Synergistic Flavor Modulation and Functional Enhancement of Douchiba via Compounding with Bacillus subtilis-Fermented Adlay
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of FADS
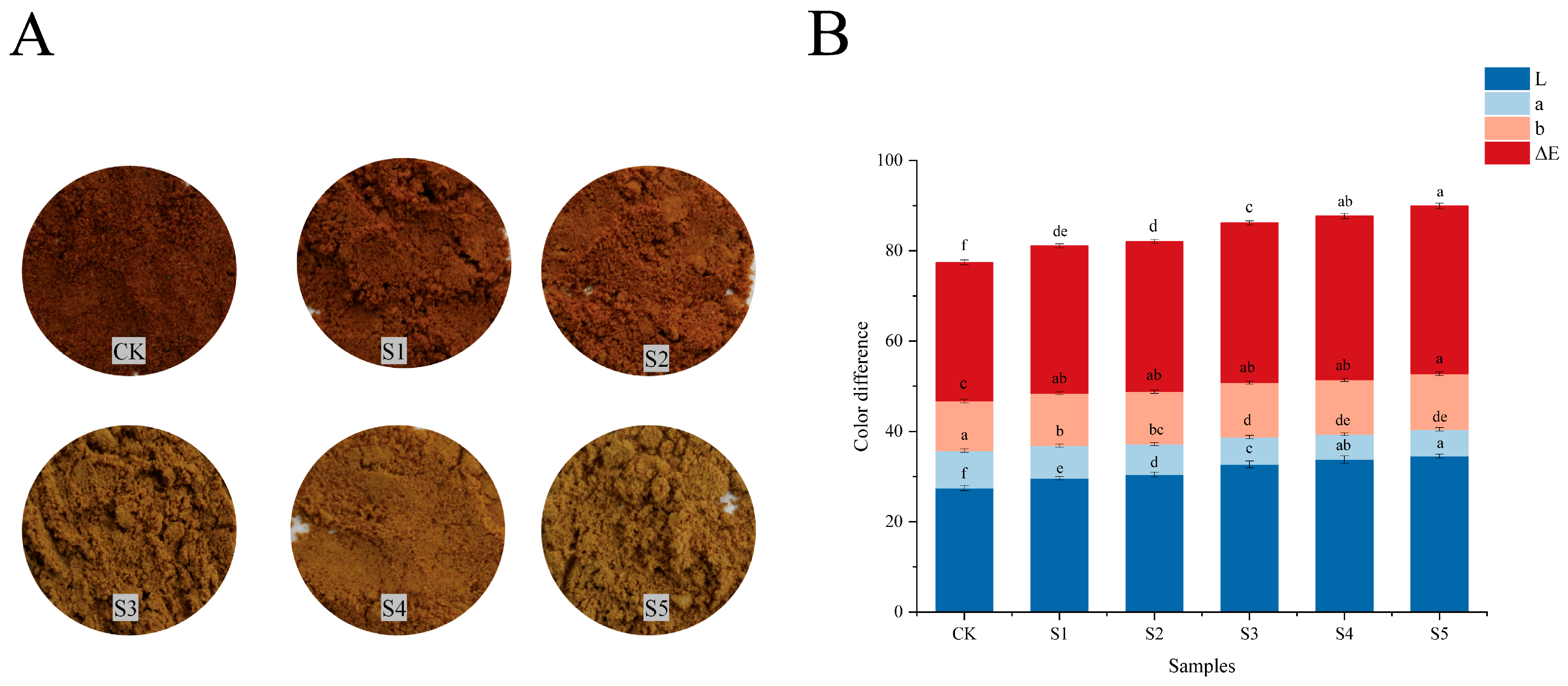
2.3. Determination of Color Difference
2.4. Determination of Water Holding Capacity (WHC) and Oil Holding Capacity (OHC)
2.5. Determination of Nutritional Components
2.6. Determination of Functional Components
2.7. Determination of the Free Amino Acid
2.8. Determination of Taste Substances
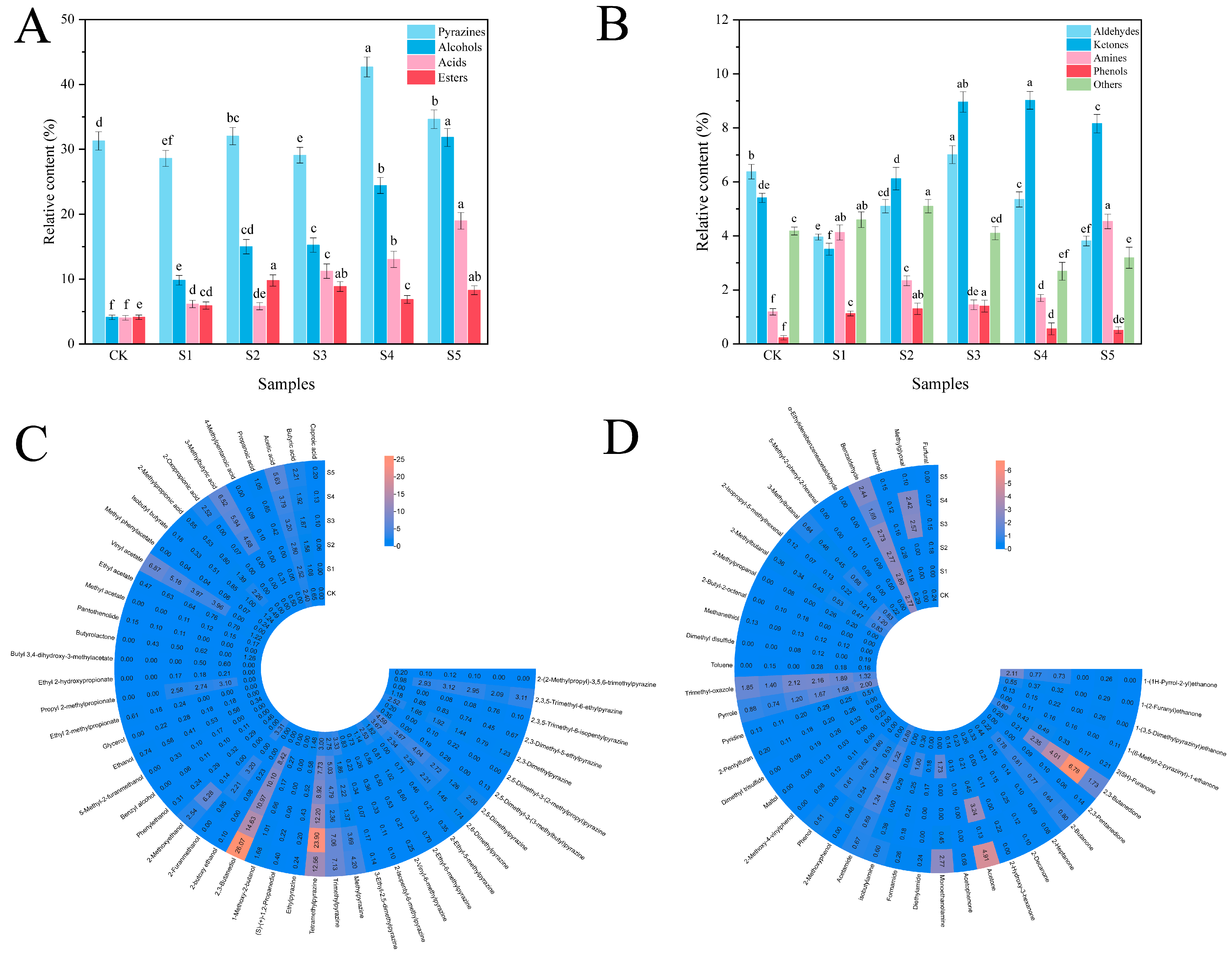
2.9. Determination of Volatile Flavor Substances
2.10. Data Analysis
3. Results and Discussion
3.1. Color Difference Analysis of FADS
3.2. Basic Quality Analysis in FADS
3.3. Contents of Active Ingredients
3.4. Contents of Free Amino Acids
3.5. Analysis of Taste Substances
3.6. Relative Content of Volatile Flavor Substances
3.7. Comprehensive Quality Evaluation of FADS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Xiang, F.; Zhang, Z.; Hou, Q.; Guo, Z. Characterization of bacterial community and flavor differences of different types of Douchi. Food Sci. Nutr. 2021, 9, 3460–3469. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; He, W.; Liao, L.; Xia, B.; Jiang, L.; Liu, Y. Analysis of microbial community and the characterization of Aspergillus flavus in Liuyang Douchi during fermentation. LWT 2022, 154, 112567. [Google Scholar] [CrossRef]
- Luo, A.; Liu, T.; Shi, S.; Liu, X.; Shi, X.; Hu, B. Characterization of Microbial Community and Flavor Compounds in Traditional Fermented Douchi Using HTS and HS-SPME-GC-MS. Food Sci. Nutr. 2025, 13, e4660. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Q.; Yang, Y.; Wen, A.; Zeng, H.; Liu, N.; Qin, L. Effects of enhanced fermentation with high-yielding strains of Tetramethylpyrazine on flavor quality of Douchiba. Food Chem. X 2025, 25, 102037. [Google Scholar] [CrossRef]
- Huang, Y.; Li, T.; Han, X.; Qiu, X. Processing of Chili Beef Paste with Flavor Douchi Cake (China). Farm Prod. Proc. 2021, 22, 1–4. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Ji, Y.; He, H.; Yang, H.; Tang, X.; Liu, Y. Characterization and correlation of dominant bacteria and volatile compounds in post-fermentation process of Ba-bao Douchi. Food Res. Int. 2022, 160, 111688. