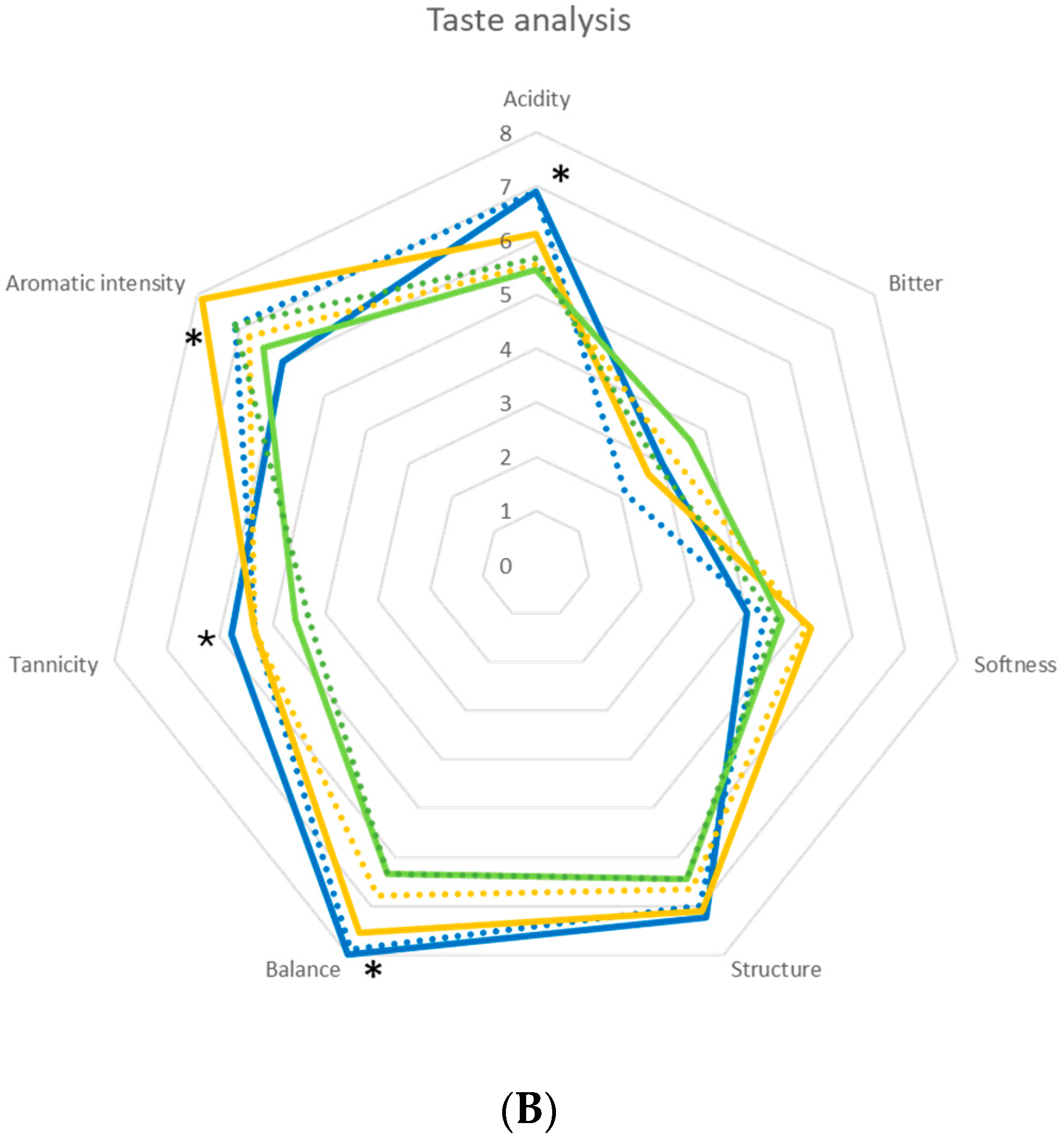
 ) and immobilized (
) and immobilized ( ); T. delbrueckii DiSVA, 130 free (
); T. delbrueckii DiSVA, 130 free ( ) and immobilized (
) and immobilized ( ); L. thermotolerans DiSVA 322, free (
); L. thermotolerans DiSVA 322, free ( ) and immobilized (
) and immobilized ( ). Note: * significantly different (Fisher ANOVA; p-value 0.05).
