Food-Grade Liposome-Loaded Delivery Systems: Current Trends and Future Perspectives
Abstract
1. Introduction
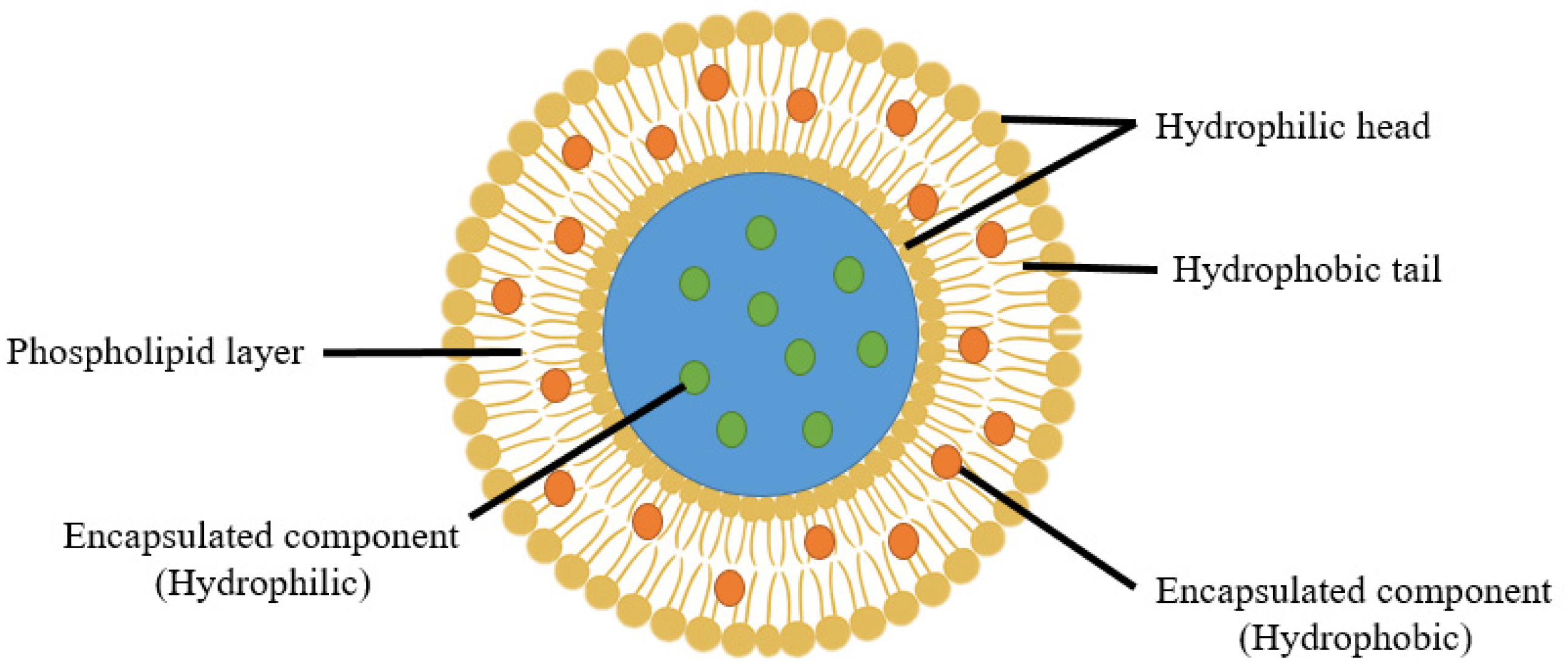
2. Liposome Structure and Composition
3. Liposome Preparation Methods
3.1. Conventional Methods
| Method | Brief Description | Advantages | Disadvantages | Vesicle Types | Ref. |
|---|---|---|---|---|---|
| Thin-film hydration | Thin lipid film is hydrated to form multilamellar vesicles | Compatible with various lipid types; allows high lipid loading; well established | Low encapsulation efficiency for hydrophilic drugs; large vesicles; solvent removal | MLV, MVV | [31] |
| Reverse-phase evaporation | Solvent-dissolved lipids mixed with aqueous phase are homogenized after solvent removal | Ease of application; adequate encapsulation efficiency | High solvent use; time-consuming | MLV, LUV | [28] |
| Ethanol injection | Ethanol-dissolved lipids are injected into aqueous phase, where vesicles form upon dilution | Simple operation; high scalability; cost-efficient | Low lipid loading; hard to load macro molecules; solvent removal | SUV, MLV | [31] |
| Supercritical fluid | Liposomes are formed using supercritical CO2, which enables lipid processing through solvent-free or low-solvent conditions | Enables solvent-free encapsulation of sensitive compounds with controlled nanoscale release | High cost; precise control; poor hydrophilic drug loading | MLV | [33,34] |
| Microfluidics | Intense fluid mixing in micro- or nanoscale channels enables the formation of uniformly sized liposomes with a narrow particle size distribution | Ease of scaling up; targeted delivery; improved stability; enhanced bioavailability due to uniform nanosized liposomes | High equipment cost; viscosity sensitive; thermal degradation risk | SUV | [33] |
3.2. Novel Methods
4. Liposomal Encapsulation of Bioactive Compounds and Application to Food Matrices
4.1. Vitamins
4.2. Polyphenols
4.3. Carotenoids
4.4. Omega-3 Fatty Acids (DHA and EPA)
4.5. Other Bioactive Components
5. Limitations of Liposomes
6. Liposome-Loaded Hybrid Delivery Systems
6.1. Liposome-Loaded Nanofibers
| Nanofiber Matrix Components | Ingredients and Size of Liposomes | Production Method of Liposome | Liposome-Encapsulated Bioactive | Results | Ref |
|---|---|---|---|---|---|
| Pectin/Pullulan | Soybean lecithin; 76 nm | Thin-film hydration + ultrasonication | Black mulberry extract (BME) | Uniform distribution of liposomes in the defect-free fiber structure; The higher cellular (Caco-2) release of anthocyanins in liposomal-BME loaded nanofiber | [17] |
| Zein/Gelatin | Soybean lecithin, polyoxyethylene sorbitan monooleate (Tween 80); 370 nm | Thin-film hydration + ultrasonication | Propolis | Enhanced mucoadhesiveness; Edible oral wound-healing material; Improved healing of oral wound and proliferation | [16] |
| Soybean lecithin; 154 nm | Ethanol injection | Curcumin | Gelatin coatings preserved fatty food simulants; Zein-based coatings preserved moisture of foods | [79] | |
| Polyethyleneoxide (PEO) | Soybean lecithin, cholesterol, stearylamine; 123–165 nm | Thin-film hydration | Basil essential oil | Maintaining the quality of chilled pork during 4 days of storage | [94] |
| Alginate/PEO-Polyvinyl alcohol (PVA), Pectin | Soybean lecithin, cholesterol; 26–32 nm | Thin-film hydration | Salmon calcitonin | Enhanced bioavailability (colon-targeted delivery) | [93] |
| Chitosan | Soybean lecithin, cholesterol; 144 nm | Thin-film hydration | Tea tree oil | Protection against Salmonella spp. in chicken and extended shelf life | [95] |
| PEO | Soybean lecithin, cholesterol; 315 nm | Thin-film hydration | SiO2-eugenol nanoparticles | Stable antioxidant activity during 60-day storage of beef | [96] |
| PEO/PVA | Hydrogenated soybean lecithin; 194 nm | Ethanol injection | β-carotene | Improved photostability | [97] |
6.2. Liposome-Loaded Cast Films
6.3. Liposome-Loaded Hydrogels
6.4. Liposome-Loaded Particles
6.5. Liposome-Loaded Emulsions
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miles, C.M.; Cullen, S.; Kenaan, H.; Gu, W.; Andrews, G.P.; Sosso, G.C.; Tian, Y. Unravelling the Interactions between Small Molecules and Liposomal Bilayers via Molecular Dynamics and Thermodynamic Modelling. Int. J. Pharm. 2024, 660, 124367. [Google Scholar] [CrossRef]
- ISO/TS 80004-1:2015; Nanotechnologies-Vocabulary-Part 1: Core Terms. International Organization for Standardization: Geneva, Switzerland, 2015.
