Exploring the Effects of Nitrogen and Potassium on the Aromatic Characteristics of Ginseng Roots Using Non-Targeted Metabolomics Based on GC-MS and Multivariate Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Determination of Ginsenoside Content
2.2.1. Monomeric Ginsenoside Quantification
2.2.2. Total Ginsenoside Determination
2.3. Determination of Mineral Elements
2.4. Measurement of Antioxidant Enzyme Activities
2.5. Sample Preparation for VOC Analysis
2.5.1. Headspace Solid-Phase Microextraction
2.5.2. GC–MS Conditions
2.6. Identification and Quantification of VOCs
2.7. Relative Odor Activity Value Calculation
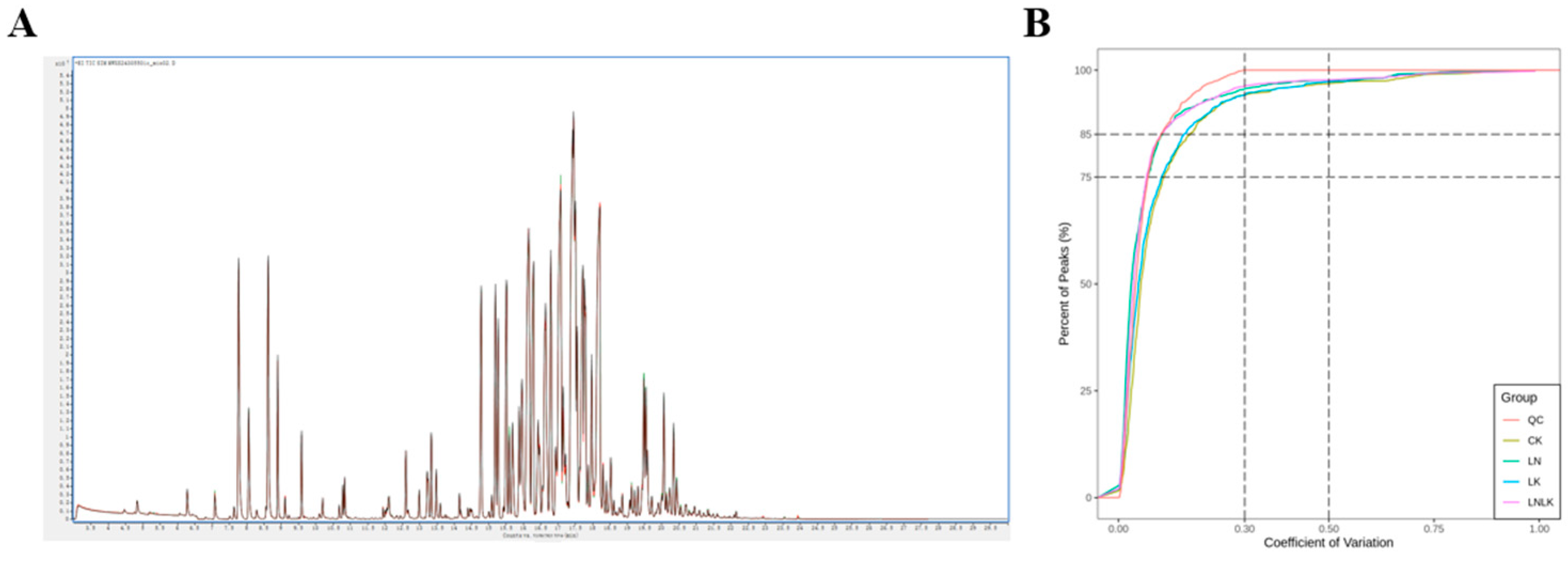
3. Statistical and Multivariate Data Analyses
4. Results
4.1. N and K Deficiency Effects on N, Phosphorus, K, and OM Contents in Ginseng Roots
4.2. Effects of N and K Deficiency on Ginsenoside Content in Ginseng Roots
4.3. Effects of N and K Deficiency on the Activity of Antioxidant Enzymes in Ginseng
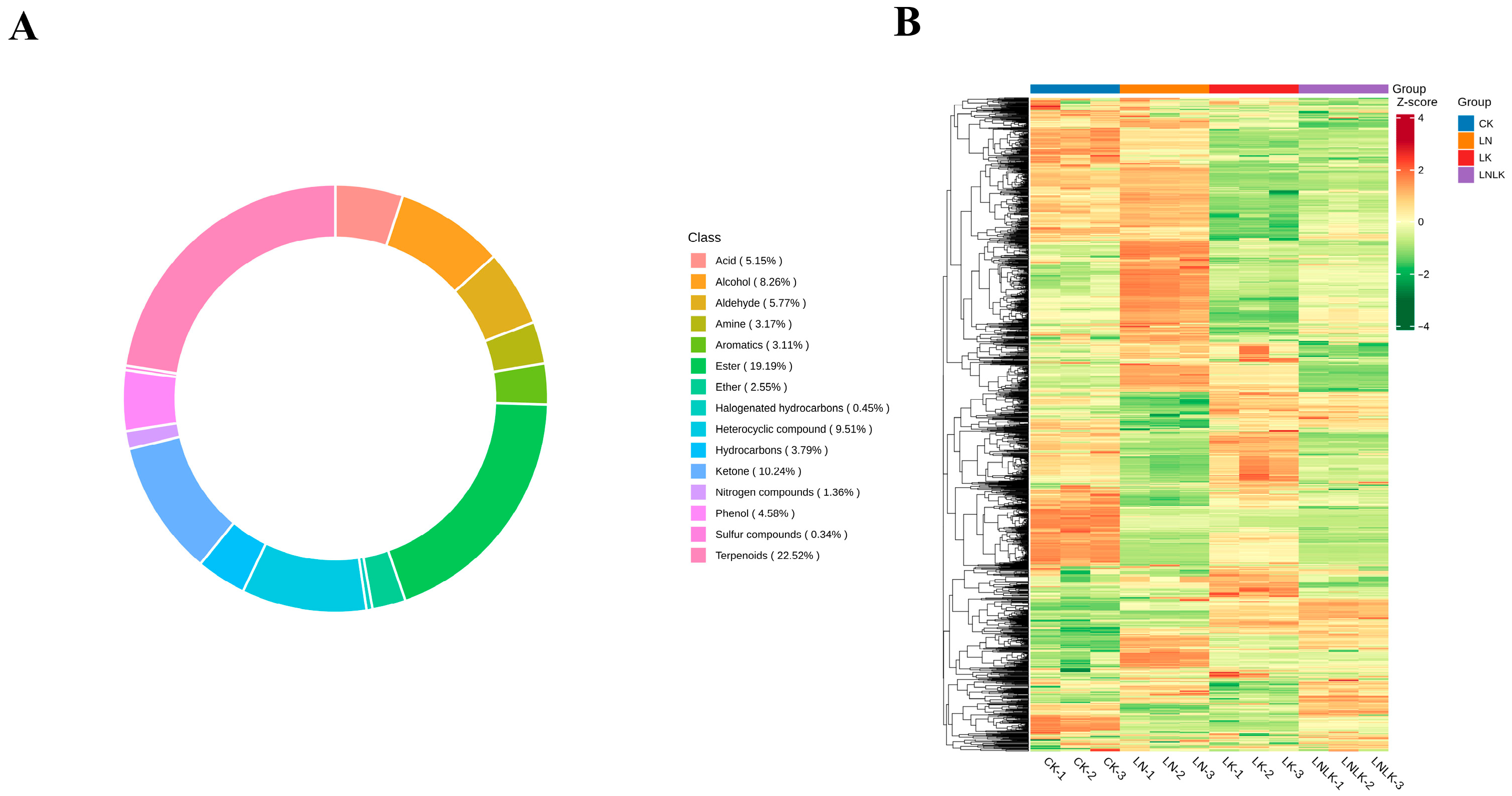
4.4. Volatile Compound Analysis
4.4.1. Volatile Compounds in Ginseng Roots Under Different Treatments
4.4.2. Multivariate Statistical Analysis of VOC Metabolic Profiles
4.5. Calculation of rOAVs of Aroma Compounds
4.6. Key Aroma Metabolite Screening
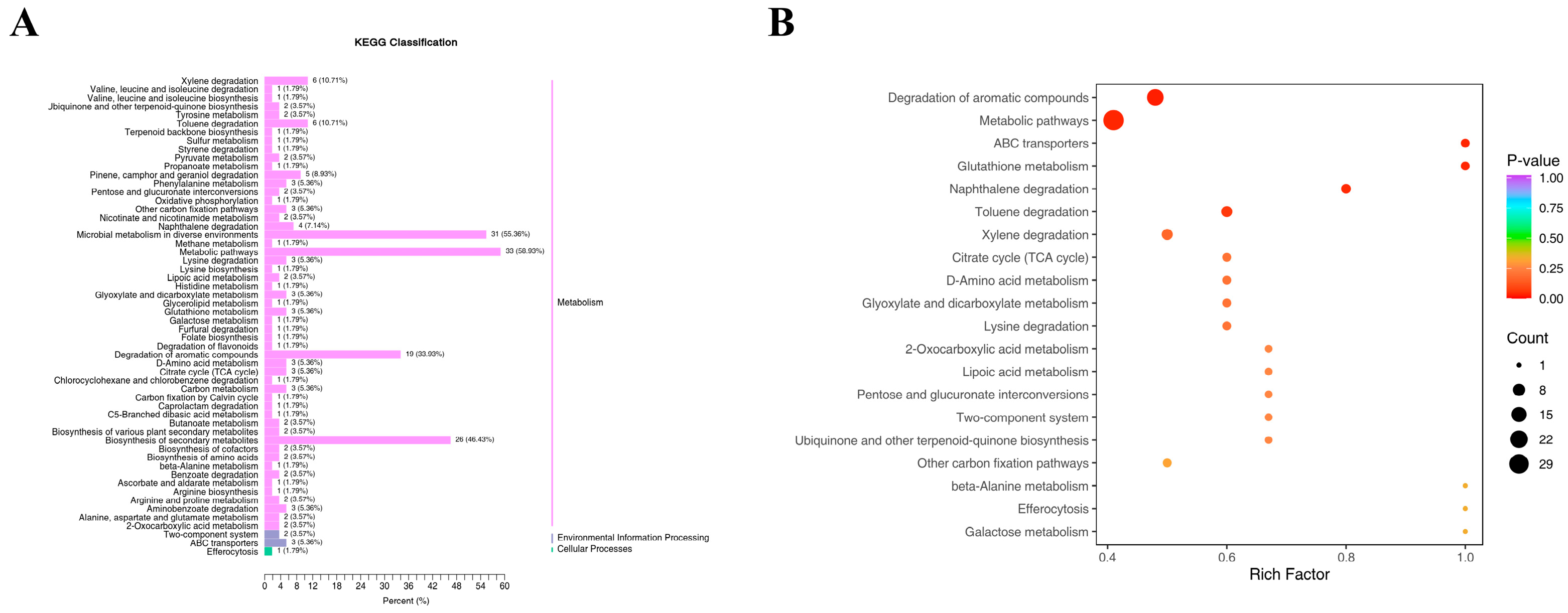
4.7. Response of Ginseng Roots to VOCs Under N and K Deficiency
4.7.1. Statistical Analysis of Intergroup Differences in VOCs
4.7.2. Response of Ginseng Roots to VOCs Under N Deficiency
4.7.3. Response of Ginseng Roots to VOCs Under LK
4.7.4. Response of Volatile Metabolites in Ginseng Roots Under Simultaneous LNLK
5. Discussion
5.1. Physiological Metabolic Disorders Drive the Reprogramming of the Aroma Pathway
5.2. N and K Co-Regulate Flavor Pathways
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, P.; Liu, J. (Eds.) Ginseng Nutritional Components and Functional Factors; Springer: Singapore, 2020; ISBN 9789811546877. [Google Scholar]
- Xu, Y.; Bian, S.; Shang, L.; Wang, X.; Bai, X.; Zhang, W. Phytochemistry, Pharmacological Effects and Mechanism of Action of Volatile Oil from Panax Ginseng C.A. Mey: A Review. Front. Pharmacol. 2024, 15, 1436624. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Panax Ginseng and Panax Quinquefolius: From Pharmacology to Toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef]
- Liu, L.; Xu, F.-R.; Wang, Y.-Z. Traditional Uses, Chemical Diversity and Biological Activities of Panax L. (Araliaceae): A Review. J. Ethnopharmacol. 2020, 263, 112792. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Su, Q.; Hu, S.; Ruan, X.; Ouyang, S. Research Progress on the Anti-Aging Potential of the Active Components of Ginseng. Nutrients 2023, 15, 3286. [Google Scholar] [CrossRef]