). Note: * significantly different (Fisher ANOVA; p-value 0.05).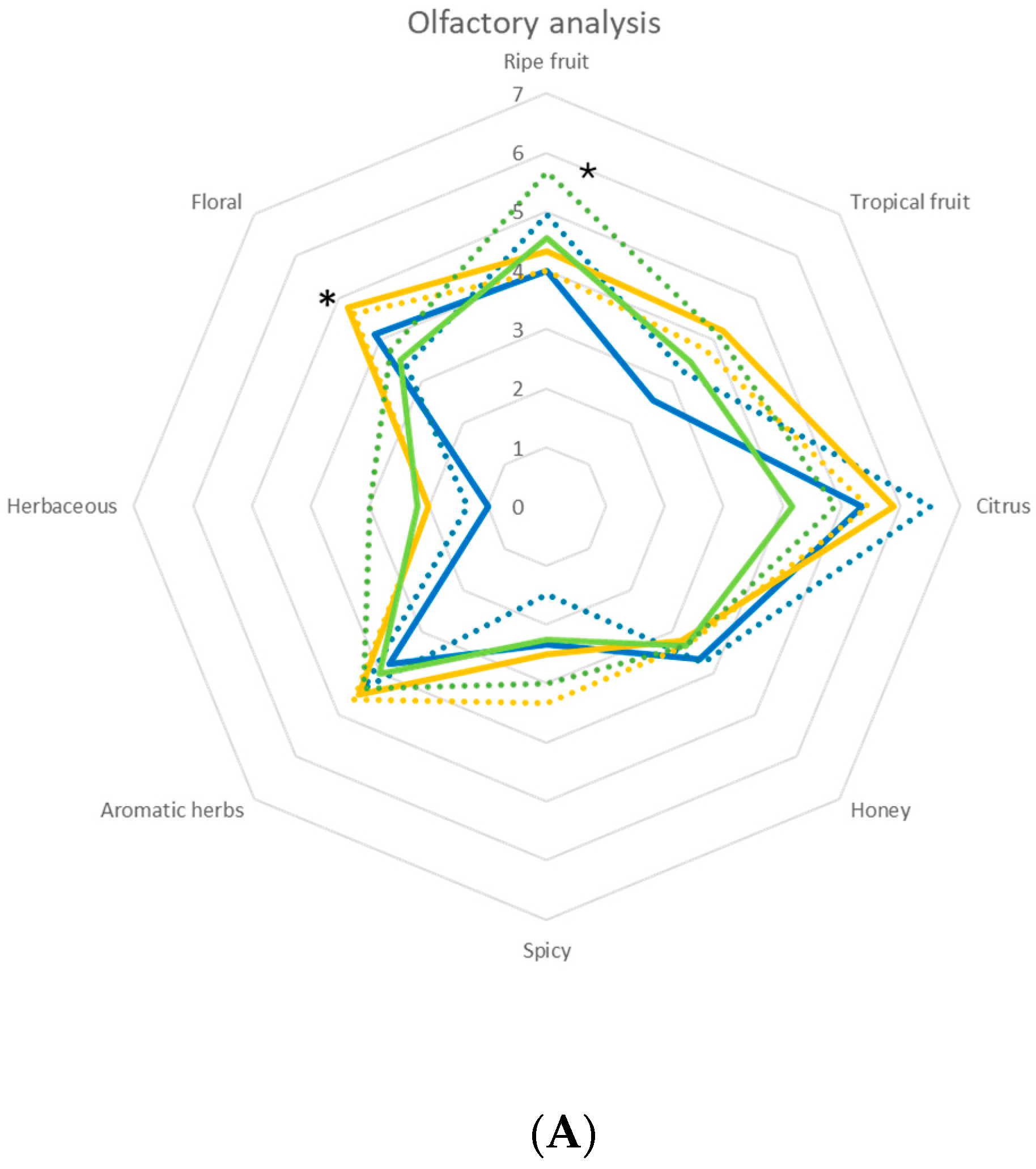
| Free Cells | Ethanol (%v/v) | Total Acidity (as Tartaric Acid g/L) | pH | Volatile Acidity (as Acetic Acid g/L) | Sugar Content (g/L) |
| S. cerevisiae | 11.92 ± 0.00 a | 4.36 ± 0.00 c | 3.57 ± 0.00 a | 0.49 ± 0.00 c | 1.04 ± 0.00 c |
| T. delbrueckii | 11.92 ± 0.00 a | 4.66 ± 0.00 b | 3.51 ± 0.00 c | 0.51 ± 0.00 b | 1.25 ± 0.00 b |
| L. thermotolerans | 11.90 ± 0.00 a | 4.61 ± 0.00 b | 3.53 ± 0.00 b | 0.51 ± 0.00 b | 1.33 ± 0.00 b |
| Immobilized Cells | Ethanol (%v/v) | Total Acidity (as Tartaric Acid g/L) | pH | Volatile Acidity (as Acetic Acid g/L) | Sugar Content (g/L) |
| S. cerevisiae | 11.91 ± 0.02 a | 5.68 ± 0.03 a | 3.42 ± 0.01 d | 0.42 ± 0.00 d | 1.32 ± 0.00 b |
| T. delbrueckii | 11.99 ± 0.03 a | 4.37 ± 0.04 c | 3.52 ± 0.00 bc | 0.54 ± 0.01 a | 3.38 ± 0.02 a |
| L. thermotolerans | 11.98 ± 0.02 a | 4.31 ± 0.04 c | 3.52 ± 0.01 b | 0.54 ± 0.01 a | 3.45 ± 0.08 a |
| Free Cells | S. cerevisiae T8 | S. cerevisiae T21 | S. cerevisiae TF |
|---|---|---|---|
| Esters (mg/L) | |||
| Ethyl butyrate | 0.133 ± 0.017 a | 0.062 ± 0.007 b | 0.042 ± 0.018 b |
| Ethyl acetate | 18.06 ± 2.30 b | 22.64 ± 0.12 a | 11.57 ± 0.20 c |
| Phenyl ethyl acetate | 0.044 ± 0.004 a | 0.014 ± 0.002 b | 0.038 ± 0.003 a |
| Ethyl hexanoate | 0.062 ± 0.004 b | 0.096 ± 0.003 a | 0.018 ± 0.000 c |
| Ethyl octanoate | 0.017 ± 0.001 a | 0.007 ± 0.001 b | 0.005 ± 0.000 b |
| Isoamyl acetate | 15.97 ± 0.06 b | 17.94 ± 0.40 a | 14.73 ± 0.15 c |
| Hexyl acetate | 0.002 ± 0.001 a | 0.001 ± 0.000 a | 0.003 ± 0.001 a |
| Diethyl succinate | 0.004 ± 0.000 a | 0.008 ± 0.000 a | 0.016 ± 0.000 a |
| Alcohols (mg/L) | |||
| n-Propanol | 28.64 ± 1.24 a | 29.50 ± 0.42 a | 22.43 ± 0.23 b |
| Isobutanol | 7.43 ± 0.16 b | 10.79 ± 0.01 a | 4.65 ± 0.26 c |
| Amyl alcohol | 3.97 ± 0.11 b | 6.25 ± 0.44 a | 3.03 ± 0.90 b |
| Isoamylic alcohol | 79.40 ± 4.70 a | 80.39 ± 0.035 a | 63.61 ± 0.10 b |
| β-Phenyl ethanol | 60.25 ± 0.13 b | 73.33 ± 0.26 a | 63.12 ± 0.29 b |