- 2011/696/EU Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial; European Commission: Brussels, Belgium, 2011.
- Ojagh, S.M.; Hasani, S. Characteristics and Oxidative Stability of Fish Oil Nano-Liposomes and Its Application in Functional Bread. J. Food Meas. Charact. 2018, 12, 1084–1092. [Google Scholar] [CrossRef]
- Liu, W.; Hou, Y.; Jin, Y.; Wang, Y.; Xu, X.; Han, J. Research Progress on Liposomes: Application in Food, Digestion Behavior and Absorption Mechanism. Trends Food Sci. Technol. 2020, 104, 177–189. [Google Scholar] [CrossRef]
- Rudzińska, M.; Grygier, A.; Knight, G.; Kmiecik, D. Liposomes as Carriers of Bioactive Compounds in Human Nutrition. Foods 2024, 13, 1814. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mao, X. Chapter Seven—Liposomes Delivery Systems of Functional Substances for Precision Nutrition. In Food-Borne Delivery Systems of Functional Substances for Precision Nutrition; Tan, M., Ed.; Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2024; Volume 112, pp. 257–300. [Google Scholar]
- Latrobdiba, Z.M.; Fulyani, F.; Anjani, G. Liposome Optimisation for Oral Delivery of Nutraceuticals in Food: A Review. Food Res. 2023, 7, 233–246. [Google Scholar] [CrossRef]
- Guldiken, B.; Linke, A.; Capanoglu, E.; Boyacioglu, D.; Kohlus, R.; Weiss, J.; Gibis, M. Formation and Characterization of Spray Dried Coated and Uncoated Liposomes with Encapsulated Black Carrot Extract. J. Food Eng. 2019, 246, 42–50. [Google Scholar] [CrossRef]
- Wu, P.; Chen, L.; Chen, M.; Chiou, B.-S.; Xu, F.; Liu, F.; Zhong, F. Use of Sodium Alginate Coatings to Improve Bioavailability of Liposomes Containing DPP-IV Inhibitory Collagen Peptides. Food Chem. 2023, 414, 135685. [Google Scholar] [CrossRef]
- Karadag, A.; Özçelik, B.; Sramek, M.; Gibis, M.; Kohlus, R.; Weiss, J. Presence of Electrostatically Adsorbed Polysaccharides Improves Spray Drying of Liposomes. J. Food Sci. 2013, 78, E206–E221. [Google Scholar] [CrossRef]
- Saroglu, O.; Atalı, B.; Yıldırım, R.M.; Karadag, A. Characterization of Nanoliposomes Loaded with Saffron Extract: In Vitro Digestion and Release of Crocin. J. Food Meas. Charact. 2022, 16, 4402–4415. [Google Scholar] [CrossRef]
- Deng, L.; Wang, Y.; Jiang, H.; Xu, X.; Han, J.; Liu, W. Specific Protection Mechanism of Oligosaccharides on Liposomes during Freeze-Drying. Food Res. Int. 2023, 166, 112608. [Google Scholar] [CrossRef]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Yuan, F. Stability and Release Performance of Curcumin-Loaded Liposomes with Varying Content of Hydrogenated Phospholipids. Food Chem. 2020, 326, 126973. [Google Scholar] [CrossRef]
- Islam Shishir, M.R.; Karim, N.; Gowd, V.; Zheng, X.; Chen, W. Liposomal Delivery of Natural Product: A Promising Approach in Health Research. Trends Food Sci. Technol. 2019, 85, 177–200. [Google Scholar] [CrossRef]
- Karakas, C.Y.; Ustundag, C.B.; Sahin, A.; Karadag, A. Co-Axial Electrospinning of Liposomal Propolis Loaded Gelatin-Zein Fibers as a Potential Wound Healing Material. J. Appl. Polym. Sci. 2023, 140, e54683. [Google Scholar] [CrossRef]
- Kalintas Caglar, N.; Saroglu, O.; Karakas, C.Y.; Tasci, C.O.; Catalkaya, G.; Yildirim, R.M.; Gultepe, E.E.; Gulec, S.; Sagdic, O.; Capanoglu, E.; et al. Liposomal Black Mulberry Extract Loaded-Nanofibers: Preparation, Characterisation, and Bioaccessibility of Phenolics by Simulated In Vitro Digestion Combined with the Caco-2 Cell Model. Int. J. Food Sci. Technol. 2024, 59, 9298–9309. [Google Scholar] [CrossRef]
- Saroglu, O.; Karakas, C.Y.; Yildirim, R.M.; Erdem, O.; Karasu, S.; Sagdic, O.; Karadag, A. Liposomal Propolis Loaded Xanthan Gum-Salep Hydrogels: Preparation, Characterization, and In Vitro Bioaccessibility of Phenolics. Int. J. Biol. Macromol. 2025, 300, 140323. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Choi, S.; Kang, B.; Yang, E.; Kim, K.; Kwak, M.K.; Chang, P.-S.; Jung, H.-S. Precise Control of Liposome Size Using Characteristic Time Depends on Solvent Type and Membrane Properties. Sci. Rep. 2023, 13, 4728. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Z.; McClements, D.J.; Zou, L.; Peng, S.; Zhou, W.; Liu, W. Improvement on Stability, Loading Capacity and Sustained Release of Rhamnolipids Modified Curcumin Liposomes. Colloids Surf. B Biointerfaces 2019, 183, 110460. [Google Scholar] [CrossRef]
- Shen, X.; He, L.; Cui, Y.; Lin, Z.; Jafari, S.M.; Tan, C. Co-Encapsulation of Bioactive Compounds in Liposomal Delivery Systems for Synergistic Effects. Food Biosci. 2025, 68, 106306. [Google Scholar] [CrossRef]
- van Nieuwenhuyzen, W.; Tomás, M.C. Update on Vegetable Lecithin and Phospholipid Technologies. Eur. J. Lipid Sci. Technol. 2008, 110, 472–486. [Google Scholar] [CrossRef]
- Gutiérrez-Méndez, N.; Chavez-Garay, D.R.; Leal-Ramos, M.Y. Lecithins: A Comprehensive Review of Their Properties and Their Use in Formulating Microemulsions. J. Food Biochem. 2022, 46, e14157. [Google Scholar] [CrossRef]
- Mangrulkar, S.V.; Kulkarni, S.S.; Nanepag, P.V.; Neje, P.S.; Chaple, D.R.; Taksande, B.G.; Umekar, M.J. A Comprehensive Review on Pleiotropic Effects and Therapeutic Potential of Soy Lecithin. Adv. Tradit. Med. 2025, 25, 145–164. [Google Scholar] [CrossRef]