- Ramesh, T.; Kim, S.-W.; Hwang, S.-Y.; Sohn, S.-H.; Yoo, S.-K.; Kim, S.-K. Panax Ginseng Reduces Oxidative Stress and Restores Antioxidant Capacity in Aged Rats. Nutr. Res. 2012, 32, 718–726. [Google Scholar] [CrossRef]
- Chan, H.-T.L.; Chan, K.-M.; Abhreet-Kaur; Sam, S.-W.; Chan, S.-W. A Review of the Pharmacological Effects of Solanum Muricatum Fruit (Pepino Melon). Foods 2024, 13, 2740. [Google Scholar] [CrossRef]
- Jung, J.H.; Kang, I.G.; Kim, D.Y.; Hwang, Y.J.; Kim, S.T. The Effect of Korean Red Ginseng on Allergic Inflammation in a Murine Model of Allergic Rhinitis. J. Ginseng Res. 2013, 37, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Tsai, C.-L.; Tseng, C.-Y.; Yu, P.-R.; Chiu, P.-Y.; Hsu, C.-C.; Chen, J.-H. Anti-Hypertensive Effect of Solanum Muricatum Aiton Leaf Extract In Vivo and In Vitro. Plant Foods Hum. Nutr. 2024, 79, 182–188. [Google Scholar] [CrossRef]
- Luo, H.-Y.; Fang, J.; Zhang, W.-H.; Chan, K.-C.; Chan, Y.-M.; Dong, C.-X.; Li, S.-L.; Lyu, A.-P.; Xu, J. Dissecting the Anti-Obesity Components of Ginseng: How Ginseng Polysaccharides and Ginsenosides Target Gut Microbiota to Suppress High-Fat Diet-Induced Obesity. J. Adv. Res. 2024; in press. [Google Scholar] [CrossRef]
- Makhlouf, E.A.; AlamElDeen, Y.K.; El-Shiekh, R.A.; Okba, M.M. Unveilling the Antidiabetic Potential of Ashwagandha (Withania Somnifera L.) and Its Withanolides—A Review. Nat. Prod. Res. 2024, 1–16. [Google Scholar] [CrossRef]
- Dai, K.; Liu, C.; Ji, H.; Liu, A. Structural Characteristics and Anti-Tumor Activity of Alkali-Extracted Acidic Polysaccharide Extracted from Panax Ginseng. Int. J. Biol. Macromol. 2025, 305, 141230. [Google Scholar] [CrossRef]
- Lv, X.; Jiang, M.-M.; Zhu, M.-T.; Yuan, H.-Y.; Gu, Z.-H.; Wang, Y.; Gong, Y.-S. Effects of Renshen Yangrong Decoction on learning and memory impairment in Alzheimer's disease mouse model. Chin. Tradit. Pat. Med. 2022, 44, 3823–3829. [Google Scholar]
- Gu, W.-T.; Li, L.-Y.; Rui, W.-J.; Diao, Z.-W.; Zhuang, G.-D.; Chen, X.-M.; Qian, Z.-M.; Wang, S.-M.; Tang, D.; Ma, H.-Y. Non-Targeted Metabolomic Analysis of Variation of Volatile Fractions of Ginseng from Different Habitats by HS-SPME-GC-MS Coupled with Chemometrics. Anal. Methods 2022, 14, 3583–3597. [Google Scholar] [CrossRef]
- Cui, L.-L.; Guan, Y.-M.; Yan, M.-X.; Hou, Z.-H.; Wang, Y.-P. Study on the supercritical CO2 extraction process of ginseng essential oil. In Proceedings of the Green Pest Control and Agricultural Product Quality Safety—Proceedings of the 2015 Academic Conference of the Chinese Society for Plant Protection, Changchun, China, 2015; p. 649. [Google Scholar]
- Cheng, R.-B.; Ge, Y.-Q.; Huang, Z. Recent Advances in the Antitumor Metastasis Function of Ginsenoside Rh2. Cent. South Pharm. 2013, 11, 211–213. [Google Scholar]