| Hexanol | 0.050 ± 0.016 b | 0.095 ± 0.006 a | 0.029 ± 0.002 b |
| Carbonyl compounds(mg/L) | |||
| Acetaldehyde | 41.13 ± 0.60 a | 14.38 ± 0.10 b | 1.40 ± 0.53 c |
| Monoterpenes | |||
| Linalool | 0.020 ± 0.013 ab | 0.043 ± 0.003 a | 0.013 ± 0.001 b |
| Geraniol | 0.023 ± 0.002 a | 0.002 ± 0.000 b | 0.004 ± 0.002 b |
| Nerol | 0.014 ± 0.008 a | 0.007 ± 0.000 a | 0.006 ± 0.000 a |
| α-terpineol | 0.071 ± 0.045 a | 0.000 ± 0.000 a | 0.080 ± 0.001 a |
| Citronellol | 0.026 ± 0.001 b | 0.030 ± 0.008 b | 0.088 ± 0.001 a |
| Norisoprenoids (mg/L) | |||
| β-damascenone | 0.006 ± 0.001 a | 0.003 ± 0.000 b | 0.003 ± 0.000 b |
| Immobilized Cells | S. cerevisiae T8 | S. cerevisiae T21 | S. cerevisiae TF |
| Esters (mg/L) | |||
| Ethyl butyrate | 0.013 ± 0.000 a | 0.020 ± 0.007 a | 0.067 ± 0.047 a |
| Ethyl acetate | 5.59 ± 0.65 b | 11.47 ± 1.58 a | 14.42 ± 0.28 a |
| Phenyl ethyl acetate | 0.013 ± 0.002 b | 0.055 ± 0.007 a | 0.020 ± 0.000 b |
| Ethyl hexanoate | 0.199 ± 0.196 a | 0.228 ± 0.017 a | 0.132 ± 0.004 a |
| Ethyl octanoate | 0.004 ± 0.000 a | 0.006 ± 0.001 a | 0.005 ± 0.000 a |
| Isoamyl acetate | 5.71 ± 1.21 b | 7.82 ± 0.20 ab | 10.14 ± 0.38 a |
| Hexyl acetate | 0.002 ± 0.000 a | 0.001 ± 0.000 a | 0.002 ± 0.000 a |
| Diethyl succinate | 0.022 ± 0.001 a | 0.018 ± 0.002 ab | 0.015 ± 0.000 b |
| Alcohols (mg/L) | |||
| n-Propanol | 25.42 ± 1.69 a | 24.94 ± 0.23 a | 23.20 ± 0.67 a |
| Isobutanol | 6.16 ± 0.08 b | 7.71 ± 0.11 a | 7.67 ± 0.16 a |
| Amyl alcohol | 15.61 ± 1.19 a | 13.55 ± 1.13 a | 4.12 ± 0.10 b |
| Isoamylic alcohol | 52.58 ± 4.42 b | 36.55 ± 0.28 c | 67.53 ± 0.63 a |
| β-Phenyl ethanol | 72.50 ± 0.73 a | 59.54 ± 0.54 a | 59.25 ± 0.48 a |
| Hexanol | 0.004 ± 0.001 c | 0.051 ± 0.003 b | 0.067 ± 0.001 a |
| Carbonyl compounds (mg/L) | |||
| Acetaldehyde | 4.44 ± 0.23 b | 8.39 ± 0.13 a | 1.80 ± 0.09 c |
| Monoterpenes | |||
| Linalool | 0.023 ± 0.007 a | 0.007 ± 0.000 b | 0.005 ± 0.000 b |
| Geraniol | 0.006 ± 0.001 b | 0.019 ± 0.001 a | 0.017 ± 0.002 a |
| Nerol | 0.024 ± 0.002 a | 0.007 ± 0.000 b | 0.004 ± 0.000 b |