- Huang, L.; Teng, W.; Cao, J.; Wang, J. Liposomes as Delivery System for Applications in Meat Products. Foods 2022, 11, 3017. [Google Scholar] [CrossRef]
- Maja, L.; Željko, K.; Mateja, P. Sustainable Technologies for Liposome Preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Giuliano, C.B.; Cvjetan, N.; Ayache, J.; Walde, P. Multivesicular Vesicles: Preparation and Applications. ChemSystemsChem 2021, 3, e2000049. [Google Scholar] [CrossRef]
- Chai, C.; Park, J. Food Liposomes: Structures, Components, Preparations, and Applications. Food Chem. 2024, 432, 137228. [Google Scholar] [CrossRef]
- Eugster, R.; Luciani, P. Liposomes: Bridging the Gap from Lab to Pharmaceuticals. Curr. Opin. Colloid Interface Sci. 2025, 75, 101875. [Google Scholar] [CrossRef]
- Dymek, M.; Sikora, E. Liposomes as Biocompatible and Smart Delivery Systems—The Current State. Adv. Colloid Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.; Tripathy, S.; Verma, D.K.; Singh, S.; Thakur, M.; Singh, A.K.; Srivastav, P.P.; Banerjee, M.; Patel, A.R.; Chávez González, M.L.; et al. Trends and Advances in Liposome Formulation Technology with an Emphasis on Ensuring Safety and Quality in Food and Drug Applications. Food Biosci. 2025, 69, 106913. [Google Scholar] [CrossRef]
- William, B.; Noémie, P.; Brigitte, E.; Géraldine, P. Supercritical Fluid Methods: An Alternative to Conventional Methods to Prepare Liposomes. Chem. Eng. J. 2020, 383, 123106. [Google Scholar] [CrossRef]
- Li, Y.; Deng, L.; Dai, T.; Li, Y.; Chen, J.; Liu, W.; Liu, C. Microfluidization: A Promising Food Processing Technology and Its Challenges in Industrial Application. Food Control 2022, 137, 108794. [Google Scholar] [CrossRef]
- Lajunen, T.; Hisazumi, K.; Kanazawa, T.; Okada, H.; Seta, Y.; Yliperttula, M.; Urtti, A.; Takashima, Y. Topical Drug Delivery to Retinal Pigment Epithelium with Microfluidizer Produced Small Liposomes. Eur. J. Pharm. Sci. 2014, 62, 23–32. [Google Scholar] [CrossRef]
- Fan, C.; Feng, T.; Wang, X.; Xia, S.; John Swing, C. Liposomes for Encapsulation of Liposoluble Vitamins (A, D, E and K): Comparation of Loading Ability, Storage Stability and Bilayer Dynamics. Food Res. Int. 2023, 163, 112264. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tian, M.; Kong, Y.; Lu, J.; Li, N.; Han, J. Multilayered Vitamin C Nanoliposomes by Self-Assembly of Alginate and Chitosan: Long-Term Stability and Feasibility Application in Mandarin Juice. LWT 2017, 75, 608–615. [Google Scholar] [CrossRef]
- Marsanasco, M.; Márquez, A.L.; Wagner, J.R.; Chiaramoni, N.S.; Alonso, S.d.V. Bioactive Compounds as Functional Food Ingredients: Characterization in Model System and Sensory Evaluation in Chocolate Milk. J. Food Eng. 2015, 166, 55–63. [Google Scholar] [CrossRef]
- Marsanasco, M.; Márquez, A.L.; Wagner, J.R.; Alonso, S.d.V.; Chiaramoni, N.S. Liposomes as Vehicles for Vitamins E and C: An Alternative to Fortify Orange Juice and Offer Vitamin C Protection after Heat Treatment. Food Res. Int. 2011, 44, 3039–3046. [Google Scholar] [CrossRef]
- Wechtersbach, L.; Poklar Ulrih, N.; Cigić, B. Liposomal Stabilization of Ascorbic Acid in Model Systems and in Food Matrices. LWT Food Sci. Technol. 2012, 45, 43–49. [Google Scholar] [CrossRef]
- Xu, X.; Tian, M.; Deng, L.; Jiang, H.; Han, J.; Zhen, C.; Huang, L.; Liu, W. Structural Degradation and Uptake of Resveratrol-Encapsulated Liposomes Using an In Vitro Digestion Combined with Caco-2 Cell Absorption Model. Food Chem. 2023, 403, 133943. [Google Scholar] [CrossRef]
- Baek, Y.; Jeong, E.W.; Lee, H.G. Encapsulation of Resveratrol within Size-Controlled Nanoliposomes: Impact on Solubility, Stability, Cellular Permeability, and Oral Bioavailability. Colloids Surf. B Biointerfaces 2023, 224, 113205. [Google Scholar] [CrossRef]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome Co-Encapsulation as a Strategy for the Delivery of Curcumin and Resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef]
- Saroglu, O.; Karadag, A. Propolis-Loaded Liposomes: Characterization and Evaluation of the In Vitro Bioaccessibility of Phenolic Compounds. ADMET DMPK 2024, 12, 209–224. [Google Scholar] [CrossRef]
- Feng, S.; Sun, Y.; Wang, P.; Sun, P.; Ritzoulis, C.; Shao, P. Co-Encapsulation of Resveratrol and Epigallocatechin Gallate in Low Methoxyl Pectin-Coated Liposomes with Great Stability in Orange Juice. Int. J. Food Sci. Technol. 2019, 55, 1872–1880. [Google Scholar] [CrossRef]
- El-Said, M.M.; El-Messery, T.M.; El-Din, H.M.F. The Encapsulation of Powdered Doum Extract in Liposomes and Its Application in Yoghurt. Acta Sci. Pol. Technol. Aliment. 2018, 17, 235–245. [Google Scholar] [CrossRef]
- Tavakoli, H.; Hosseini, O.; Jafari, S.M.; Katouzian, I. Evaluation of Physicochemical and Antioxidant Properties of Yogurt Enriched by Olive Leaf Phenolics within Nanoliposomes. J. Agric. Food Chem. 2018, 66, 9231–9240. [Google Scholar] [CrossRef] [PubMed]