- Lee, I.-A.; Hyam, S.R.; Jang, S.-E.; Han, M.J.; Kim, D.-H. Ginsenoside Re Ameliorates Inflammation by Inhibiting the Binding of Lipopolysaccharide to TLR4 on Macrophages. J. Agric. Food Chem. 2012, 60, 9595–9602. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A. Adaptogenic Herb Ginseng (Panax) as Medical Food: Status Quo and Future Prospects. Biomed. Pharmacother. 2017, 85, 120–127. [Google Scholar] [CrossRef]
- Chung, H.S.; Lee, Y.-C.; Rhee, Y.K.; Lee, S.-Y. Consumer Acceptance of Ginseng Food Products. J. Food Sci. 2011, 76, S516–S522. [Google Scholar] [CrossRef]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the Consequence of Environmental Stress for Accumulation of Secondary Metabolites in Medicinal and Aromatic Plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Kimball, B.A. Volatile Metabolome: Problems and Prospects. Bioanalysis 2016, 8, 1987–1991. [Google Scholar] [CrossRef]
- Cui, S.; Wang, J.; Yang, L.; Wu, J.; Wang, X. Qualitative and Quantitative Analysis on Aroma Characteristics of Ginseng at Different Ages Using E-Nose and GC–MS Combined with Chemometrics. J. Pharm. Biomed. Anal. 2015, 102, 64–77. [Google Scholar] [CrossRef]
- Cho, I.H. Volatile Compounds of Ginseng (Panax sp.): A Review. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 67–75. [Google Scholar] [CrossRef]
- Abbas, F.; O’Neill Rothenberg, D.; Zhou, Y.; Ke, Y.; Wang, H. Volatile Organic Compounds as Mediators of Plant Communication and Adaptation to Climate Change. Physiol. Plant. 2022, 174, e13840. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Mancini, I. The Role of Volatile Organic Compounds in Plant Response to Environmental Stresses. Ph.D. Thesis, University of Insubria, Lombardy, Italy, 2021. [Google Scholar]
- Zahoor, R.; Zhao, W.; Abid, M.; Dong, H.; Zhou, Z. Title: Potassium Application Regulates Nitrogen Metabolism and Osmotic Adjustment in Cotton (Gossypium hirsutum L.) Functional Leaf under Drought Stress. J. Plant Physiol. 2017, 215, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Xu, C.; Rogers, A.; Fisher, R.A.; Wullschleger, S.D.; Massoud, E.C.; Vrugt, J.A.; Muss, J.D.; McDowell, N.G.; Fisher, J.B.; et al. A Global Scale Mechanistic Model of Photosynthetic Capacity (LUNA V1.0). Geosci. Model Dev. 2016, 9, 587–606. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Du, M.; Shou, G.; Wang, Z.; Xu, G. Nitrogen as a Regulator for Flowering Time in Plant. Plant Soil 2022, 480, 1–29. [Google Scholar] [CrossRef]