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, P.; Wen, A.; Zeng, H.; Liu, N.; Qin, L.; Zhou, P. Dynamic Correlation Between Bacterial Communities and Volatile Compounds During Douchiba Fermentation. Food Sci. Nutr. 2025, 13, e70153. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, A.; Qin, L.; Hu, Y.; Zhu, Y. Study on Characteristic Flavor Substances in Traditional Fermented Douchiba. Food Ferment. Sci. Technol. 2023, 59, 62–72. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Y.; Long, Z.; Fu, X.; Ren, K. Study on the taste active compounds in Douchi using metabolomics method. Food Chem. 2023, 412, 135343. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Q.; He, L.; Dong, L.; Liu, Z.; Wang, X.; Li, C.; Qiao, S. Fermentation with Bacillus natto and Bifidobacterium improves the functional, physicochemical properties, and taste qualities of coix seed-natto. Food Res. Int. 2024, 196, 115074. [Google Scholar] [CrossRef]
- Fu, H.; Wang, L. Study on Production Process of Coix seed Natto. China Condiment 2016, 41, 79–83. [Google Scholar] [CrossRef]
- Wen, A.; Xie, C.; Mazhar, M.; Wang, C.; Zeng, H.; Qin, L.; Zhu, Y. Tetramethylpyrazine from adlay (Coix lacryma-jobi) biotransformation by Bacillus subtilis and its quality characteristics. J. Food Sci. Technol. 2020, 57, 4092–4102. [Google Scholar] [CrossRef]
- Wen, A.; Zhu, Y.; Mazhar, M.; Qin, L.; Zeng, H.; Zhu, Y. Enhancement of Anti-Proliferative Activity of the Extracts from Dehulled Adlay by Fermentation with Bacillus subtilis. Foods 2021, 10, 2959. [Google Scholar] [CrossRef] [PubMed]
- Subtain, M.; Pasha, I.; Ahmad, F.; Rasheed, H.; Ansar, S.; Fordos, S.; Ayub, H. Phytochemical characterization and end use evaluation of native and fermented cereal brans. J. Food Meas. Charact. 2024, 18, 5552–5563. [Google Scholar] [CrossRef]
- Fu, J.; Wu, Y.; Yang, Y.; Zhang, W.; Zhang, S.; Ge, P.; Wen, A.; Wu, Q.; Zeng, H.; Qin, L. Quality and safety of natto fermented with Bacillus subtilis BJ3-2 using kidney beans from Guizhou. Int. J. Food Sci. Technol. 2025, 60, vvae097. [Google Scholar] [CrossRef]