- Altin, G.; Gültekin-Özgüven, M.; Ozcelik, B. Liposomal Dispersion and Powder Systems for Delivery of Cocoa Hull Waste Phenolics via Ayran (Drinking Yoghurt): Comparative Studies on In-Vitro Bioaccessibility and Antioxidant Capacity. Food Hydrocoll. 2018, 81, 364–370. [Google Scholar] [CrossRef]
- Kalajahi, S.E.M.; Amjadi, S.; Ghandiha, S. Development of the Spray-Dried Nettle (Urtica dioica L.) Extract-Loaded Nanoliposome Powder for Application as a Natural Additive in Cake. J. Food Process. Preserv. 2022, 46, e17229. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Thys, R.C.S.; Brandelli, A. Antifungal Properties of Phosphatidylcholine-Oleic Acid Liposomes Encapsulating Garlic against Environmental Fungal in Wheat Bread. Int. J. Food Microbiol. 2019, 293, 72–78. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.; Lou, X.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.; Xia, S. Liposomes as Delivery Systems for Carotenoids: Comparative Studies of Loading Ability, Storage Stability and In Vitro Release. Food Funct. 2014, 5, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- da Veiga de Mattos, M.V.C.; Michelon, M.; de Medeiros Burkert, J.F. Production and Stability of Food-Grade Liposomes Containing Microbial Carotenoids from Rhodotorula Mucilaginosa. Food Struct. 2022, 33, 100282. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zou, Y.-X.; Luo, Z.-G.; Tamer, T.M. Co-Encapsulation of Vitamin C and β-Carotene in Liposomes: Storage Stability, Antioxidant Activity, and In Vitro Gastrointestinal Digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef]
- Dutta, D.; Das Gupta, B.; Dutta, D. β-Cryptoxanthin Encapsulated Nanoliposome in Milk with an Approach to Enhance Vitamin A through Fortification. Int. Dairy J. 2025, 166, 106231. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-Encapsulation of Fish Oil in Nano-Liposomes and Its Application in Fortification of Yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef]
- Ramezanzade, L.; Hosseini, S.F.; Akbari-Adergani, B.; Yaghmur, A. Cross-Linked Chitosan-Coated Liposomes for Encapsulation of Fish-Derived Peptide. LWT 2021, 150, 112057. [Google Scholar] [CrossRef]
- Niu, Z.; Acevedo-Fani, A.; McDowell, A.; Barnett, A.; Loveday, S.M.; Singh, H. Nanoemulsion Structure and Food Matrix Determine the Gastrointestinal Fate and In Vivo Bioavailability of Coenzyme Q10. J. Control. Release 2020, 327, 444–455. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Liu, S.; Lu, Y.; Guo, C.; Cao, J.; Wang, X.; Wang, X. Liposome-Mediated Encapsulation of Probiotics: Current Status, Challenges and Future Directions. Crit. Rev. Food Sci. Nutr. 2025, 1–18. [Google Scholar] [CrossRef]
- Ko, J.; Yoo, C.; Xing, D.; Gonzalez, D.E.; Jenkins, V.; Dickerson, B.; Leonard, M.; Nottingham, K.; Kendra, J.; Sowinski, R.; et al. Pharmacokinetic Analyses of Liposomal and Non-Liposomal Multivitamin/Mineral Formulations. Nutrients 2023, 15, 3073. [Google Scholar] [CrossRef] [PubMed]
- Batinić, P.M.; Đorđević, V.B.; Stevanović, S.I.; Balanč, B.D.; Marković, S.B.; Luković, N.D.; Mijin, D.Ž.; Bugarski, B.M. Formulation and Characterization of Novel Liposomes Containing Histidine for Encapsulation of a Poorly Soluble Vitamin. J. Drug Deliv. Sci. Technol. 2020, 59, 101920. [Google Scholar] [CrossRef]
- Maiti, T.K.; Parvate, S.; Dixit, P.; Singh, J.; Reddy, V.J.; Bhuvanesh, E.; Chattopadhyay, S. Chapter 6—Liposome for Encapsulation of Essential Oil and Fatty Acids. In Liposomal Encapsulation in Food Science and Technology; Anandharamakrishnan, C., Dutta, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 113–124. ISBN 978-0-12-823935-3. [Google Scholar]
- Huang, R.; Song, H.; Wang, X.; Shen, H.; Li, S.; Guan, X. Fatty Acids-Modified Liposomes for Encapsulation of Bioactive Peptides: Fabrication, Characterization, Storage Stability and In Vitro Release. Food Chem. 2024, 440, 138139. [Google Scholar] [CrossRef] [PubMed]
- Homayonpour, P.; Jalali, H.; Shariatifar, N.; Amanlou, M. Effects of Nano-Chitosan Coatings Incorporating with Free /Nano-Encapsulated Cumin (Cuminum cyminum L.) Essential Oil on Quality Characteristics of Sardine Fillet. Int. J. Food Microbiol. 2021, 341, 109047. [Google Scholar] [CrossRef]
- Laridi, R.; Kheadr, E.E.; Benech, R.-O.; Vuillemard, J.C.; Lacroix, C.; Fliss, I. Liposome Encapsulated Nisin Z: Optimization, Stability and Release during Milk Fermentation. Int. Dairy J. 2003, 13, 325–336. [Google Scholar] [CrossRef]
- Khanniri, E.; Bagheripoor-Fallah, N.; Sohrabvandi, S.; Mortazavian, A.M.; Khosravi-Darani, K.; Mohammad, R. Application of Liposomes in Some Dairy Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 484–493. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Singh, H. Chapter 8—Progress in Applications of Liposomes in Food Systems. In Microencapsulation and Microspheres for Food Applications; Sagis, L.M.C., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 151–170. ISBN 978-0-12-800350-3. [Google Scholar]