- Lebaudy, A.; Véry, A.-A.; Sentenac, H. K+ Channel Activity in Plants: Genes, Regulations and Functions. FEBS Lett. 2007, 581, 2357–2366. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Han, W.; Chen, J.; Lai, J.; Wang, Y. Potassium Nutrition of Maize: Uptake, Transport, Utilization, and Role in Stress Tolerance. Crop J. 2023, 11, 1048–1058. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in Plants: Growth Regulation, Signaling, and Environmental Stress Tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, X.; Mao, Z.; Qin, S.; Lv, D. Integration of Root Architecture, Root Nitrogen Metabolism, and Photosynthesis of ‘Hanfu’ Apple Trees under the Cross-Talk between Glucose and IAA. Hortic. Plant J. 2023, 9, 631–644. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Ni, W.; Liu, C.; Qin, H.; Guan, Y.; Liu, J.; Feng, Z.; Xing, Y.; Tian, G.; et al. Nitrogen–Potassium Balance Improves Leaf Photosynthetic Capacity by Regulating Leaf Nitrogen Allocation in Apple. Hortic. Res. 2024, 11, uhad253. [Google Scholar] [CrossRef]
- Shah, I.H.; Jinhui, W.; Li, X.; Hameed, M.K.; Manzoor, M.A.; Li, P.; Zhang, Y.; Niu, Q.; Chang, L. Exploring the Role of Nitrogen and Potassium in Photosynthesis Implications for Sugar: Accumulation and Translocation in Horticultural Crops. Sci. Hortic. 2024, 327, 112832. [Google Scholar] [CrossRef]
- Qian, J.-Q.; Sun, H.; Ruan, Y.; Wu, H.-P.; Zhang, Y.-Y. Effects of magnesium supply level on growth, nutrient element absorption and distribution, and quality of Panax quinquefolium. China J. Chin. Mater. Medica 2022, 47, 1205–1214. [Google Scholar] [CrossRef]
- Zuo, X.; Sun, H.; Qian, J.; Wu, C.; Zhang, Y. Correlation Analysis of Ginseng Quality and Ginseng Root Mineral Elements Cultivated in Farmland of Different Producing Areas in Northeast China. J. Jilin Agric. Univ. 2022, 44, 307–312. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, G.; Xie, H.; Zhang, J.; Huang, J.; Liu, Z.; Wang, C. Characterization of the Key Differential Aroma Compounds in Five Dark Teas from Different Geographical Regions Integrating GC–MS, ROAV and Chemometrics Approaches. Food Res. Int. 2024, 194, 114928. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Li, R.; Sun, P.; Chen, D.; Shen, J.; Feng, T. Characteristic Aroma Analysis of Finger Citron in Four Different Regions Based on GC–MS-HS-SPME and ROAV. J. Food Process. Preserv. 2022, 46, e17191. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Liu, Y.; Wang, D. Analyzing Volatile Compounds of Young and Mature Docynia Delavayi Fruit by HS-SPME-GC-MS and rOAV. Foods 2022, 12, 59. [Google Scholar] [CrossRef]
- Huang, W.; Fang, S.; Wang, J.; Zhuo, C.; Luo, Y.; Yu, Y.; Li, L.; Wang, Y.; Deng, W.-W.; Ning, J. Sensomics Analysis of the Effect of the Withering Method on the Aroma Components of Keemun Black Tea. Food Chem. 2022, 395, 133549. [Google Scholar] [CrossRef]
- Xue, J.; Liu, P.; Yin, J.; Wang, W.; Zhang, J.; Wang, W.; Le, T.; Ni, D.; Jiang, H. Dynamic Changes in Volatile Compounds of Shaken Black Tea during Its Manufacture by GC × GC-TOFMS and Multivariate Data Analysis. Foods 2022, 11, 1228. [Google Scholar] [CrossRef]