- Almaghlouth, B.J.; Alqahtani, N.K.; Alnabbat, K.I.; Mohamed, H.A.; Alnemr, T.M.; Habib, H.M. Valorization of Second-Grade Date Fruit Byproducts and Nonstandard Sweet Potato Tubers to Produce Novel Biofortified Functional Jam. Foods 2023, 12, 1906. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Guo, X.; Chen, Y.; Li, H.; Zhou, G.; Sun, S.; Ren, Q.; Gandara, J.S.; Sun, J.; et al. Extraction, purification and anticancer activity studies on triterpenes from pomegranate peel. Food Funct. 2024, 15, 6914–6928. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Li, Y.; Cui, F.; Zhang, W.; Zhang, Z.; Li, H. Nutrition, Flavor, and Microbial Communities of Two Traditional Bacterial Douchi from Gansu, China. Foods 2024, 13, 3519. [Google Scholar] [CrossRef]
- Liu, N.; Qin, L.; Lu, X.; Zhao, Y.; Miao, S. Physicochemical components and flavor properties of acid rice soup (rice-acid) fermented with Lactobacillus paracasei and/or Kluyveromyces marxianus. Food Biosci. 2021, 43, 101278. [Google Scholar] [CrossRef]
- Zhu, Z.; Anthony, P.B.; Cao, Y.; Du, X.; Huang, T.; Cheng, Y.; Huang, M. Meat quality and flavor evaluation of Nanjing water boiled salted duck (NWSD) produced by different Muscovy duck (Cairina moschata) ingredients. Food Chem. 2022, 397, 133833. [Google Scholar] [CrossRef]
- Li, M.; Huang, J.; Chen, Y.; Liu, C.; Wu, X. Protein from red adzuki bean: Extraction optimization, glycosylation modification and physicochemical properties of glycation products. J. Food Meas. Charact. 2024, 18, 4229–4245. [Google Scholar] [CrossRef]
- Xue, H.; Tu, Y.; Zhang, G.; Xin, X.; Hu, H.; Qiu, W.; Ruan, D.; Zhao, Y. Mechanism of ultrasound and tea polyphenol assisted ultrasound modification of egg white protein gel. Ultrason. Sonochem. 2021, 81, 105857. [Google Scholar] [CrossRef]
- Sayem, A.S.M.; Talukder, S.; Akter, S.S.; Alam, M.; Rana, M.R.; Alam, M.M. Utilization of fruits and vegetables wastes for the dietary fiber enrichment of biscuits and its quality attributes. J. Agric. Food Res. 2024, 15, 101077. [Google Scholar] [CrossRef]
- Li, Z.; Deng, P.; He, Z.; Wang, Z.; Chen, Q.; Chen, J.; Wang, X.; Zeng, M. Effects of enzymatic hydrolysis, ball milling, and extrusion on the physical and functional properties of dietary fibers from sweet potatoes. Food Res. Int. 2025, 203, 115883. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, C.; Wang, T.; Sun, L.; Wu, F.; Yu, D. Combined multispectral analysis and molecular docking to research the interaction of soybean isolate protein with different kinds of phospholipid liposomes and its effect on liposome properties. Food Chem. 2025, 474, 143160. [Google Scholar] [CrossRef]