- Khorasani, S.; Danaei, M.; Mozafari, M.R. Nanoliposome Technology for the Food and Nutraceutical Industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Xia, S.; Xu, S. Ferrous Sulfate Liposomes: Preparation, Stability and Application in Fluid Milk. Food Res. Int. 2005, 38, 289–296. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Harty, P.S.; Stratton, M.T.; Siedler, M.R.; Rodriguez, C. Liposomal Mineral Absorption: A Randomized Crossover Trial. Nutrients 2022, 14, 3321. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting Liposomes for Oral Drug Delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Ferreira, L.S.; da Silva, B.B.; Chaves, M.A.; Pinho, S.C. Physicochemical Characterization of Liposomes Produced by Ultrasonication and Coated with Pectin for the Coencapsulation of Vitamins D3 and B12. Food Chem. 2025, 485, 144441. [Google Scholar] [CrossRef]
- Hassane Hamadou, A.; Zhang, J.; Chao, C.; Xu, B. Stability of Rutin Using Pectin-Chitosan Dual Coating Nanoliposomes. LWT 2022, 170, 114084. [Google Scholar] [CrossRef]
- Sharma, V.M.; Valsaraj, T.V.; Sudeep, H.V.; Kodimule, S.; Jacob, J. Sustained Release of Liposomal Caffeine Using Novel Natural Fiber Interlaced Liposomal Technology: Development and Structural Characterisation. J. Pharm. Innov. 2024, 19, 65. [Google Scholar] [CrossRef]
- Pan, L.; Li, H.; Hou, L.; Chang, Z.; Li, Y.; Li, X. Gastrointestinal Digestive Fate of Whey Protein Isolate Coated Liposomes Loading Astaxanthin: Lipolysis, Release, and Bioaccessibility. Food Biosci. 2022, 45, 101464. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Karadağ, A.; Duman, Ş.; Özkal, B.; Özçelik, B. Fortification of Dark Chocolate with Spray Dried Black Mulberry (Morus nigra) Waste Extract Encapsulated in Chitosan-Coated Liposomes and Bioaccessability Studies. Food Chem. 2016, 201, 205–212. [Google Scholar] [CrossRef]
- Li, Z.; Paulson, A.T.; Gill, T.A. Encapsulation of Bioactive Salmon Protein Hydrolysates with Chitosan-Coated Liposomes. J. Funct. Foods 2015, 19, 733–743. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Sun, B. Biopolymer-Liposome Hybrid Systems for Controlled Delivery of Bioactive Compounds: Recent Advances. Biotechnol. Adv. 2021, 48, 107727. [Google Scholar] [CrossRef]
- Alehosseini, A.; Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; López-Rubio, A. Electrospun Curcumin-Loaded Protein Nanofiber Mats as Active/Bioactive Coatings for Food Packaging Applications. Food Hydrocoll. 2019, 87, 758–771. [Google Scholar] [CrossRef]
- Feng, X.; Li, Y.; Cui, Z.; Tang, R. Sodium Alginate/Carboxymethyl Cellulose Films Embedded with Liposomes Encapsulated Green Tea Extract: Characterization, Controlled Release, Application. RSC Adv. 2024, 14, 245–254. [Google Scholar] [CrossRef]
- Luo, B.; Xuan, S.; Wang, X.; Ding, K.; Jin, P.; Zheng, Y.; Wu, Z. Liposome/Chitosan Coating Film Bioplastic Packaging for Litchi Fruit Preservation. Food Chem. 2025, 464, 141850. [Google Scholar] [CrossRef]
- Liang, W.; Ge, X.; Lin, Q.; Zhao, W.; Niu, L.; Muratkhan, M.; Li, W. PH-Responsive Liposomes for Controlled Release of Alpinia Galanga Essential Oil: Investigating Characteristics, Stability, Control Release Behavior, and Functionality. Ind. Crops Prod. 2024, 209, 117978. [Google Scholar] [CrossRef]
- Najafi, Z.; Kahn, C.J.F.; Bildik, F.; Arab-Tehrany, E.; Şahin-Yeşilçubuk, N. Pullulan Films Loading Saffron Extract Encapsulated in Nanoliposomes; Preparation and Characterization. Int. J. Biol. Macromol. 2021, 188, 62–71. [Google Scholar] [CrossRef]
- Sepúlveda, C.T.; Alemán, A.; Zapata, J.E.; Montero, M.P.; Gómez-Guillén, M.C. Characterization and Storage Stability of Spray Dried Soy-Rapeseed Lecithin/Trehalose Liposomes Loaded with a Tilapia Viscera Hydrolysate. Innov. Food Sci. Emerg. Technol. 2021, 71, 102708. [Google Scholar] [CrossRef]
- Farahmand, A.; Mousavi, S.F.; Emadzadeh, B.; Ghorani, B. Liposome-Fluidic Method for Aroma Masking of Cinnamon Essential Oil in Beverage. J. Polym. Environ. 2025, 33, 717–729. [Google Scholar] [CrossRef]
- Mousavi, S.F.; Koocheki, A.; Ghorani, B.; Mohebbi, M. Physicochemical Characteristics of Liposomal Curcumin Immobilized in Hybrid Alginate/ Alyssum Homocarpum Seed Gum Hydrogels by Electro-Hydrodynamic Atomization. Food Hydrocoll. 2025, 163, 111081. [Google Scholar] [CrossRef]
- Liu, H.; Meng, X.; Li, L.; Xia, Y.; Hu, X.; Fang, Y. The Incorporated Hydrogel of Chitosan-Oligoconjugated Linoleic Acid Vesicles and the Protective Sustained Release for Curcumin in the Gel. Int. J. Biol. Macromol. 2023, 227, 17–26. [Google Scholar] [CrossRef]
- Liu, W.; Kong, Y.; Ye, A.; Shen, P.; Dong, L.; Xu, X.; Hou, Y.; Wang, Y.; Jin, Y.; Han, J. Preparation, Formation Mechanism and In Vitro Dynamic Digestion Behavior of Quercetin-Loaded Liposomes in Hydrogels. Food Hydrocoll. 2020, 104, 105743. [Google Scholar] [CrossRef]
- Al-Moghazy, M.; El-sayed, H.S.; Salama, H.H.; Nada, A.A. Edible Packaging Coating of Encapsulated Thyme Essential Oil in Liposomal Chitosan Emulsions to Improve the Shelf Life of Karish Cheese. Food Biosci. 2021, 43, 101230. [Google Scholar] [CrossRef]
- Cengiz, A.; Schroën, K.; Berton-Carabin, C. Towards Oxidatively Stable Emulsions Containing Iron-Loaded Liposomes: The Key Role of Phospholipid-to-Iron Ratio. Foods 2021, 10, 1293. [Google Scholar] [CrossRef]