| α-terpineol | 0.064 ± 0.007 a | 0.015 ± 0.003 b | 0.029 ± 0.012 b |
| Citronellol | 0.015 ± 0.003 ab | 0.015 ± 0.001 b | 0.050 ± 0.019 a |
| Norisoprenoids (mg/L) | |||
| β-damascenone | 0.005 ± 0.000 b | 0.013 ± 0.002 a | 0.010 ± 0.001 a |
| Free Cells | T. delbrueckii T8 | T. delbrueckii T21 | T. delbrueckii TF |
| Esters (mg/L) | |||
| Ethyl butyrate | 0.105 ± 0.043 a | 0.021 ± 0.001 b | 0.000 ± 0.000 b |
| Ethyl acetate | 19.29 ± 0.12 c | 23.59 ± 0.24 a | 20.54 ± 0.60 b |
| Phenyl ethyl acetate | 0.040 ± 0.002 b | 0.056 ± 0.001 a | 0.023 ± 0.006 c |
| Ethyl hexanoate | 0.049 ± 0.039 a | 0.022 ± 0.002 a | 0.010 ± 0.001 a |
| Ethyl octanoate | 0.001 ± 0.000 c | 0.003 ± 0.000 b | 0.006 ± 0.001 a |
| Isoamyl acetate | 25.65 ± 0.31 a | 18.83 ± 0.16 b | 8.79 ± 0.30 c |
| Hexyl acetate | 0.003 ± 0.000 a | 0.001 ± 0.000 a | 0.002 ± 0.000 a |
| Diethyl succinate | 0.003 ± 0.000 c | 0.009 ± 0.003 b | 0.017 ± 0.001 a |
| Alcohols (mg/L) | |||
| n-Propanol | 21.85 ± 0.51 b | 27.83 ± 0.42 a | 22.52 ± 0.08 b |
| Isobutanol | 7.37 ± 0.26 a | 5.53 ± 0.37 b | 4.26 ± 0.23 c |
| Amyl alcohol | 4.34 ± 2.28 a | 4.08 ± 0.01 a | 3.77 ± 0.32 a |
| Isoamylic alcohol | 53.63 ± 0.36 c | 79.38 ± 0.56 a | 64.12 ± 0.59 b |
| β-Phenyl ethanol | 80.34 ± 0.00 b | 91.68 ± 0.00 a | 69.27 ± 0.00 c |
| Hexanol | 0.020 ± 0.011 b | 0.026 ± 0.008 b | 0.078 ± 0.001 a |
| Carbonyl Compounds (mg/L) | |||
| Acetaldehyde | 31.37 ± 0.53 b | 93.78 ± 0.42 a | 3.10 ± 0.59 c |
| Monoterpens | |||
| Linalool | 0.036 ± 0.003 a | 0.007 ± 0.000 c | 0.014 ± 0.001 b |
| Geraniol | 0.030 ± 0.000 a | 0.023 ± 0.001 b | 0.000 ± 0.000 c |
| Nerol | 0.055 ± 0.004 a | 0.027 ± 0.001 b | 0.000 ± 0.000 c |
| α-terpineol | 0.051 ± 0.001 b | 0.091 ± 0.010 a | 0.063 ± 0.013 ab |
| Citronellol | 0.025 ± 0.004 c | 0.068 ± 0.012 b | 0.132 ± 0.011 a |
| Norisoprenoids (mg/L) | |||
| β-damascenone | 0.021 ± 0.001 a | 0.010 ± 0.001 b | 0.003 ± 0.000 c |
| Immobilized Cells | T. delbrueckii T8 | T. delbrueckii T21 | T. delbrueckii TF |
| Esters (mg/L) | |||
| Ethyl butyrate | 0.024 ± 0.001 b | 0.089 ± 0.003 a | 0.016 ± 0.022 b |