- Sui, L.; Wang, S.; Wang, X.; Su, L.; Xu, H.; Xu, W.; Chen, L.; Li, H. Analysis of Different Strains Fermented Douchi by GC×GC-TOFMS and UPLC–Q-TOFMS Omics Analysis. Foods 2024, 13, 3521. [Google Scholar] [CrossRef] [PubMed]
- Cempaka, L.; Eliza, N.; Ardiansyah, A.; Handoko, D.D.; Astuti, R.M. Proximate Composition Total Phenolic Content and Sensory Analysis of Rice Bran Tempeh. Makara J. Sci. 2018, 22, 89–94. [Google Scholar] [CrossRef]
- Chao, F.; Yang, H.; Fan, D.; Deng, J. Dietary Triterpenoids in Functional Food and Drug Ingredients: A review of structure-activity relationships, biosynthesis, applications, and AI-driven strategies. Trends Food Sci. Technol. 2025, 159, 104961. [Google Scholar] [CrossRef]
- Dong, L.; Yang, Y.; Zhao, Y.; Liu, Z.; Li, C.; He, L.; Liu, L. Effect of different conditions on the germination of coix seed and its characteristics analysis. Food Chem. X 2024, 22, 101332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, H.; Wang, S.; Fu, Y.; Wang, M. Gut Microbiota and Neurotransmitter Regulation: Functional Effects of Four Traditional Chinese Fermented Soybean (Sojae Semen Praeparatum). Foods 2025, 14, 671. [Google Scholar] [CrossRef] [PubMed]
- Chitisankul, W.T.; Itabashi, M.; Son, H.; Takahashi, Y.; Ito, A.; Varanyanond, W.; Tsukamoto, C. Soyasaponin composition complexities in soyfoods relating nutraceutical properties and undesirable taste characteristics. LWT 2021, 146, 111337. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Li, T.; Zhang, X.; Li, W. Enhance Production of γ-Aminobutyric Acid (GABA) and Improve the Function of Fermented Quinoa by Cold Stress. Foods 2022, 11, 3908. [Google Scholar] [CrossRef] [PubMed]
- Golubova, D.; Salmon, M.; Su, H.; Tansley, C.; Kaithakottil, G.G.; Linsmith, G.; Schudoma, C.; Swarbreck, D.; Connell, M.O.; Patron, N.J. Biosynthesis and bioactivity of anti-inflammatory triterpenoids in Calendula officinalis. Nat. Commun. 2025, 16, 6941. [Google Scholar] [CrossRef]
- Shen, L.; Chen, X.; Zhou, Y.; Pan, M.; Zhou, X.; Hu, Y.; Zhao, L. Multi-dimensional evaluation of the impacts of heat treatment processes on the flavor quality of antarctic krill. J. Food Meas. Charact. 2024, 18, 6419–6432. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Zhu, Y.; He, Y.; Qin, L.; Liang, Y. Quality evaluation of Guizhou kidney beans based on principal component analysis. Food Mach. 2020, 36, 48–53. [Google Scholar] [CrossRef]