- Pattnaik, M.; Mishra, H.N. Effect of Ultrasonication and Wall Materials on the Stability, Rheology, and Encapsulation Efficiency of Vitamins in a Lipid-Based Double Emulsion Template. J. Food Process Eng. 2023, 46, e14201. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, W.; Pu, C.; Li, R.; Sun, Q.; Wang, H. Improved Stability of Liposome-Stabilized Emulsions as a Co-Encapsulation Delivery System for Vitamin B2{,} Vitamin E and β-Carotene. Food Funct. 2022, 13, 2966–2984. [Google Scholar] [CrossRef]
- Feng, K.; Li, C.; Wei, Y.-S.; Zong, M.-H.; Wu, H.; Han, S.-Y. Development of a Polysaccharide Based Multi-Unit Nanofiber Mat for Colon-Targeted Sustained Release of Salmon Calcitonin. J. Colloid Interface Sci. 2019, 552, 186–195. [Google Scholar] [CrossRef]
- Li, C.; Bai, M.; Chen, X.; Hu, W.; Cui, H.; Lin, L. Controlled Release and Antibacterial Activity of Nanofibers Loaded with Basil Essential Oil-Encapsulated Cationic Liposomes against Listeria Monocytogenes. Food Biosci. 2022, 46, 101578. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of Chitosan Nanofibers Containing Tea Tree Oil Liposomes against Salmonella spp. in Chicken. LWT 2018, 96, 671–678. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Li, W.; Lin, L. Antioxidant Property of SiO2-Eugenol Liposome Loaded Nanofibrous Membranes on Beef. Food Packag. Shelf Life 2017, 11, 49–57. [Google Scholar] [CrossRef]
- de Freitas Zômpero, R.H.; López-Rubio, A.; de Pinho, S.C.; Lagaron, J.M.; de la Torre, L.G. Hybrid Encapsulation Structures Based on β-Carotene-Loaded Nanoliposomes within Electrospun Fibers. Colloids Surf. B Biointerfaces 2015, 134, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Sarraf-Ov, N.; Awlqadr, F.H.; Abdalla, K.R.; Hashemi, H.; Rouhi, M.; Mohammadi, R.; Ebrahimi, B. Characterization of Gelatin-Chitosan Films Incorporated with Nicotiana Tabacum Extract Nanoliposomes for Food Packaging Applications. Int. J. Biol. Macromol. 2025, 311, 143701. [Google Scholar] [CrossRef]
- Tagrida, M.; Gulzar, S.; Nilsuwan, K.; Prodpran, T.; Ma, L.; Benjakul, S. Properties of Gelatin/Chitosan Blend Films Incorporated with Betel Leaf Ethanolic Extract Loaded in Liposomes and Their Use as Pouches for Shrimp Oil Packaging. Int. J. Food Sci. Technol. 2022, 58, 1108–1119. [Google Scholar] [CrossRef]
- Montero, P.; Mosquera, M.; Marín-Peñalver, D.; Alemán, A.; Martínez-Álvarez, Ó.; Gómez-Guillén, M.C. Changes in Structural Integrity of Sodium Caseinate Films by the Addition of Nanoliposomes Encapsulating an Active Shrimp Peptide Fraction. J. Food Eng. 2019, 244, 47–54. [Google Scholar] [CrossRef]
- Tu, Y.; Ning, Y.; Wang, H.; Li, Y.; Yao, Z.; Tao, S.; Yang, W.; Li, B.; Li, X.; He, H.; et al. Liposomal Nano-Encapsulation of BFGF Combined with Injectable BSA Hydrogel for Efficient Burn Wound Healing. Colloids Surf. B Biointerfaces 2025, 245, 114263. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Wang, S.; Zhu, Y.; Wang, Y.; Chen, Y.; Xia, M.; Yang, M.; Yi, H.; Su, K. PH-Responsive Liposome–Hydrogel Composite Accelerates Nasal Mucosa Wound Healing. Pharmaceutics 2025, 17, 690. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Johannessen, M.; Gravningen, K.; Puolakkainen, M.; Acharya, G.; Basnet, P.; Škalko-Basnet, N. Liposomes-In-Hydrogel Delivery System Enhances the Potential of Resveratrol in Combating Vaginal Chlamydia Infection. Pharmaceutics 2020, 12, 1203. [Google Scholar] [CrossRef] [PubMed]
- Saroglu, O.; Karadag, A.; Çakmak, Z.H.T.; Karasu, S. The Formulation and Microstructural, Rheological, and Textural Characterization of Salep-Xanthan Gum-Based Liposomal Gels. Polym. Bull. 2023, 80, 9941–9962. [Google Scholar] [CrossRef]
- Pasban, A.; Mousavi, S.F.; Abdollahi, S.; Hesarinejad, M.A. Evaluating the Potential of Soy Protein Isolate /Alginate Hydrogel as Polyphenolic Liposome Carrier during Gastrointestinal Tract: A Case Study on Sumac Extract. LWT 2024, 211, 116883. [Google Scholar] [CrossRef]
- Eugster, R.; Ganguin, A.A.; Seidi, A.; Aleandri, S.; Luciani, P. 3D Printing Injectable Microbeads Using a Composite Liposomal Ink for Local Treatment of Peritoneal Diseases. Drug Deliv. Transl. Res. 2024, 14, 1567–1581. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tagami, T.; Ozeki, T. Fabrication of 3D-Printed Fish-Gelatin-Based Polymer Hydrogel Patches for Local Delivery of PEGylated Liposomal Doxorubicin. Mar. Drugs 2020, 18, 325. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Pérez-García, A.; Alemán, A.; Gómez-Guillén, M.C.; Montero, P. Drying Soy Phosphatidylcholine Liposomal Suspensions in Alginate Matrix: Effect of Drying Methods on Physico-Chemical Properties and Stability. Food Hydrocoll. 2021, 111, 106357. [Google Scholar] [CrossRef]
- Kasapoğlu, K.N.; Gültekin-Özgüven, M.; Kruger, J.; Frank, J.; Bayramoğlu, P.; Barla-Demirkoz, A.; Özçelik, B. Effect of Spray Drying on Physicochemical Stability and Antioxidant Capacity of Rosa Pimpinellifolia Fruit Extract-Loaded Liposomes Conjugated with Chitosan or Whey Protein During In Vitro Digestion. Food Bioprocess Technol. 2024, 17, 3162–3176. [Google Scholar] [CrossRef]