| Ethyl acetate | 21.73 ± 1.26 a | 18.30 ± 0.80 b | 12.73 ± 0.25 c |
| Phenyl ethyl acetate | 0.078 ± 0.001 a | 0.053 ± 0.002 b | 0.030 ± 0.002 c |
| Ethyl hexanoate | 0.104 ± 0.055 b | 0.167 ± 0.012 a | 0.018 ± 0.001 c |
| Ethyl octanoate | 0.007 ± 0.001 a | 0.004 ± 0.001 ab | 0.003 ± 0.000 b |
| Isoamyl acetate | 4.53 ± 0.00 c | 7.03 ± 0.13 b | 10.80 ± 0.51 a |
| Hexyl acetate | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.000 ± 0.000 b |
| Diethyl succinate | 0.001 ± 0.000 c | 0.017 ± 0.002 b | 0.022 ± 0.003 a |
| Alcohols (mg/L) | |||
| n-Propanol | 29.22 ± 1.12 a | 23.10 ± 0.13 b | 19.04 ± 0.47 c |
| Isobutanol | 10.47 ± 0.30 b | 11.37 ± 0.26 a | 4.97 ± 0.11 c |
| Amyl alcohol | 4.16 ± 0.37 b | 12.70 ± 2.69 a | 3.05 ± 0.25 b |
| Isoamylic alcohol | 75.96 ± 0.06 a | 46.30 ± 0.42 c | 69.33 ± 0.70 b |
| β-Phenyl ethanol | 67.86 ± 0.00 b | 69.27 ± 0.00 a | 59.06 ± 0.00 c |
| Hexanol | 0.033 ± 0.007 a | 0.042 ± 0.007 a | 0.037 ± 0.004 a |
| Carbonyl Compounds (mg/L) | |||
| Acetaldehyde | 1.90 ± 0.14 c | 28.14 ± 0.04 a | 3.77 ± 3.58 b |
| Monoterpenes | |||
| Linalool | 0.016 ± 0.006 a | 0.007 ± 0.002 a | 0.015 ± 0.000 a |
| Geraniol | 0.007 ± 0.001 b | 0.017 ± 0.003 a | 0.021 ± 0.001 a |
| Nerol | 0.011 ± 0.000 a | 0.005 ± 0.001 b | 0.004 ± 0.000 b |
| α-terpineol | 0.055 ± 0.006 a | 0.052 ± 0.007 ab | 0.032 ± 0.008 b |
| Citronellol | 0.026 ± 0.016 a | 0.055 ± 0.010 a | 0.063 ± 0.011 a |
| Norisoprenoids (mg/L) | |||
| β-damascenone | 0.010 ± 0.000 a | 0.011 ± 0.000 a | 0.003 ± 0.000 b |
| Free Cells | L. thermotolerans T8 | L. thermotolerans T21 | L. thermotolerans TF |
| Esters (mg/L) | |||
| Ethyl butyrate | 0.000 ± 0.000 b | 0.034 ± 0.010 b | 0.225 ± 0.017 a |
| Ethyl acetate | 24.68 ± 0.22 a | 17.84 ± 0.12 c | 19.89 ± 0.06 b |
| Phenyl ethyl acetate | 0.037 ± 0.001 b | 0.065 ± 0.001 a | 0.015 ± 0.002 c |
| Ethyl hexanoate | 0.011 ± 0.001 b | 0.023 ± 0.001 a | 0.019 ± 0.001 a |
| Ethyl octanoate | 0.005 ± 0.001 a | 0.006 ± 0.000 a | 0.002 ± 0.000 b |
| Isoamyl acetate | 22.69 ± 1.56 a | 17.46 ± 0.30 b | 11.84 ± 0.29 c |