- Adeyinka, A.J.; Berka, N.P.; Sefater, G.; Bamikole, O.A.; Mayowa, O.O.; Adeoye, O.S.; Ayodeji, A.O. Fermentation of Cereals and Legumes: Impact on Nutritional Constituents and Nutrient Bioavailability. Fermentation 2022, 8, 63. [Google Scholar] [CrossRef]
- Wen, A.; Yang, Z.; Liu, N.; Zeng, H.; Qin, L. Dynamic correlation between tetramethylpyrazine and influencing factors in Bacillus subtilis-fermented dehulled adlay. Food Biosci. 2023, 52, 102355. [Google Scholar] [CrossRef]
- Han, F.; James, M.P.; Juntao, L.; Natascha, S.; Pang, S. The Complementarity of Amino Acids in Cooked Pulse/Cereal Blends and Effects on DIAAS. Plants 2021, 10, 1999. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, C.; Xu, M.; Chen, J.; Jiang, G.; Lou, D.; Zhao, H. Isolation, Identification and Taste Characteristics of Flavor Peptides from Douchi. Food Sci. 2024, 45, 176–182. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, X.; Dong, L.; Zhao, Y.; He, L.; Li, C.; Wang, X.; Zeng, X. Effects of dual-strain fermentation on physicochemical properties of Rosa roxburghii Tratt and coix seed beverage. LWT 2024, 194, 115813. [Google Scholar] [CrossRef]
- Suo, H.; Zhao, X.; Qian, Y.; Chen, J.; Li, J.; Zhang, Y.; Wang, Y.; Kan, J. Changes in Total Sugar and Amino Acids, and Formation of Taste Compounds in Yongchuan Douchi during Fermentation. Food Sci. 2015, 36, 100–104. [Google Scholar] [CrossRef]
- Li, C.; Yang, D.; Li, L.; Wang, Y.; Chen, S.; Zhao, Y.; Lin, W. Comparison of the taste mechanisms of umami and bitter peptides from fermented mandarin fish (Chouguiyu) based on molecular docking and electronic tongue technology. Food Funct. 2023, 14, 9671–9680. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.; Qin, Y.; Jiang, L.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Li, G.; Sng, K.S.; Shu, B.; Wang, Y.; Yao, M.; Cui, X. Effects of tetramethylpyrazine treatment in a rat model of spinal cord injury: A systematic review and meta-analysis. Eur. J. Pharmacol. 2023, 945, 175524. [Google Scholar] [CrossRef]