- Song, F.; Tian, S.; Chen, X.; Sun, S. Development of Liposomes Stabilized Pickering Emulsions as a Potential Delivery System for Flaxseed Oil. LWT 2024, 195, 115794. [Google Scholar] [CrossRef]
- Pascual-Silva, C.; Alemán, A.; López-Caballero, M.E.; Montero, M.P.; Gómez-Guillén, M.d.C. Physical and Oxidative Water-in-Oil Emulsion Stability by the Addition of Liposomes from Shrimp Waste Oil with Antioxidant and Anti-Inflammatory Properties. Antioxidants 2022, 11, 2236. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Antioxidant Loaded Emulsions Entrapped in Liposomes Produced Using a Supercritical Assisted Technique. J. Supercrit. Fluids 2019, 154, 104626. [Google Scholar] [CrossRef]


| Delivery Matrix | Liposome-Encapsulated Compounds | Functional Improvements | Application Areas | Advantages | Limitations/Challenges | Ref. |
|---|---|---|---|---|---|---|
| Electrospun nanofibers | Anthocyanins; propolis; curcumin | Bioaccessibility; mucoadhesion; sustained release; antioxidant stability | Oral delivery; wound healing; active food packaging | Very high surface area; tunable release profiles; inherent mucoadhesiveness | Scale-up difficulties, low loading of active compound, limited variety of food-grade polymers | [16,17,79] |
| Edible films | Green tea extract; essential oils; saffron extract | Mechanical and barrier properties; shelf-life extension; aroma masking | Coatings of perishable fruits and meats; active packaging | Biodegradable; consumer and environment-friendly format | Potential odor migration; moisture sensitivity; limited loading capacity | [80,81,82,83] |
| Spray-dried particles | Protein hydrolysate; anthocyanins; essential oils | Stability; good rehydration properties; maintained bioactivity | Functional ingredient incorporation into dry foods | Extended shelf life; good rehydration | Thermal degradation risk of heat-labile bioactives | [9,84,85] |
| Hydrogels | Propolis; curcumin; quercetin | Structural integrity; mechanical strength; viscoelasticity; gastrointestinal stability; bioaccessibility | Oral delivery in food matrices; customized nutrition; edible food gels/sauces | High water content; tunable rheology | Limited mechanical stability under stress; syneresis and potential microbial growth (without preservatives) during storage | [18,86,87,88] |
| Emulsion | Essential oils: iron; vitamins; β-carotene | Oxidative and physical stability; bioaccessibility; protection during storage and thermal processing; reduced lipolysis | Dairy products; beverages; sauces; bakery applications | Especially suited for lipophilic bioactives; easy to scale up | Prone to phase separation over time; requires surfactant/emulsifier compatibility with food matrix | [89,90,91,92] |
| Cast Film Matrix Components | Ingredients and Size of Liposomes | Production Method of Liposome | Liposome-Encapsulated Bioactive | Results | Ref |
|---|---|---|---|---|---|
| Chitosan | Soybean lecithin, cholesterol; 190 nm | Thin-film hydration | Copper nanoparticles, thyme essential oil | Better fresh-keeping effect on litchi | [81] |
| Bovine gelatin (B type)/Chitosan | Soybean lecithin, Tween 80; 110 nm | Thin-film hydration | Nicotiana tabacum (Tobacco) extract | Improved antibacterial efficacy against Salmonella enterica and Pseudomonas aerogenosan | [98] |
| Esterified konjac glucomannan (KGM)/Polyvinyl alcohol (PVA) | Soybean lecithin, cholesterol, hexadecyl trimethyl ammonium bromide (CTAB), γ-polyglutamic acid (γ-PGA, coating material); 100–300 nm | Thin-film hydration | Alpina galangal essential oil | pH-responsive liposomes endow films with intelligent controlled-release behavior; Extended the shelf life of citrus fruits | [82] |
| Sodium alginate/Carboxymethyl cellulose | Soybean lecithin, Tween 80; 810 nm | Thin-film hydration | Green tea extract | Improved mechanical strength and antioxidant capacity; Extended shelf life in blueberry packaging | [80] |
| Gelatin/Chitosan | Soybean lecithin, cholesterol; 195–450 nm | Thin-film hydration + ethanol injection | Betel leaf extract | Improved antioxidant activity and UV transmittance; Reduced visible light transmittance; Reduced release rate | [99] |
| Pullulan | Rapeseed lecithin; 119–138 nm | Thin-film hydration + ultrasonication | Saffron extract | Reduced oxygen permeability; Slightly weakened mechanical strength | [83] |
| Sodium caseinate | Soybean lecithin; 94–99 nm | Thin-film hydration+ ultrasonication | Shrimp peptide fraction | More water soluble and mucoadhesive films; Enhanced sensory properties | [100] |
| Hydrogel Matrix Components | Ingredients and Size of Liposomes | Production Method of Liposome | Liposome-Encapsulated Bioactive | Results | Ref |
|---|---|---|---|---|---|
| Xanthan gum/Salep | Soybean lecithin (70% phosphatidylcholine, PC); 94.21 nm | Thin-film hydration | - | Liposome incorporation to gel enhanced viscoelastic behavior; gel hardness and structural stability | [104] |