- Mou, Z.; Cai, X.; Liu, J.; Deng, R.; Liu, Z.; Fan, R.; Tang, J.; Luo, A. Elucidation of the Key Aroma Compounds of Floral and Fruity Aroma in Sauce-Flavored Baijiu by Pervaporative Membrane Separation, GC-IMS, GC-MS, and Aroma Omission Studies. Food Biosci. 2025, 66, 106270. [Google Scholar] [CrossRef]
- Yu, L.; Pang, Y.; Shen, G.; Bai, B.; Yang, Y.; Zeng, M. Identification and Selection of Volatile Compounds Derived from Lipid Oxidation as Indicators for Quality Deterioration of Frozen White Meat and Red Meat Using HS-SPME-GC–MS Combined with OPLS-DA. Food Chem. 2025, 463, 141112. [Google Scholar] [CrossRef]
- Xie, F.; Hao, L.; Wang, Z.-J.; Jia, J.; Shao, Y.-T. Advances in understanding how branches of the phenylpropanoid pathway respond to biotic stress. China Plant Prot. 2023, 43, 23–30. [Google Scholar]
- Shang, J.; Wu, W.-Z.; Ma, Y.-G. Phenylpropanoid Metabolism Pathway in Plants. Chin. J. Biochem. Mol. Biol. 2022, 38, 1467–1476. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Fuchs, G.; Boll, M.; Heider, J. Microbial Degradation of Aromatic Compounds—From One Strategy to Four. Nat. Rev. Microbiol. 2011, 9, 803–816. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Huang, L.-Q.; Guo, L.-P. Secondary metabolites accumulating and geoherbs formation under enviromental stress. China J. Chin. Mater. Medica 2007, 32, 277–280. [Google Scholar]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in Plants: An Integrated Overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Hiradate, S.; Ma, J.F.; Matsumoto, H. Strategies of Plants to Adapt to Mineral Stresses in Problem Soils. Adv. Agron. 2007, 96, 65–132. [Google Scholar]
- Coskun, D.; Britto, D.T.; Kronzucker, H.J. The Nitrogen–Potassium Intersection: Membranes, Metabolism, and Mechanism. Plant Cell Environ. 2017, 40, 2029–2041. [Google Scholar] [CrossRef]
- Zuo, X.-X. Soil Characteristics of the Cultivated Ginseng in Main Producing Areas and Its Influence on the Quality of Ginseng. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2021. [Google Scholar]
- Duan, Y.; Yang, H.; Yang, H.; Wei, Z.; Che, J.; Wu, W.; Lyu, L.; Li, W. Physiological and Morphological Responses of Blackberry Seedlings to Different Nitrogen Forms. Plants 2023, 12, 1480. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.I.; Van Breusegem, F. Improving Oxidative Stress Resilience in Plants. Plant J. 2022, 109, 359–372. [Google Scholar] [CrossRef]
- Schultz, J.C.; Appel, H.M.; Ferrieri, A.P.; Arnold, T.M. Flexible Resource Allocation during Plant Defense Responses. Front. Plant Sci. 2013, 4, 324. [Google Scholar] [CrossRef] [PubMed]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef] [PubMed]