- Zhong, H.; Shen, J.; Meng, Z.; Zhao, J.; Xiao, Z. Tetramethylpyrazine production from edible materials by the probiotic Bacillus coagulans. Prep. Biochem. Biotechnol. 2020, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, R.; Yang, F.; Xie, Y.; Guo, Y.; Yang, W.; Zhou, W. Control strategies of pyrazines generation from Maillard reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Sun, Y.; Guo, C.; Zhu, J.; Niu, X.; Gao, M. Comparative analysis of physicochemical properties, sensory characteristics, and volatile flavor compounds in five types of potato chips. Front. Nutr. 2025, 12, 1525480. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Huang, X.; Tong, S.; Li, C.; Wu, Y. Bacillus subtilis BJ3-2 lspA Affects the Synthesis of Tetramethylpyrazine in Bacterial-type Douchi. Food Ferment. Sci. Technol. 2022, 58, 9–15. [Google Scholar] [CrossRef]
- Lei, X.; Chen, Y.; Gao, B.; Zhao, X.; Sun, Q.; Qin, Y.; Song, Y.; Jiang, J.; Liu, Y. Adaptive laboratory evolution of Lachancea thermotolerans for enhanced production of 2-Phenylethanol and 2-Phenylethyl acetate in wine. Food Chem. X 2025, 27, 102483. [Google Scholar] [CrossRef]
- Li, J.; Peng, B.; Huang, L.; Zhong, B.; Yu, C.; Hu, X.; Wang, W.; Tu, Z. Association between flavors and microbial communities of traditional Aspergillus-Douchi produced by a typical industrial-scale factory. LWT 2023, 176, 114532. [Google Scholar] [CrossRef]




| Sample | CK | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|---|
| Umami | 0.46 ± 0.09 a | 0.46 ± 0.06 a | 0.43 ± 0.15 ab | 0.42 ± 0.11 ab | 0.40 ± 0.09 ab | 0.36 ± 0.04 ab |
| Asp | 0.03 ± 0.03 d | 0.10 ± 0.07 bc | 0.08 ± 0.01 bc | 0.10 ± 0.02 bc | 0.11 ± 0.01 bc | 0.12 ± 0.03 ab |
| Glu | 0.43 ± 0.08 a | 0.36 ± 0.11 ab | 0.35 ± 0.03 ab | 0.32 ± 0.01 c | 0.29 ± 0.21 cd | 0.24 ± 0.07 cd |
| Sweet | 0.65 ± 0.10 a | 0.57 ± 0.05 ab | 0.60 ± 0.11 ab | 0.58 ± 0.08 ab | 0.56 ± 0.06 ab | 0.53 ± 0.20 ab |
| Thr | 0.02 ± 0.01 a | 0.01 ± 0.01 ab | 0.01 ± 0.01 ab | 0.01 ± 0.01 ab | 0.01 ± 0.01 ab | 0.01 ± 0.00 ab |
| Ser | 0.17 ± 0.02 a | 0.11 ± 0.01 b | 0.11 ± 0.01 b | 0.09 ± 0.01 bc | 0.07 ± 0.00 d | 0.06 ± 0.02 de |
| Gly | 0.12 ± 0.05 a | 0.09 ± 0.06 ab | 0.09 ± 0.04 ab | 0.08 ± 0.02 ab | 0.07 ± 0.03 ab | 0.06 ± 0.03 ab |
| Ala | 0.34 ± 0.09 bc | 0.36 ± 0.05 bc | 0.39 ± 0.05 ab | 0.40 ± 0.15 ab | 0.41 ± 0.04 ab | 0.41 ± 0.15 ab |
| Bitter | 1.31 ± 0.23 bc | 1.66 ± 0.24 bc | 1.63 ± 0.16 bc | 1.72 ± 0.41 ab | 1.78 ± 0.22 ab | 1.75 ± 0.31 ab |
| Val | 0.23 ± 0.02 ab | 0.25 ± 0.03 ab | 0.24 ± 0.03 ab | 0.25 ± 0.14 ab | 0.26 ± 0.02 ab | 0.24 ± 0.03 ab |