| Soybean lecithin (70% PC), Ammonium phosphatide (AMP), Tween 80; 80.97–179.30 nm | Thin-film hydration | Propolis extract | Liposome-loaded gels had higher mucoadhesion; AMP-based liposomal gels exhibited better structural and functional properties; The bioaccessibility of phenolics significantly increased compared to liposomes | [18] | |
| Alginate/Alyssum homocarpum seed gum | Soybean lecithin, cholesterol; 0.06–0.13 mm (size of liposomal gel) | Ethanol injection | Curcumin | Improved thermal stability and curcumin bioaccessibility; Delayed release with increasing hydrogel ratio | [86] |
| Soy protein isolate (SPI)/Alginate | Soybean lecithin, cholesterol; 218.5–266.5 nm | Hydration + heating | Sumac extract | Ratio of SPI to alginate had a substantial impact on the release of phenolic compounds from liposomes; In intestinal conditions, denser matrices offered more protection and slower release for liposomes | [105] |
| Chitosan | OCLAVs (oligo-conjugated linoleic acid vesicles) | Thin-film hydration | Curcumin | Sustained release over 96 h (51.23% vs. 93.37% in control); Increased antioxidant activity up to 148.1%. | [87] |
| Chitosan/Gelatin | L-a-PC (Soybean > 94%) | Thin-film hydration | Quercetin | Liposome-loaded gels provided better gastric protection for quercetin (~40% less release) compared to free liposomes; Texture of gels influenced release behavior | [88] |
| Particle Matrix Components | Ingredients and Size of Liposomes | Production Method of Liposome | Liposome-Encapsulated Bioactive | Results | Ref |
|---|---|---|---|---|---|
| Alginate | Soybean lecithin, cholesterol; 252 nm | Ethanol injection | Cinnamon essential oil (CEO) | Masking the odor and taste of CEO in an acidic beverage | [85] |
| Soybean lecithin; 98–612 nm | Thin-film hydration + ultrasonication | - | Enhanced stability of liposomes | [108] | |
| Maltodextrin | Soybean lecithin, cholesterol, Tween 80, chitosan and whey protein (surface coating) uncoated: 86–95 nm; coated: 167 nm, 326 nm | Ethanol injection + high pressure homogenization | Rosa pimpinellifolia fruit extract (Rosa extract) | Protection of polyphenols against processing and digestive tract conditions | [109] |
| Soybean lecithin, chitosan (surface coating); 82 nm | Thin-film hydration + ultrasonication | Black carrot anthocyanin extract (BCE) | Elevated physical and chemical stability of BCE | [9] | |
| Trehalose | Soybean/rapeseed lecithin; 215–250 nm | Thin-film hydration + ultrasonication | Tilapia viscera hydrolysate | Enhanced structural and functional stability of liposomes | [84] |
| Emulsion Matrix Components | Ingredients and Size of Liposomes | Production Method of Liposome | Liposome-Encapsulated Bioactive | Results | Ref |
|---|---|---|---|---|---|
| W/O/W emulsion: Vegetable oil blend, milk protein isolate, trehalose | Soybean lecithin; 143–396 nm | Thin-film hydration + ultrasonication | Multivitamins (A, D, B2, B9, B12) | Vitamin A, D, B12 had >96% encapsulation; Improved stability and viscosity Freeze-dried liposomes enabled better vitamin retention | [91] |
| W/O emulsion: Sunflower oil | Phospholipids from shrimp waste; 143.13–190.5 nm | Thin-film hydration + ultrasonication | Antioxidant lipids | Enhanced oxidative and physical stability | [111] |
| O/W emulsion: Corn oil | Soybean lecithin, cholesterol, Tween 80; 91.3 3–339.5 nm | Thin-film hydration | Vitamin B2, Vitamin E, β-carotene | Improved stability; Higher vitamin B2 bioaccessibility; Smaller particle size | [92] |
| Chitosan, essential oil | Soybean lecithin; 42–175 μm | Reverse-phase evaporation | Thyme essential oil | Extended shelf life (up to 4 weeks); Reduced microbial growth; Stability of thymol and p-cymene | [89] |
| O/W emulsion: Rapeseed oil, Tween 20 | Egg-yolk PC (~60%), cholesterol; 129–154 nm | Reverse phase evaporation + ultrasonication | Soluble iron (ferrous-Fe sulfate) | Free iron (Fe) and low PC:Fe ratio increased lipid oxidation | [90] |
| O/W emulsion: Isopropyl myristate, Tween 80 | Egg-yolk PC (99%); 116–230 nm | Supercritical-assisted production | Lipophilic antioxidants (farnesol, limonene, linalool) | Higher encapsulation efficiency; Submicron particle size; Improved antioxidant stability | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mankan, E.; Karakas, C.Y.; Saroglu, O.; Mzoughi, M.; Sagdic, O.; Karadag, A. Food-Grade Liposome-Loaded Delivery Systems: Current Trends and Future Perspectives. Foods 2025, 14, 2978. https://doi.org/10.3390/foods14172978
Mankan E, Karakas CY, Saroglu O, Mzoughi M, Sagdic O, Karadag A. Food-Grade Liposome-Loaded Delivery Systems: Current Trends and Future Perspectives. Foods. 2025; 14(17):2978. https://doi.org/10.3390/foods14172978
Chicago/Turabian StyleMankan, Erkan, Canan Yagmur Karakas, Oznur Saroglu, Mondher Mzoughi, Osman Sagdic, and Ayse Karadag. 2025. "Food-Grade Liposome-Loaded Delivery Systems: Current Trends and Future Perspectives" Foods 14, no. 17: 2978. https://doi.org/10.3390/foods14172978
APA StyleMankan, E., Karakas, C. Y., Saroglu, O., Mzoughi, M., Sagdic, O., & Karadag, A. (2025). Food-Grade Liposome-Loaded Delivery Systems: Current Trends and Future Perspectives. Foods, 14(17), 2978. https://doi.org/10.3390/foods14172978