| Hexyl acetate | 0.003 ± 0.000 a | 0.001 ± 0.000 b | 0.004 ± 0.000 a |
| Diethyl succinate | 0.015 ± 0.001 a | 0.007 ± 0.001 c | 0.011 ± 0.000 b |
| Alcohols (mg/L) | |||
| n-Propanol | 24.92 ± 0.32 b | 27.92 ± 0.86 a | 26.72 ± 0.21 a |
| Isobutanol | 5.51 ± 1.34 a | 5.94 ± 0.87 a | 4.67 ± 0.03 a |
| Amyl alcohol | 4.43 ± 0.11 a | 3.91 ± 0.52 a | 4.18 ± 0.11 a |
| Isoamylic alcohol | 84.78 ± 0.23 a | 79.95 ± 0.34 b | 56.13 ± 0.88 c |
| β-Phenyl ethanol | 76.69 ± 0.00 a | 78.54 ± 0.48 a | 57.96 ± 0.17 b |
| Hexanol | 0.040 ± 0.002 b | 0.078 ± 0.015 a | 0.032 ± 0.000 b |
| Carbonyl Compounds (mg/L) | |||
| Acetaldehyde | 94.56 ± 2.97 a | 56.71 ± 0.03 b | 5.99 ± 0.58 c |
| Monoterpenes | |||
| Linalool | 0.011 ± 0.000 a | 0.006 ± 0.000 a | 0.009 ± 0.006 a |
| Geraniol | 0.029 ± 0.001 a | 0.002 ± 0.000 c | 0.017 ± 0.005 b |
| Nerol | 0.005 ± 0.001 a | 0.003 ± 0.000 a | 0.004 ± 0.001 a |
| α-terpineol | 0.018 ± 0.006 a | 0.040 ± 0.025 a | 0.011 ± 0.008 a |
| Citronellol | 0.062 ± 0.023 a | 0.057 ± 0.032 a | 0.055 ± 0.021 a |
| Norisoprenoids (mg/L) | |||
| β-damascenone | 0.005 ± 0.001 b | 0.005 ± 0.000 b | 0.013 ± 0.001 a |
| Immobilized Cells | L. thermotolerans T8 | L. thermotolerans T21 | L. thermotolerans TF |
| Esters (mg/L) | |||
| Ethyl butyrate | 0.005 ± 0.000 b | 0.007 ± 0.001 b | 0.568 ± 0.007 a |
| Ethyl acetate | 11.08 ± 1.09 b | 14.53 ± 0.02 a | 13.18 ± 0.23 a |
| Phenyl ethyl acetate | 0.022 ± 0.001 b | 0.048 ± 0.006 a | 0.040 ± 0.002 a |
| Ethyl hexanoate | 0.107 ± 0.005 b | 0.198 ± 0.021 a | 0.018 ± 0.000 c |
| Ethyl octanoate | 0.005 ± 0.000 a | 0.002 ± 0.000 b | 0.002 ± 0.000 b |
| Isoamyl acetate | 6.82 ± 0.27 b | 9.98 ± 0.21 a | 8.53 ± 1.66 ab |
| Hexyl acetate | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.001 ± 0.000 a |
| Diethyl succinate | 0.002 ± 0.000 b | 0.018 ± 0.002 a | 0.018 ± 0.003 a |
| Alcohols (mg/L) | |||
| n-Propanol | 25.64 ± 0.46 a | 16.80 ± 0.18 b | 18.44 ± 1.72 b |
| Isobutanol | 7.69 ± 0.33 a | 5.37 ± 0.22 b | 4.45 ± 0.59 b |