- Monson, R.K.; Trowbridge, A.M.; Lindroth, R.L.; Lerdau, M.T. Coordinated Resource Allocation to Plant Growth–Defense Tradeoffs. New Phytol. 2022, 233, 1051–1066. [Google Scholar] [CrossRef] [PubMed]










| Compounds | Class I | Aroma Description | LN | CK | Type |
|---|---|---|---|---|---|
| 3,4,5-Trichloro-1,2-benzenediol | Alcohol | / | 299,114 ± 10,978 | 6294 ± 675 | Upregulated |
| N-Acetyl-L-phenylalanine ethyl ester | Ester | / | 480,395 ± 23,484 | 10,291 ± 364 | Upregulated |
| 1,2,3,3a,8,9,9a,9b-Octahydrocyclopenta[def]phenanthrene | Aromatics | / | 21,046 ± 229 | 785 ± 506 | Upregulated |
| 2-Naphthyl methyl ketone | Ketone | / | 60,453 ± 39,320 | 3013 | Upregulated |
| Diallyl tetrasulfide, di-2-propenyl tetrasulfide | Sulfur compounds | Strong garlic, onion | 65,058 ± 3368 | 3478 | Upregulated |
| 5-Methyl-4-hexen-3-one | Ketone | / | 4419 ± 913 | 242 | Upregulated |
| 1-Methylpiperidine | Heterocyclic compound | / | 2064 ± 2043 | 31,688 ± 2792 | Downregulated |
| Isoborneol | Terpenoids | Balsamic, camphor, herbal, woody | 205,135 ± 9803 | 3296,923 ± 602,517 | Downregulated |
| 1-Methyl-2-nitrobenzene | Nitrogen compounds | / | 1948 ± 69 | 33,174 ± 625 | Downregulated |
| 2-Isopropyl-5-methylcyclohexan | Terpenoids | Minty | 1051 ± 88 | 18,311 ± 1088 | Downregulated |
| 2-Isopropenyl-3-methylenecyclohexanol | Terpenoids | / | 1406 ± 71 | 24,753 ± 1538 | Downregulated |
| 1-(2-hydroxyphenyl)ethanone | Ketone | Phenol, sweet, hawthorn, tobacco, honey, herbal | 12,547 ± 355 | 263,503 ± 41,314 | Downregulated |
| 3,5-Octadien-2-one, (E,E)- | Ketone | Fruity, green, grassy | 1048 | 23,155 ± 701 | Downregulated |
| 3,5-Octadien-2-one | Ketone | Fruity, fatty, mushroom | 1048 | 23,155 ± 701 | Downregulated |
| 2-methoxy-4-propyl-Phenol | Phenol | Clove, sharp, spicy, sweet, phenol, powdery, allspice | 1403 | 53,602 ± 2355 | Downregulated |
| 1-Butyl-3,5-dimethyl-1H-pyrazole | Heterocyclic compound | / | 3402 | 208,237 ± 11,490 | Downregulated |
| 1H-pyrrole-2-carbonitrile | Heterocyclic compound | / | 1325 | 86,783 ± 13,060 | Downregulated |
| 1H-pyrrole-3-carbonitrile | Heterocyclic compound | / | 1325 | 86,783 ± 13,060 | Downregulated |
| 2,6,6-Trimethylcyclohexa-1,4-dienecarbaldehyde | Aldehyde | / | 856 | 84,078 ± 2016 | Downregulated |
| Butoxybenzene | Ether | Anisic, licorice | 1279 | 157,085 ± 6795 | Downregulated |
| Compounds | Class I | Aroma Description | LK | CK | Type |
|---|---|---|---|---|---|
| 2-Naphthyl methyl ketone | Ketone | Sweet, neroli, orange, blossom, neroli, powdery | 76,556 ± 6746 | 3013 | Upregulated |
| Spiro [4.5]dec-7-ene, 1,8-dimethyl-4-(1-methylethenyl)-, [1S-(1.alpha.,4.beta.,5.alpha.)]- | Terpenoids | / | 11,157,201 ± 194,332 | 1254,135 | Upregulated |
| Phenol, 3-ethyl- 3-Ethylphenol | Phenol | / | 24,131 ± 1730 | 2754 | Upregulated |