| Met | 0.01 ± 0.01 e | 0.05 ± 0.02 cd | 0.05 ± 0.01 cd | 0.06 ± 0.03 bc | 0.07 ± 0.01 bc | 0.08 ± 0.01 b |
| Ile | 0.19 ± 0.03 a | 0.17 ± 0.07 ab | 0.17 ± 0.09 ab | 0.16 ± 0.07 ab | 0.15 ± 0.07 ab | 0.14 ± 0.02 ab |
| Leu | 0.34 ± 0.08 d | 0.59 ± 0.11 bc | 0.58 ± 0.04 bc | 0.63 ± 0.09 ab | 0.68 ± 0.09 ab | 0.68 ± 0.02 ab |
| Tyr | 0.27 ± 0.01 bc | 0.28 ± 0.02 bc | 0.28 ± 0.08 bc | 0.29 ± 0.08 ab | 0.28 ± 0.03 bc | 0.28 ± 0.12 bc |
| Phe | 0.21 ± 0.09 bc | 0.24 ± 0.02 bc | 0.24 ± 0.02 bc | 0.25 ± 0.02 bc | 0.26 ± 0.11 ab | 0.26 ± 0.11 ab |
| His | 0.02 ± 0.01 ab | 0.03 ± 0.01 ab | 0.03 ± 0.02 ab | 0.04 ± 0.05 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a |
| Arg | 0.04 ± 0.03 ab | 0.05 ± 0.02 a | 0.04 ± 0.02 ab | 0.04 ± 0.01 ab | 0.04 ± 0.02 ab | 0.03 ± 0.01 ab |
| Odourless | 0.25 ± 0.12 a | 0.16 ± 0.08 ab | 0.16 ± 0.11 ab | 0.14 ± 0.12 ab | 0.12 ± 0.04 ab | 0.10 ± 0.05 ab |
| Cys | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Lys | 0.24 ± 0.02 a | 0.15 ± 0.04 b | 0.15 ± 0.06 b | 0.13 ± 0.07 bc | 0.11 ± 0.12 bc | 0.09 ± 0.04 bc |
| Essential Amino Acids | 1.24 ± 0.03 c | 1.46 ± 0.15 ab | 1.45 ± 0.15 ab | 1.49 ± 0.13 ab | 1.54 ± 0.23 ab | 1.50 ± 0.20 ab |
| Total Amino Acids | 2.67 ± 0.31 ab | 2.85 ± 0.35 ab | 2.82 ± 0.46 ab | 2.86 ± 0.38 ab | 2.86 ± 0.32 ab | 2.74 ± 0.37 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.; Wu, Y.; Wen, A.; Zeng, H.; Qin, L. Synergistic Flavor Modulation and Functional Enhancement of Douchiba via Compounding with Bacillus subtilis-Fermented Adlay. Foods 2025, 14, 2976. https://doi.org/10.3390/foods14172976
Peng L, Wu Y, Wen A, Zeng H, Qin L. Synergistic Flavor Modulation and Functional Enhancement of Douchiba via Compounding with Bacillus subtilis-Fermented Adlay. Foods. 2025; 14(17):2976. https://doi.org/10.3390/foods14172976
Chicago/Turabian StylePeng, Lian, Yongjun Wu, Anyan Wen, Haiying Zeng, and Likang Qin. 2025. "Synergistic Flavor Modulation and Functional Enhancement of Douchiba via Compounding with Bacillus subtilis-Fermented Adlay" Foods 14, no. 17: 2976. https://doi.org/10.3390/foods14172976
APA StylePeng, L., Wu, Y., Wen, A., Zeng, H., & Qin, L. (2025). Synergistic Flavor Modulation and Functional Enhancement of Douchiba via Compounding with Bacillus subtilis-Fermented Adlay. Foods, 14(17), 2976. https://doi.org/10.3390/foods14172976