| Amyl alcohol | 3.57 ± 0.32 a | 3.46 ± 0.40 a | 1.92 ± 1.04 a |
| Isoamylic alcohol | 61.58 ± 0.32 b | 67.42 ± 0.13 a | 56.75 ± 0.14 c |
| β-Phenyl ethanol | 54.78 ± 0.52 a | 65.51 ± 0.57 a | 58.22 ± 0.16 a |
| Hexanol | 0.014 ± 0.000 b | 0.017 ± 0.001 b | 0.059 ± 0.004 a |
| Carbonyl Compounds (mg/L) | |||
| Acetaldehyde | 2.26 ± 0.003 b | 35.72 ± 3.37 a | 1.18 ± 0.26 b |
| Monoterpenes | |||
| Linalool | 0.029 ± 0.006 b | 0.033 ± 0.002 b | 0.103 ± 0.006 a |
| Geraniol | 0.003 ± 0.001 c | 0.016 ± 0.001 b | 0.022 ± 0.000 a |
| Nerol | 0.015 ± 0.002 a | 0.004 ± 0.001 b | 0.000 ± 0.000 c |
| α-terpineol | 0.035 ± 0.003 a | 0.036 ± 0.002 a | 0.007 ± 0.004 b |
| Citronellol | 0.023 ± 0.007 b | 0.045 ± 0.008 a | 0.046 ± 0.004 a |
| Norisoprenoids (mg/L) | |||
| β-damascenone | 0.019 ± 0.002 a | 0.010 ± 0.001 b | 0.010 ± 0.001 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canonico, L.; Moretti, L.; Agarbati, A.; Comitini, F.; Ciani, M. Free and Immobilized Cells of Torulaspora delbrueckii and Lachancea thermotolerans in Sparkling Wine: Innovative Application in Secondary Bottle Fermentation. Foods 2025, 14, 3007. https://doi.org/10.3390/foods14173007
Canonico L, Moretti L, Agarbati A, Comitini F, Ciani M. Free and Immobilized Cells of Torulaspora delbrueckii and Lachancea thermotolerans in Sparkling Wine: Innovative Application in Secondary Bottle Fermentation. Foods. 2025; 14(17):3007. https://doi.org/10.3390/foods14173007
Chicago/Turabian StyleCanonico, Laura, Laura Moretti, Alice Agarbati, Francesca Comitini, and Maurizio Ciani. 2025. "Free and Immobilized Cells of Torulaspora delbrueckii and Lachancea thermotolerans in Sparkling Wine: Innovative Application in Secondary Bottle Fermentation" Foods 14, no. 17: 3007. https://doi.org/10.3390/foods14173007
APA StyleCanonico, L., Moretti, L., Agarbati, A., Comitini, F., & Ciani, M. (2025). Free and Immobilized Cells of Torulaspora delbrueckii and Lachancea thermotolerans in Sparkling Wine: Innovative Application in Secondary Bottle Fermentation. Foods, 14(17), 3007. https://doi.org/10.3390/foods14173007