| Phenol, 2-ethyl- 2-Ethylphenol | Phenol | Phenol | 24,131 ± 1730 | 2754 | Upregulated |
| Phenol, 2,4-dimethyl- 2,4-Dimethylphenol; | Phenol | Weak, smoky, roasted, dark | 24,131 ± 1730 | 2754 | Upregulated |
| Phenol, 2,5-dimethyl- 2,5-Dimethylphenol; | Phenol | Sweet, naphthyl, phenol, smoky, bacon | 24,131 ± 1730 | 2754 | Upregulated |
| Phenol, (1,1-dimethylethyl)-4-methoxy- (CAS) | Phenol | Mild, rubbery | 8860 | 78,641 ± 15,419 | Downregulated |
| 4-Penten-1-ol, propanoate | Ester | / | 35,659 | 322,004 ± 27,654 | Downregulated |
| Phenol, 4-chloro-5-methyl-2-(1-methylethyl)- | Phenol | / | 334,552 | 3388,867 ± 275,723 | Downregulated |
| 2(1H)-naphthalenone, 3,4,5,6,7,8-hexahydro- | Ketone | / | 109,080 | 1110,076 ± 228,5650 | Downregulated |
| 4-Aminobenzoic acid | Acid | / | 1667 | 17,418 ± 971 | Downregulated |
| Butane, 1-nitro- 1-Nitrobutane | Nitrogen compounds | / | 3069 | 34,387 ± 1206 | Downregulated |
| Cyclopentanone, 2-methyl- 2-Methylcyclopentanone | Ketone | Roasted, beefy | 10,013 | 113,024 ± 772 | Downregulated |
| Cyclopentanone, 3-methyl- 3-Methylcyclopentanone | Ketone | Roasted, beefy | 10,013 | 113,024 ± 772 | Downregulated |
| Benzene, (1-nitropropyl)- (1-Nitropropyl)benzene | Nitrogen compounds | / | 8571 | 101,042 ± 9400 | Downregulated |
| 1,2-Benzenediol, 4-methyl- 4-Methyl-1,2-benzenediol | Alcohol | / | 11,048 ± 10,959 | 133,103 ± 5893 | Downregulated |
| 1,2-Benzenediol, 3-methyl- 3-Methyl-1,2-benzenediol | Alcohol | / | 11,048 ± 10,959 | 133,103 ± 5893 | Downregulated |
| 3-Hexanone | Ketone | Sweet, fruity, waxy, rummy, grape | 571 | 9003 ± 594 | Downregulated |
| 2-Hexanone | Ketone | Fruity, fungal, meaty, buttery | 571 | 9003 ± 594 | Downregulated |
| Butanedioic acid, hydroxy-, diethyl ester Hydroxybutanedioic acid diethyl | Ester | Brown, sugar, sweet, wine, fruity, herbal | 2359 | 51,612 ± 931 | Downregulated |
| Compounds | Class I | Aroma Description | LNLK | CK | Type |
|---|---|---|---|---|---|
| 2-Naphthyl methyl ketone | Ketone | Sweet, neroli, orange, blossom, neroli, powdery | 66,718 ± 5049 | 3013 | Upregulated |
| 4-Hexen-3-one, 5-methyl- 5-Methyl-4-hexen-3-one | Ketone | / | 3293 ± 250 | 242 | Upregulated |
| Acetic acid, phenylmethyl ester | Ester | Sweet, floral, fruity, jasmine, fresh | 16,299 ± 1803 | 215,710 ± 5917 | Downregulated |
| Butyramide, 2-cyano-2-ethyl- | Amine | / | 411 | 5472 ± 1295 | Downregulated |
| Phenol, 2-(1-methylpropyl)- | Phenol | / | 3703 ± 162 | 51,069 ± 845 | Downregulated |
| Tricyclo [2.2.1.0(2,6)]heptane-3-methanol, 2,3-dimethyl- | Terpenoids | / | 36,940 ± 686 | 521,286 ± 44,237 | Downregulated |
| 2,3-Dihydroxybenzaldehyde | Aldehyde | Almond, vanilla | 27,327 ± 683 | 389,349 ± 15,554 | Downregulated |
| Ethyl 2-octynoate | Ester | Violet, leafy, oily, waxy | 3912 ± 168 | 56,235 ± 799 | Downregulated |
| Memantine | Amine | / | 90,870 ± 2709 | 1,372,610 ± 21,391 | Downregulated |
| Cyclohexanone, 5-methyl-2-(1-methylethyl)- 2-Isopropyl-5-methylcyclohexan | Terpenoids | / | 1177 ± 40 | 18,311 ± 1088 | Downregulated |
| 3-Hexanone | Ketone | Sweet, fruity, waxy, rummy, grape | 571 | 9003 ± 594 | Downregulated |
| 2-Hexanone | Ketone | Fruity, fungal, meaty, buttery | 571 | 9003 ± 594 | Downregulated |
| 2,6,6-Trimethylcyclohexa-1,4-dienecarbaldehyde | Aldehyde | / | 4465 ± 226 | 84,078 ± 2016 | Downregulated |
| Resorcinol | Phenol | Nutty, creamy, phenol, hawthorn, musty | 1652 | 32,340 ± 2717 | Downregulated |
| Butanedioic acid, hydroxy-, diethyl ester Hydroxybutanedioic acid diethy | Ester | Brown, sugar, sweet, wine, fruity, herbal | 2359 | 51,612 ± 931 | Downregulated |
| Benzene, 1-methyl-2-nitro- 1-Methyl-2-nitrobenzene | Nitrogen compounds | / | 1482 ± 99 | 33,174 ± 625 | Downregulated |
| Benzenepropanoic acid, methyl ester | Ester | Honey, fruity, wine, balsamic, floral | 4482 | 105,109 ± 2242 | Downregulated |
| 4-Octenoic acid, ethyl ester, (Z)- | Ester | Fruity, fatty, green, pineapple, pear | 1230 ± 51 | 33,135 ± 1314 | Downregulated |
| Hexadecanoic acid, ethyl ester | Ester | Mild, waxy, fruity, creamy, milky, balsamic, greasy, oily | 3857 ± 315 | 110,683 ± 10,046 | Downregulated |
| Benzene, butoxy- Butoxybenzene | Ether | Anisic, licorice | 2985 ± 2954 | 157,085 ± 6795 | Downregulated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Sun, H.; Shao, C.; Long, H.; Cui, Y.; Sun, C.; Zhang, Y. Exploring the Effects of Nitrogen and Potassium on the Aromatic Characteristics of Ginseng Roots Using Non-Targeted Metabolomics Based on GC-MS and Multivariate Analysis. Foods 2025, 14, 2981. https://doi.org/10.3390/foods14172981
Cao W, Sun H, Shao C, Long H, Cui Y, Sun C, Zhang Y. Exploring the Effects of Nitrogen and Potassium on the Aromatic Characteristics of Ginseng Roots Using Non-Targeted Metabolomics Based on GC-MS and Multivariate Analysis. Foods. 2025; 14(17):2981. https://doi.org/10.3390/foods14172981
Chicago/Turabian StyleCao, Weiyu, Hai Sun, Cai Shao, Hongjie Long, Yanmei Cui, Changwei Sun, and Yayu Zhang. 2025. "Exploring the Effects of Nitrogen and Potassium on the Aromatic Characteristics of Ginseng Roots Using Non-Targeted Metabolomics Based on GC-MS and Multivariate Analysis" Foods 14, no. 17: 2981. https://doi.org/10.3390/foods14172981
APA StyleCao, W., Sun, H., Shao, C., Long, H., Cui, Y., Sun, C., & Zhang, Y. (2025). Exploring the Effects of Nitrogen and Potassium on the Aromatic Characteristics of Ginseng Roots Using Non-Targeted Metabolomics Based on GC-MS and Multivariate Analysis. Foods, 14(17), 2981. https://doi.org/10.3390/foods14172981






